Introduction
Rift Valley fever (RVF) is a viral zoonosis that primarily affects farmed animals and humans, (WHO, 2004) . The disease is notifiable and predominant in East Africa (Martin et al., 2008). The WHO listed the disease as an emerging disease in 2015, indicating that it requires international attention. If not addressed, the disease outbreaks, which can sometimes be extremely severe, can have significant economic, health, and agricultural impacts (Anyamba et al., 2012). Proper prediction and analysis of the outbreaks is crucial for early preparedness and control by the government and public health agencies.
RVF modeling has previously relied on the traditional time series methods, which only rely on raw data to capture the time series components: secular trends, seasonal variations, and patterns, (Munyua et al., 2016). Time series analysis comprises methods for analyzing time series data to extract meaningful statistics and other characteristics of the data (Ochieng et al., 2016). The ability of time series models to capture temporal dependencies and extract meaningful insights from disease outbreaks has recently led to their use for analysis. However, these models often face challenges in handling complex data sets to explore non-linear relationships and integrate them (Lee dale et al., 2016).
Recently, the use of machine learning approaches to model, classify, and predict disease outbreaks in the fields of epidemiology and public health has been on the rise (Redding et al., 2017). This is because of their ability to handle large and complex datasets, unveil hidden patterns, and make reliable predictions using advanced algorithms (Kariuki Njenga & Bett, 2019). We have adopted these machine learning models as the most suitable for modeling the interactions between the climatic predictors that drive RVF outbreaks. This study is to conduct a systematic literature review comparing and contrasting the effectiveness of the use of time series and machine learning models in RVF modeling, prediction, and analysis from 1930 to 2024.
This study deals with to evaluate and integrate all of the models and compare their accuracy, speed, efficiency, and interpretability with close screening and analysis of existing studies on RVF. Furthermore, the review will provide an opportunity to combine both machine learning and time series models to understand RVF epidemiology. A thorough understanding of hybrid modeling approaches will be critical in developing robust techniques that can clearly inform the public and other stakeholders. Identifying literature gaps and current approaches will improve ways of managing and controlling future RVF shocks.
Methodology
Literature Search and Data Collection
To achieve a comprehensive and thorough search, a relevant list of search terms was developed. These search terms were tailored for five major scientific databases including; PubMed, JSTOR, PLOS ONE, SCOPUS, Google Scholar and Web of Science. Majorly, the terms used in the search focused on the studies on RVF, time series analyses and methodologies, machine learning models and techniques.
Databases and electronic search of Terms
The literature search was conducted in PubMed, PLOS ONE, JSTOR, Web of Science, Google Scholar and SCOPUS, covering publications from the last 90 years to maintain a manageable and relevant scope with the key words as highlighted in
Table 1. The search included general and specific terms such as “Rift Valley fever prediction”, “ time series prediction of RVF cases,” “time series prediction of diseases,” “machine learning classification of RVF diseases,” and “RVF outbreak and Climatic changes”.
Screening Process
In the review process, literature screening is critical. We used the COVIDENCE platform in our study. We conducted screening in two stages to ensure efficiency in the title, abstracts, and full text. Two impartial reviewers assessed the titles and abstracts during the first round, designating each entry as “include” or “exclude.” The reviewers resolved minor disagreements through dialogue until they reached a consensus.
Full-Text Review and PICOS
We reviewed the shortlisted papers again, concentrating on their full texts. During this phase, we manually uploaded other papers and used the COVIDENCE platform to automatically retrieve freely available full texts. The study used the PICOS framework, which stands for population, intervention, comparison, outcome, and study design. It provides a structured way to assess the literature and ensures that the resulting conclusions are based on comprehensive and well-defined criteria
This review included specific studies on RVF-affected animals and humans, as well as vector species associated with RVF transmission. In relation to the emergence, transmission, and dissemination of RVF, the intervention element took into account the impact of climate change and the strategies used to adapt to it. Due to the nature of the research, the study did not consider comparisons, as its primary goal was to understand the overall effects of climate change on RVF outbreaks, rather than to compare individual interventions. The incidence, geographic distribution, and transmission patterns of RVF, as well as the efficacy of any attempts at climate adaptation, were of interest.
The analysis concluded with the inclusion criteria, which included original research articles, review articles, theses, conference paper proceedings, and research policy papers published between 2004 and 2024. It is important to note that the study period encompassed several RVF outbreaks, leading to a wealth of information and publications. The study excluded resources such as editorials, opinion views, and new articles from studies that were not in English. This comprehensive PICOS approach ensured that our review remained focused and relevant, capturing the most pertinent and relevant studies related to RVF and climate change. The final extracted data included various categories, as detailed in
Table 2.
Data Extraction and Categorization
We sorted the extracted data into multiple important categories, including categorization tasks, publisher/journal, study objectives, article type, and application domain. We meticulously documented the input data, output labels, data sources, population size, exclusion criteria, and tested algorithms. There were three types of classification tasks: window-based classification, sequence-to-sequence (point wise) classification, and whole-series classification.
Evaluation Metrics
The performance of the algorithm was evaluated using common measures. The F1-score indicated the harmonic mean of precision and recall, whereas accuracy quantified the percentage of properly predicted RVF cases. The model’s ability to discriminate between RVF and non-RVF cases was assessed using the AUC-ROC. True positive and true negative rates were defined by sensitivity and specificity, respectively, while inter-rate reliability was evaluated using Cohen’s Kappa. The probabilities of true positive and true negative outcomes were represented by PPV and NPV, respectively, and the frequency of inaccurate positive predictions was evaluated by the false positive rate.
