1. Introduction
The Aryl hydrocarbon receptor (AHR) is a cytosolic receptor for low molecular weight molecules which acts as an environmental sensor and influences several signalling pathways, most significantly the cytochrome P450 1A1, 1A2, 1B1 monooxygenases which introduce functional groups prior to conjugation with water soluble molecules by the phase 2 detoxification enzymes [
1]. In addition, it has immunomodulatory effects and plays an important role in the cutaneous response to UV light and pigmentation.
It is emerging as an important therapeutic target. Tar preparations are an established dermatological modality known to be active at the AHR [
2] and tapinarof is an AHR agonist currently available for atopic dermatitis [
3] and psoriasis [
4].
6-formylindolo[3,2-
b] carbazole (FICZ) is a potent endogenous ligand of the AHR produced by the photo-oxidation of L-tryptophan. It is immunoinhibitory at high concentrations and immunostimulatory at low concentrations [
5].
L-tryptophan is metabolized to nicotinamide via the kynurenine pathway (KP). Several of the metabolic intermediates are active at the AHR [
1] and contribute to AHR modulation.
Fibrosing cutaneous disorders such as morphea and scleroderma remain incompletely understood. The initial or inflammatory phase begins with vascular endothelial damage and the upregulation of adhesion molecules such as E-cadherin and VCAM-1[
6] and the subsequent recruitment of pro-inflammatory Th1 and TH 17 cells. At this stage the cytokine signature profile is TH 1 weighted [
7]. In the second or fibrotic phase, a TH2 cytokine signature predominates [
8] mediating tissue fibrosis. In morphea fibrosis resolves over 2-5 years but may be followed by persistent tissue atrophy [
9].
