Submitted:
28 August 2024
Posted:
30 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
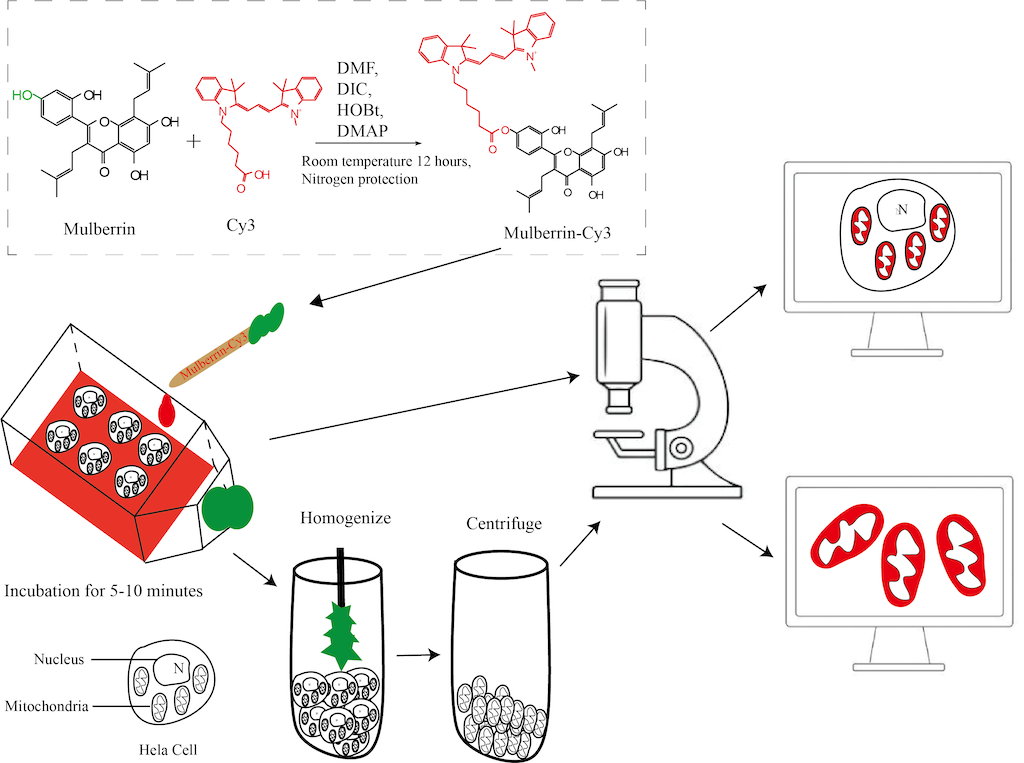
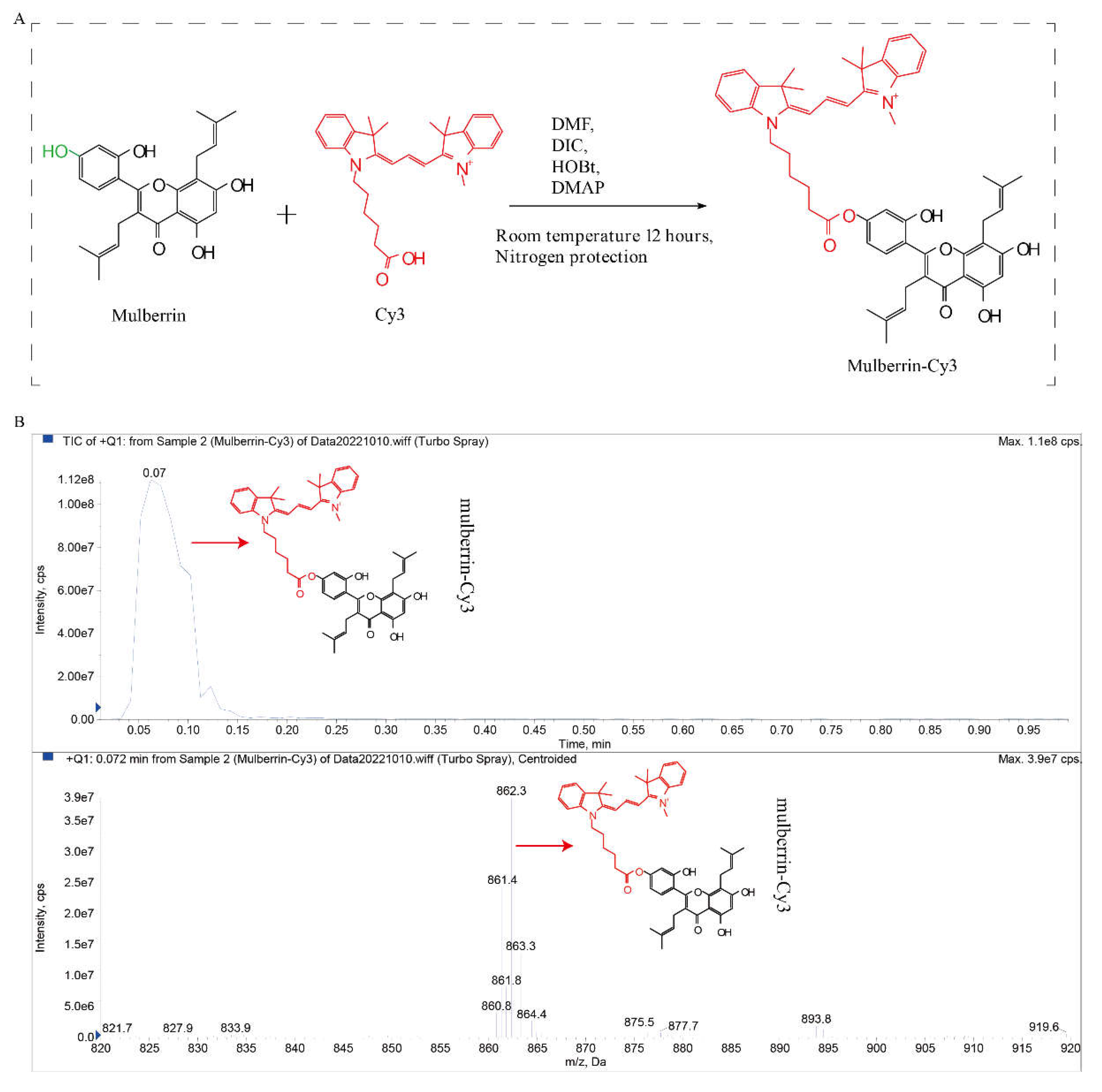
2.1. Synthesis of Mulberrin-Cy3
2.2. Mitochondrial Labeling Solution Mulberrin-Cy3 Storage Solution Preparation
2.3. Cellular Mitochondrial Extraction