Results
Overview of Screening Process
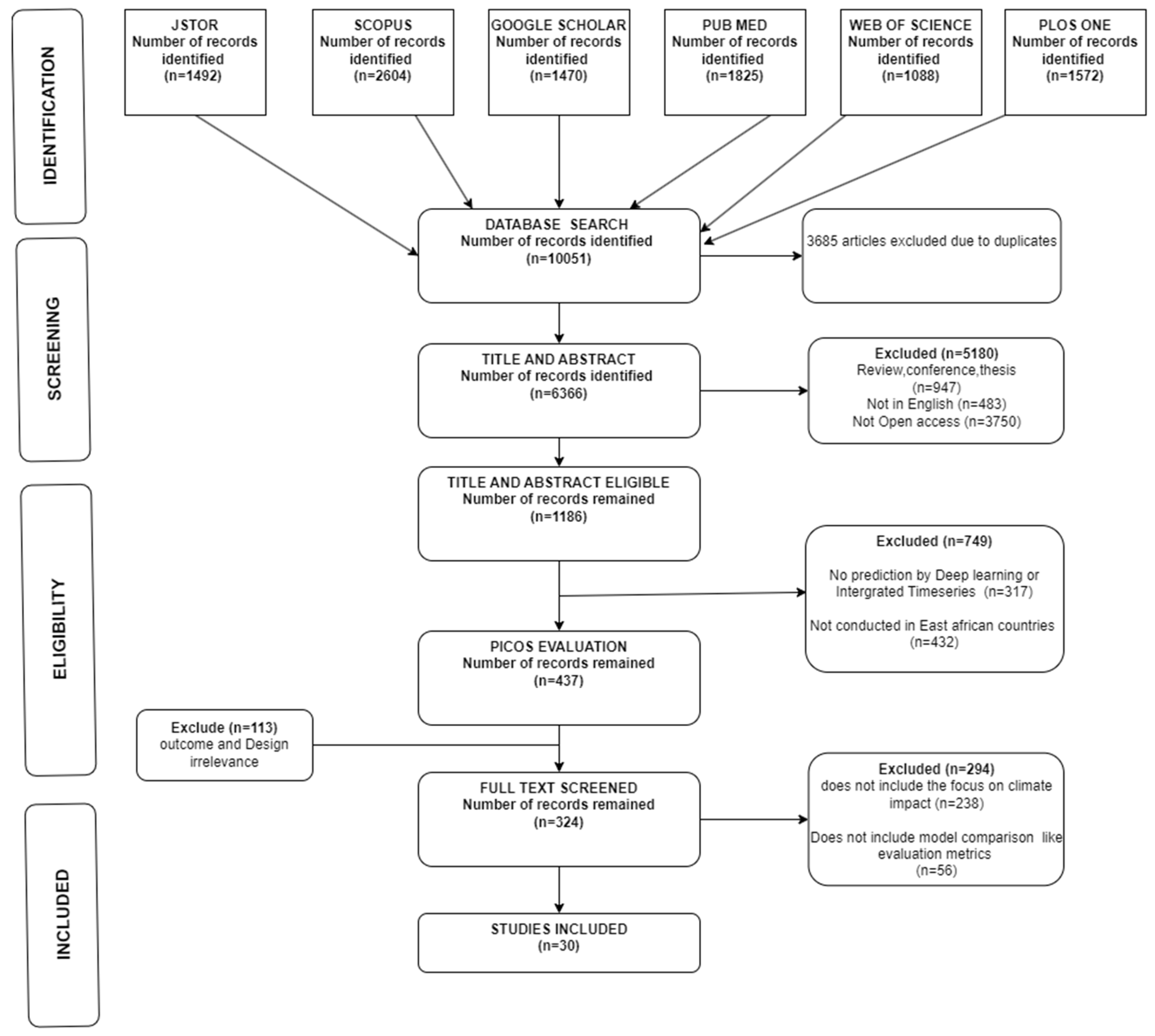
The Prisma Chart on
Figure 1 shows the overall steps taken for the systematic review of Rift Valley fever (RVF) studies by illustrating the meticulous process undertaken to identify, screen, and select relevant articles for inclusion. Initially, a comprehensive search was conducted across multiple databases, including JSTOR, SCOPUS, Google Scholar, PubMed, Web of Science, and PLOS ONE, yielding a total of 10,015 records. During the identification phase, duplicate records (3,685) were removed, leaving 6,366 unique records for further screening. This initial phase ensured that all potential studies were accounted for, covering a broad spectrum of literature pertinent to RVF and climate impacts.
In the screening phase, the titles and abstracts of the remaining 6,366 records were evaluated against the predefined inclusion and exclusion criteria. A significant number of articles (5,180) were excluded due to being review articles, conference papers, theses, or not available in English, and not being open access. This rigorous screening process narrowed the pool to 1,186 eligible records. The focus then shifted to a more detailed evaluation using the PICOS framework, which further refined the selection by excluding studies that did not meet specific criteria related to population, intervention, outcomes, and study design. Studies that did not utilize deep learning or integrated time series predictions (317) or were not conducted in East African countries (432) were excluded, resulting in 437 records for detailed full-text screening.
The eligibility phase involved a thorough review of the full texts of the 437 remaining articles. During this phase, studies were excluded if their outcomes and design were deemed irrelevant, resulting in 113 exclusions. Of the 324 records that progressed to the final eligibility check, an additional 294 were excluded because they did not focus on climate impact or did not include model comparison evaluation metrics. Ultimately, 30 studies were included in the review, representing a focused selection of research that addresses the intersection of climate change and RVF. This PRISMA flow chart underscores the importance of a systematic and transparent approach in conducting literature reviews, ensuring that the final selection of studies is both comprehensive and relevant to the research question at hand.