The primary aim of this study was to establish that a graduated increase in natural ultraviolet light (UV) exposure combined with a topical preparation of L-tryptophan formulated to favor the generation of FICZ demonstrated activity at the AHR.
As sunflower lecithin was incorporated in the preparation and lecithin-based multilamellar liposomes have been reported to offer a biodegradable alternative to traditional sunscreen preparations with equal efficacy [
10] the UV absorbance of natural sunlight was determined via UV integrator. Additional values were also determined utilizing 365 and 395 UV light sources to assess UV absorbance in the ultraviolet A1 spectrum (340-400nm) as UVA1 phototherapy is recommended for fibrosing cutaneous disorders. An ideal agent would demonstrate strong activity at the AHR and UV protection comparable to traditional sunscreens whilst permissive of transmission within the UVA 1 spectrum.
2. Results
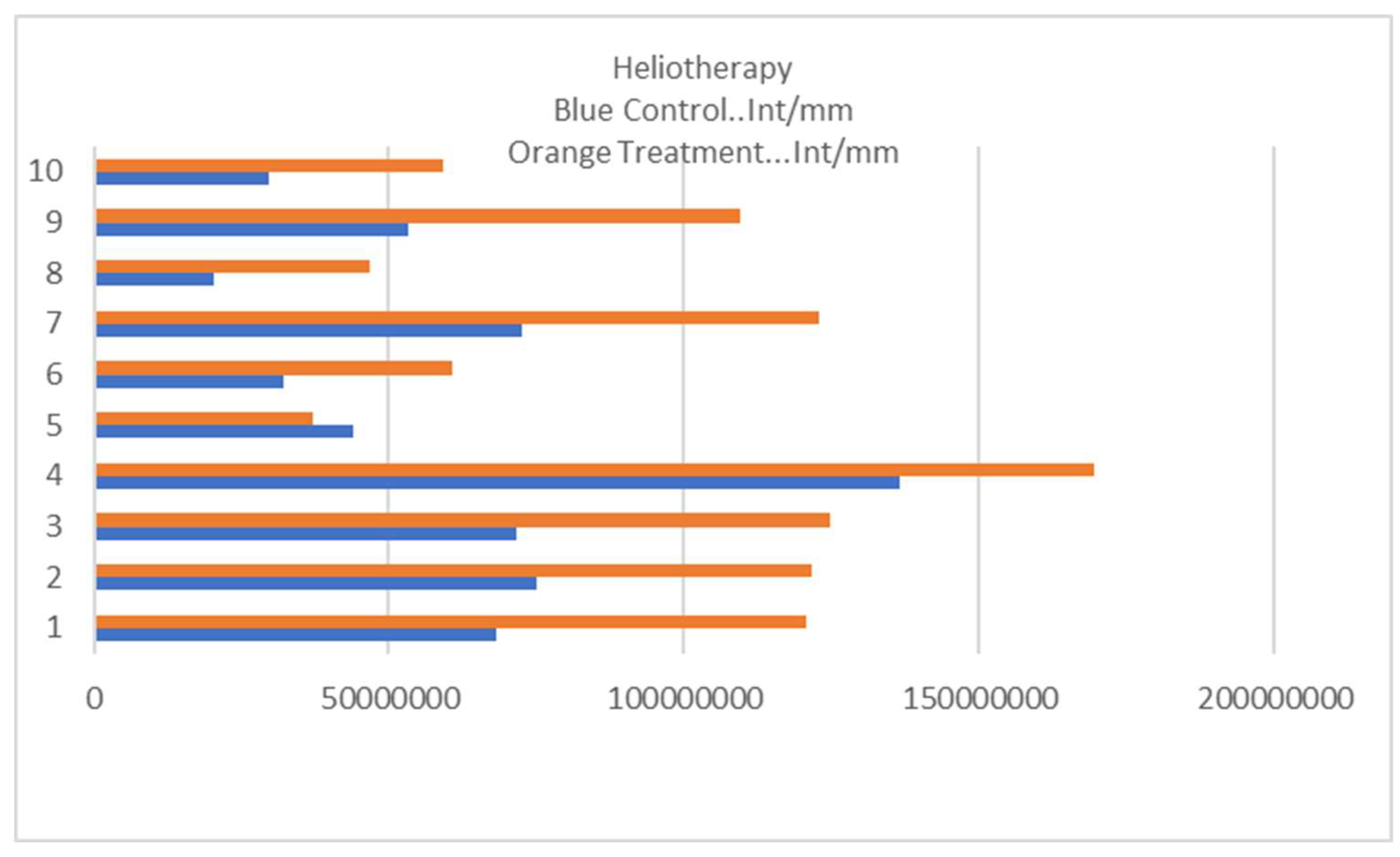
Ten participants applied a trial agent consisting of L- tryptophan, lecithin, polyvinyl alcohol and ethanol followed by 36 sessions of graduated sun exposure increasing to a total of 20 Joules per square centimetre. At the end of the trial, biopsies were taken from a treated site with photo-protected buttock skin used as a control. Assessment was via intensity of cytochrome P450 1A2 staining.
The results are outlined in
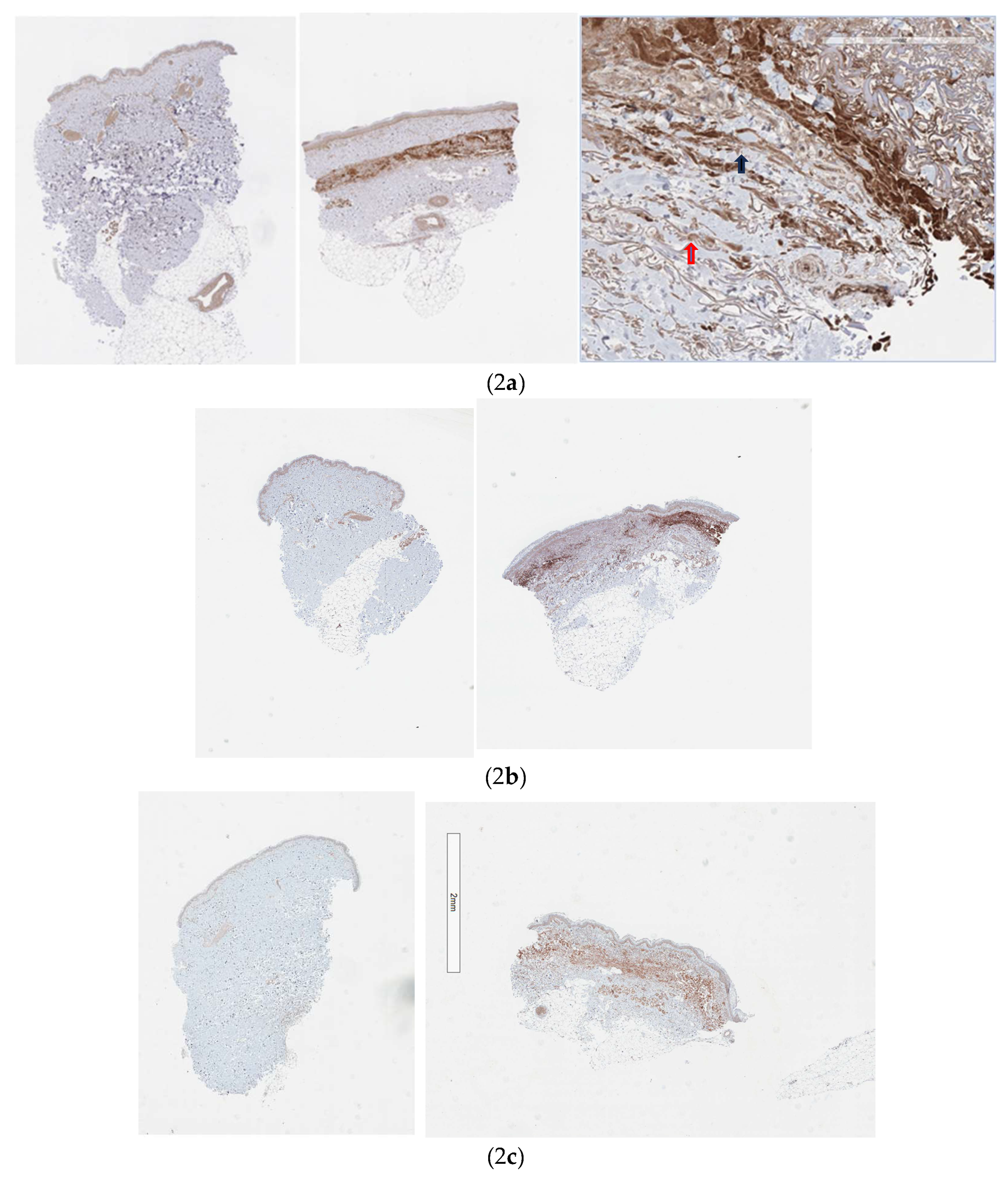
Figure 1. The Kolmogorov-Smirnov Test confirms the distribution as normal with a mean of 60319716 at the control site and 972214294 at the treatment site with a p value of 0.047 supporting a statistically significant result. Sample slides are shown in
Figure 2 a, b, and c. Although all cells in the integument express the AHR [
