Submitted:
27 August 2024
Posted:
29 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
1. Spine and Sacroiliac Joints
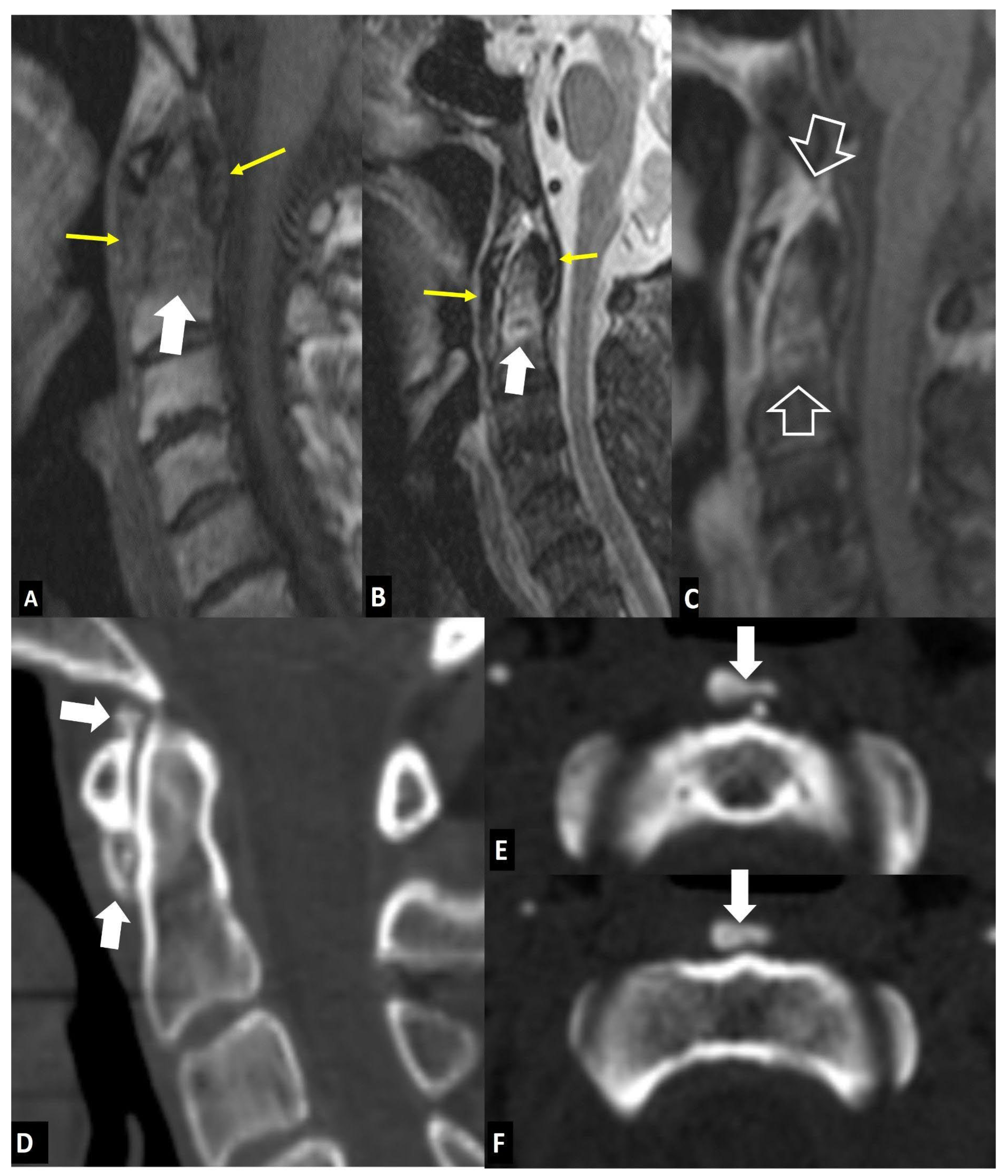
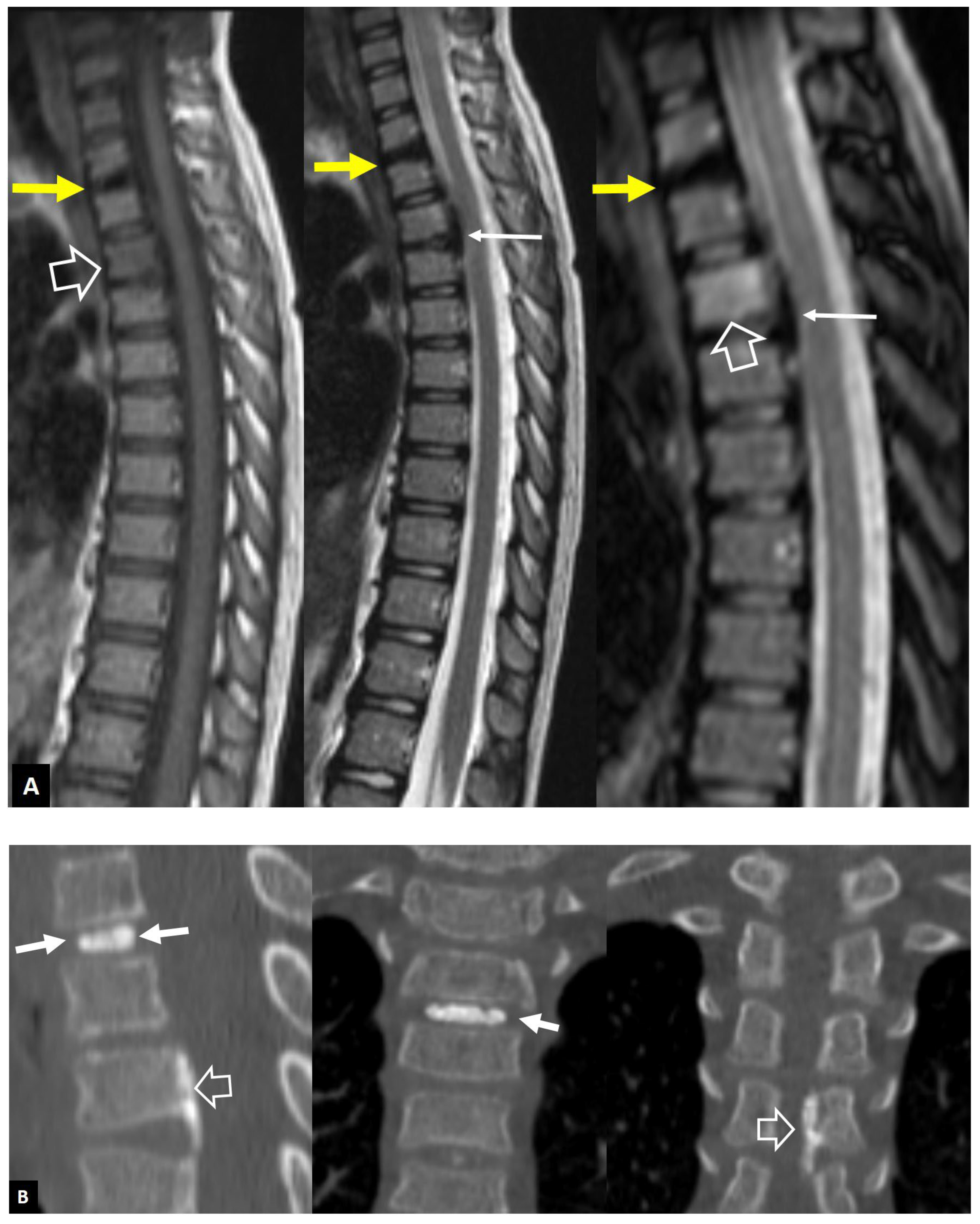
1. a. Non Infectious Discitis and Spondylodiscitis
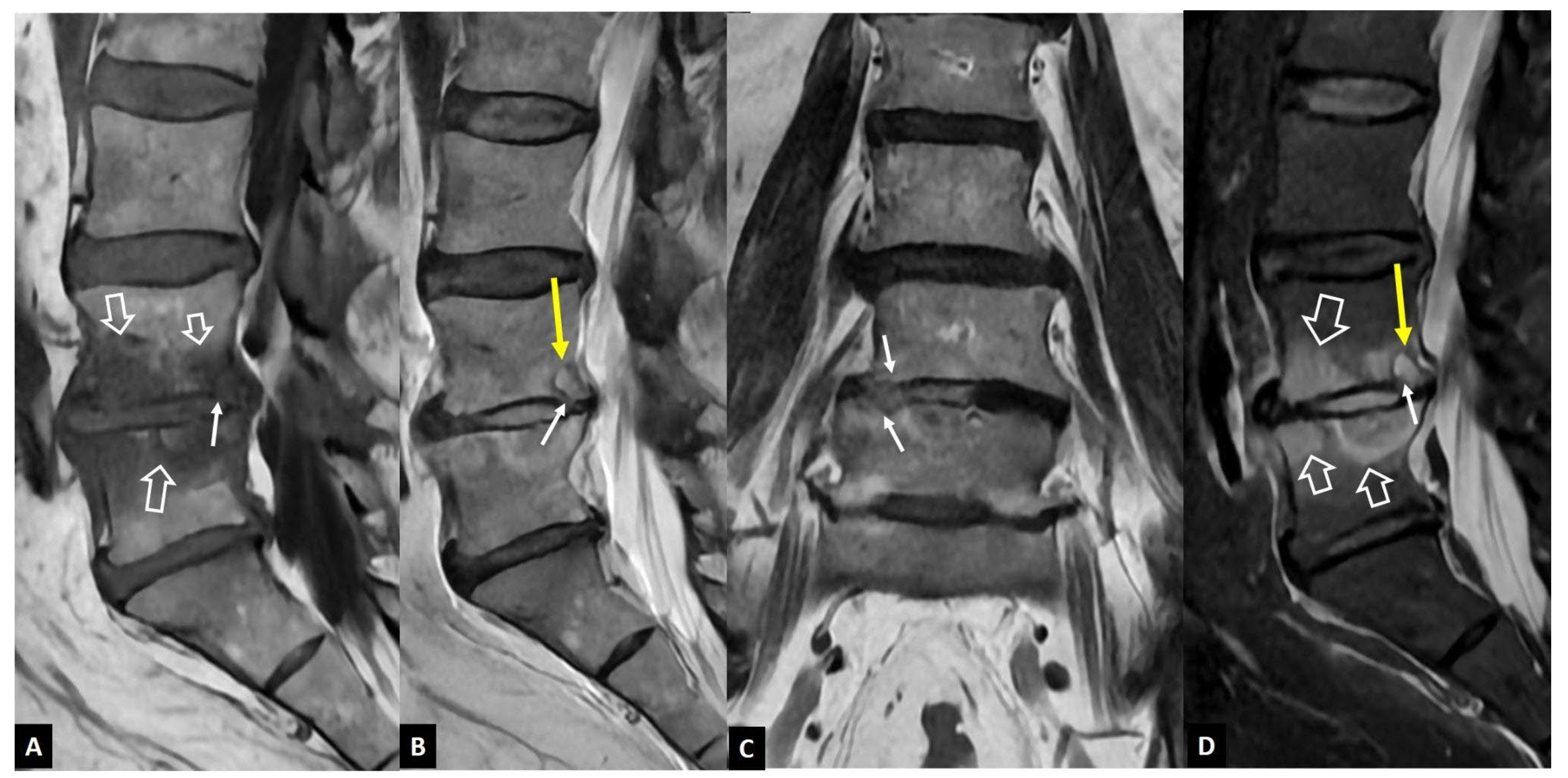