Publication Year
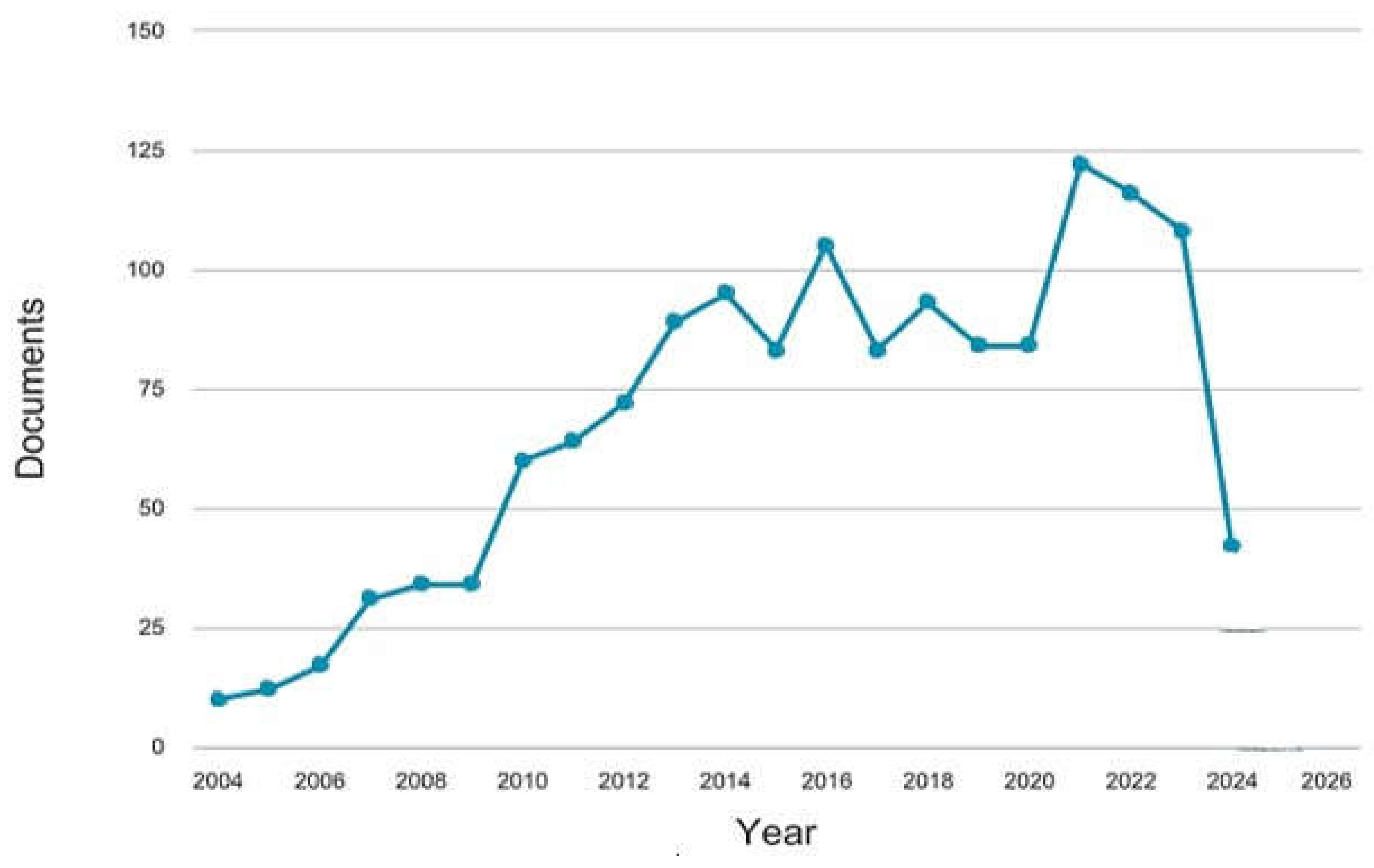
The chart on
Figure 2 detailing documents by year from 2004 to 2026 provides an insightful look into the trends of research publication over time. Between 2004 and 2010, the number of documents remained relatively low but showed a gradual increase. This period likely represents the foundational years of research, with initial studies being conducted to understand the basics of Rift Valley fever (RVF) and its impacts. The growth during these years could be attributed to the rising awareness of the disease and its implications, leading to more focused research efforts.
From 2010 to 2016, there is a marked increase in publications, peaking around 2016. This surge can be linked to several factors, including heightened global health initiatives, increased funding for infectious disease research, and the advent of new technologies and methodologies in studying RVF. The peak in 2016 might reflect the culmination of extensive research projects, international collaborations, and significant outbreaks that prompted deeper investigations. The years following 2016 show a somewhat fluctuating trend, with the number of documents stabilizing yet experiencing periodic peaks and troughs. These variations could be influenced by the cyclical nature of research funding, shifting focus towards other emerging health threats, and the publication cycles of major research projects. Despite these fluctuations, the overall trend remains high, indicating sustained interest and continuous advancements in RVF research. A notable spike is observed around 2021-2022, which could be attributed to increased global attention on zoonotic diseases due to the COVID-19 pandemic. This period likely saw a surge in studies exploring the connections between climate change, vector-borne diseases, and public health, contributing to a significant number of publications.
However, the sharp decline in 2024 and projected into 2026 raises questions. This decrease might be a result of a temporary shift in research priorities, completion of major research initiatives, or delays in publication processes. It could also reflect a natural ebb following a peak period of intense research activity. Overall, the chart illustrates a dynamic and evolving field of study, driven by global health needs, scientific advancements, and environmental changes. The data underscores the importance of continuous support for research in RVF to address its ongoing and emerging challenges, ensuring that knowledge and strategies keep pace with the disease’s development and impact.
