Submitted:
31 August 2024
Posted:
02 September 2024
Read the latest preprint version here
Abstract
Keywords:
Introduction
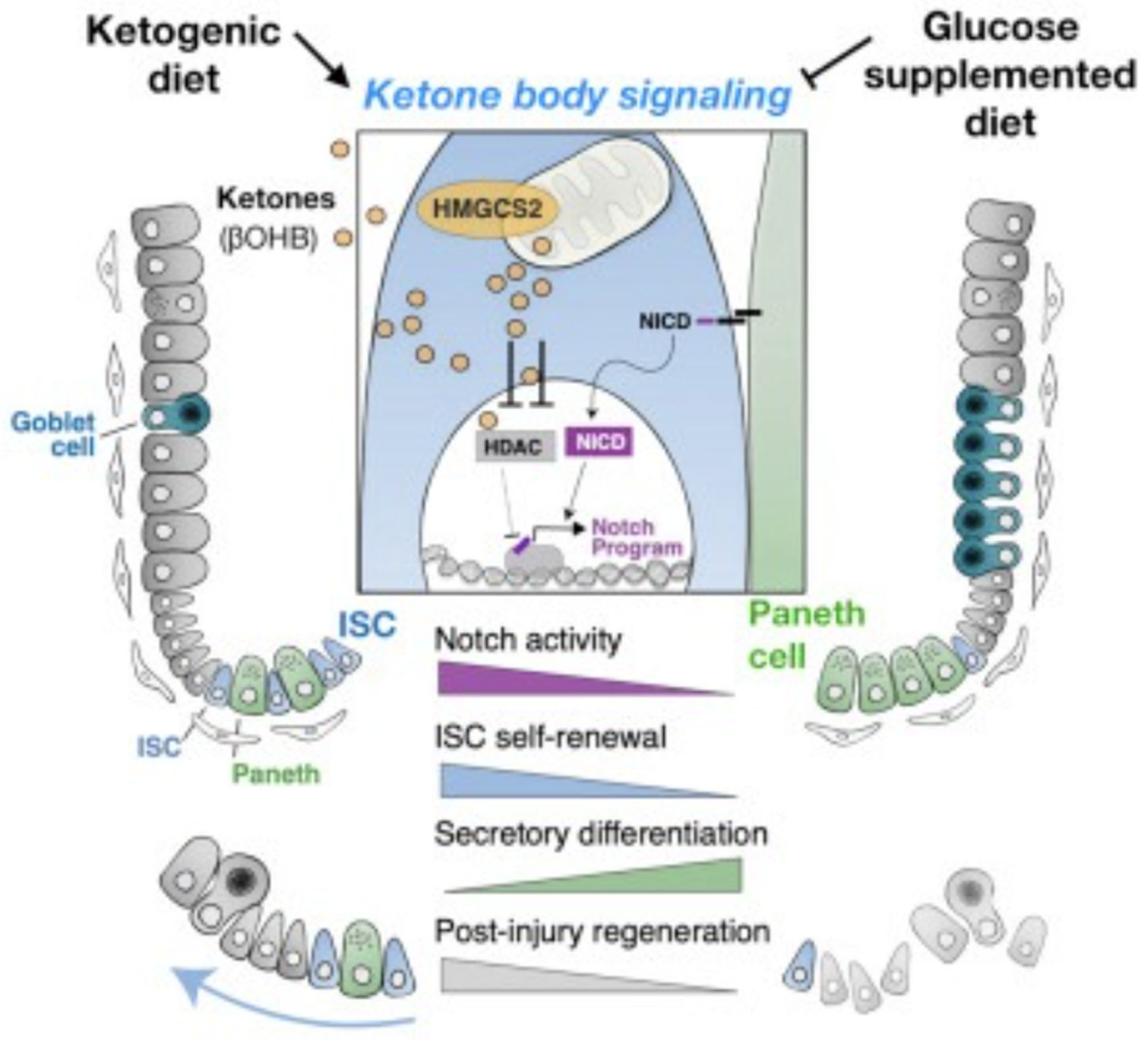
Carnivore Diet as a Regenerative Immunotherapy
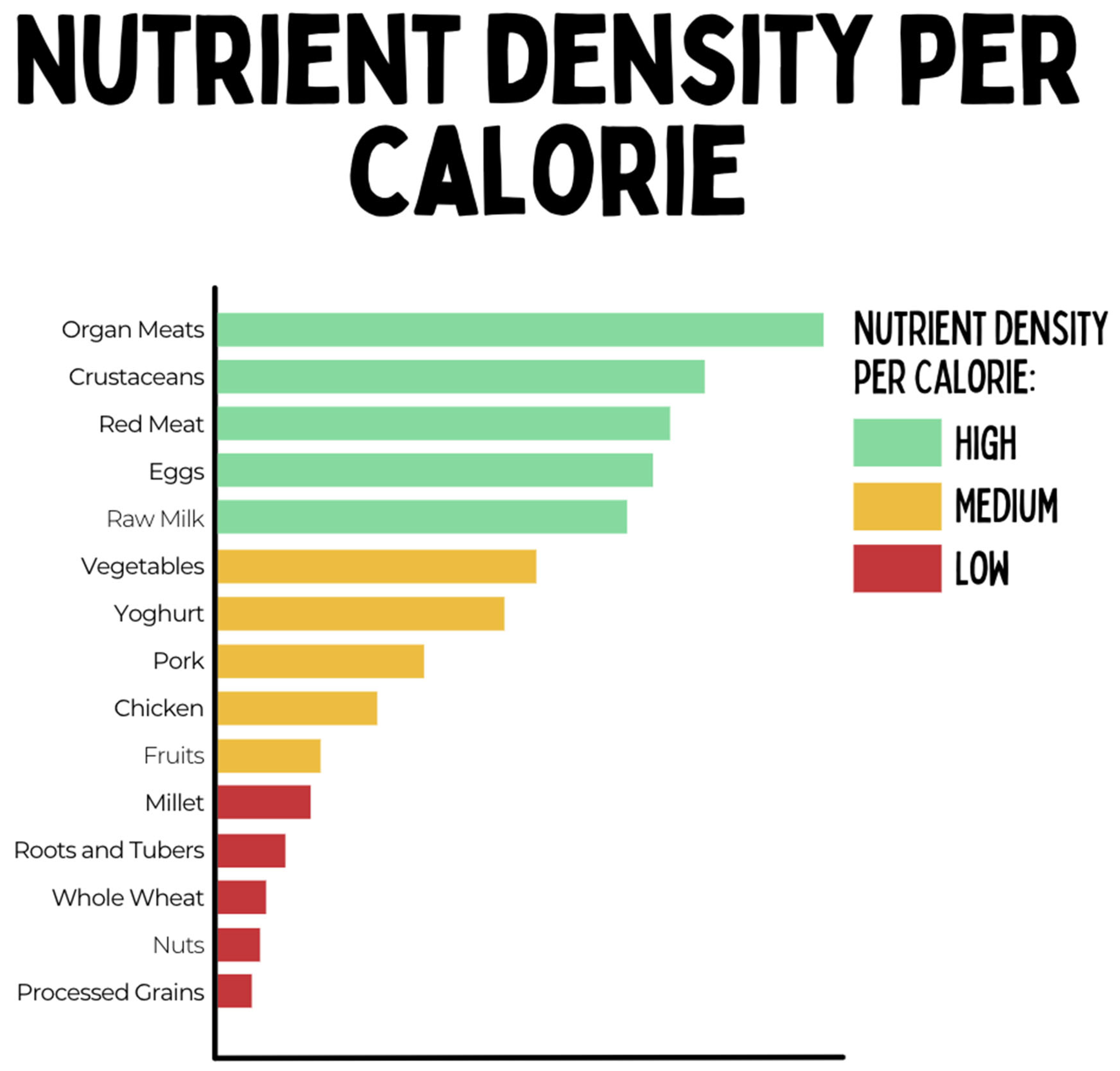
Hypothesized Mechanisms of the Carnivore Diet
- Decreased Plant Toxins: Plants contain various toxins. Lectins, solanines, and saponins are associated with autoimmunity and inflammation (Kuang et al., 2023, Konijeti et al.,2017, and Iablokov et al., 2010).
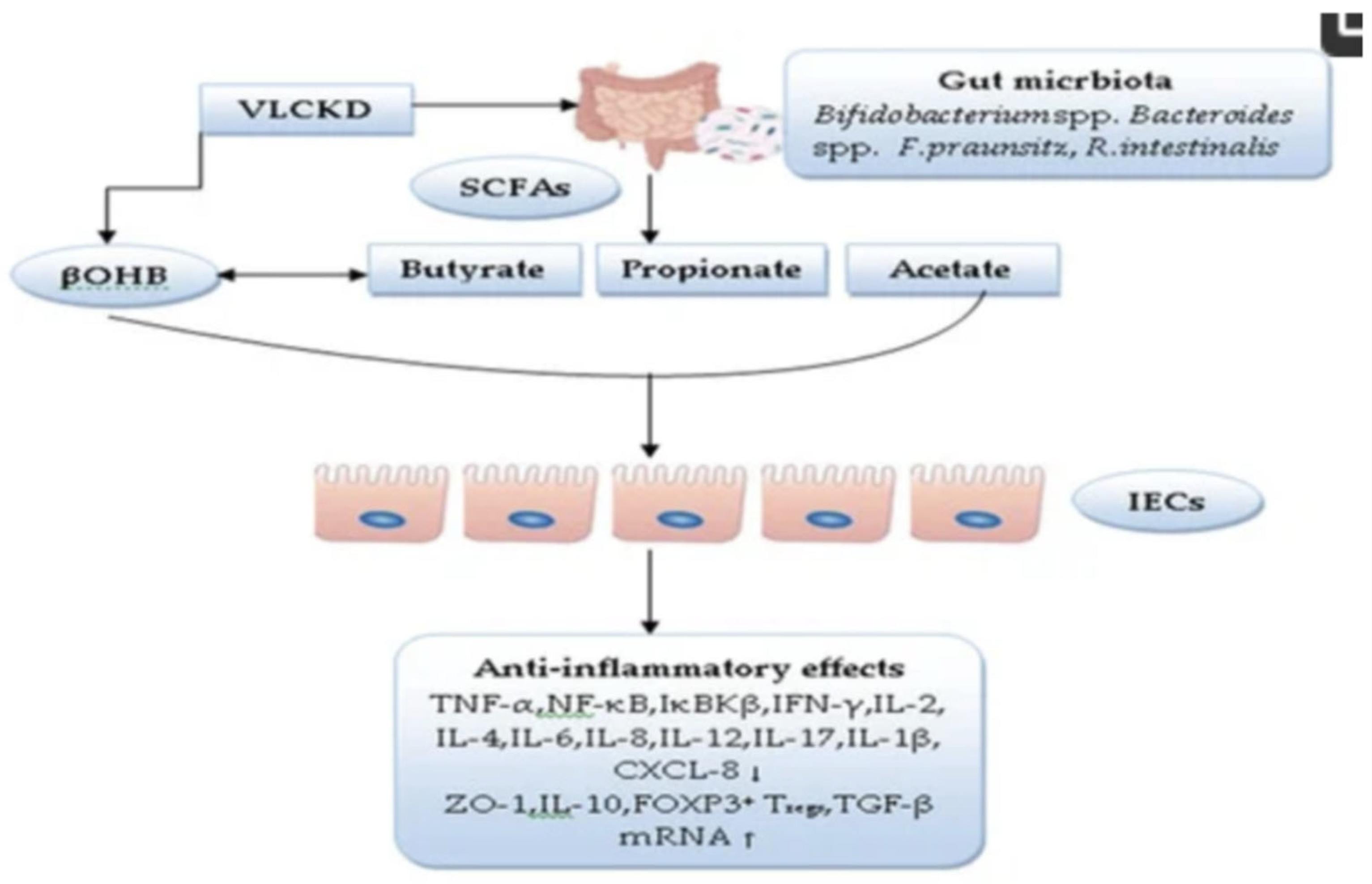
- Direct SCFA Supply: With the direct provision of SCFAs and the growth stimulation of SCFA-producing bacteria, the carnivore diet may bypass pre-existing dysbiosis, which is highly prevalent in the IBD population. (Alsharairi et al., 2021, Parada Venegas et al., 2019 and Kaur et al. 2011).
- 3.