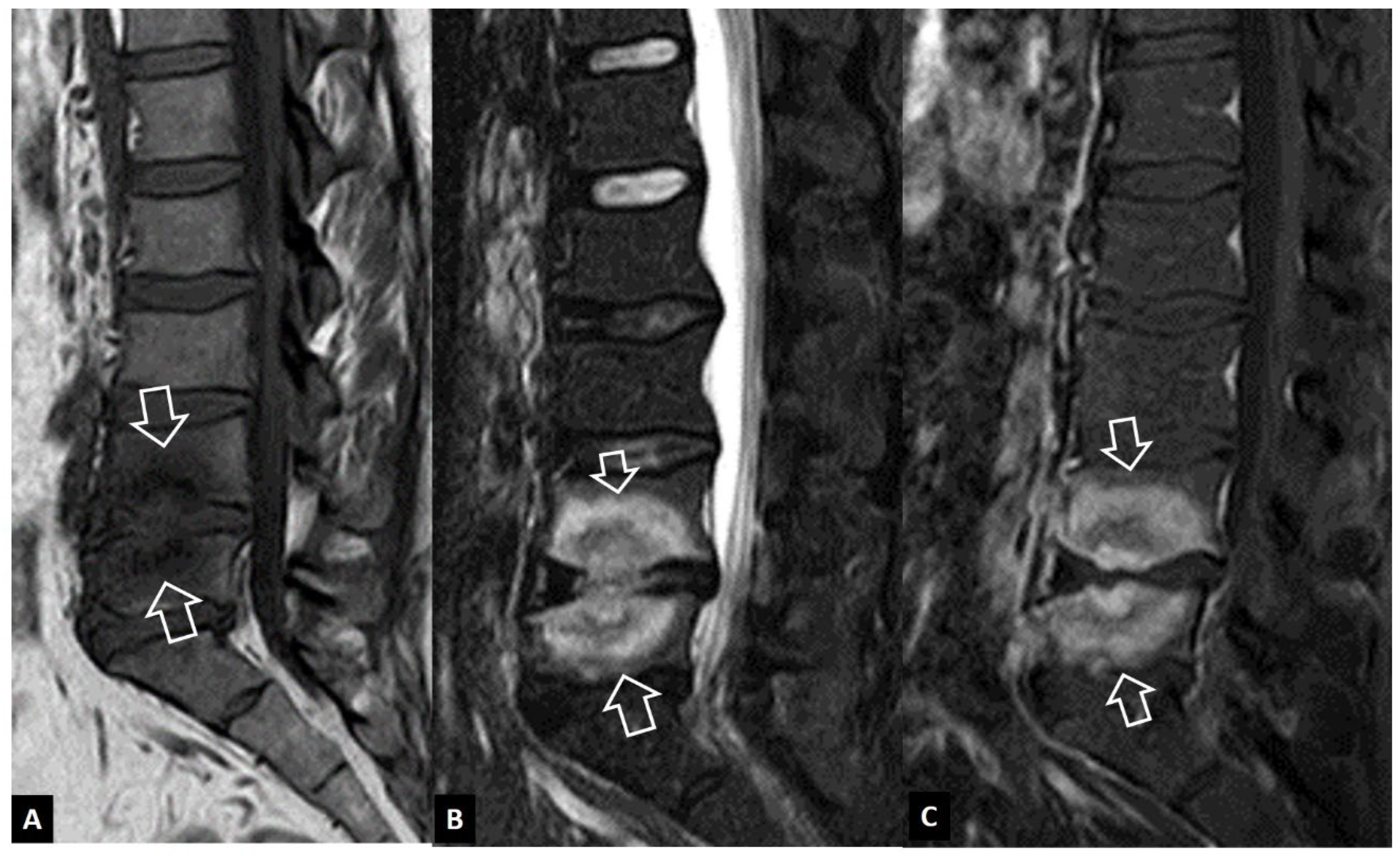
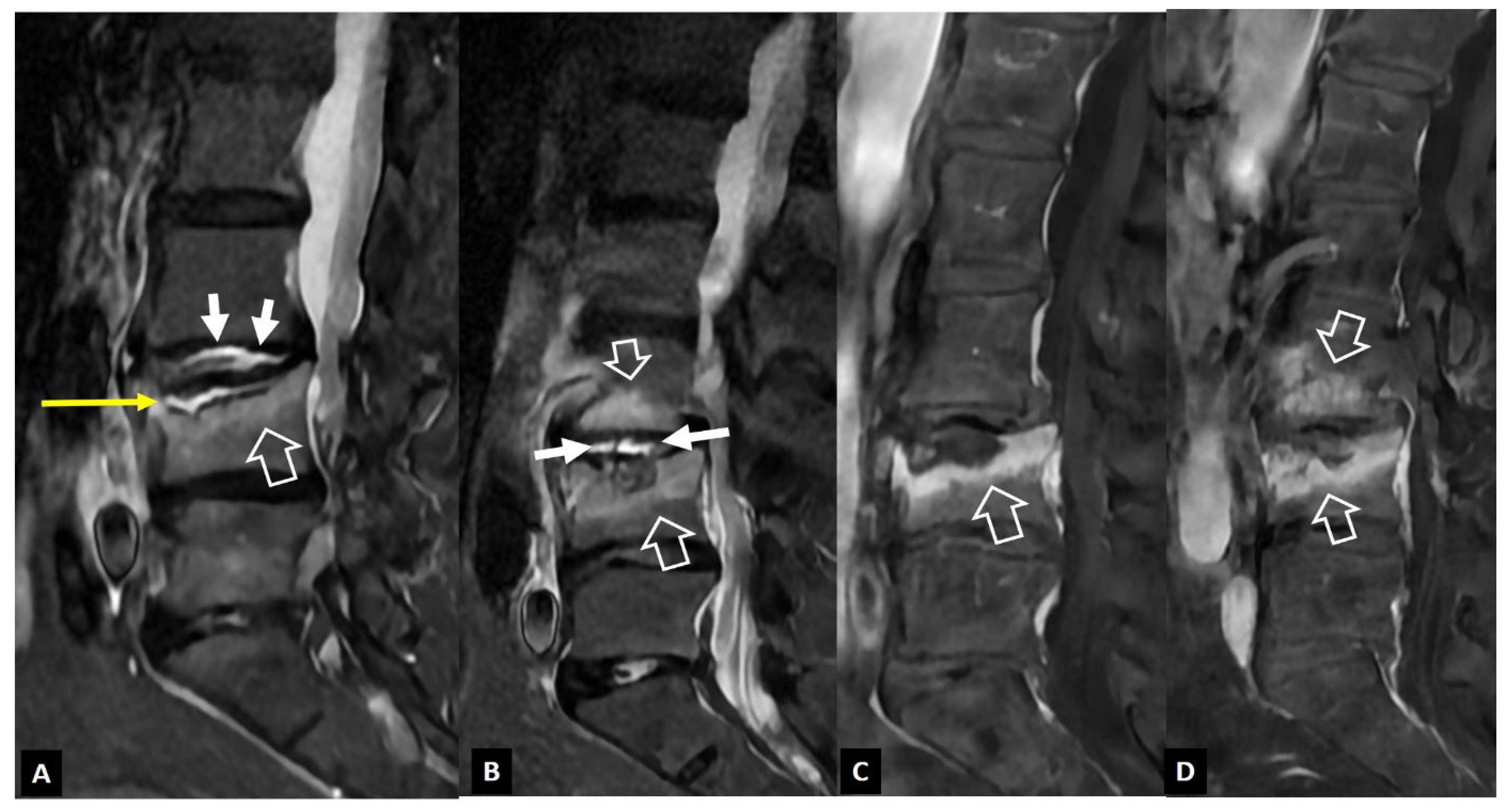
1. a.i. Modic I Changes
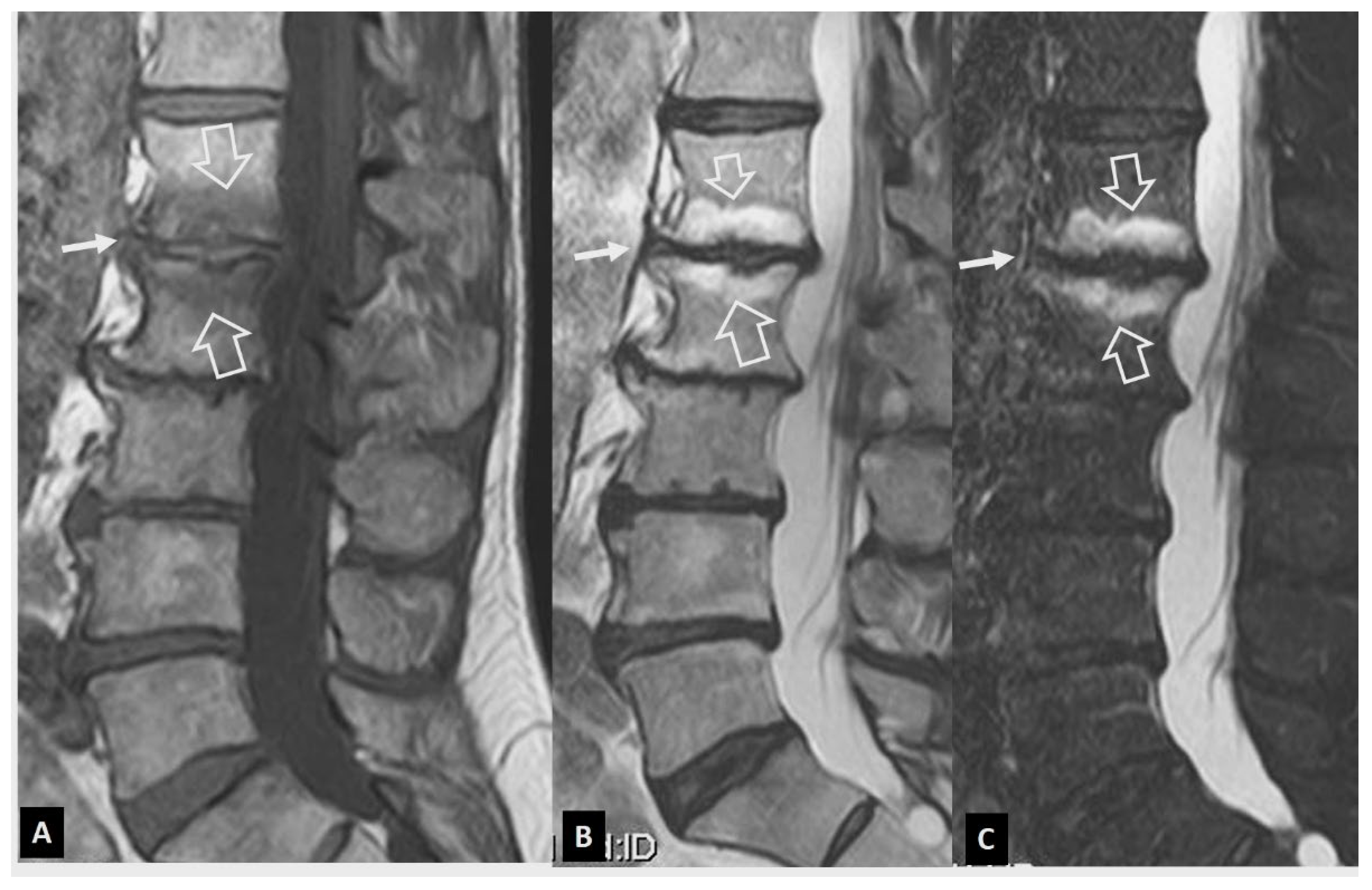
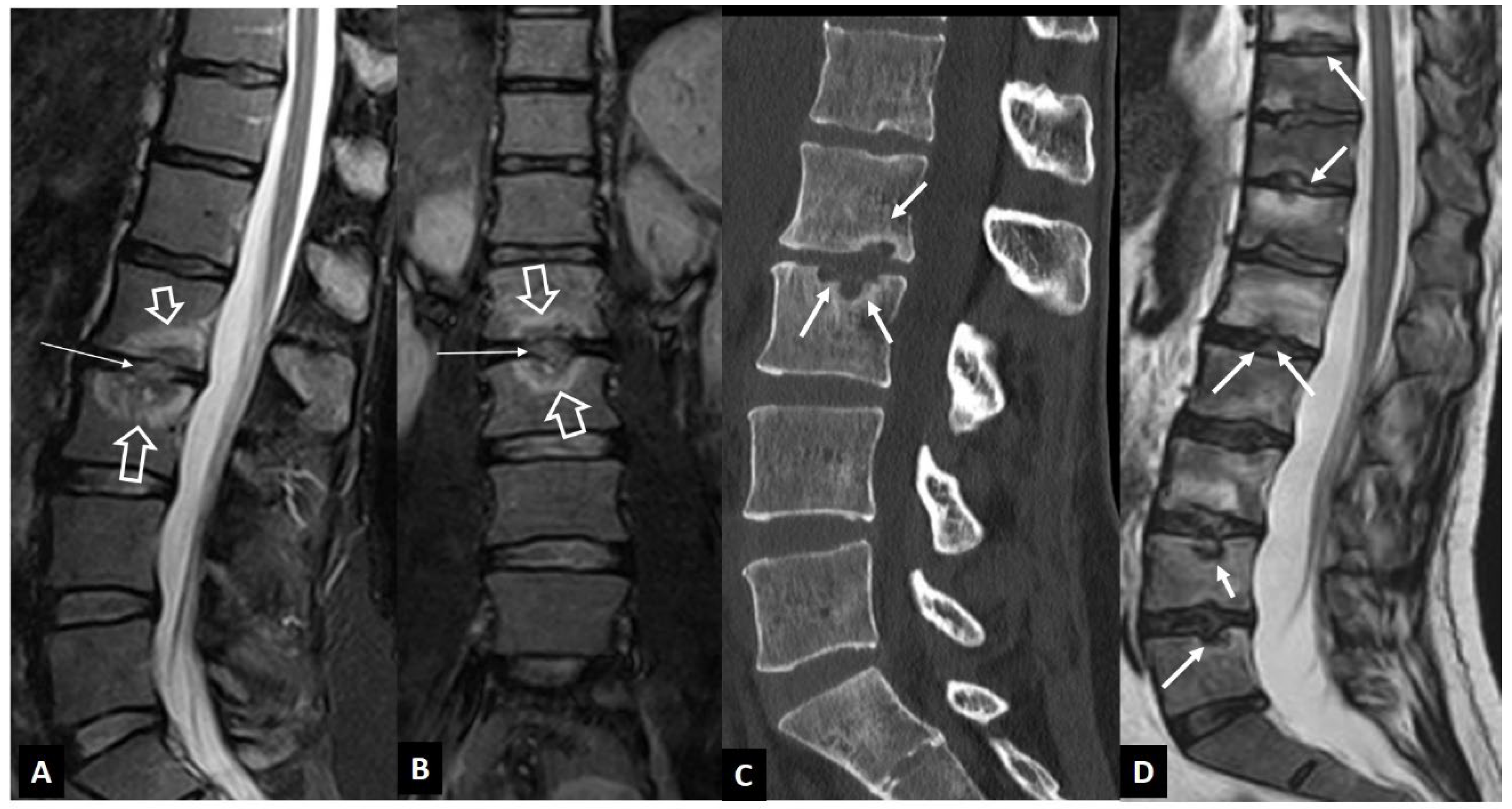
1. a.ii. Aseptic Spondylodiscitis
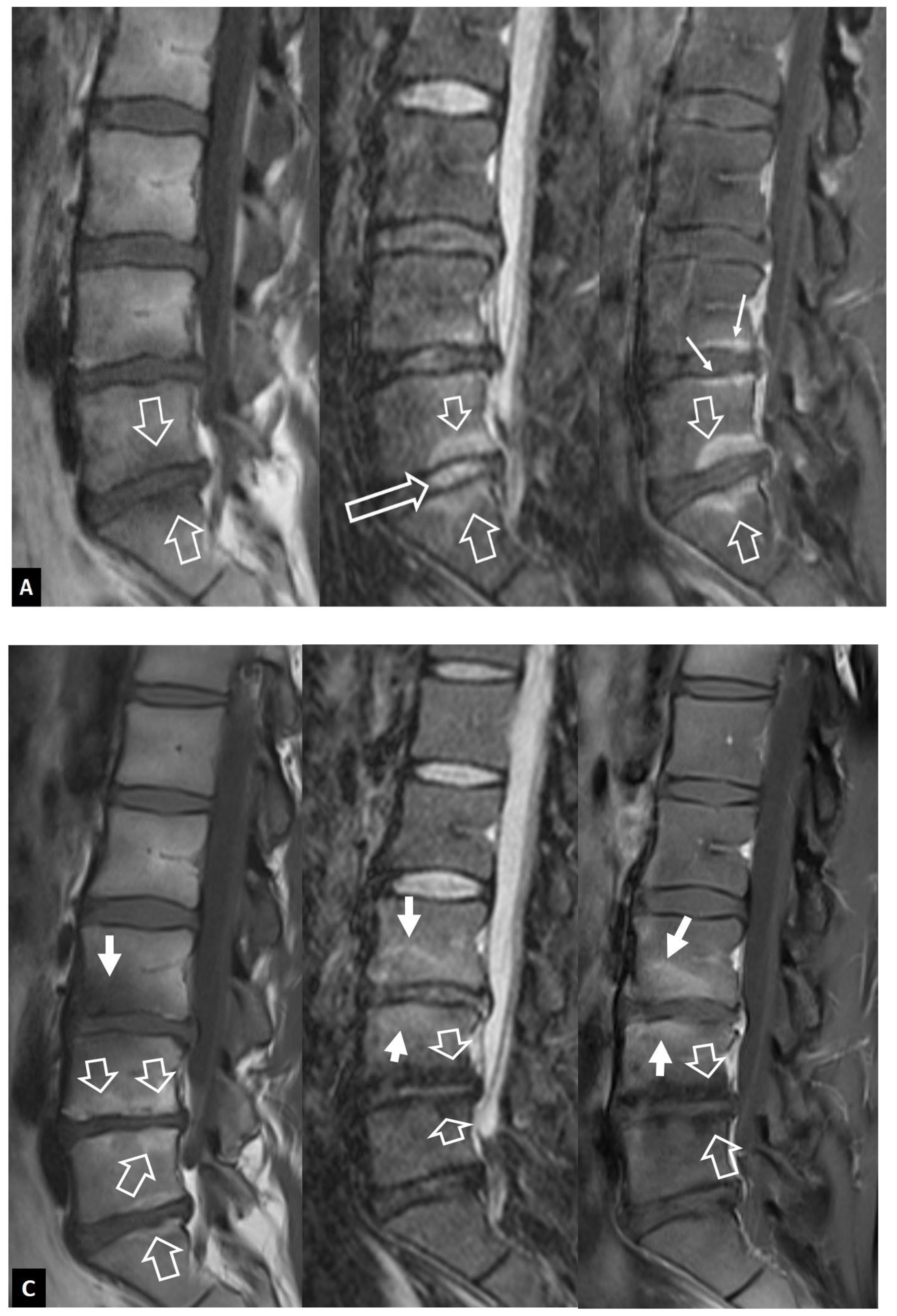
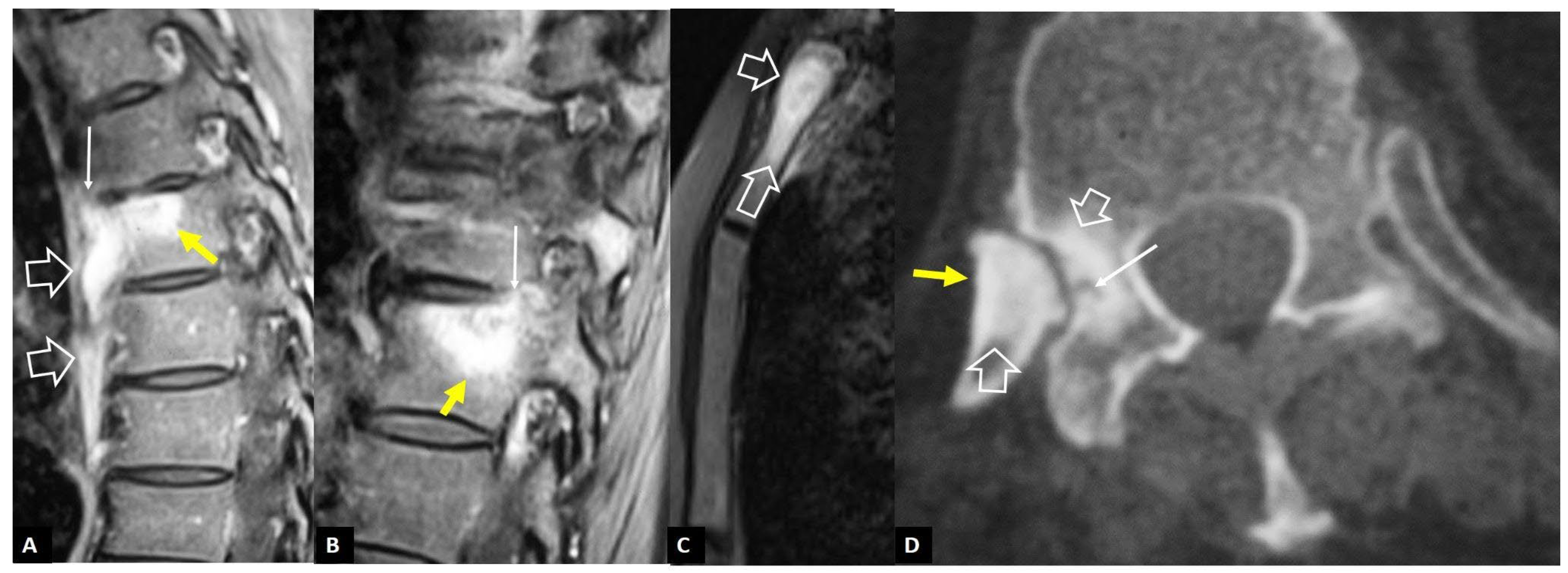