1], Cytochrome P450 induction was noted to be strongest amongst fibroblasts and infiltrating mononuclear cells (
Figure 2a).
Due to the strong induction of Cytochrome P4501A2 the Hematoxylin and Eosin sections were examined for evidence of cellular apoptosis. No significant increase was noted.
2.1. Tryptophan Toxicity
The ingestion of contaminated L tryptophan has been reported to have an association with eosinophilia myalgia syndrome although other authors have suggested that kynurenine metabolites themselves may have been responsible for this disorder [
11]. Eosinophilia myalgia syndrome was reported with daily doses of L tryptophan of 1.2-2.4 G [
12] equating to the use of 60-120G of the trial preparation daily, well beyond the recommended maximum daily dose of 20G based on the fingertip unit scale. No participants reported symptoms or displayed and signs of eosinophilia myalgia syndrome.
2.2. UV Absorbance Natural Sunlight
The Sun Protection factor (SPF) of a sunscreens is traditionally determined using ten human volunteers and is based on the UV dose required to produce cutaneous erythema on skin without the photoprotective agent compared to skin with the photoprotective agent, the ratio providing the value. In this study, as an alternative, ten in vitro measurements were made using a UV integrator. The trial agent was applied to adhesive tape mounted over the sensor component UV-SPEEDRE UV Integrator immediately prior to sun exposure at a concentration of 2.5mg per cm
2 and the UV dose measured after 1 hour of sun exposure. A second UV-SPEEDRE UV Integrator with the sensor covered with adhesive tape was used as a control. The results are outlined in
Table 1 and the setup in
Figure 3. They are consistent with a 50 plus value previously reported for a lecithin-based sunscreen [
10].