Publication across Countries
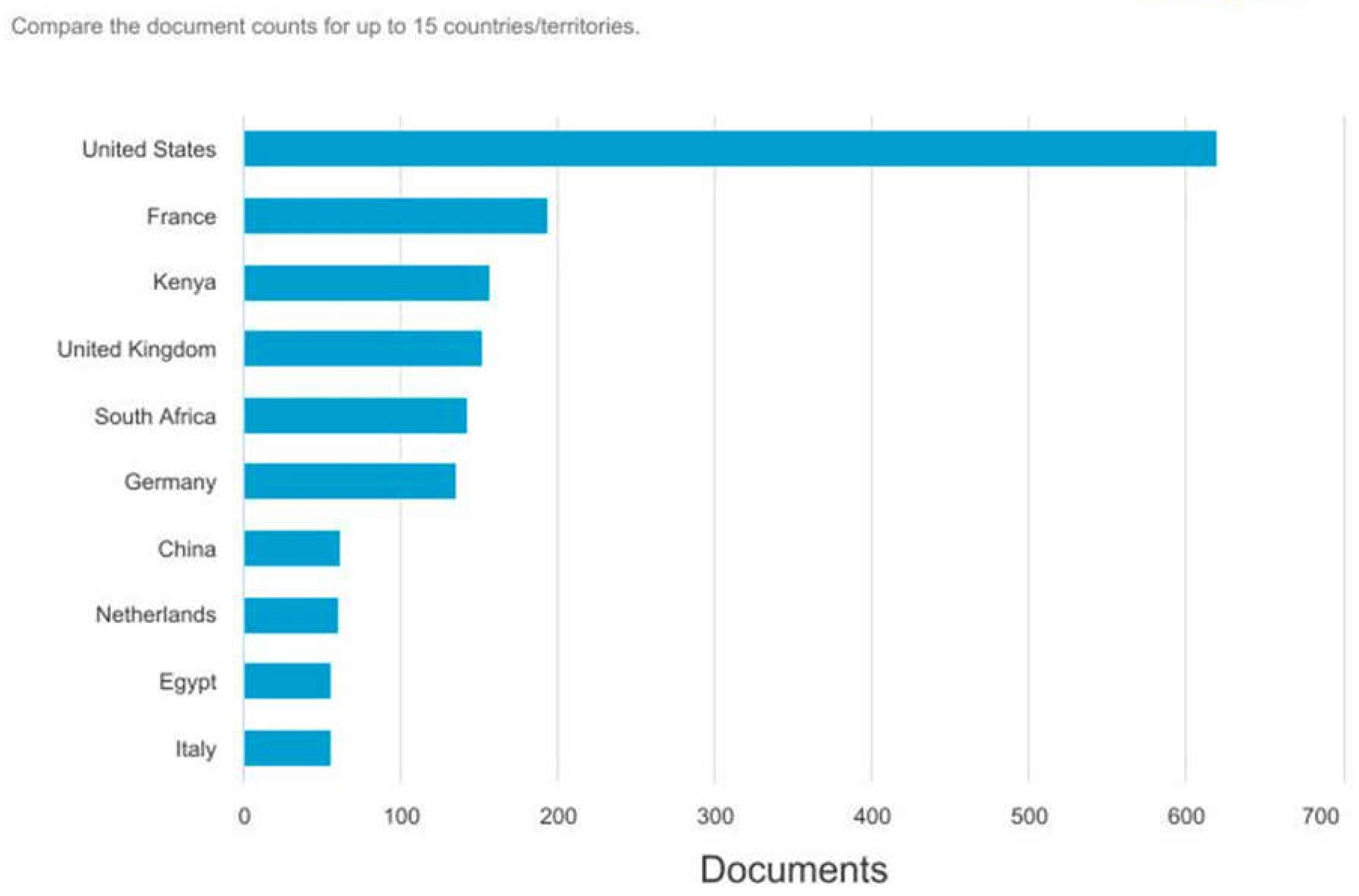
The chart in
Figure 3 depicts the number of documents by country, revealing significant geographical trends in RVF-related research. The United States leads overwhelmingly, with approximately 600 documents demonstrating its robust contribution to this field. The US’s substantial research funding and resources, along with its advanced technological infrastructure, enable extensive scientific investigations and publications, contributing to its dominance (Mearns & Norton, 2010).
France is the second-highest contributor, with approximately 250 documents. This is indicative of France’s active role in global health research, particularly in diseases that have implications for public health and agriculture. Kenya’s position, almost equal to France’s, highlights the country’s critical role in RVF research. Given that RVF is endemic to East Africa, Kenyan institutions are on the frontlines of studying and managing this disease, contributing valuable insights and data directly from affected regions.(Godoy et al., 2014).
The Rift Valley is part of the East African Rift System, which extends from the Afar Triangle in Ethiopia down through Kenya and into Tanzania. While the Rift Valley also passes through other East African countries like Ethiopia, Tanzania, and Uganda, Kenya’s segment is the most internationally recognized due to its distinct geological features, archaeological significance, and its economic and cultural importance. These countries’ involvement underscores the global recognition of RVF as a critical issue that transcends regional boundaries, necessitating a concerted international research effort.
This data highlights the diverse geographical spread of RVF research, reflecting both the disease’s impact and the global scientific community’s commitment to understanding and combating it. The concentration of research in specific countries emphasizes the importance of international collaboration to effectively address the challenges posed by RVF. This also emphasizes the need for continued support and funding for research in both high-contributing countries and regions directly affected by RVF, ensuring a comprehensive approach to studying and mitigating this significant health threat (A et al., 2022).
Discussion
This discussion integrates findings from a comprehensive systematic review of studies on RVF and explores the role of climate, machine learning, and time series methodologies in understanding and controlling the disease.
Climate and RVF Epidemiology
Climate plays a critical role in RVF epidemiology. The World Health Organization (2004) emphasizes the importance of using climatic data to predict infectious disease outbreaks. Climate variables such as rainfall, temperature, and humidity directly influence mosquito vector breeding and survival, which are crucial for RVF transmission. Martin et al. (2008) further elaborate on how climate change impacts RVF epidemiology and control, particularly through its effects on the vectors’ lifecycle and distribution.
Anyamba et al. (2009) utilized remote sensing data to predict RVF outbreaks, demonstrating the feasibility of integrating climatic data into predictive models. This study revealed that specific climatic conditions, such as increased rainfall and subsequent flooding, create ideal breeding conditions for mosquitoes, leading to heightened RVF transmission. The integration of remote sensing and climate data has significantly improved the ability to forecast RVF outbreaks, providing vital lead times for public health interventions.
Machine Learning in RVF Prediction
Machine learning has emerged as a powerful tool in epidemiological modeling and disease prediction. We have employed machine learning algorithms in the context of RVF to analyze large datasets, identify risk factors, and predict outbreaks. For example, Mulwa et al. (2024) applied XGBoost, a gradient-boosting algorithm, to predict RVF outbreaks in Kenya using climatic factors. The model demonstrated high accuracy in forecasting outbreaks, highlighting the potential of machine learning in enhancing predictive capabilities. Pedro et al. (2016) utilized a stochastic host-vector model to predict RVF inter-epidemic activities. This study integrated various climatic variables and host-vector dynamics, providing a comprehensive understanding of RVF transmission.