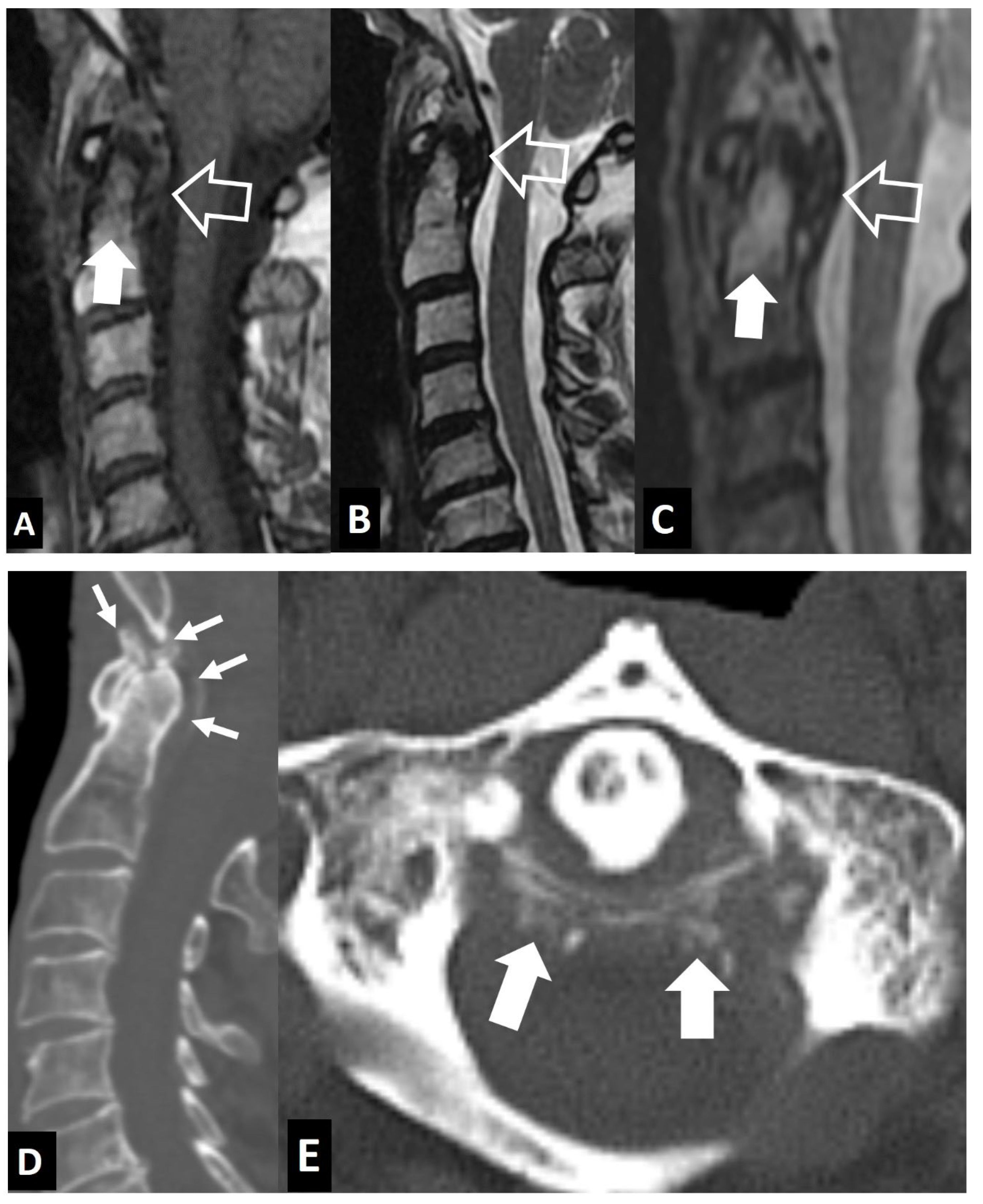
1. a.iii. SAPHO Syndrome
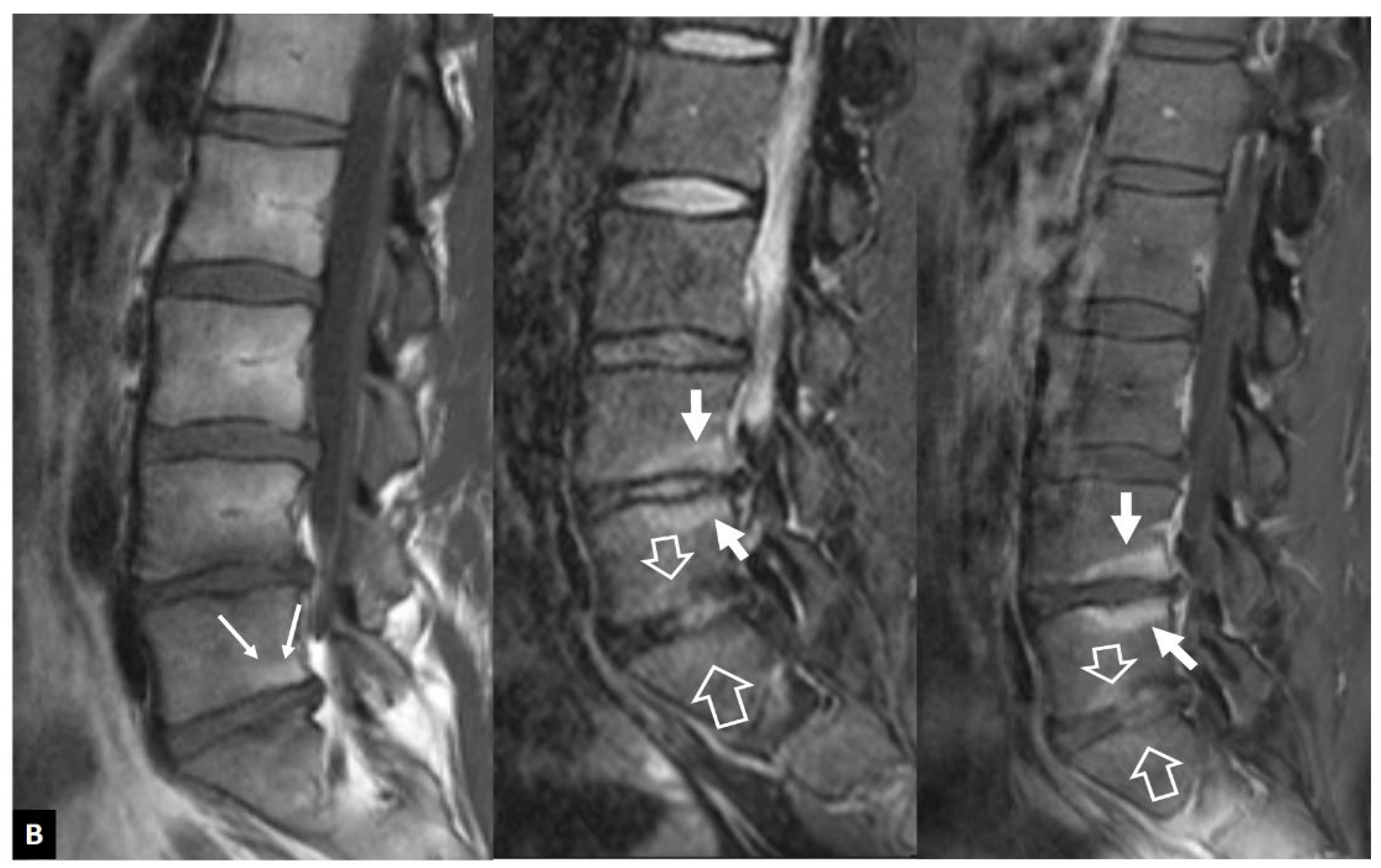
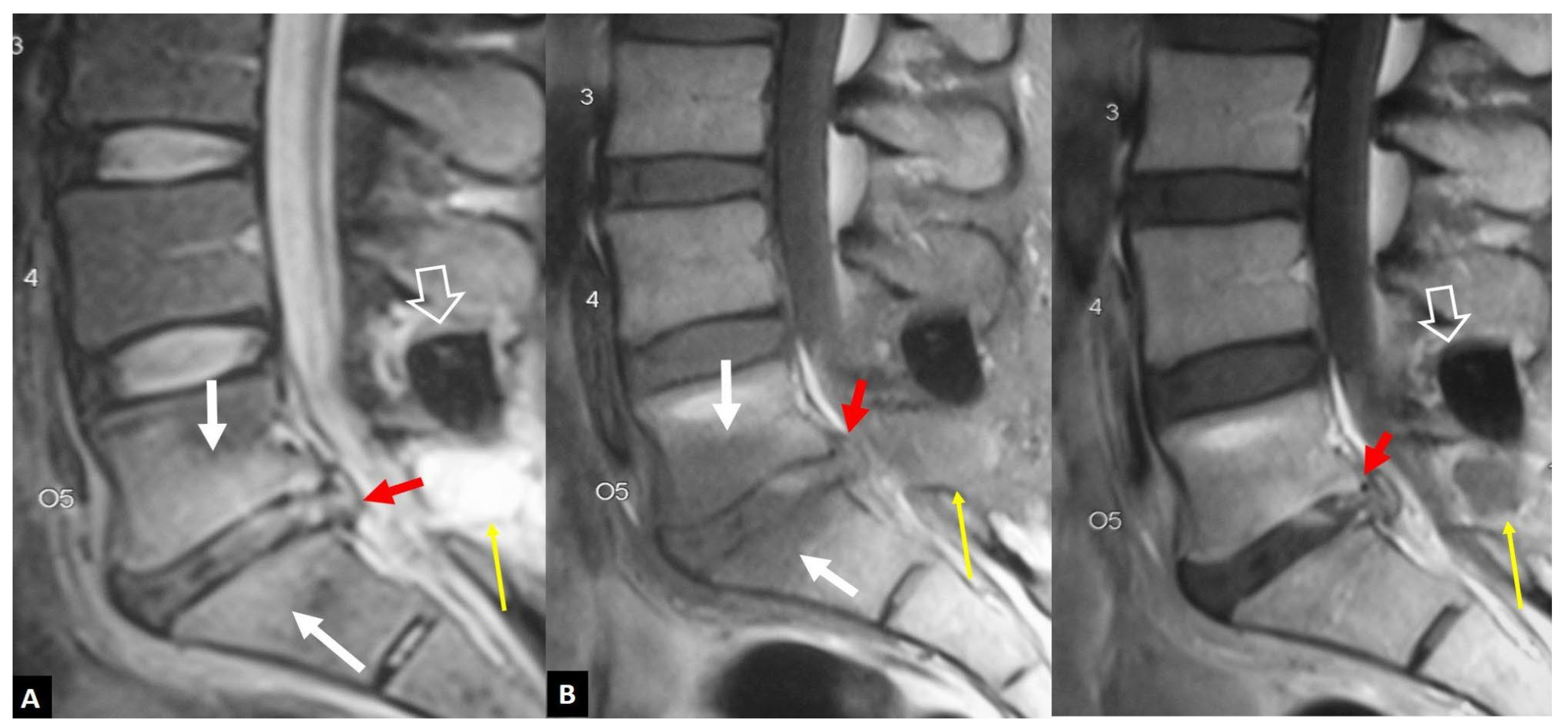
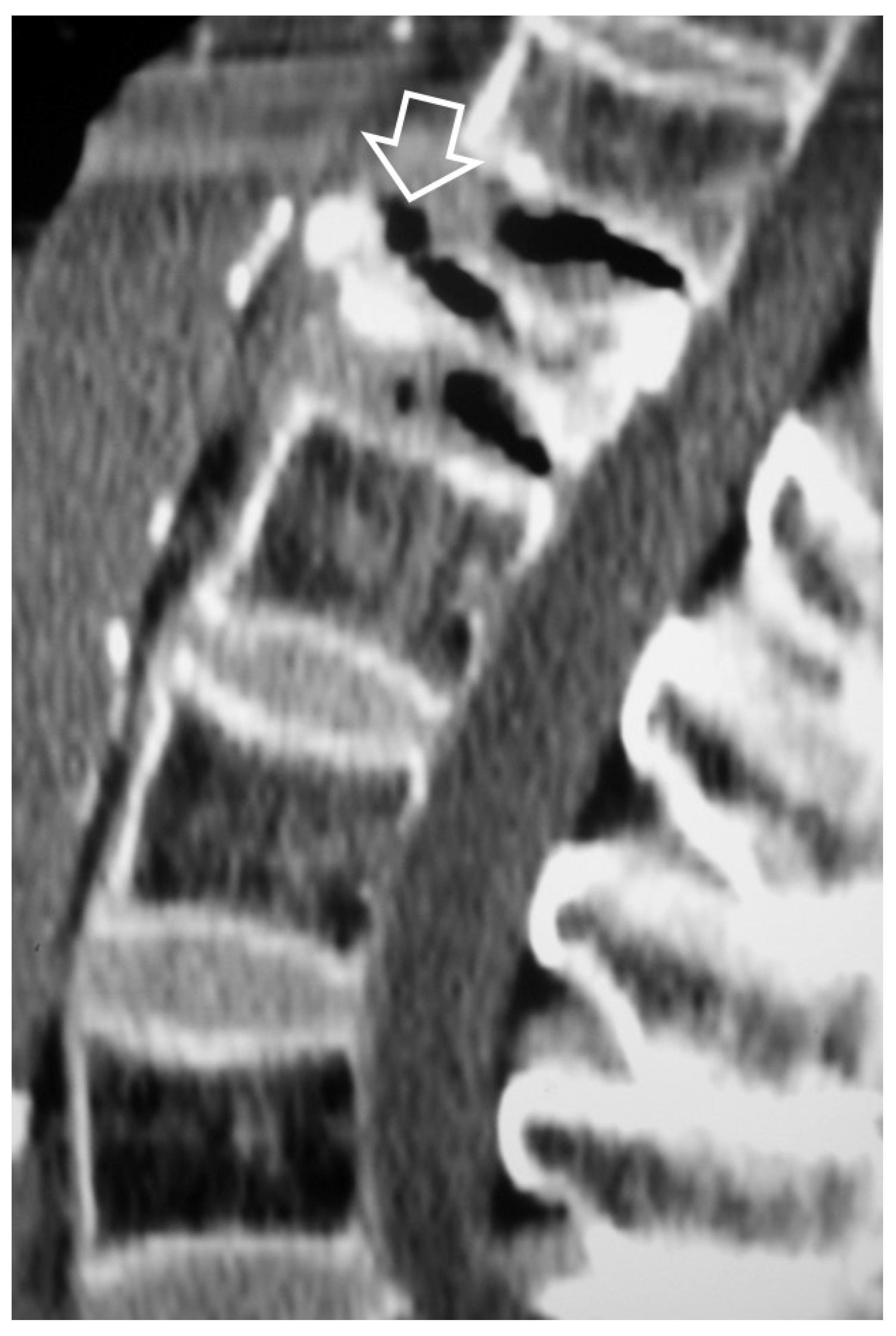
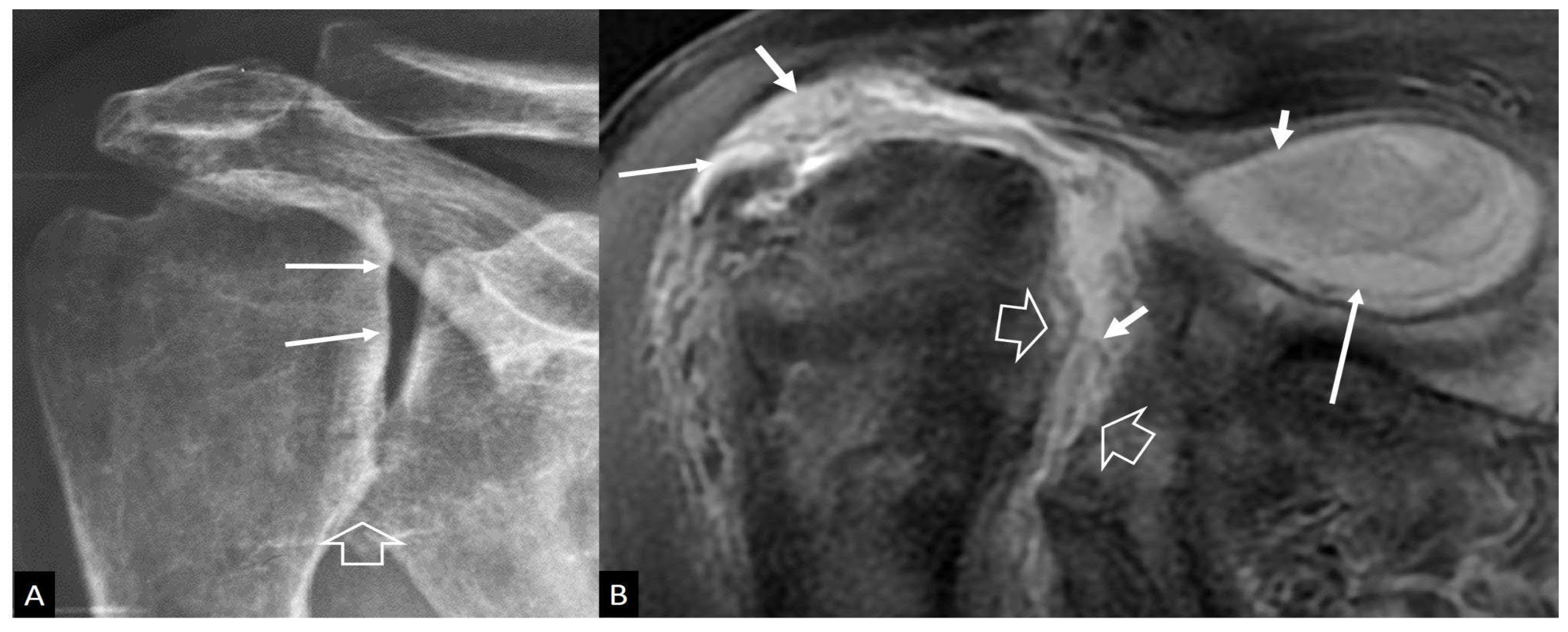
1. a.iv. Destructive Spondyloarthropathy
1. a.v. Crystal Deposition