2.3. UV Absorbance 395 nm
This protocol involved a 60 second illumination via a 395nm 3-watt light source applied directly over the sensor of a UV-SPEEDRE UV Integrator covered with adhesive tape to which the trial agent had been applied at a concentration of 2.5mg per cm
2. The control value was determined utilizing the same sensor covered with adhesive tape without the trial agent. The results are outlined in
Table 2 and demonstrate approximately a 75 percent reduction in UV transmission at 395nm consistent with the known absorbance spectrum of lecithin [
13] and supportive of the concept that this agent permits partial transmission in the UVA 1 spectrum.
2.4. UV Absorbance 365 nm
This protocol involved a 60 second illumination via a 365 nm light source applied directly over the sensor of a UV-SPEEDRE UV Integrator covered with adhesive tape to which the trial agent had been applied at a concentration of 2.5mg per cm
2. The control value was determined utilizing the same sensor covered with adhesive tape without the trial agent. The results are outlined in
Table 3.
3. Discussion
Fibrosis is characterized by the aberrant deposition of extracellular matrix leading to organ dysfunction. Although the exact pathogenesis is yet to be elucidated transforming growth factor beta (TGF beta) signalling, myofibroblast activation and immunological disequilibrium play key roles.
As AHR signalling is involved in all the above processes, it is an attractive therapeutic target.
Myofibroblast differentiation is central to the fibrotic process. Progenitor cells undergoing epithelial to mesenchymal transition (EMT), mesothelial to mesenchymal transition (MMT) and endothelial to mesenchymal transition (EndoMT) in response to cytokines released by infiltrating immune cells differentiate into active myofibroblasts. TGF beta is pivotal in promoting EMT, MMT and EndoMT, the secretion of various extracellular matrix (ECM) components and matrix metalloproteinase (MMP) activity. TGF beta expression is negatively influenced by AHR signalling [
14].
The AHR and KP intermediates serve several important functions in myofibroblast activation. The KP metabolite kynurenine has been reported to modulate the expression of several ECM components via AHR signalling [
15,
16] and the archetypical AHR ligand TCDD induces the induction of MMP-1 in keratinocytes [
17]. In a similar fashion the tryptophan photoproduct and AHR ligand FICZ has been demonstrated to upregulate MMP-1 expression in dermal fibroblasts offering an insight into the therapeutic mechanism of phototherapy in scleroderma [
18].
An imbalance between TH17 cells and Treg cells has been reported in systemic sclerosis [
19] and the AHR is known to promote T reg differentiation [
20] which supresses the profibrotic immune response [
21].
The inclusion of lecithin in this preparation is significant. Lecithin absorbs UV light in a broad band from 200-380nm with peak absorbance at 235, 271 and 355 nanometres [
13] and thus has potential as a biodegradable and ecofriendly sunscreen with an efficacy equivalent to traditional sunscreens [
10]. As such, preparations of the kind used in this trial may form the basis for therapeutic sunscreens, that is preparations which offer both photoprotection and the immunomodulatory benefits of ultraviolet light-based therapy without the harmful effects associated with ultraviolet (UV) irradiation of the skin [
22].
L-tryptophan absorbs maximally at 280nm with absorbance falling rapidly to 310nm and is thus expected to provide strong photoprotection in the UVB spectrum.
UVA1 phototherapy utilizing UV radiation in the 340-400nm band is considered a promising treatment for cutaneous fibrosing disorders [
23], although it is unlikely that all the antifibrotic effects of UVA 1 therapy are mediated via the AHR [
24]. Specifically, immediate T cell apoptosis through the activation of the FAS/FAS ligand system appears to be a specific property of UVA1 phototherapy, since it is not observed with UVB [
25] and thus likely to be AHR independent. As such an agent which is permissive of partial UV transmission in the 340-400nm range would be advantageous.
The results are consistent with previous studies that lecithin displays a 50 plus SPF [
10] (98% UV absorption) in the UVB and UVA-2 spectrum with absorbance falling to 87% at 365 nm and 80% at 395nm.
In summary this is a promising preparation which provides strong UV protection via filtering ultraviolet B (290-310 nm} and ultraviolet A2 (320-340 nm) light yet retains strong activity at the AHR, a potential therapeutic target in fibrosing skin disorders. In addition, it allows partial UVA 1 transmission (340-400nm) theoretically providing convenient and cost-effective antifibrotic therapy. Finally, it is biodegradable and ecofriendly with a lower potential impact on coral reefs and marine plankton [
10]. The availability of UVA-1 units is often restricted to major population centres and remain non accessible to rural and remote populations and an alternative means of delivering UVA-1 therapy would represent meaningful progress.