- Reduced Omega-6 (Linoleic Acid) consumption: Linoleic Acid may directly induce inflammation in the intestinal epithelium via formation of oxidative linoleic acid metabolites (OXLAMs) and consequent dysregulation of the Endocannabinoid System (Deol et al., 2023). Carnivore diets more closely resemble the pre-modern consumption of <2g/day linoleic acid vs. the modern consumption of 29g/day (Mercola et al., 2023).
- 4.
- Higher Micronutrient Density: Animal Foods are more dense in most micronutrients (vitamins and minerals) compared with plant foods and lack anti-nutrients such as phytates (O'Hearn, 2020, Beal et al., 2022), which may improve immune regulation and regenerative capacity of intestinal epithelial cells.
- 5.
- Reduced Dietary Fiber: Soluble fiber inhibits activity of pancreatic enzymes and protein sequestration while insoluble fiber increases bloating and tension possibly contributing to intestinal pathologies (Tan et al., 2007, Tan et al., 2012).
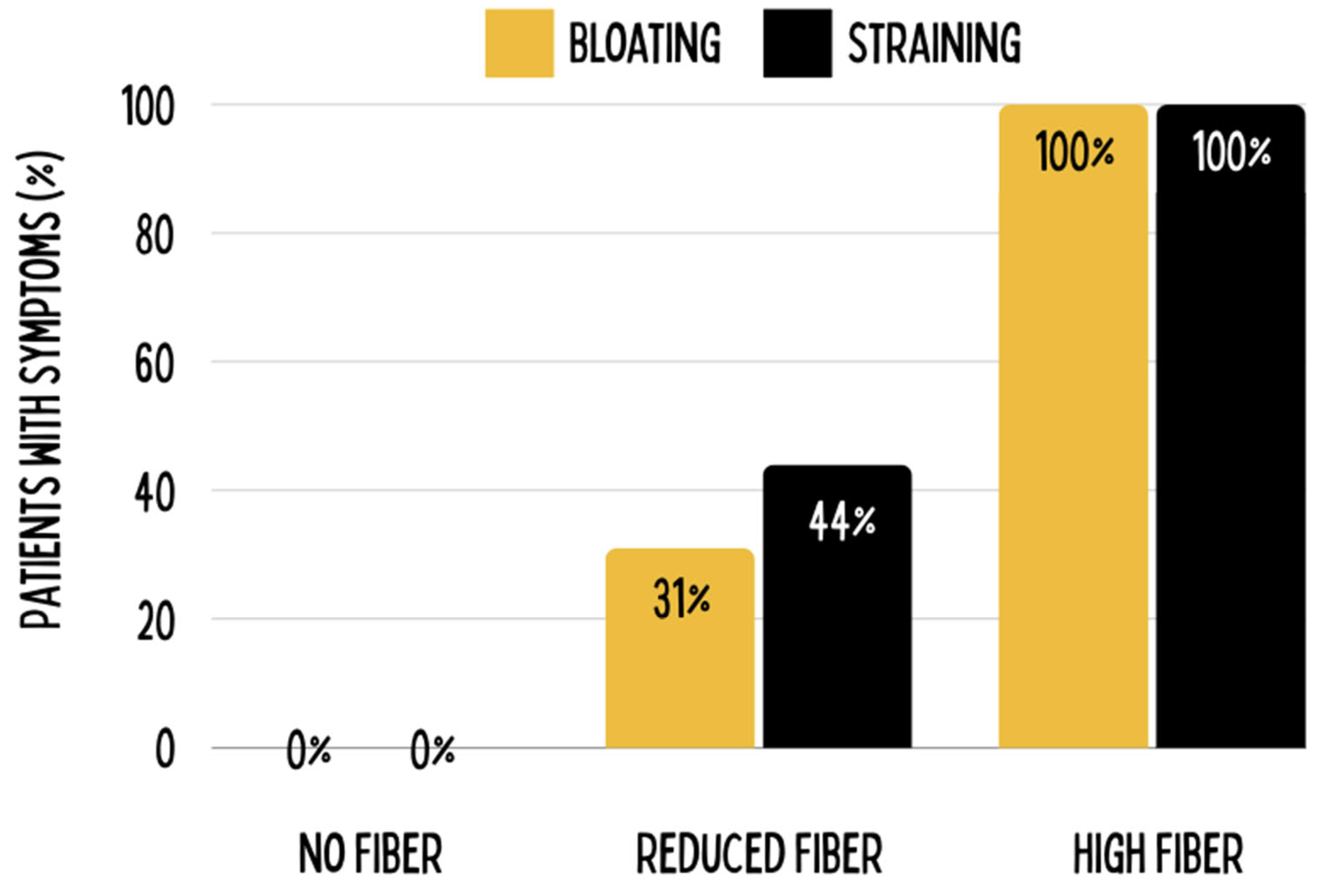
Possible Concerns