1. a.vi. Postop Aseptic Discitis
1. a.vii. Trauma
1. a.viii. Rheumatoid Arthritis
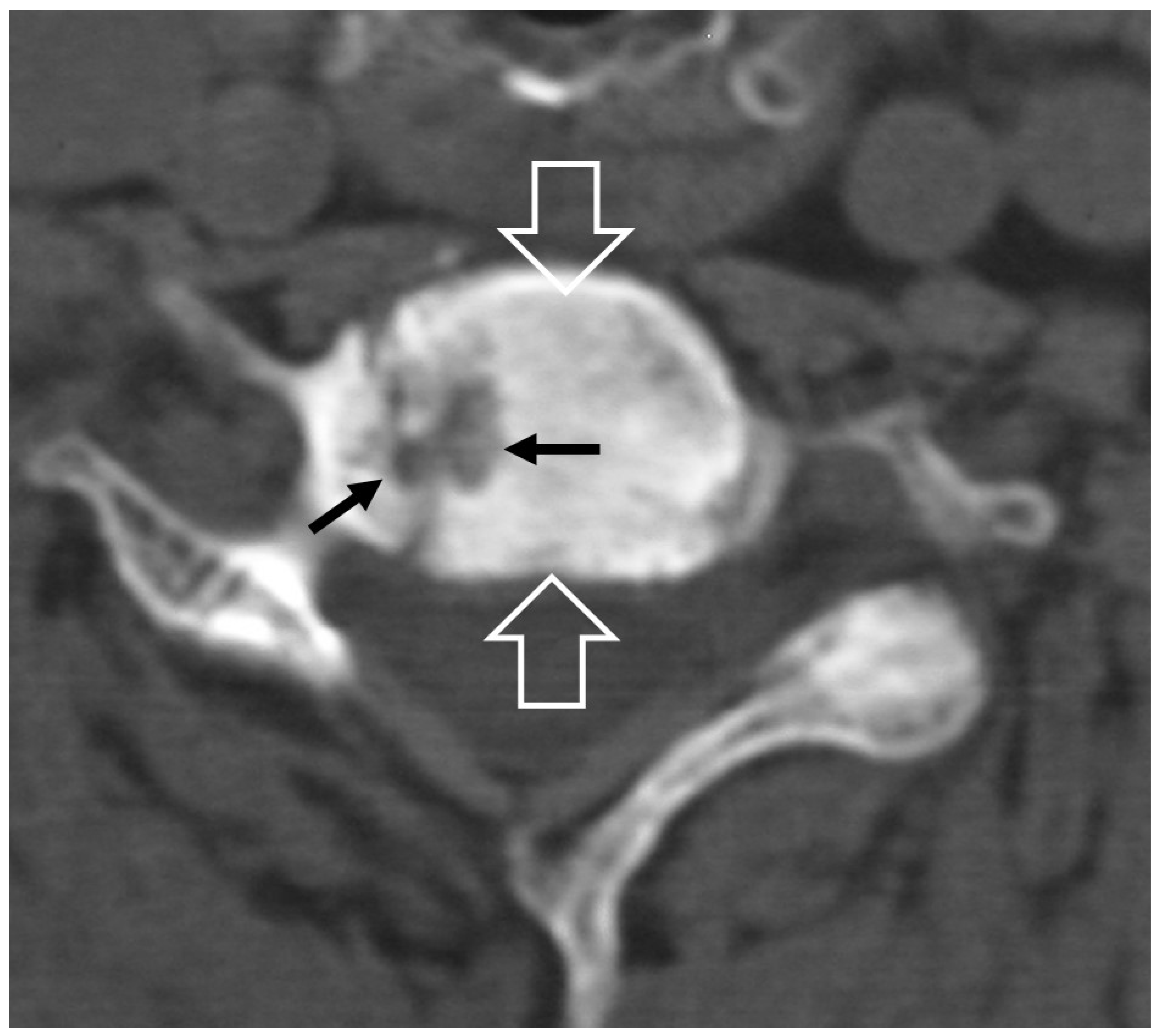
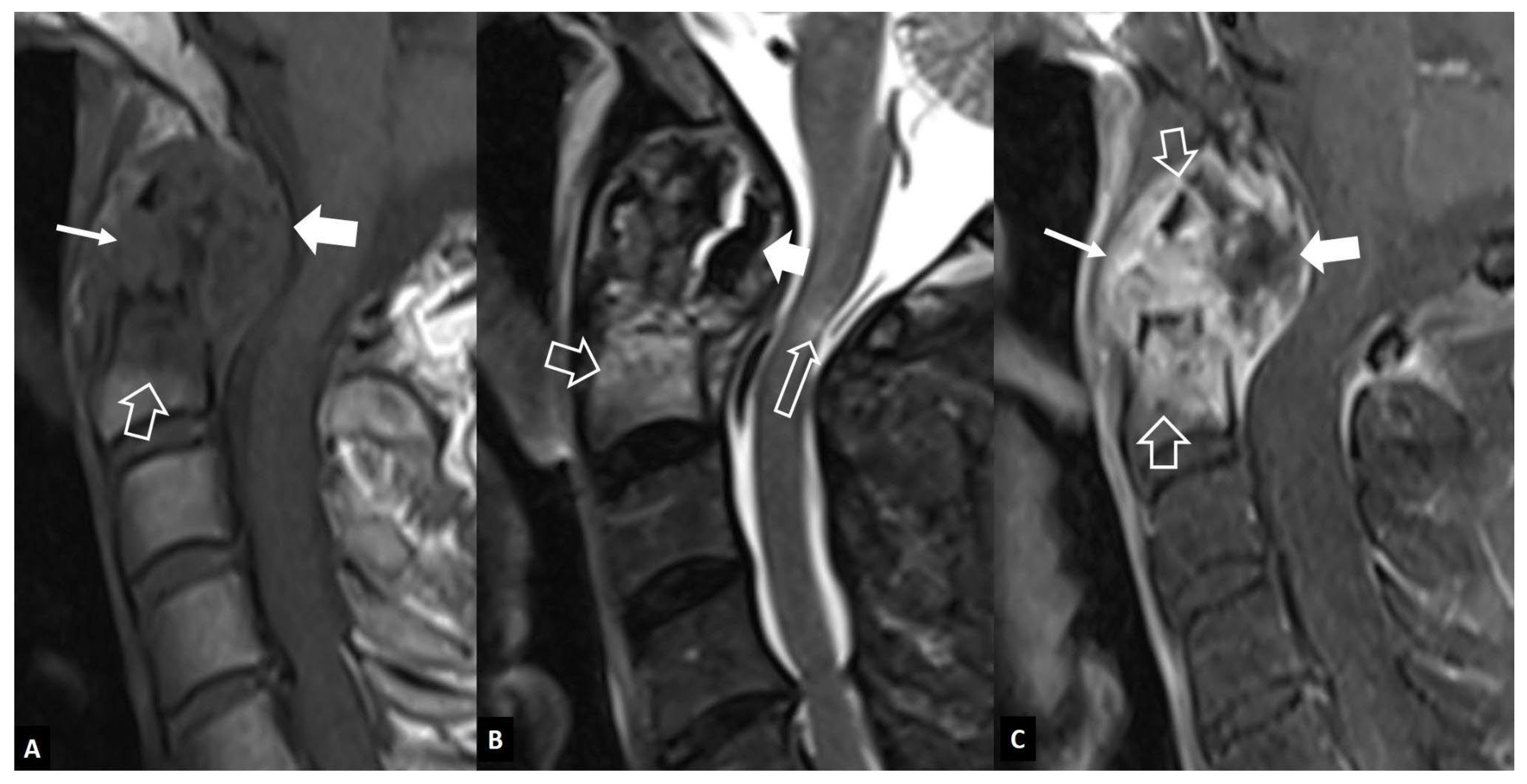
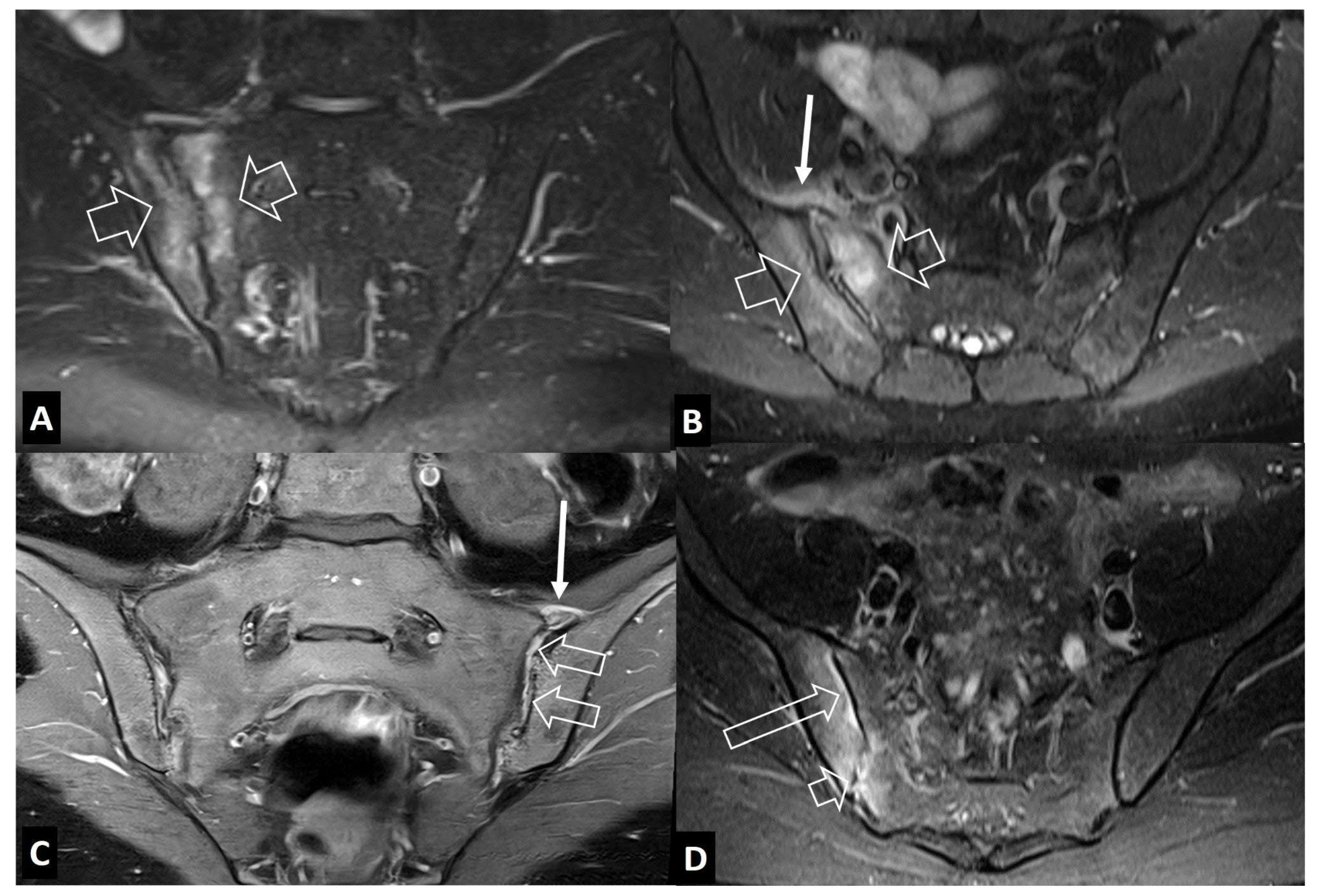
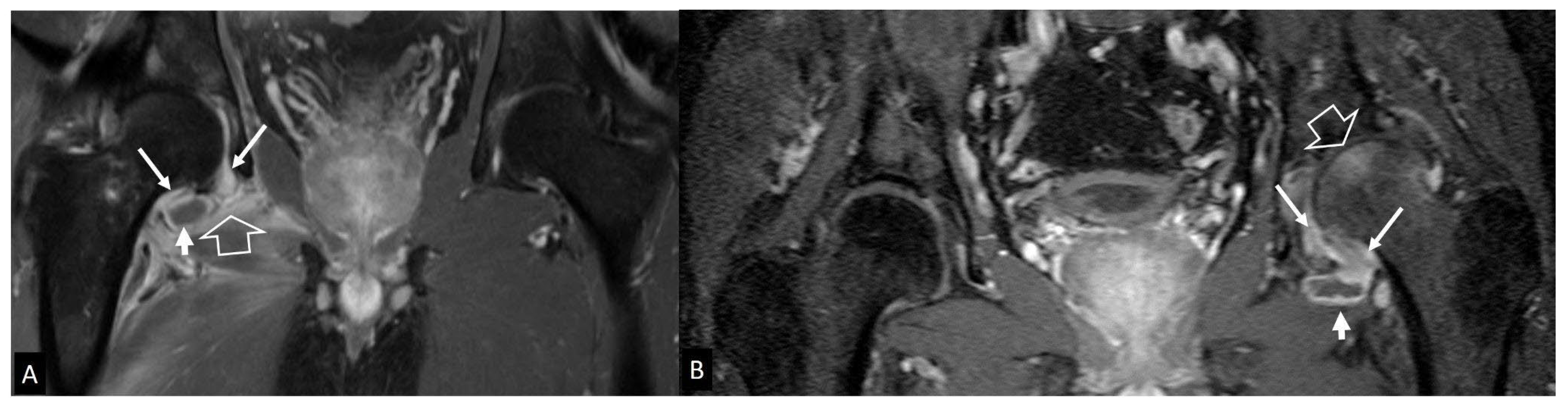
1. b. Non Infectious Sacroiliitis
2. Long Bones