Limitations of this study include the fact that this is a proof-of-concept study only, designed to confirm this agent displays activity at the AHR whilst offering photoprotection and partial transmission within the UVA 1 spectrum and may thus act as an antifibrotic agent. Although displaying statistically significant activity at the AHR, the small cohort would necessitate repeat studies in a larger trial group.
Additionally, a demonstration of efficacy in the management of fibrosing cutaneous disorders would need to be made and outcomes would need to be assessed against UVA-I therapy. The localized scleroderma cutaneous assessment tool (LoSCAT) is used to measure the therapeutic response in morphea [
26] and the modified Rodnan skin score in scleroderma [
27]. No scoring tool exists for nephrogenic systemic fibrosis and the clinical scoring of chronic graft versus host disease incorporates visceral disease.
Assessing this agent against UVA-1 phototherapy is difficult as fibrosing skin conditions are uncommon making it difficult to source a large cohort and previous studies with phototherapy have been reported to be based on low quality evidence [
28]. None the less improvement is reported [
28].
Another limiting factor is the relative contributions of AHR activation and immediate T cell apoptosis in the antifibrotic effect. UVA-1 therapy is traditionally divided into low dose (10-20J/cm
2), medium dose (50-60J/cm
2) and high dose (up to 130J/cm
2) and although various protocols have been used the Cochrane metanalysis has been reported as showing no difference in outcomes [
28]. The summer (December to February), the peak daily UVA exposure has been reported as 205 J/cm
2 in the subtropics [
29]. UVA1-absorption of 87% at 365nm and 80% at 395 nm would equate to a full day’s sun exposure at subtropical latitudes in summer utilizing this agent as a sunscreen to achieve low dose UVA-1 equivalence and thus this preparation may be of less value in the winter months at lower latitudes, but this would require further evaluation.
4. Materials and Methods
4.1. The Generation of FICZ from Tryptophan
This is an oxidative deamination reaction mediated by either an oxidizing agent or UV light. The intermediate is indole 3 acetaldehyde (I3A), two molecules of which undergo condensation under acid or alkaline conditions [
30].
Lecithin, a mixture of the glycerophospholipids phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, phosphatidylinositol, and phosphatidic acid was included in the trial agent. Ethanol was used in the preparation to promote the aggregation of the phosphatidylcholine molecules as they are more soluble in ethanol [
31]. L-Tryptophan plays an important role in anchoring proteins to cell membranes [
32] and it was proposed that aggregation of phosphatidyl choline, the principal component of cell membranes would bring individual L tryptophan molecules into proximity facilitating the deamination reaction.
The UV exposure protocol employed is outlined in
Table 4. Dosage was determined by a UV-SPEEDRE UV Integrator. If the recommended dose had not been achieved at 2 hrs sun exposure was ceased. The dose escalation paralleled that used in Narrowband UVB (NBUVB) and was designed to allow “hardening” of the skin to minimize the risk of UV burns. The final total dose achieved corresponded to a summative low dose NBUVB (1000mJ) and low dose UVA 1 therapy (~ 20J).
The trial agent was applied to involved sites immediately prior to sun exposure using the fingertip unit (250mg covering a palm sized area with the fingers together).
The decision to limit sun exposure to 2hrs was based on considerations of practicality and evidence from studies on “Dead Sea therapy” which it was felt may have shared sufficient similarities to allow data to be extrapolated [
33].
4.2. Histological Analysis
At the completion of therapy, a 4mm punch biopsy was taken form a treated site and another which acted as a control from photo-protected non treated site (buttock). Specimens were stained for hematoxylin and eosin. Cytochrome P450 1A2 staining was via immunohistochemistry. Cytochrome P450 1A2 (Cytochrome P450 1A2 Polyclonal Antibody, Biotin Conjugated) was used as the marker for AHR activity.
Slides were dewaxed sequentially with xylene (one 3-minute cycle followed by two 1-minute cycles) and ethanol (two 1minute cycles using 100% ethanol, one 1-minute cycle using 90% ethanol and one 1-minute cycle using a 70% ethanol) before being washed under running water for 3 minutes.
Endogenous peroxide activity was blocked by incubation with 3% hydrogen peroxide for 5 minutes before being washed in running water for 2 minutes.