The stochastic model’s ability to incorporate randomness and variability in climatic conditions makes it particularly suited for predicting RVF outbreaks, which are influenced by complex and dynamic environmental factors (Park et al., 2021). Chemison et al. (2024) demonstrated the effectiveness of a dynamic climate-sensitive disease model in reproducing historical RVF outbreaks across Africa. This study highlights the importance of using climate-sensitive models to capture the intricate relationship between climatic conditions and RVF transmission. These models’ ability to simulate historical outbreaks gives us confidence in their predictive capabilities and usefulness in guiding public health interventions (Chemison et al., 2024).
Time Series Analysis in RVF Research
Time series analysis is a statistical method used to analyse temporal data and identify patterns and trends over time. In RVF research, time series analysis has been instrumental in understanding the cyclical nature of outbreaks and developing predictive models. Anyamba et al. (2010) used time series models to analyse historical RVF data, identifying periodic patterns that precede outbreaks. This approach has been essential in developing early warning systems for RVF. Mpeshe et al. (2014) modelled the impact of climate change on the dynamics of RVF using time series analysis. This study demonstrated how changes in climatic conditions over time affect the risk of RVF outbreaks.
By incorporating climate change projections into time series models, researchers can predict future outbreak patterns and inform long-term public health strategies. The combination of time series analysis and machine learning has further enhanced the predictive capabilities of RVF models (Anyamba et al., 2012). Munyua et al. (2016) used time series data and machine learning techniques to map RVF risk areas in Kenya. The study integrated various data sources, including climatic, geographical, and epidemiological data, to develop a comprehensive risk map. This approach provides valuable insights for targeting surveillance and control measures in high-risk areas (Redding et al., 2017).
Case Studies and Model Validation
Several case studies illustrate the practical application of predictive models in managing RVF outbreaks. For example, Anyamba et al. (2012) used remote sensing data to predict RVF outbreaks in East and Southern Africa from 2006 to 2008. The study’s predictions were validated by subsequent outbreaks, demonstrating the model’s accuracy and reliability. This validation is crucial for gaining confidence in predictive models and their use in public health decision-making (Glancey et al., 2015). Munyua et al. (2016) developed a dynamic risk model for RVF outbreaks in Kenya based on climate and disease outbreak data.
The model was validated using historical outbreak data, showing high predictive accuracy. This case study highlights the importance of validating predictive models using real-world data to ensure their effectiveness in guiding public health interventions. The work by Mulwa et al. (2024) on predictive modelling of RVF outbreaks using XGBoost also underscores the need for model validation. The study used historical outbreak data to train and test the model, demonstrating its ability to accurately predict future outbreaks. The validation process is critical for identifying model limitations and refining predictive algorithms (Gikungu et al., 2016).
Challenges and Limitations
Despite the advancements in predictive modelling for RVF, several challenges and limitations remain. One major challenge is the variability in data quality and availability. Accurate and comprehensive data are essential for developing reliable predictive models. However, data gaps and inconsistencies can affect model accuracy and reliability. Efforts should be made to improve data collection and integration, particularly in resource-limited settings where RVF is prevalent.
Another challenge is the complexity of RVF transmission dynamics. RVF involves multiple hosts and vectors, each influenced by various environmental and biological factors. Modelling these complex interactions requires sophisticated algorithms and extensive data. Simplified models may not capture the full complexity of RVF transmission, leading to inaccurate predictions. Therefore, researchers should strive to develop more comprehensive models that incorporate a wide range of factors.
The study by Martin et al. (2008) highlights the impact of climate change on RVF control strategies. Climate change introduces additional uncertainty and variability, making it challenging to predict future outbreak patterns. Predictive models must account for these uncertainties and provide flexible strategies that can adapt to changing climatic conditions. This requires ongoing research and model refinement to keep pace with evolving climate patterns
Implications of the Study
The integration of climate data, machine learning, and time series analysis into RVF research has significant implications for public health. Predictive models provide valuable tools for early warning systems, enabling timely and targeted interventions to prevent and control RVF outbreaks. By identifying high-risk areas and periods, public health authorities can allocate resources more efficiently and implement proactive measures to mitigate the impact of RVF.
For instance, the predictive models developed by Anyamba et al. (2009) and Munyua et al. (2016) have been used to guide vector control strategies and livestock vaccination campaigns in high-risk areas. These interventions have been instrumental in reducing the incidence and spread of RVF, demonstrating the practical benefits of predictive modelling in public health.
Moreover, the use of machine learning and time series analysis in RVF research provides a framework for addressing other vector-borne diseases. The methodologies and findings from RVF studies can be applied to similar diseases, enhancing the overall capacity for disease prediction and control. This cross-disciplinary approach fosters collaboration between climatologists, epidemiologists, and data scientists, leading to more integrated and effective public health strategies.
Summary
The integration of machine learning and time series analysis into RVF research has significantly advanced the field of disease prediction and control. By leveraging large datasets from climatic, ecological, and geographical sources, these models provide accurate and timely predictions of RVF outbreaks. The studies reviewed demonstrate that ML models, particularly when combined with remote sensing and time series analysis, offer powerful tools for understanding and mitigating the impacts of RVF. Machine learning models like logistic regression, XGBoost, and stochastic models have proven effective in predicting the distribution of RVF vectors and outbreak patterns.
Time series analysis techniques, such as ARIMA models, have further enhanced the ability to forecast RVF outbreaks based on historical trends. These advancements are particularly crucial in the context of climate change, which significantly influences the epidemiology of RVF. The application of remote sensing data in ML models has enabled continuous monitoring and early warning systems, which are essential for timely interventions. Enhanced surveillance during high-risk periods, as demonstrated by Oyas et al. (2018), and the use of ecological niche modelling to map RVF risk areas, as shown by Kiunga (2015) and Mosomtai et al. (2016), highlight the practical implications of these technologies.