- Gout Risks: The supersaturation of uric acid can -under the wrong circumstances- lead to the deposition of monosodium urate monohydrate crystals in the tissues, with resultant gout arthritis. Gout can be manifested by the elevation of serum urate, acute gouty arthritic attacks, the formation of tophi, gouty nephropathy, and uric acid stones. Meat itself has not been established as a causative agent, but the high amount of purine within it can serve as a triggering factor in causing episodes of gout arthritis in a pre-existing metabolic dysregulation. Our own clinical experience shows that a ketogenic/carnivore diet can even alleviate gout medium term. Hypothetically, this could be due to reduced oxidative stress since uric acid acts as an antioxidant, reduced availability of dietary monosodium (glutamate), or perhaps increased exercise in our patient population since muscle activity induces myokine secretion, hence helping in the conversion of uric acid to allantoin for excretion through the kidneys (Roman 2023). Indeed, recent reviews have confirmed our observation of reduced uric acid in very low carbohydrate ketogenic diets (Gohari et al., 2023).
- Carcinogenicity: The World Healths Organizations (WHO) International Agency for the Research of Cancer (IARC) has classified Processed Meat as carcinogenic (Class I), and unprocessed Red Meat as possibly carcinogenic (Class IIa). No causal relationships have been established and no causal agents in red meat have been identified to date. By the classification standards of IARC, the classification is to be based on an associative relationship and does not establish the magnitude of risk. Recent systematic reviews have argued that evidence even for the proposed associative relationship between unprocessed red meat and negative health outcomes (including cancer) is lacking, and recommendations for reduced consumption of unprocessed red meat are not backed by scientific data (Lescinsky et al., 2022 and Bradley et al., 2019).