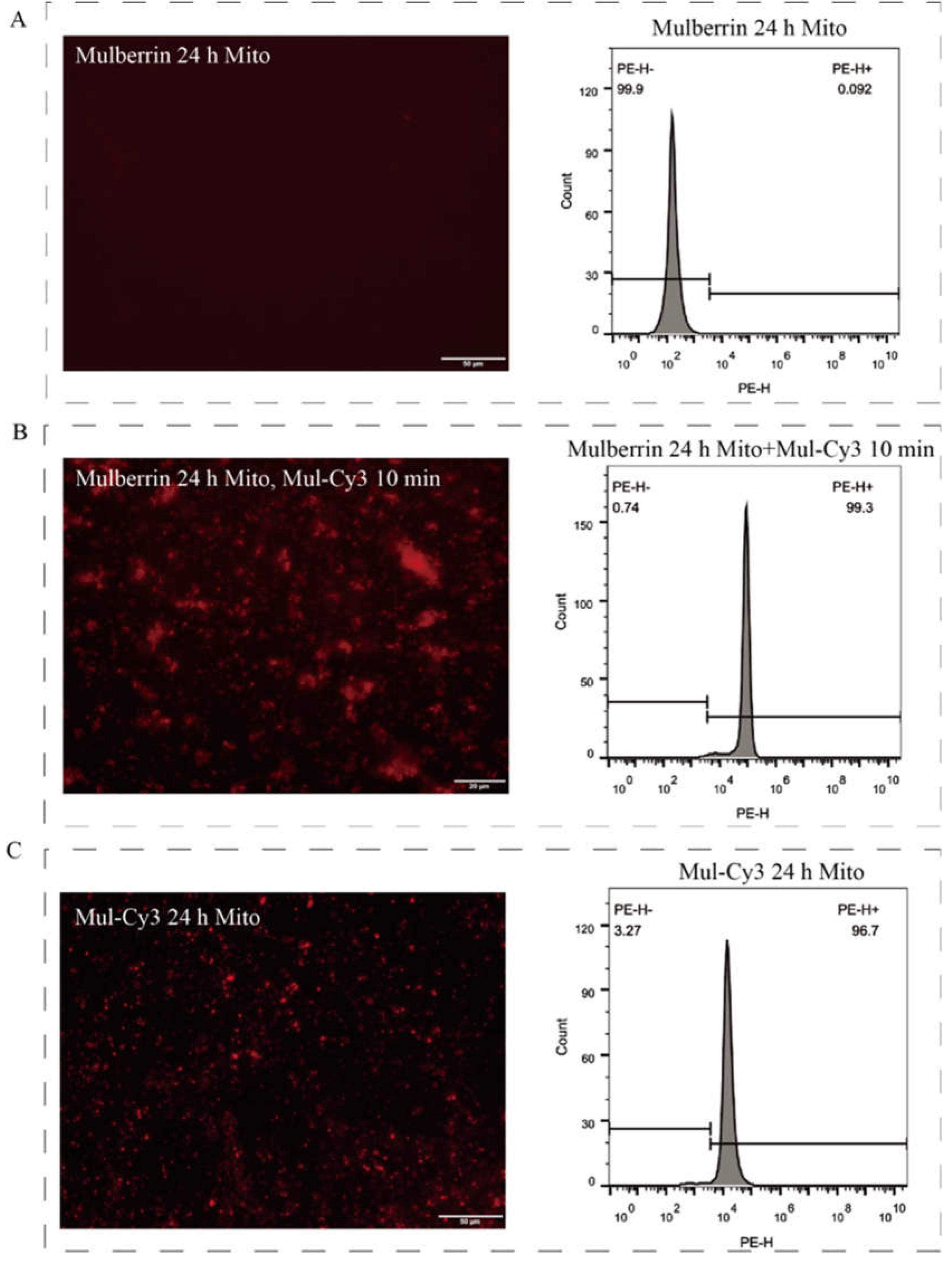
2.4. Mitochondrial Identification
2.5. Comparison of Mulberrin-Cy3 with Commercially Available Mitochondrial Labeling Probes
2.6. Determination of the Lowest Concentration of Mulberrin-Cy3 Labeling
3. Results
3.1. Synthesis Route of the Mitochondrial Red Fluorescent Labeling Compound Mulberrin-Cy3