The slides were placed Dako Epitope Retrieval buffer (pH 9.0) and heat induced epitope retrieval performed in a Biocare Medical Decloaker for 5 minutes at 125C before being allowed to cool for 20 minutes.
The slides were then washed three times in Tris Buffer Saline and 0.02% Tween 20 (TBSTW) for 2 minutes before applying Vector Biotin Blocking solutions (Streptavidin and Biotin) for 10 minutes each, washing between applications. The slides were than washed three times in TBSTW for 2 minutes.
The tissue was then covered with the blocking solution, Biocare Medical Background Sniper + 2% BSA for 15 min. The blocking solution was than aspirated and the primary antibody, Bioss Rabbit anti-Cytochrome P450 1A2 diluted 1:300 in Da Vinci Green antibody diluent added to each slide and incubated for 2 hours at room temperature. The slides were than washed three times in TBSTW for 2 minutes.
Following this the tissue was incubated with Jackson Immunoresearch streptavidin conjugated HRP diluted 1:600 in TBSTW for 60 minutes before being washed three times in TBSTW for 2 minutes.
Biocare DAB was applied for 5 mins and the slides washed with water for 5 minutes after which a coverslip was applied.
Analysis was via Aperio Imagescope using Positive Pixel Count v9. Values for total intensity/mm2 were used to assess enzyme activity.
4.3. Preparation of the Trial Agent
3 grams of L-tryptophan were dissolved in 100 mls of sterile water at pH 2.8 via continuous stirring at room temperature. The solution was then heated to 50C, and 45 grams of sunflower lecithin slowly added whilst stirring. 5G of polyvinylalcohol were then added with continued stirring until dissolved.
The solution was then allowed to cool to room temperature and100% ethanol added to 20% of the final volume, stirring until into solution. The pH was than adjusted to 5.8. Final volume 150mls.
The preparation was prepared by a commercial compounding pharmacy (Formulae Compounding Lab Albion) utilizing pharmaceutical grade ingredients.
4.4. Determination of UV Absorption (Natural Sunlight)
The trial agent was applied to adhesive tape mounted over the sensor component UV-SPEEDRE UV Integrator immediately prior to sun exposure at a concentration of 2.5mg per cm
2 and the UV dose measured after 1 hour of sun exposure. A second UV-SPEEDRE UV Integrator with the sensor covered with adhesive tape was used as a control. Measurements were taken at various times during the day as outlined in
Table 1. Cloud cover was variable. Measurements were taken at latitude was 26 degrees south during the winter months. The setup used is shown in
Figure 3.
4.5. Determination of UV Absorption at 395 nm
The trial agent was applied to adhesive tape mounted over the sensor component UV-SPEEDRE UV Integrator immediately prior to exposure at a concentration of 2.5mg per cm2 and the UV dose measured after 60 seconds of UVB exposure. Illumination was via a 395nm light source applied directly over the sensor. The manufacturers reported fluence was 1500mJ/cm 2 at the source. The control value was determined utilizing the same sensor covered with adhesive tape without the trial agent.
4.6. UV Absorbance 365 nm
This protocol involved a 60 second illumination via a 365 nm light source applied directly over the sensor of a UV-SPEEDRE UV Integrator covered with adhesive tape to which the trial agent had been applied at a concentration of 2.5mg per cm2. The manufacturers reported fluence at the source was 455 mJ/cm2. The control value was determined utilizing the same sensor covered with adhesive tape without the trial agent.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee the Ramsay Health Care QLD HREC, Newdegate St, Greenslopes QLD 4120.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The author would like to thank the nursing and administrative staff at the Qld Institute of Dermatology for their assistance with this project.
Conflicts of Interest
The author reports no conflicts of interest.
References
- Noakes R. The aryl hydrocarbon receptor: a review of its role in the physiology and pathology of the integument and its relationship to the tryptophan metabolism. International Journal of Tryptophan Research. 2015 Jan;8:IJTR-S19985. [CrossRef]
- Merk, H. F. (2019) Merk HF. The aryl hydrocarbon receptor as the target structure for new drugs in psoriasis and atopic dermatitis. Der Hautarzt. 2019 Dec;70:942-7.