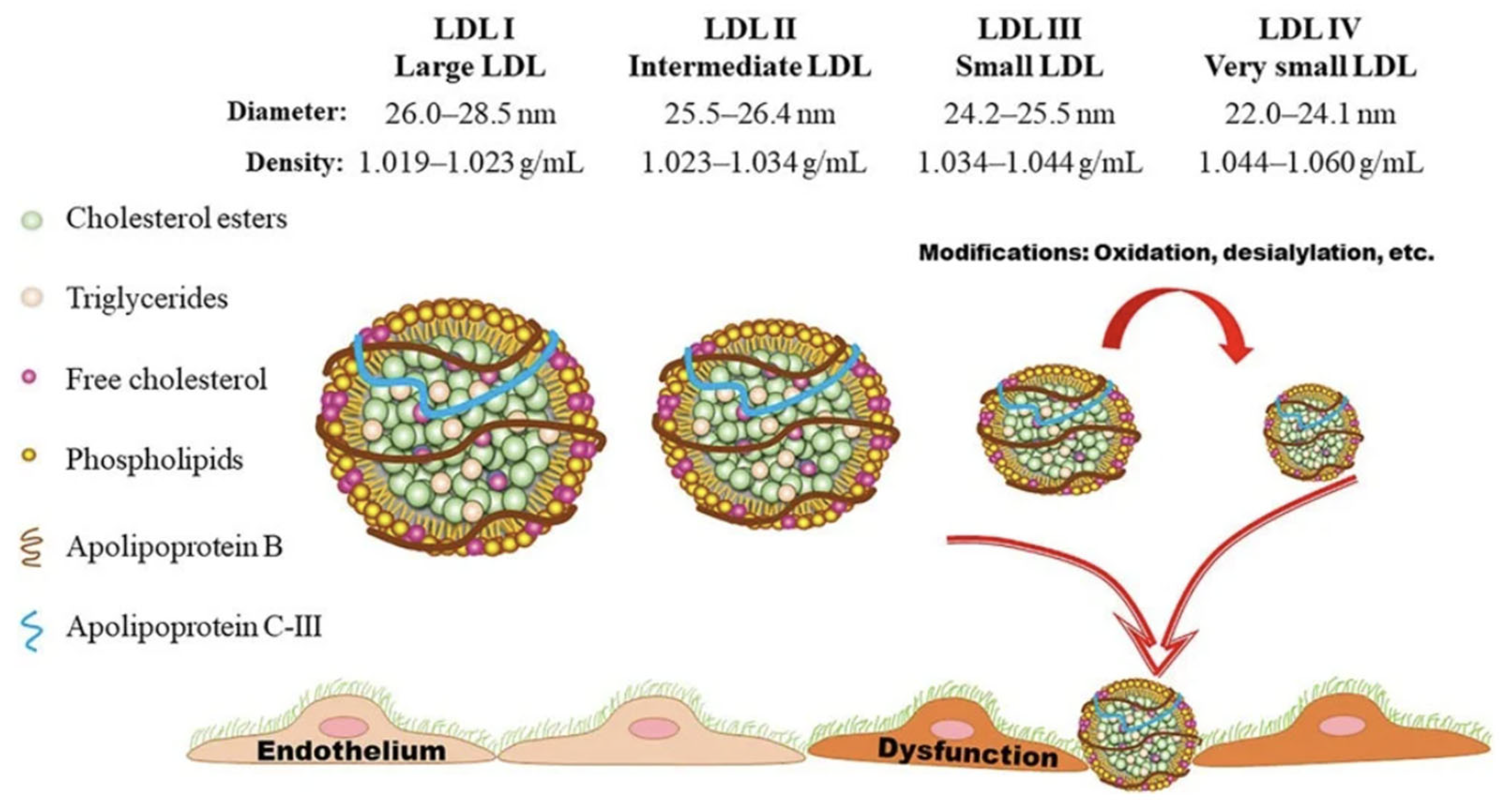
- Dyslipidemia and Cardiovascular Risks: For a given population on a standard diet, increased serum total LDL Lipoprotein molecular mass (measured in mg/dl) has been considered causal in the progression of atherosclerosis (Libby 2021). On ketogenic diets, total serum LDL lipoprotein mass can and most likely will increase; however, the size of the LDL particle becomes larger, thereby reducing the number of atherogenic particles (Westman et al. 2006, Falkenhain et al., 2021, and Froyen 2021, and Qiao et al., 2022). A reduction in the number of the atherogenic small and dense LDL lipoproteins and concurrent increase in the lipoprotein size is associated with improved cardiovascular risk markers such as reduced BMI, body weight, inflammatory markers, sdLDL, Triglycerides, Lipoprotein A, Apolipoprotein B, Blood Glucose, HbA1c, Insulin, and Blood Pressure, and increased HDL. During ketogenic diets LDL Lipoproteins serve other functions as in standard diets and are not to be interpreted as signs of metabolic dysfunction (Norwitz et al., 2022). Therefore, increased serum LDL on a ketogenic diet has to be evaluated differently than increased serum LDL on a standard diet and statin therapy is usually not warranted in a low-carbohydrate ketogenic diet (Diamond et al., 2022)
Testing the Hypothesis – Study Design and Methodology
Study Objectives
|
Alkaline Phosphatase Bilirubin (Serum) Calcium (Serum) Chloride (Serum) Cholesterol (Serum) HDL (Serum) LDL (Serum) CK (Serum) CK-MB (Serum) Iron (Serum) Protein Electrophoresis (Serum) Total Protein (Serum) GOT (Serum) GPT (Serum) Uric Acid (Serum) Urea (Serum) HbA1 (EDTA) Potassium (Serum) Creatinine (Serum) LDH (Serum) Sodium (Serum) Inorganic Phosphate (Serum) Transferrin (Serum) Triglycerides (Serum) |
Full Blood Count (EDTA) Reticulocytes (EDTA) Quick/INR (Citrate) PTT (Citrate) Thrombin Time (Citrate) Indirect Bilirubin (Serum) Minerals 11+4 (Heparin) hsCRP (Serum) TNF-alpha (Serum) Ferritin (Serum) Vitamin B1 bioactive (Serum) Vitamin B2 bioactive (Serum) Vitamin B6 bioactive (Serum) Vitamin B9 bioactive (EDTA) Vitamin B12 bioactive (Serum) 25-OH-Vitamin D (Serum) Amino Acids Metabolism (EDTA Plasma) Amino Acids Neuro (EDTA Plasma) Lactate/Pyruvate (Fluoride 3x) Nitrotyrosine (Serum) Carnitine (Serum) Fatty Acids of Erythrocyte Membrane (EDTA) |
Lipoprotein (a) (Serum) TSH Basal (Serum) Apo-Lipoprotein B (Serum) Homocysteine (Serum centrifuged) Cortisol Awake Response (Saliva) Molecular Genetic Profile Microbiota (Stool) SCFA (Short-Chain Fatty Acids) (Serum) SCFA (Short-Chain Fatty Acids) (Stool Pancreatic Elastase (Stool) Bile Acids (Stool) Alpha-1-Antitrypsin (Stool) Zonulin (Stool) Calprotectin (Stool) MDA-LDL (Serum) AGE (Serum) IL-6 (Serum) BDNF (Serum) Lipopolysaccharide Binding Protein (LBP) (Serum centrifuged) IFABP (Serum) |
Study Design
Participant Criteria
- Diagnosis of IBD as per Montreal classification
- Aged 18-70 years.