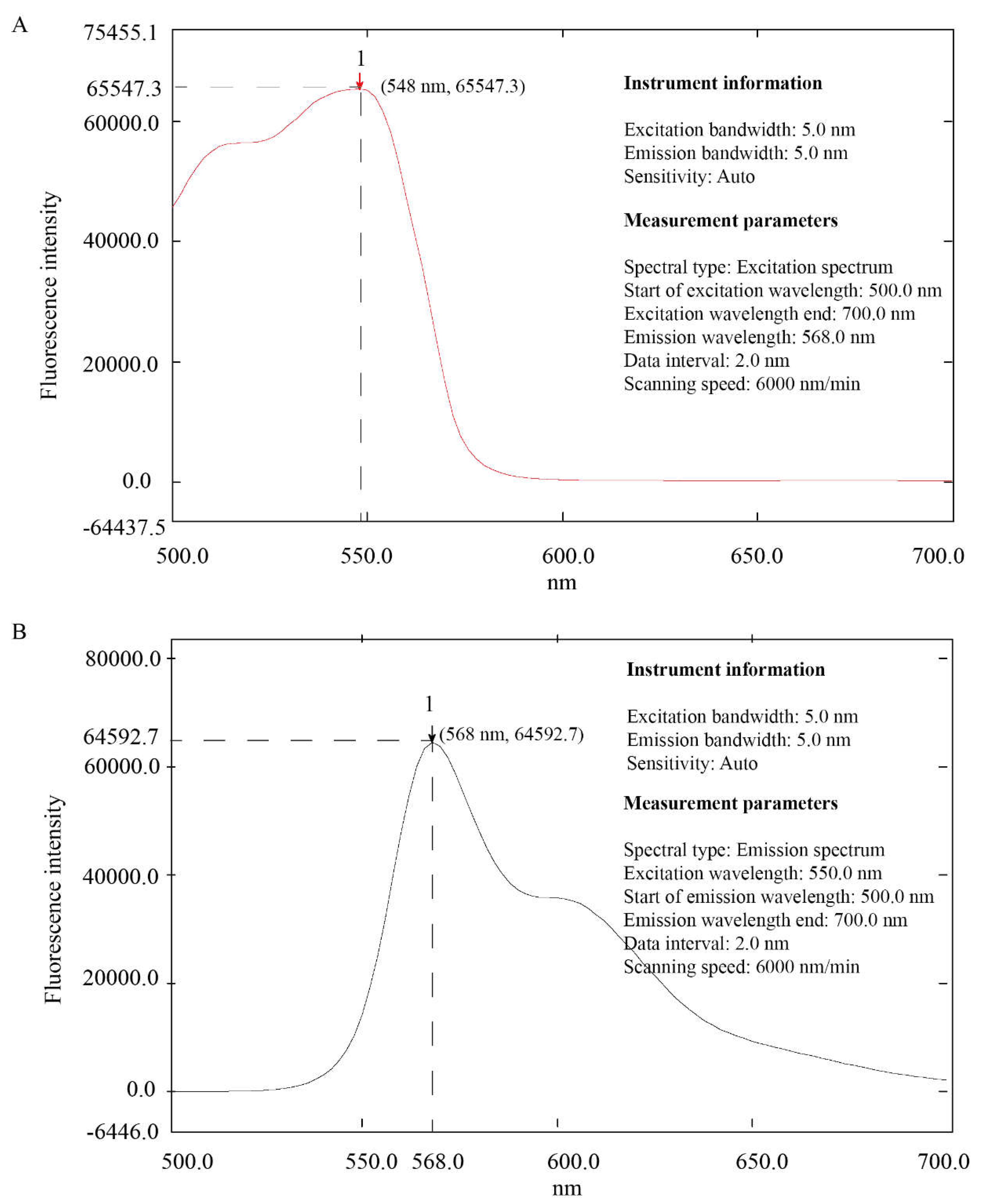
3.2. Excitation Wavelength and Emission Wavelength of Mulberrin-Cy3
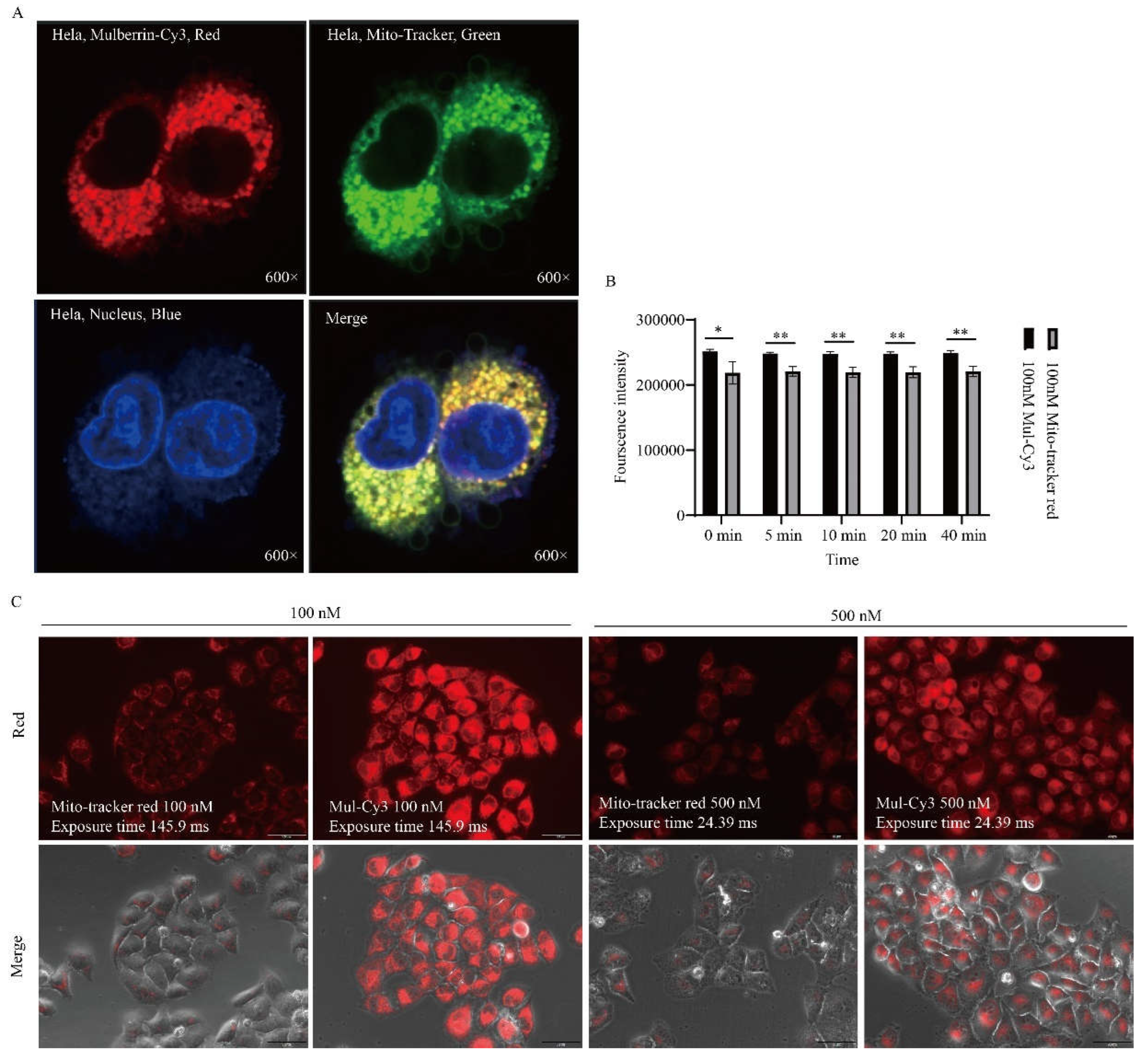
3.3. Mitochondrial Labeling of HeLa Cells
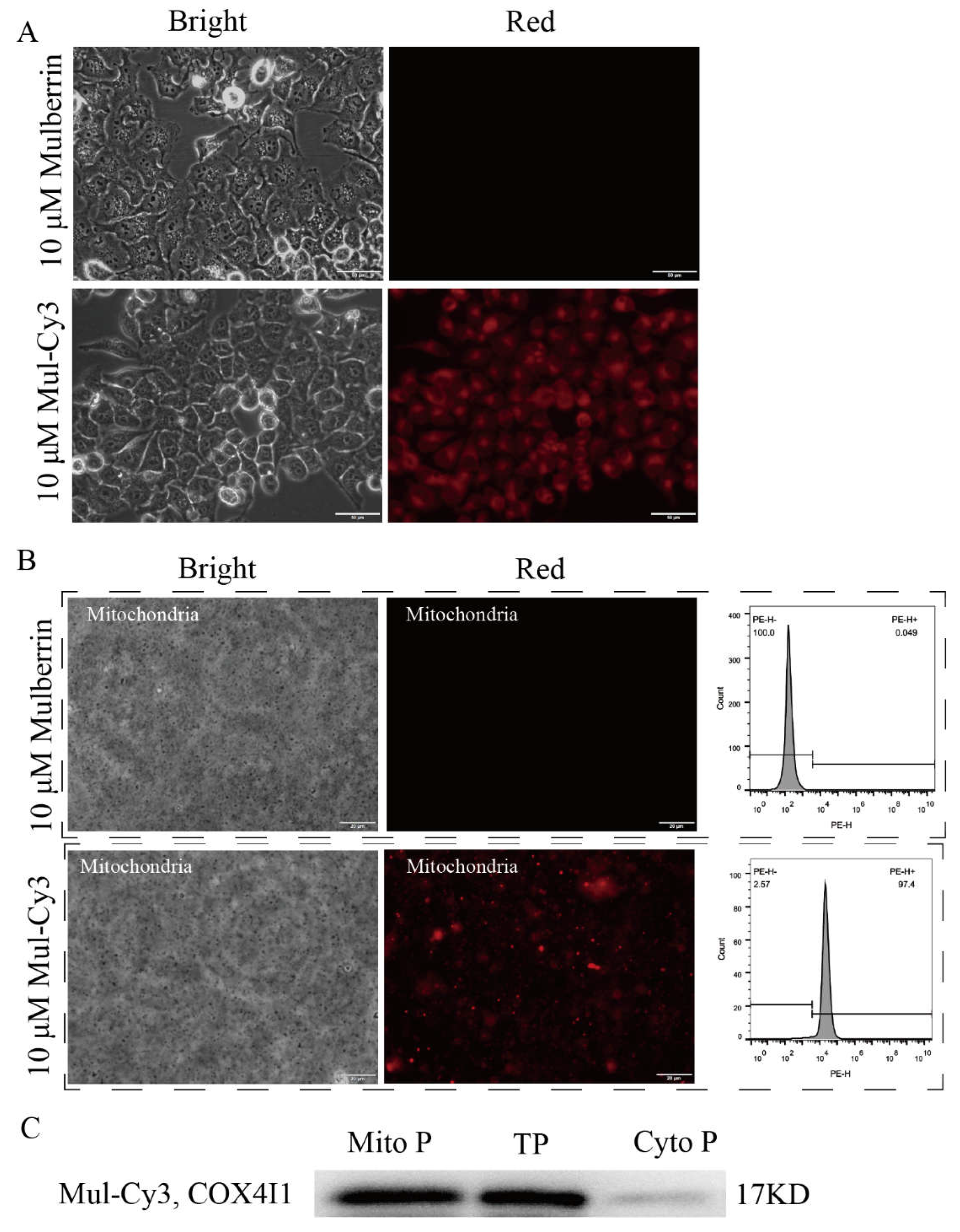
3.4. Comparison of Mulberrin-Cy3 and Mito-Tracker Labeled Mitochondria
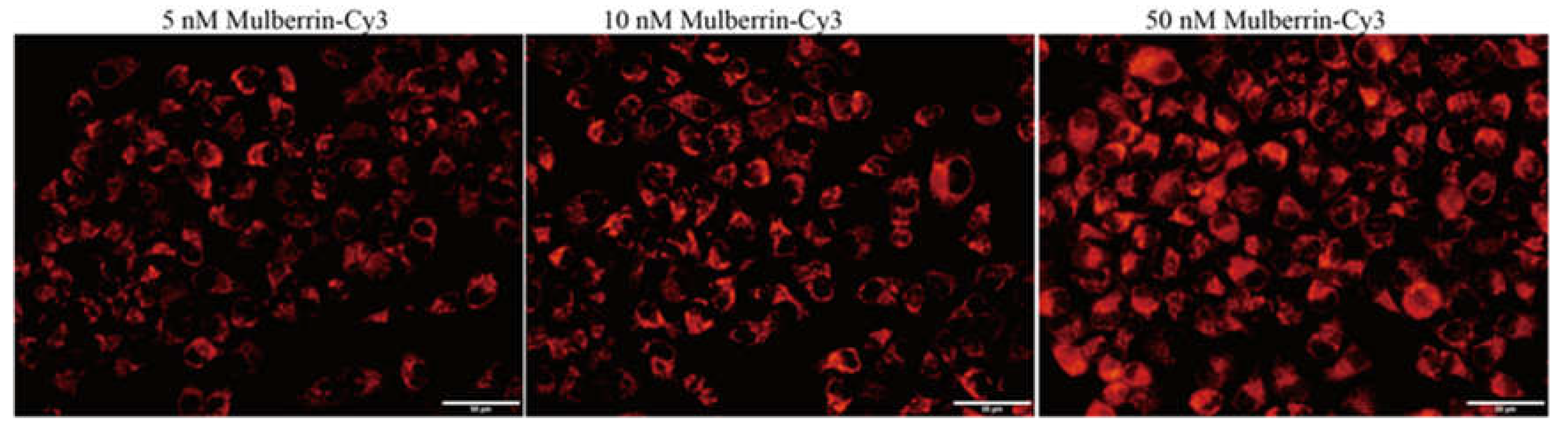
3.5. Mulberrin-Cy3 Labeling Concentration Measurement
4. Discussion
- 1)
- Simple labeling process, do not pre-treat the cells, directly add mulberrin-CY3 solution to cell culture plates/cell culture flasks (adherent cells) or cell suspension (suspended cells);
- 2)
- Fast labeling, stable red fluorescence, not easy to quench;
- 3)
- Short incubation time (5 minutes is enough), room temperature can be incubated;
- 4)
- Stable after staining, it can be labeled with other stains without fear of fixation or permeabilization; It is not necessary to add an anti-fluorescence quencher to prevent fluorescence quenching before taking pictures of the microscope.