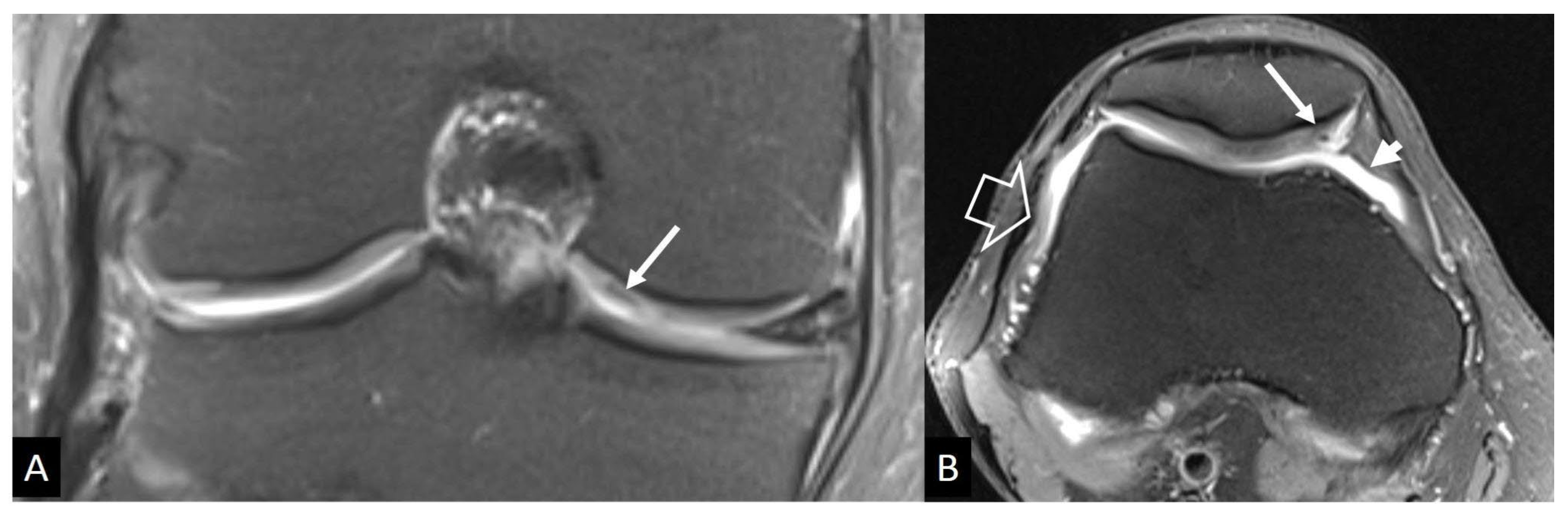
2. a. Stress Injuries
2. b. Neoplasms
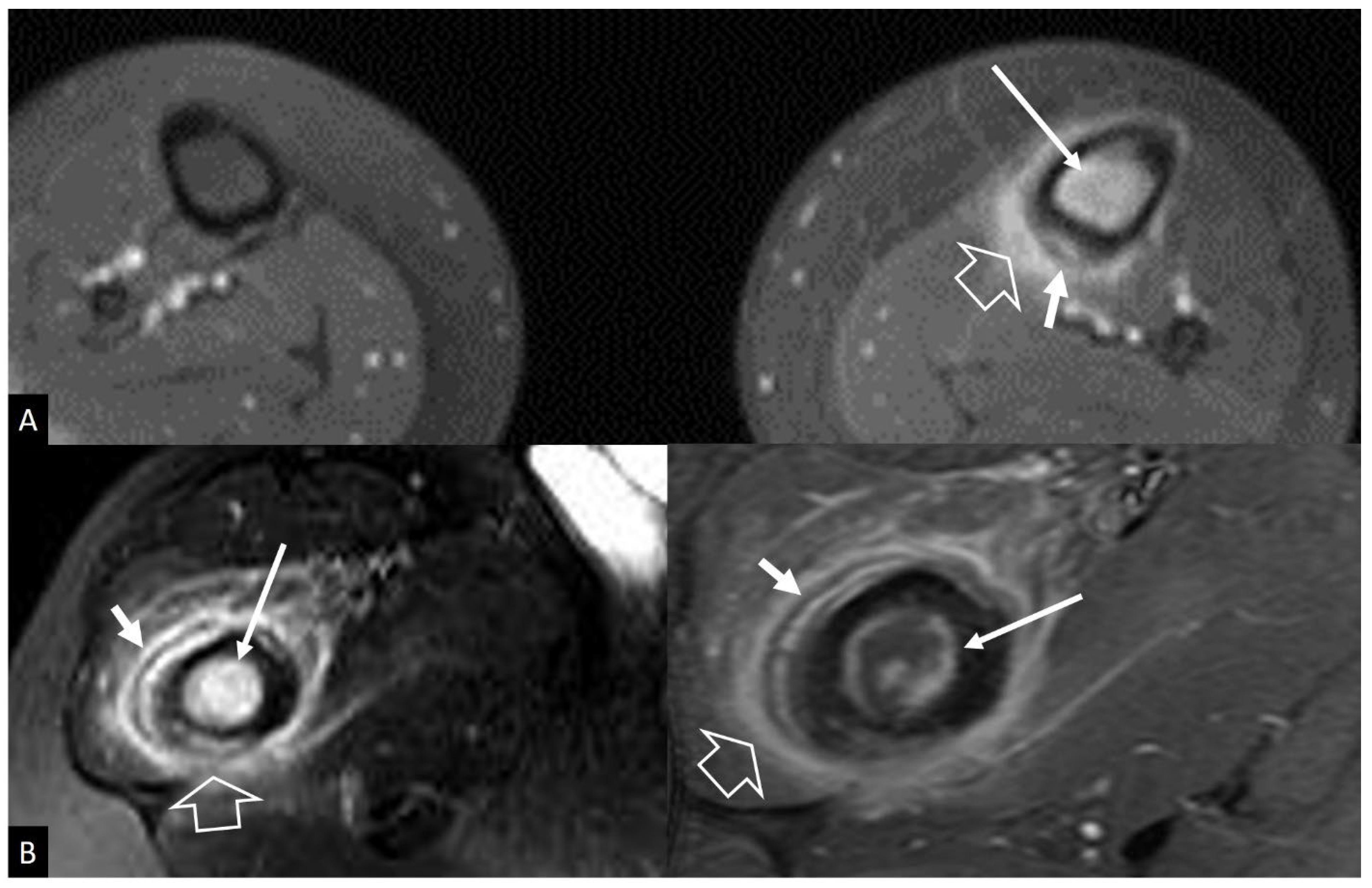
2. b.i. Ewing’s Sarcoma
2. b.ii. Lymphoma
2. c. Radiation Osteiitis - Iatrogenic
3. Peripheral Joints
3. a. Imaging Features of Septic Arthritis
3. b. Imaging Features of Septic Arthritis Mimickers