- Peppers J, Paller AS, Maeda-Chubachi T, Wu S, Robbins K, Gallagher K, Kraus JE. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. Journal of the American Academy of Dermatology. 2019 Jan 1;80(1):89-98. [CrossRef]
- Lebwohl MG, Stein Gold L, Strober B, Papp KA, Armstrong AW, Bagel J, Kircik L, Ehst B, Hong HC, Soung J, Fromowitz J. Phase 3 trials of tapinarof cream for plaque psoriasis. New England Journal of Medicine. 2021 Dec 9;385(24):2219-29. [CrossRef]
- Rannug A, Rannug U. The tryptophan derivative 6-formylindolo [3, 2-b] carbazole, FICZ, a dynamic mediator of endogenous aryl hydrocarbon receptor signaling, balances cell growth and differentiation. Critical reviews in toxicology. 2018 Aug 9;48(7):555-74. [CrossRef]
- Lee JS, Park HS, Yoon HS, Chung JH, Cho S. CD 34 stromal expression is inversely proportional to smooth muscle actin expression and extent of morphea. Journal of the European Academy of Dermatology and Venereology. 2018 Dec;32(12):2208-16. [CrossRef]
- Torok KS, Kurzinski K, Kelsey C, Yabes J, Magee K, Vallejo AN, Medsger Jr T, Feghali-Bostwick CA. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Seminars in arthritis and rheumatism 2015 Dec 1 (Vol. 45, No. 3, pp. 284-293). WB Saunders.
- Papara C, De Luca DA, Bieber K, Vorobyev A, Ludwig RJ. Morphea: the 2023 update. Frontiers in medicine. 2023 Feb 13;10:1108623. [CrossRef]
- O’Brien JC, Nymeyer H, Green A, Jacobe HT. Changes in disease activity and damage over time in patients with morphea. JAMA dermatology. 2020 May 1;156(5):513-20. [CrossRef]
- Bernasqué A, Faure C, Rezvani H, Cario M. A new eco-friendly and water-resistant sunscreen agent: Lecithin-based multilamellar liposomes. Journal of Cosmetic Dermatology. 2024 Mar;23(3):918-25.
- Noakes R, Spelman L, Williamson R. Is the L-tryptophan metabolite quinolinic acid responsible for eosinophilic fasciitis? Clinical and Experimental Medicine. 2006 Jun; 6:60-4.
- Hertzman PA, Blevins WL, Mayer J, Greenfield B, Ting M, Gleich GJ. Association of the eosinophilia–myalgia syndrome with the ingestion of tryptophan. New England journal of medicine. 1990 Mar 29;322(13):869-73. [CrossRef]
- Latif, M.H., Alsouz, M.A. and Taher, M.B., 2014. Quantification of the components of the Iraqi Chicken wet egg yolk, and characterization of Lecithin. Chemistry and Materials Research, 6(6).
- Shi Y, Zeng Z, Yu J, Tang B, Tang R, Xiao R. The aryl hydrocarbon receptor: An environmental effector in the pathogenesis of fibrosis. Pharmacological Research. 2020 Oct 1;160:105180. [CrossRef]
- Poormasjedi-Meibod MS, Salimi Elizei S, Leung V, Baradar Jalili R, Ko F, Ghahary A. Kynurenine Modulates MMP-1 and Type-I Collagen Expression Via Aryl Hydrocarbon Receptor Activation in Dermal Fibroblasts. J Cell Physiol. 2016 Dec;231(12):2749-60. [CrossRef]
- Murai M, Yamamura K, Hashimoto-Hachiya A, Tsuji G, Furue M, Mitoma C. Tryptophan photo-product FICZ upregulates AHR/MEK/ERK-mediated MMP1 expression: Implications in anti-fibrotic phototherapy. J Dermatol Sci. 2018 Jul;91(1):97-103. Epub 2018 Apr 21. PMID: 29703420. [CrossRef]
- Murphy KA, Villano CM, Dorn R, White LA. Interaction between the aryl hydrocarbon receptor and retinoic acid pathways increases matrix metalloproteinase-1 expression in keratinocytes. Journal of Biological Chemistry. 2004 Jun 11;279(24):25284-93. [CrossRef]
- Murai M, Yamamura K, Hashimoto-Hachiya A, Tsuji G, Furue M, Mitoma C. Tryptophan photo-product FICZ upregulates AHR/MEK/ERK-mediated MMP1 expression: Implications in anti-fibrotic phototherapy. Journal of Dermatological Science. 2018 Jul 1;91(1):97-103. [CrossRef]
- Mo C, Zeng Z, Deng Q, Ding Y, Xiao R. Imbalance between T helper 17 and regulatory T cell subsets plays a significant role in the pathogenesis of systemic sclerosis. Biomedicine & Pharmacotherapy. 2018 Dec 1; 108:177-83. [CrossRef]
- Noakes R. The role of kynurenine pathway aryl hydrocarbon receptor axis in autoimmune diseases of the skin. Translational Autoimmunity. 2023 Jan 1:79-90. Elsevier.