- 5)
- Lower cost; the price of developing into a commercial product is much lower than the mainstream products in the current market, which is helpful for reducing the cost of research and development;
- 6)
- Established mulberrin-Cy3 mitochondria staining solution is very stable and can be stored at 4°C or -20°C. Repeated freezing and thawing will not affect the mitochondrial labeling effect and can be stored for a long period of time. It can be stored for a long time without affecting the mitochondrial labeling effect. Thus, it helps to simplify the sample preparation for cell analysis workflow.
- 7)
- Mulberrin-Cy3 are safe for human body.
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green, D. E., Mitochondria--structure, function, and replication. The New England journal of medicine 1983, 309, (3), 182-3. [CrossRef] [PubMed]
- Pullman, M. E.; Schatz, G., Mitochondrial oxidations and energy coupling. Annual review of biochemistry 1967, 36, 539-611. [CrossRef]
- Houten, S. M.; Violante, S.; Ventura, F. V.; Wanders, R. J., The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annual review of physiology 2016, 78, 23-44. [CrossRef] [PubMed]
- Long, J. Z.; Svensson, K. J.; Bateman, L. A.; Lin, H.; Kamenecka, T.; Lokurkar, I. A.; Lou, J.; Rao, R. R.; Chang, M. R.; Jedrychowski, M. P.; Paulo, J. A.; Gygi, S. P.; Griffin, P. R.; Nomura, D. K.; Spiegelman, B. M., The Secreted Enzyme PM20D1 Regulates Lipidated Amino Acid Uncouplers of Mitochondria. Cell 2016, 166, (2), 424-435. [CrossRef]
- Verkerke, A. R. P.; Wang, D.; Yoshida, N.; Taxin, Z. H.; Shi, X.; Zheng, S.; Li, Y.; Auger, C.; Oikawa, S.; Yook, J. S.; Granath-Panelo, M.; He, W.; Zhang, G. F.; Matsushita, M.; Saito, M.; Gerszten, R. E.; Mills, E. L.; Banks, A. S.; Ishihama, Y.; White, P. J.; McGarrah, R. W.; Yoneshiro, T.; Kajimura, S., BCAA-nitrogen flux in brown fat controls metabolic health independent of thermogenesis. Cell 2024, 187, (10), 2359-2374.e18. [CrossRef]
- Fang, W.; Jiang, L.; Zhu, Y.; Yang, S.; Qiu, H.; Cheng, J.; Liang, Q.; Tu, Z. C.; Ye, C., Methionine restriction constrains lipoylation and activates mitochondria for nitrogenic synthesis of amino acids. Nature communications 2023, 14, (1), 2504. [CrossRef] [PubMed]
- Haskins, N.; Bhuvanendran, S.; Anselmi, C.; Gams, A.; Kanholm, T.; Kocher, K. M.; LoTempio, J.; Krohmaly, K. I.; Sohai, D.; Stearrett, N.; Bonner, E.; Tuchman, M.; Morizono, H.; Jaiswal, J. K.; Caldovic, L., Mitochondrial Enzymes of the Urea Cycle Cluster at the Inner Mitochondrial Membrane. Frontiers in physiology 2020, 11, 542950. [CrossRef]
- Zhou, Y. X.; Wei, J.; Deng, G.; Hu, A.; Sun, P. Y.; Zhao, X.; Song, B. L.; Luo, J., Delivery of low-density lipoprotein from endocytic carriers to mitochondria supports steroidogenesis. Nat Cell Biol 2023, 25, (7), 937-949. [CrossRef]
- Nicholls, D. G.; Crompton, M., Mitochondrial calcium transport. FEBS letters 1980, 111, (2), 261-8. [CrossRef]