3. b.i. Inflammatory Arthritis
3. b.ii. Neuropathic Arthropathy
3. b.iii Crystal-Induced Arthropathies
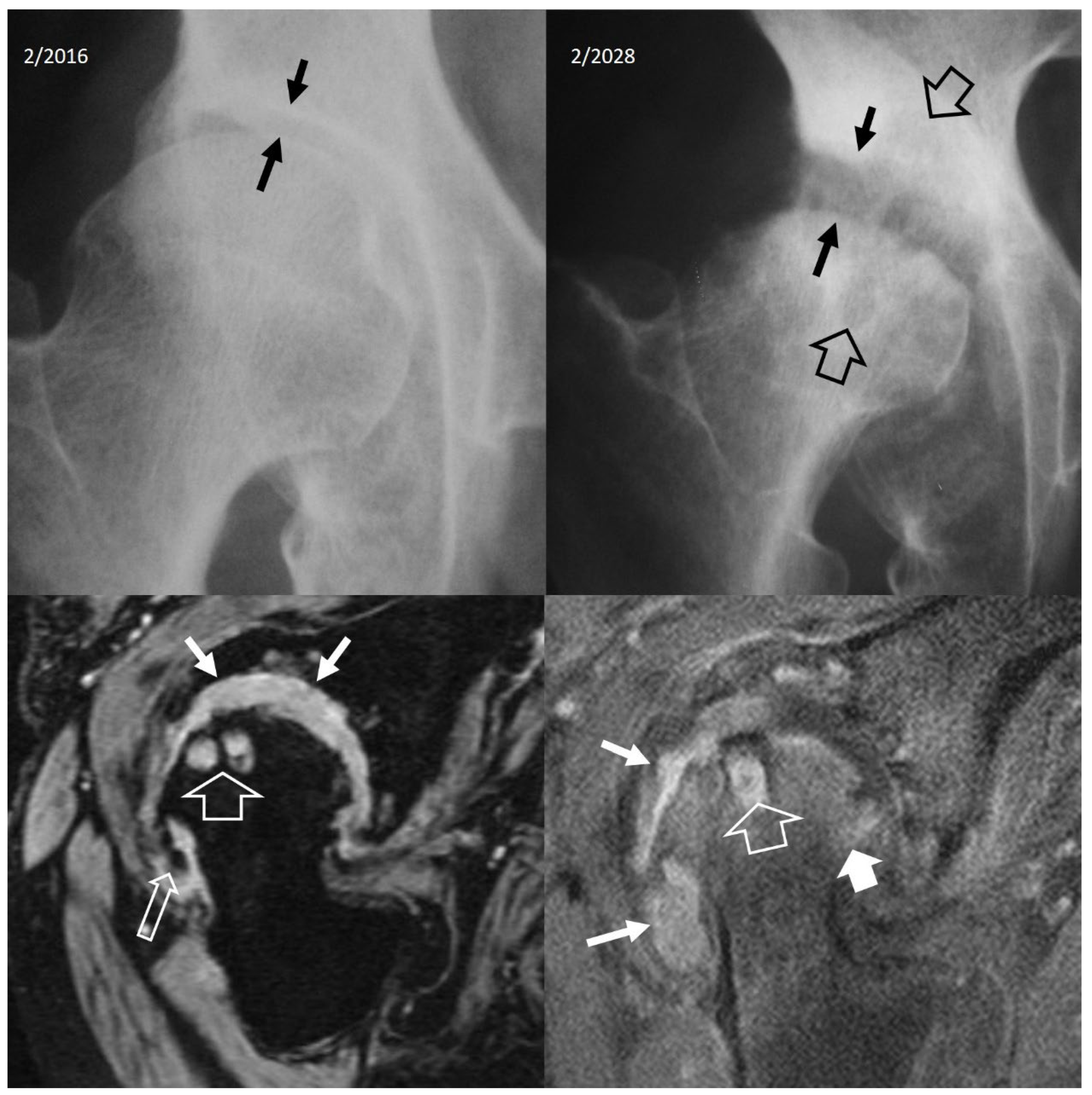
3. b.iv. Rapidly Destructive Osteoarthritis of the Hip
3. b.v. Neoplasms
4. Muscles and Soft Tissues
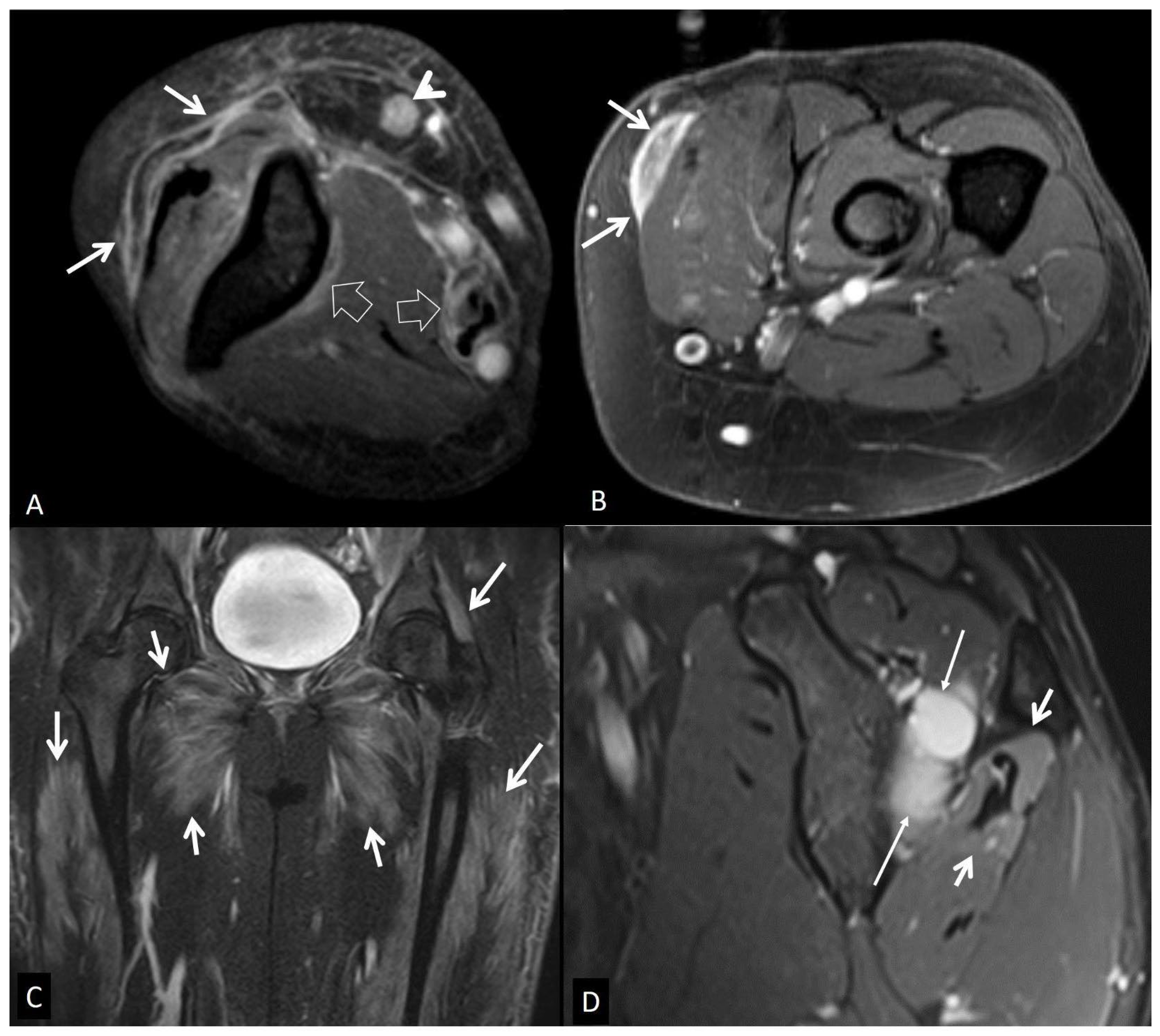