- Pregnant or intending to become pregnant within the next 3 months.
- Currently abusing substances.
- On ketogenic or carnivore diet in last 6 months.
- Currently Vegan or vegetarian diet and unwilling to switch to carnivore diet.
- Hospitalization during the last 3 months.
- Participation in another research project.
- Inability to fill out the initial questionnaires.
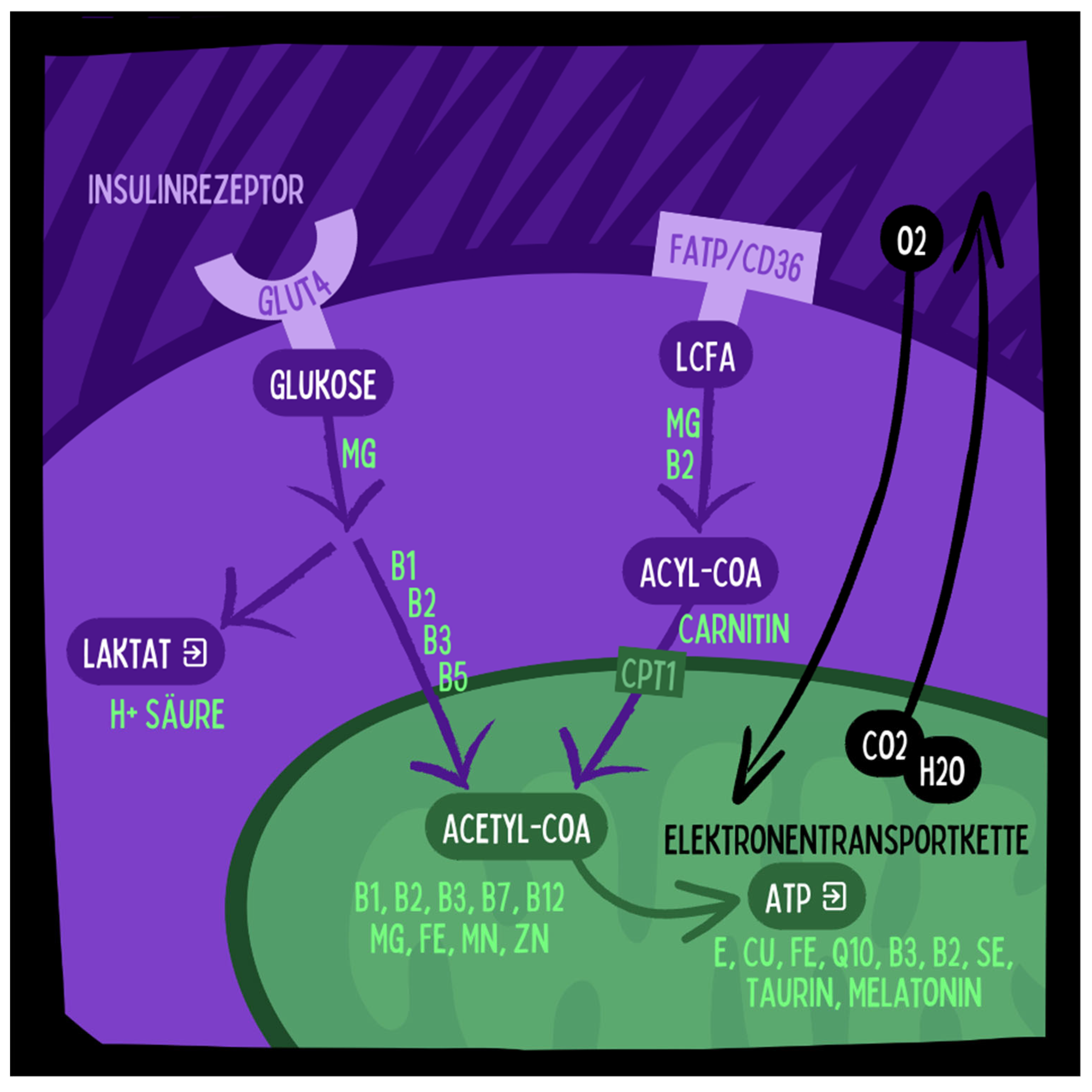
- Active liver, kidney, or cardiovascular diseases, kidney stones, severe hyperlipidemia.
- Glycogen storage disease type 1 (von Gierke disease).