- Boyman, L.; Karbowski, M.; Lederer, W. J., Regulation of Mitochondrial ATP Production: Ca(2+) Signaling and Quality Control. Trends in molecular medicine 2020, 26, (1), 21-39. [CrossRef]
- Carneiro, B. A.; El-Deiry, W. S., Targeting apoptosis in cancer therapy. Nature reviews. Clinical oncology 2020, 17, (7), 395-417. [CrossRef]
- Willems, P. H.; Rossignol, R.; Dieteren, C. E.; Murphy, M. P.; Koopman, W. J., Redox Homeostasis and Mitochondrial Dynamics. Cell metabolism 2015, 22, (2), 207-18. [CrossRef] [PubMed]
- Mishra, P.; Chan, D. C., Metabolic regulation of mitochondrial dynamics. Journal of Cell Biology 2016, 212, (4), 379-387. [CrossRef]
- Sekine, Y.; Houston, R.; Eckl, E. M.; Fessler, E.; Narendra, D. P.; Jae, L. T.; Sekine, S., A mitochondrial iron-responsive pathway regulated by DELE1. Molecular cell 2023, 83, (12), 2059-2076.e6. [CrossRef]
- Grotehans, N.; McGarry, L.; Nolte, H.; Xavier, V.; Kroker, M.; Narbona-Pérez Á, J.; Deshwal, S.; Giavalisco, P.; Langer, T.; MacVicar, T., Ribonucleotide synthesis by NME6 fuels mitochondrial gene expression. The EMBO journal 2023, 42, (18), e113256. [CrossRef]
- Nunnari, J.; Suomalainen, A., Mitochondria: in sickness and in health. Cell 2012, 148, (6), 1145-59. [CrossRef] [PubMed]
- Marchi, S.; Guilbaud, E.; Tait, S. W. G.; Yamazaki, T.; Galluzzi, L., Mitochondrial control of inflammation. Nature reviews. Immunology 2023, 23, (3), 159-173. [CrossRef] [PubMed]
- Bargiela, D.; Burr, S. P.; Chinnery, P. F., Mitochondria and Hypoxia: Metabolic Crosstalk in Cell-Fate Decisions. Trends in endocrinology and metabolism: TEM 2018, 29, (4), 249-259. [CrossRef]
- Floryk, D.; Houstĕk, J., Tetramethyl rhodamine methyl ester (TMRM) is suitable for cytofluorometric measurements of mitochondrial membrane potential in cells treated with digitonin. Bioscience reports 1999, 19, (1), 27-34. [CrossRef] [PubMed]
- Crowley, L. C.; Christensen, M. E.; Waterhouse, N. J., Measuring Mitochondrial Transmembrane Potential by TMRE Staining. Cold Spring Harbor protocols 2016, 2016, (12). [CrossRef]
- Garner, D. L.; Thomas, C. A.; Joerg, H. W.; DeJarnette, J. M.; Marshall, C. E., Fluorometric assessments of mitochondrial function and viability in cryopreserved bovine spermatozoa. Biology of reproduction 1997, 57, (6), 1401-6. [CrossRef]
- Logan, D. C.; Leaver, C. J., Mitochondria-targeted GFP highlights the heterogeneity of mitochondrial shape, size and movement within living plant cells. Journal of experimental botany 2000, 51, (346), 865-71. [CrossRef]
- Ball, E. H.; Singer, S. J., Mitochondria are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proceedings of the National Academy of Sciences of the United States of America 1982, 79, (1), 123-6. [CrossRef]
- Garner, D. L.; Thomas, C. A., Organelle-specific probe JC-1 identifies membrane potential differences in the mitochondrial function of bovine sperm. Molecular reproduction and development 1999, 53, (2), 222-9. [CrossRef]
- Gutscher, M.; Pauleau, A. L.; Marty, L.; Brach, T.; Wabnitz, G. H.; Samstag, Y.; Meyer, A. J.; Dick, T. P., Real-time imaging of the intracellular glutathione redox potential. Nat Methods 2008, 5, (6), 553-9. [CrossRef] [PubMed]