Conclusion
In conclusion, the studies reviewed underscore the importance of integrating machine learning and time series analysis into RVF research. These approaches provide valuable insights into the temporal and spatial dynamics of RVF outbreaks, aiding in the development of effective control strategies. As climate change continues to impact the epidemiology of RVF, the role of advanced predictive modelling will become increasingly vital in safeguarding public health. The ongoing research and technological advancements in this field promise to enhance our understanding and management of RVF, ultimately reducing the disease’s impact on affected populations. The systematic review and meta-analysis of RVF research underscore the critical role of climatic variables, machine learning, and time series analysis in understanding and predicting RVF outbreaks. The integration of these methodologies has provided significant advancements in predictive modelling, enabling more accurate and timely predictions of RVF outbreaks. However, challenges such as data variability and transmission complexity remain, necessitating ongoing research and model refinement.
The practical applications of predictive models in public health interventions highlight their value in preventing and controlling RVF. By providing early warning systems and identifying high-risk areas, these models enable targeted and proactive measures, reducing the disease’s impact on public health and agriculture. Future research should continue to explore the potential of advanced machine learning techniques and real-time data integration. Developing comprehensive, multi-disciplinary models will enhance our understanding of RVF transmission and improve our ability to predict and control outbreaks. Through collaborative efforts and technological advancements, we can mitigate the impact of RVF and protect public health.
Future Directions
The future of RVF research lies in the continued integration of advanced technologies and methodologies. Emerging machine learning techniques, such as deep learning and neural networks, offer new possibilities for predictive modelling. These techniques can handle large and complex datasets, providing more accurate and robust predictions. Future research should explore the application of these advanced algorithms to RVF prediction and control.
Additionally, the use of real-time data and internet of things (IoT) devices can enhance data collection and monitoring. IoT devices, such as weather stations and mosquito traps, can provide real-time data on climatic conditions and vector activity. Integrating this real-time data into predictive models can improve their accuracy and responsiveness, enabling more timely interventions.
The study by Pedro et al. (2016) on stochastic host-vector models highlights the potential of integrating various data sources and methodologies. Future research should focus on developing multi-disciplinary models that incorporate climatic, geographical, biological, and social factors. These comprehensive models can provide a more holistic understanding of RVF transmission and inform more effective control strategies.
Data Availability Statement
Acknowledgments
The authors extend their appreciation to their universities for supporting their research work.
Conflicts of Interest
The authors declare no conflicts of interest.
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Anyamba, A., Chretien, J.-P., Small, J., Tucker, C. J., Formenty, P. B., Richardson, J. H., Britch, S. C., Schnabel, D. C., Erickson, R. L., & Linthicum, K. J. (2009). Prediction of a Rift Valley fever outbreak. Proceedings of the National Academy of Sciences, 106(3), 955–959. [CrossRef]
- Anyamba, A., Linthicum, K. J., Small, J., Britch, S. C., Pak, E., de La Rocque, S., Formenty, P., Hightower, A. W., Breiman, R. F., & Chretien, J.-P. (2010). Prediction, assessment of the Rift Valley fever activity in East and Southern Africa 2006–2008 and possible vector control strategies. The American Journal of Tropical Medicine and Hygiene, 83(2 Suppl), 43. [CrossRef]
- Anyamba, A., Linthicum, K. J., Small, J., Britch, S. C., & Tucker, C. J. (2012). Remote sensing contributions to prediction and risk assessment of natural disasters caused by large-scale Rift Valley fever outbreaks. Proceedings of the IEEE, 100(10), 2824–2834. [CrossRef]
- Anyamba, A., Linthicum, K. J., & Tucker, C. J. (2001). Climate-disease connections: Rift Valley fever in Kenya. Cadernos de Saude Publica, 17, S133–S140. [CrossRef]
- Baba, M., Masiga, D. K., Sang, R., & Villinger, J. (2016). Has Rift Valley fever virus evolved with increasing severity in human populations in East Africa? Emerging Microbes & Infections, 5(1), 1–10. [CrossRef]
- Bett, B., Lindahl, J., & Delia, G. (2019). Climate change and infectious livestock diseases: the case of rift valley fever and tick-borne diseases. The Climate-Smart Agriculture Papers: Investigating the Business of a Productive, Resilient and Low Emission Future, 29–37. [CrossRef]
- Chemison, A., Ramstein, G., Jones, A., Morse, A., & Caminade, C. (2024). Ability of a dynamical climate sensitive disease model to reproduce historical Rift Valley Fever outbreaks over Africa. Scientific Reports, 14(1), 3904. [CrossRef]
- Gikungu, D., Wakhungu, J., Siamba, D., Neyole, E., Muita, R., & Bett, B. (2016). Dynamic risk model for Rift Valley fever outbreaks in Kenya based on climate and disease outbreak data. Geospatial Health, 11(2). [CrossRef]
- Githeko, A. K. (2021). Health related vulnerabilities and enabling institutions to facilitate responses to climate change in East Africa. East Africa Science, 3(1), 1–18. [CrossRef]
- Hassan, A. A., & Jin, S. (2014). Lake level change and total water discharge in East Africa Rift Valley from satellite-based observations. Global and Planetary Change, 117, 79–90. [CrossRef]
- Hightower, A., Kinkade, C., Nguku, P. M., Anyangu, A., Mutonga, D., Omolo, J., Njenga, M. K., Feikin, D. R., Schnabel, D., & Ombok, M. (2012). Relationship of climate, geography, and geology to the incidence of Rift Valley fever in Kenya during the 2006–2007 outbreak. The American Journal of Tropical Medicine and Hygiene, 86(2), 373. [CrossRef]