- Carnitine palmitoyltransferase deficiencies (CPT I/II).
- Primary carnitine deficiency.
- Carnitine-acylcarnitine translocase deficiency.
- Pyruvate carboxylase deficiency.
- Succinyl-CoA acetoacetate transferase deficiency.
- Various fatty acid oxidation disorders.
- Acute intermittent porphyria.
Implementation and Follow-Up
Participant Training
- Nutritional science behind the ketogenic and carnivore diets.
- Appropriate foods and sample recipes.
- Targeted ketone and glucose levels.
- Food measurement-grams.
- Preparation for diet initiation across environments.
- Overcoming obstacles-quality, procurement, and preparation.
- Dining out, traveling, and illness guidelines.
- Medication guidelines.
- Prevention/management of potential side effects; for example hypoglycemia or hyperketosis.
- Why diets may fail.
- Modifications for illness-more water, no concern for ketone level.
- Fitting the diet into larger ecological, spiritual, and economic contexts.
Ketone Monitoring
Discussion
- for gout risk, check uric acid levels on a regular basis.
- for cancer risk, perform long-term monitoring of biomarkers for cancer.
- for cardiovascular risk, evaluate lipid profiles including Apolipoprotein B as a measure of particle number, blood pressure, visceral body fat and markers of systemic inflammation such as hsCRP.
References
- Alsharairi, N.A. (2021). The Role of Short-Chain Fatty Acids in Mediating Very Low-Calorie Ketogenic Diet-Infant Gut Microbiota Relationships and Its Therapeutic Potential in Obesity. Nutrients, 13(11), 3702. [CrossRef] [PubMed]
- Andersen, O.E., Poulsen, J.V., Farup, J. & de Morree, A. (2023). Regulation of adult stem cell function by ketone bodies. Frontiers in Cell and Developmental Biology, 11, 1246998. [CrossRef] [PubMed]
- Beal, T. & Ortenzi, F. (2022). Priority Micronutrient Density in Foods. Frontiers in Nutrition, 9: 806566. [CrossRef]
- Ben-Dor, M., Sirtoli, R. & Barkai, R. (2021). The evolution of the human trophic level during the Pleistocene. Yearbook of Physical Anthropology, 175(Suppl. 72), 27–56. [CrossRef]
- Bradley C. Johnston, Dena Zeraatkar, Mi Ah Han, et al. Unprocessed Red Meat and Processed Meat Consumption: Dietary Guideline Recommendations From the Nutritional Recommendations (NutriRECS) Consortium. Ann Intern Med.2019;171:756-764. [Epub 1 October 2019]. [CrossRef]
- Bohnen, J.L.B., Albin, R.L. & Bohnen, N.I. (2023). Ketogenic interventions in mild cognitive impairment, Alzheimer's disease, and Parkinson's disease: A systematic review and critical appraisal. Frontiers in Neurology. Volume 14. [CrossRef]
- Cheng, C.-W., Biton, M., Haber, A.L., Gunduz, N., Eng, G., Gaynor, L.T., Tripathi, S., Calibasi-Kocal, G., Rickelt, S., Butty, V.L., Moreno-Serrano, M., Iqbal, A.M., Bauer-Rowe, K.E., Imada, S., Ulutas, M.S., Mylonas, C., Whary, M.T., Levine, S.S., Basbinar, Y., Hynes, R.O., Mino-Kenudson, M., Deshpande, V., Boyer, L.A., Fox, J.G., Terranova, C., Rai, K., Piwnica-Worms, H., Mihaylova, M.M., Regev, A. & Yilmaz, Ö.H. (2019). Ketone Body Signaling Mediates Intestinal Stem Cell Homeostasis and Adaptation to Diet. Cell, 178(5), 1115-1131.e15. [CrossRef]
- Diamond DM, Bikman BT, Mason P. Statin therapy is not warranted for a person with high LDL-cholesterol on a low-carbohydrate diet. Curr Opin Endocrinol Diabetes Obes. 2022 Oct 1;29(5):497-511. [CrossRef] [PubMed]
- Gohari, S., Ghobadi, S., Jafari, A. et al. The effect of dietary approaches to stop hypertension and ketogenic diets intervention on serum uric acid concentration: a systematic review and meta-analysis of randomized controlled trials. Sci Rep 13, 10492 (2023). [CrossRef]
- Kaja Falkenhain, Lauren A Roach, Sara McCreary, Eric McArthur, Ethan J Weiss, Monique E Francois, Jonathan P Little, Effect of carbohydrate-restricted dietary interventions on LDL particle size and number in adults in the context of weight loss or weight maintenance: a systematic review and meta-analysis, The American Journal of Clinical Nutrition, Volume 114, Issue 4, 2021, Pages 1455-1466, ISSN 0002-9165. [CrossRef]
- Deol, P.; et al. (2023). Diet high in linoleic acid dysregulates the intestinal endocannabinoid system and increases susceptibility to colitis in Mice. Gut Microbes, 15(1), 2229945. [CrossRef]
- Gauree, G., Konijeti, G., Kim, N., Lewis, J.D., Groven, S., Chandrasekaran, A., Grandhe, S., Diamant, C., Singh, E., Oliveira, G., Wang, X., Molparia, B., Torkamani, A. (2017). Efficacy of the Autoimmune Protocol Diet for Inflammatory Bowel Disease. Inflammatory Bowel Diseases, 23(11), 2054–2060. [CrossRef]
- Iablokov, V., Sydora, B.C., Foshaug, R. et al. (2010). Naturally Occurring Glycoalkaloids in Potatoes Aggravate Intestinal Inflammation in Two Mouse Models of Inflammatory Bowel Disease. Digestive Diseases and Sciences, 55, 3078–3085. [CrossRef]
- Kaur, N., Chen, C.C., Luther, J. & Kao, J.Y. (2011). Intestinal dysbiosis in inflammatory bowel disease. Gut Microbes, 2(4), 211–216. [CrossRef]
- Kossoff, E.H., Turner, Z., Cervenka, M. & Barron, B. (2021). Ketogenic Diet Therapies for Epilepsy and Other Conditions. New York: Demos Health.