- Chazotte, B., Labeling mitochondria with MitoTracker dyes. Cold Spring Harbor protocols 2011, 2011, (8), 990-2. [CrossRef]
- Haugland, R. P., Probes for Mitochondria. In Molecular Probes Handbook, A Guide to Fluorescent Probes and Labeling Technologies, 11th ed.; Life Technologies: 2010; pp 510-511.
- Patti, M. E.; Corvera, S., The role of mitochondria in the pathogenesis of type 2 diabetes. Endocrine reviews 2010, 31, (3), 364-95. [CrossRef]
- Lin, M. T.; Beal, M. F., Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, (7113), 787-95. [CrossRef]
- Dai, D. F.; Rabinovitch, P. S.; Ungvari, Z., Mitochondria and cardiovascular aging. Circulation research 2012, 110, (8), 1109-24. [CrossRef]
- Porporato, P. E.; Filigheddu, N.; Pedro, J. M. B.; Kroemer, G.; Galluzzi, L., Mitochondrial metabolism and cancer. Cell research 2018, 28, (3), 265-280. [CrossRef]
- Beaulant, A.; Dia, M.; Pillot, B.; Chauvin, M. A.; Ji-Cao, J.; Durand, C.; Bendridi, N.; Chanon, S.; Vieille-Marchiset, A.; Da Silva, C. C.; Patouraux, S.; Anty, R.; Iannelli, A.; Tran, A.; Gual, P.; Vidal, H.; Gomez, L.; Paillard, M.; Rieusset, J., Endoplasmic reticulum-mitochondria miscommunication is an early and causal trigger of hepatic insulin resistance and steatosis. Journal of hepatology 2022, 77, (3), 710-722. [CrossRef]
- Kanellopoulos, A. K.; Mariano, V.; Spinazzi, M.; Woo, Y. J.; McLean, C.; Pech, U.; Li, K. W.; Armstrong, J. D.; Giangrande, A.; Callaerts, P.; Smit, A. B.; Abrahams, B. S.; Fiala, A.; Achsel, T.; Bagni, C., Aralar Sequesters GABA into Hyperactive Mitochondria, Causing Social Behavior Deficits. Cell 2020, 180, (6), 1178-1197.e20. [CrossRef]
- Javani, G.; Babri, S.; Farajdokht, F.; Ghaffari-Nasab, A.; Mohaddes, G., Mitochondrial transplantation improves anxiety- and depression-like behaviors in aged stress-exposed rats. Mechanisms of ageing and development 2022, 202, 111632. [CrossRef]
- Jain, R.; Begum, N.; Tryphena, K. P.; Singh, S. B.; Srivastava, S.; Rai, S. N.; Vamanu, E.; Khatri, D. K., Inter and intracellular mitochondrial transfer: Future of mitochondrial transplant therapy in Parkinson’s disease. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2023, 159, 114268. [CrossRef]
- Lin, R. Z.; Im, G. B.; Luo, A. C.; Zhu, Y.; Hong, X.; Neumeyer, J.; Tang, H. W.; Perrimon, N.; Melero-Martin, J. M., Mitochondrial transfer mediates endothelial cell engraftment through mitophagy. Nature 2024, 629, (8012), 660-668. [CrossRef]
- Chang, J. C.; Chang, H. S.; Wu, Y. C.; Cheng, W. L.; Lin, T. T.; Chang, H. J.; Kuo, S. J.; Chen, S. T.; Liu, C. S., Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. Journal of experimental & clinical cancer research : CR 2019, 38, (1), 30. [CrossRef]
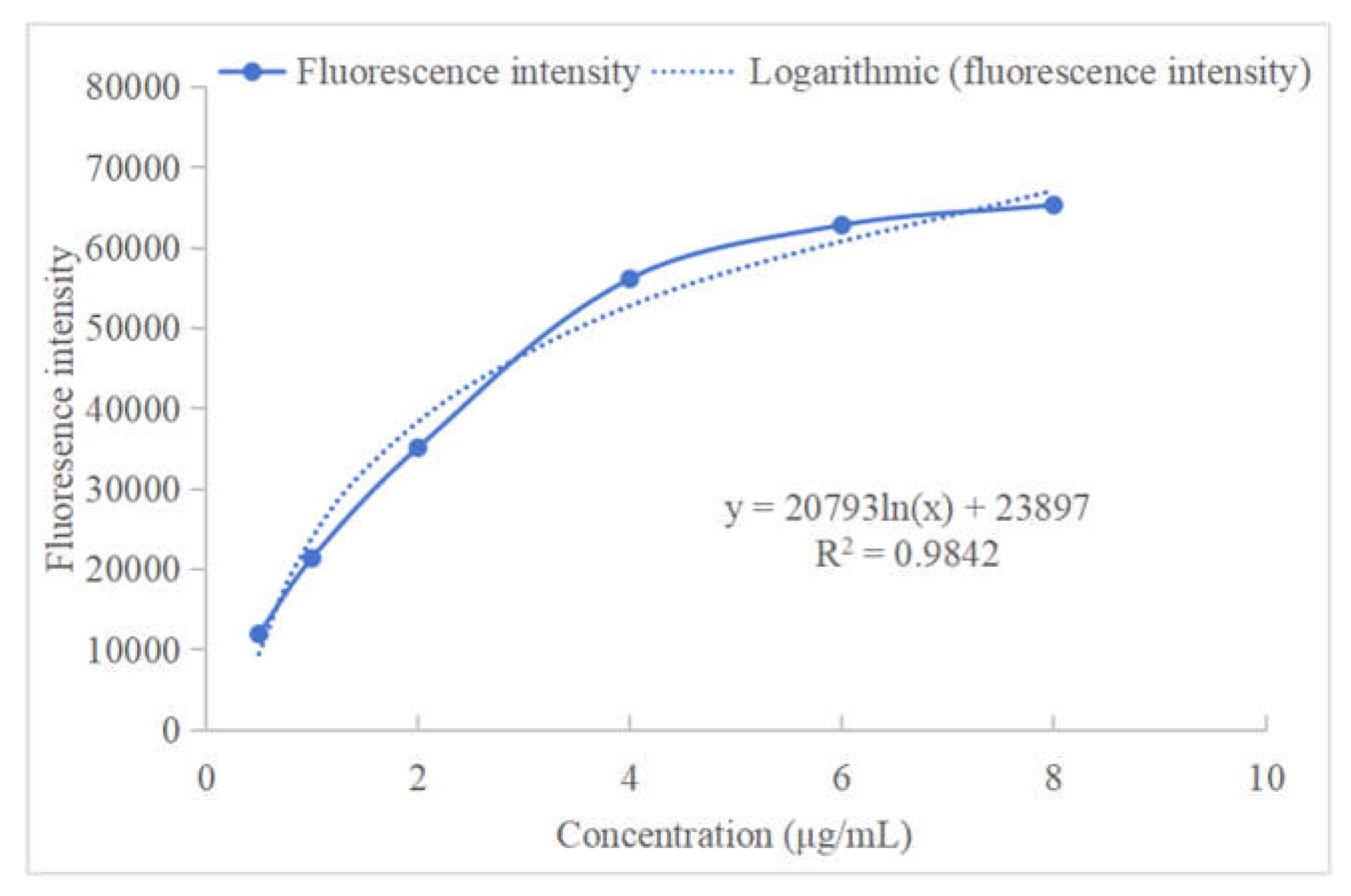
| Concentration (μg/ml) | 0.5 | 1 | 2 | 4 | 6 | 8 |
| Fluorescence intensity | 11944.766 | 21375.713 | 35119.884 | 56129.718 | 62816.1 | 65311.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