- Himeidan, Y. E., Kweka, E. J., Mahgoub, M. M., El Rayah, E. A., & Ouma, J. O. (2014). Recent outbreaks of rift valley fever in East Africa and the Middle East. Frontiers in Public Health, 2, 169. [CrossRef]
- Leedale, J., Jones, A. E., Caminade, C., & Morse, A. P. (2016). A dynamic, climate-driven model of Rift Valley fever. Geospatial Health, 11(s1). [CrossRef]
- Linthicum, K. J., Anyamba, A., Tucker, C. J., Kelley, P. W., Myers, M. F., & Peters, C. J. (1999). Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science, 285(5426), 397–400. [CrossRef]
- Martin, V., Chevalier, V., Ceccato, P., Anyamba, A., De Simone, L., Lubroth, J., De La Rocque, S., & Domenech, J. (2008). The impact of climate change on the epidemiology and control of Rift Valley fever. [CrossRef]
- Mosomtai, G., Evander, M., Sandström, P., Ahlm, C., Sang, R., Hassan, O. A., Affognon, H., & Landmann, T. (2016). Association of ecological factors with Rift Valley fever occurrence and mapping of risk zones in Kenya. International Journal of Infectious Diseases, 46, 49–55. [CrossRef]
- Mpeshe, S. C., Luboobi, L. S., & Nkansah-Gyekye, Y. (2014). Modeling the impact of climate change on the dynamics of Rift Valley fever. Computational and Mathematical Methods in Medicine, 2014(1), 627586. [CrossRef]
- Mulwa, D., Kazuzuru, B., Misinzo, G., & Bett, B. (2024). An XGBoost Approach to Predictive Modelling of Rift Valley Fever Outbreaks in Kenya Using Climatic Factors. [CrossRef]
- Munyua, P. M., Murithi, R. M., Ithondeka, P., Hightower, A., Thumbi, S. M., Anyangu, S. A., Kiplimo, J., Bett, B., Vrieling, A., & Breiman, R. F. (2016). Predictive factors and risk mapping for Rift Valley fever epidemics in Kenya. PLoS One, 11(1), e0144570. [CrossRef]
- Mweya, C. N., Kimera, S. I., Kija, J. B., & Mboera, L. E. G. (2013). Predicting distribution of Aedes aegypti and Culex pipiens complex, potential vectors of Rift Valley fever virus in relation to disease epidemics in East Africa. Infection Ecology & Epidemiology, 3(1), 21748. [CrossRef]
- Nanyingi, M. O., Munyua, P., Kiama, S. G., Muchemi, G. M., Thumbi, S. M., Bitek, A. O., Bett, B., Muriithi, R. M., & Njenga, M. K. (2015). A systematic review of Rift Valley Fever epidemiology 1931–2014. Infection Ecology & Epidemiology, 5(1), 28024. [CrossRef]
- Nguku, P. M., Sharif, S. K., Mutonga, D., Amwayi, S., Omolo, J., Mohammed, O., Farnon, E. C., Gould, L. H., Lederman, E., & Rao, C. (2010). An investigation of a major outbreak of Rift Valley fever in Kenya: 2006–2007. The American Journal of Tropical Medicine and Hygiene, 83(2 Suppl), 05. [CrossRef]
- Ochieng, A. O., Nanyingi, M., Kipruto, E., Ondiba, I. M., Amimo, F. A., Oludhe, C., Olago, D. O., Nyamongo, I. K., & Estambale, B. B. A. (2016). Ecological niche modelling of Rift Valley fever virus vectors in Baringo, Kenya. Infection Ecology & Epidemiology, 6(1), 32322. [CrossRef]
- Organization, W. H. (2004). Using climate to predict infectious disease outbreaks: A review.
- Oyas, H., Holmstrom, L., Kemunto, N. P., Muturi, M., Mwatondo, A., Osoro, E., Bitek, A., Bett, B., Githinji, J. W., & Thumbi, S. M. (2018). Enhanced surveillance for Rift Valley Fever in livestock during El Niño rains and threat of RVF outbreak, Kenya, 2015-2016. PLoS Neglected Tropical Diseases, 12(4), e0006353. [CrossRef]
- Pedro, S. A., Abelman, S., & Tonnang, H. E. Z. (2016). Predicting Rift Valley fever inter-epidemic activities and outbreak patterns: insights from a stochastic host-vector model. PLoS Neglected Tropical Diseases, 10(12), e0005167. [CrossRef]
- Redding, D. W., Tiedt, S., Lo Iacono, G., Bett, B., & Jones, K. E. (2017). Spatial, seasonal and climatic predictive models of Rift Valley fever disease across Africa. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1725), 20160165. [CrossRef]
- Taylor, D., Hagenlocher, M., Jones, A. E., Kienberger, S., Leedale, J., & Morse, A. P. (2016). Environmental change and Rift Valley fever in eastern Africa: projecting beyond HEALTHY FUTURES. Geospatial Health, 11(s1). [CrossRef]
- Vilaly, A. E. El, Arora, M., Butterworth, M. K., Vilaly, M. A. salam M. El, Jarnagin, W., & Comrie, A. C. (2013). Climate, environment and disease: The case of Rift Valley fever. Progress in Physical Geography, 37(2), 259–269. [CrossRef]
- A, O. C., O, A. G., C, O. O., I, O. S., J, O. I., & C, A. C. (2022). ORIGIN, STRUCTURE AND ASSOCIATED MINERAL RESOURCES OF EAST AFRICAN RIFT SYSTEM: AN OVERVIEW World Journal of Engineering Research and Technology WJERT. 8(1), 1–23. www.wjert.org.
- Anyamba, A. Anyamba, A., Linthicum, K. J., Small, J., Britch, S. C., & Tucker, C. J. (2012). Remote sensing contributions to prediction and risk assessment of natural disasters caused by large-scale rift valley fever outbreaks. Proceedings of the IEEE, 100(10), 2824–2834. [CrossRef]
- Chemison, A., Ramstein, G., Jones, A., Morse, A., & Caminade, C. (2024). Ability of a dynamical climate sensitive disease model to reproduce historical Rift Valley Fever outbreaks over Africa. Scientific Reports, 14(1), 1–10. [CrossRef]
- Gikungu, D., Wakhungu, J., Siamba, D., Neyole, E., Muita, R., & Bett, B. (2016). Dynamic risk model for Rift Valley fever outbreaks in Kenya based on climate and disease outbreak data. Geospatial Health, 11(2), 95–103. [CrossRef]
- Glancey, M. M., Anyamba, A., & Linthicum, K. J. (2015). Epidemiologic and Environmental Risk Factors of Rift Valley Fever in Southern Africa from 2008 to 2011. Vector-Borne and Zoonotic Diseases, 15(8), 502–511. [CrossRef]
- Godoy, D., Dewbre, J., PIN, Amegnaglo, C. J., Soglo, Y. Y., Akpa, A. F., Bickel, M., Sanyang, S., Ly, S., Kuiseu, J., Ama, S., Gautier, B. P., Officer, E. S., Officer, E. S., Eberlin, R., Officer, P., Branch, P. A., Oduro-ofori, E., Aboagye Anokye, P., … Swanson, B. E. (2014). The future of food and agriculture: trends and challenges. In The future of food and agriculture: trends and challenges (Vol. 4, Issue 4).