- Kuang, R., Levinthal, D.J., Ghaffari, A.A. et al. (2023). Nightshade Vegetables: A Dietary Trigger for Worsening Inflammatory Bowel Disease and Irritable Bowel Syndrome?. Digestive Diseases and Sciences, 68, 2853–2860. [CrossRef]
- Lennerz, B.S., Mey, J.T., Henn, O.H. & Ludwig, D.S. (2021). Behavioral Characteristics and Self-Reported Health Status among 2029 Adults Consuming a "Carnivore Diet". Current Developments in Nutrition, 5(12), nzab133. [CrossRef]
- Lescinsky, H., Afshin, A., Ashbaugh, C. et al. Health effects associated with consumption of unprocessed red meat: a Burden of Proof study. Nat Med 28, 2075–2082 (2022). [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021). [CrossRef]
- Martin-McGill, K.J., Bresnahan, R., Levy, R.G. & Cooper, P.N. (2020). Ketogenic diets for drug-resistant epilepsy. Cochrane Database of Systematic Reviews, 6, CD001903.
- Mercola J, D’Adamo CR. Linoleic Acid: A Narrative Review of the Effects of Increased Intake in the Standard American Diet and Associations with Chronic Disease. Nutrients. 2023; 15(14):3129. [CrossRef]
- Needham, N., Campbell, I., Grossi, H., Kamenska, I., Rigby, B., Simpson, S.,... & Smith, D. (2023). Pilot study of a ketogenic diet in bipolar disorder. BJPsych Open, 9(6), E176.
- Norwitz NG, Soto-Mota A, Kaplan B, Ludwig DS, Budoff M, Kontush A, Feldman D. The Lipid Energy Model: Reimagining Lipoprotein Function in the Context of Carbohydrate-Restricted Diets. Metabolites. 2022; 12(5):460. [CrossRef]
- O'Hearn, A. (2020). Can a carnivore diet provide all essential nutrients? Current Opinion in Endocrinology, Diabetes and Obesity, 27(5), 312-316. [CrossRef]
- Parada Venegas, D.; et al. (2019). Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Frontiers in Immunology, 10, 277. [CrossRef]
- Roman, Y.M. The Role of Uric Acid in Human Health: Insights from the Uricase Gene. J. Pers. Med. 2023, 13, 1409. [Google Scholar] [CrossRef] [PubMed]
- Sourbron, J., Klinkenberg, S., van Kuijk, S.M.J., Lagae, L., Lambrechts, D. & Braakman, H.M.H. (2020). Ketogenic diet for the treatment of pediatric epilepsy: review and meta-analysis. Child's Nervous System, 36(6), 1099-1110.
- Srivastava, S., Pawar, V.A., Tyagi, A., Sharma, K.P., Kumar, V. & Shukla, S.K. (2023). Immune Modulatory Effects of Ketogenic Diet in Different Disease Conditions. Immuno, 3, 1-15. [CrossRef]
- Tan, K.Y. & Seow-Choen, F. (2007). Fiber and colorectal diseases: separating fact from fiction. World Journal of Gastroenterology, 13(31), 4161-4167. [CrossRef] [PubMed]
- Tan, C., Daud, M. & Seow-Choen, F. (2012). Stopping or reducing dietary fiber intake reduces constipation and its associated symptoms. World Journal of Gastroenterology, 18(33), 4593-4596. [CrossRef]
- Tóth, C., Dabóczi, A., Howard, M., Miller, N.J. & Clemens, Z. (2016). Crohn’s disease successfully treated with the paleolithic ketogenic diet. International Journal of Case Reports and Images, 7(10), 570–578.
- Qiao Ya-Nan , Zou Yan-Li , Guo Shou-Dong (2022). Low-density lipoprotein particles in atherosclerosis, Frontiers in Physiology, Volume 13, 2022.
- Westman Eric, William S. Yancy, Maren K. Olsen, Tara Dudley, John R. Guyton, Effect of a low-carbohydrate, ketogenic diet program compared to a low-fat diet on fasting lipoprotein subclasses, International Journal of Cardiology, Volume 110, Issue 2, 2006, Pages 212-216, ISSN 0167-5273. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