- Kalekar LA, Cohen JN, Prevel N, Sandoval PM, Mathur AN, Moreau JM, Lowe MM, Nosbaum A, Wolters PJ, Haemel A, Boin F. Regulatory T cells in skin are uniquely poised to suppress profibrotic immune responses. Science immunology. 2019 Sep 6;4(39). [CrossRef]
- Noakes R (2024) Photoprotective, Immunomodulatory and Eco-Friendly: On the road to the Holy Grail of Sunscreen Development. J Clin Dermatol Ther 10: 0138. [CrossRef]
- Keyal, U., Bhatta, A.K. and Wang, X.L., 2017. UVA1 a promising approach for scleroderma. American Journal of Translational Research, 9(9), p.4280.
- Tognetti, L., Marrocco, C., Carraro, A., Guerrini, G., Mariotti, G., Cinotti, E. and Rubegni, P., 2022. Clinical and laboratory characterization of patients with localized scleroderma and response to UVA-1 phototherapy: In vivo and in vitro skin models. Photodermatology, Photoimmunology & Photomedicine, 38(6), pp.531-540. [CrossRef]
- Morita, A., Werfel, T., Stege, H., Ahrens, C., Karmann, K., Grewe, M., Grether-Beck, S., Ruzicka, T., Kapp, A., Klotz, L.O. and Sies, H., 1997. Evidence that singlet oxygen-induced human T helper cell apoptosis is the basic mechanism of ultraviolet-A radiation phototherapy. The Journal of experimental medicine, 186(10), pp.1763-1768. [CrossRef]
- Noakes R. Assessing the response of morphea and limited scleroderma to tranilast: a small prospective study comparing topical corticosteroids to a combination of topical corticosteroids and tranilast. Clinical, Cosmetic and Investigational Dermatology. 2018 Jul 4:321-6. [CrossRef]
- Low, A.H., Ng, S.A., Berrocal, V., Brennan, B., Chan, G., Ng, S.C. and Khanna, D., 2019. Evaluation of Scleroderma Clinical Trials Consortium training recommendations on modified Rodnan skin score assessment in scleroderma. International journal of rheumatic diseases, 22(6), pp.1036-1040. [CrossRef]
- de Albuquerque, J.V., Andriolo, B.N., Vasconcellos, M.R., Civile, V.T., Lyddiatt, A. and Trevisani, V.F., 2019. Interventions for morphea. Cochrane Database of Systematic Reviews, (7).
- Kimlin, M.G., Parisi, A.V., Sabburg, J. and Downs, N.J., 2002. Understanding the UVA environment at a sub-tropical site and its consequent impact on human UVA exposure. Photochemical & Photobiological Sciences, 1(7), pp.478-482. [CrossRef]
- Smirnova A, Wincent E, Vikström Bergander L, Alsberg T, Bergman J, Rannug A, Rannug U. Evidence for new light-independent pathways for generation of the endogenous aryl hydrocarbon receptor agonist FICZ. Chemical research in toxicology. 2016 Jan 19;29(1):75-86. [CrossRef]
- Cabezas DM, Diehl B, Tomás MC. Effect of processing parameters on sunflower phosphatidylcholine-enriched fractions extracted with aqueous ethanol. European journal of lipid science and technology. 2009 Oct;111(10):993-1002. [CrossRef]
- de Jesus AJ, Allen TW. The role of tryptophan side chains in membrane protein anchoring and hydrophobic mismatch. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2013 Feb 1;1828(2):864-76. [CrossRef]
- Even-Paz Z, Efron D, Kipnis V, Abels DJ. How much Dead Sea sun for psoriasis? Journal of dermatological treatment. 1996 Jan 1;7(1):17-9.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).