- Kenmoe, S., Abanda, N. N., Ebogo-belobo, J. T., Bowo-ngandji, A., Mbaga, D. S., Magoudjou-pekam, J. N., Kame-ngasse, G. I., Tchatchouang, S., Zeuko, E., Okobalemba, E. A., Noura, E. A., Meta-djomsi, D., Kenfack-momo, R., Mbacham, W. F., Njouom, R., Maïdadi-foudi, M., Kengne-nde, C., Kenfack-zanguim, J., Esemu, S. N., … Sadeuh-mba, S. A. (2023). Contemporary epidemiological data of Rift Valley fever virus in humans, mosquitoes and other animal species in Africa : A systematic review and meta-analysis. 2309–2328. [CrossRef]
- Mearns, R., & Norton, A. (2010). Social Dimensions of Climate Change: Equity and Vulnerablity in a Warming World. In Social Dimensions of Climate Change: Equity and Vulnerability in a Warming World. [CrossRef]
- Park, D. J., Park, M. W., Lee, H., Kim, Y. J., Kim, Y., & Park, Y. H. (2021). Development of machine learning model for diagnostic disease prediction based on laboratory tests. Scientific Reports, 11(1), 1–11. [CrossRef]
- Redding, D. W., Tiedt, S., Lo Iacono, G., Bett, B., & Jones, K. E. (2017). Spatial, seasonal and climatic predictive models of rift valley fever disease across Africa. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1725), 1–9. [CrossRef]
- A, O. C., O, A. G., C, O. O., I, O. S., J, O. I., & C, A. C. (2022). ORIGIN, STRUCTURE AND ASSOCIATED MINERAL RESOURCES OF EAST AFRICAN RIFT SYSTEM: AN OVERVIEW World Journal of Engineering Research and Technology WJERT. 8(1), 1–23. www.wjert.org.
- Anyamba, A., Linthicum, K. J., Small, J., Britch, S. C., & Tucker, C. J. (2012). Remote sensing contributions to prediction and risk assessment of natural disasters caused by large-scale rift valley fever outbreaks. Proceedings of the IEEE, 100(10), 2824–2834. [CrossRef]
- Chemison, A., Ramstein, G., Jones, A., Morse, A., & Caminade, C. (2024). Ability of a dynamical climate sensitive disease model to reproduce historical Rift Valley Fever outbreaks over Africa. Scientific Reports, 14(1), 1–10. [CrossRef]
- Gikungu, D., Wakhungu, J., Siamba, D., Neyole, E., Muita, R., & Bett, B. (2016). Dynamic risk model for Rift Valley fever outbreaks in Kenya based on climate and disease outbreak data. Geospatial Health, 11(2), 95–103. [CrossRef]
- Glancey, M. M., Anyamba, A., & Linthicum, K. J. (2015). Epidemiologic and Environmental Risk Factors of Rift Valley Fever in Southern Africa from 2008 to 2011. Vector-Borne and Zoonotic Diseases, 15(8), 502–511. [CrossRef]
- Godoy, D., Dewbre, J., PIN, Amegnaglo, C. J., Soglo, Y. Y., Akpa, A. F., Bickel, M., Sanyang, S., Ly, S., Kuiseu, J., Ama, S., Gautier, B. P., Officer, E. S., Officer, E. S., Eberlin, R., Officer, P., Branch, P. A., Oduro-ofori, E., Aboagye Anokye, P., … Swanson, B. E. (2014). The future of food and agriculture: trends and challenges. In The future of food and agriculture: trends and challenges (Vol. 4, Issue 4). www.fao.org/publications%0Ahttp://www.fao.org/3/a-i6583e.pdf%0Ahttp://siteresources.worldbank.org/INTARD/825826-1111044795683/20424536/Ag_ed_Africa.pdf%0Awww.fao.org/cfs%0Ahttp://www.jstor.org/stable/4356839%0Ahttps://ediss.uni-goettingen.de/bitstream/han.
- Kenmoe, S., Abanda, N. N., Ebogo-belobo, J. T., Bowo-ngandji, A., Mbaga, D. S., Magoudjou-pekam, J. N., Kame-ngasse, G. I., Tchatchouang, S., Zeuko, E., Okobalemba, E. A., Noura, E. A., Meta-djomsi, D., Kenfack-momo, R., Mbacham, W. F., Njouom, R., Maïdadi-foudi, M., Kengne-nde, C., Kenfack-zanguim, J., Esemu, S. N., … Sadeuh-mba, S. A. (2023). Contemporary epidemiological data of Rift Valley fever virus in humans, mosquitoes and other animal species in Africa : A systematic review and meta-analysis. 2309–2328. [CrossRef]
- Mearns, R., & Norton, A. (2010). Social Dimensions of Climate Change: Equity and Vulnerablity in a Warming World. In Social Dimensions of Climate Change: Equity and Vulnerability in a Warming World. [CrossRef]
- Park, D. J., Park, M. W., Lee, H., Kim, Y. J., Kim, Y., & Park, Y. H. (2021). Development of machine learning model for diagnostic disease prediction based on laboratory tests. Scientific Reports, 11(1), 1–11. [CrossRef]
- Redding, D. W., Tiedt, S., Lo Iacono, G., Bett, B., & Jones, K. E. (2017). Spatial, seasonal and climatic predictive models of rift valley fever disease across Africa. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1725), 1–9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).