4. a. Non - Infectious Subcutaneous Edema
4. b. Non - Infectious Fsciitis
4. b.i. Eosinophilic Fasciitis
4. b.ii. Paraneoplastic Fasciitis
4. b.iii. Nodular Fasciitis
4. b. Inflammatory Myopathies
4. c. Muscle Denervation
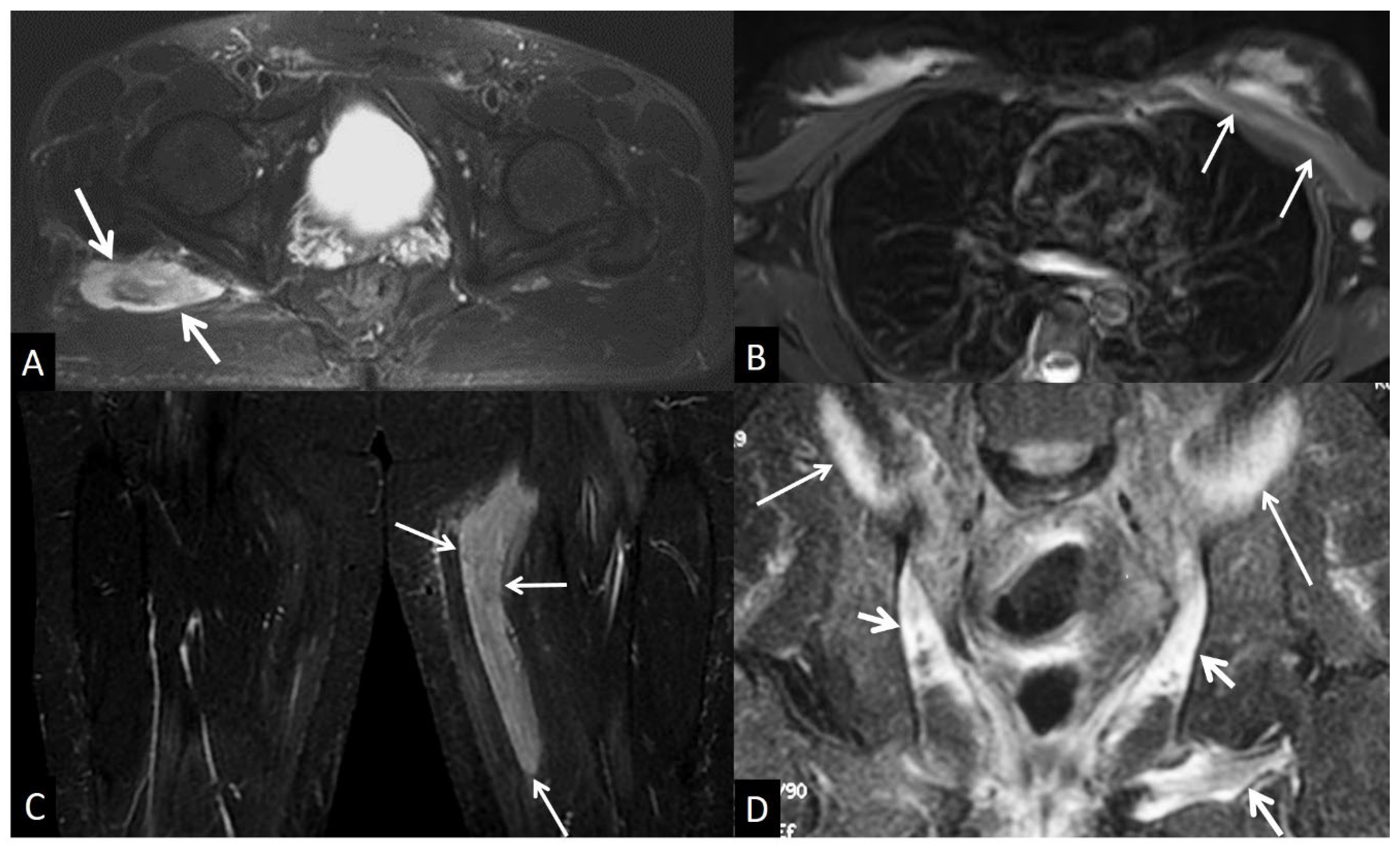
4. d. Traumatic Soft Tissue Injury
4. d.i. Muscle Contusion
4. d.ii. Muscle Strain
4. d.iii. Delayed Onset Muscle Soreness
4. d.iv. Rhabdomyolysis
4. d.v. Myositis Ossificans
4. e. Neoplasm and Post Therapy Soft-Tissue Changes
4. e.i. Neoplasms
4. e.ii. Post Therapy Soft-Tissue Changes
Conclusions
Figures
References
- Shih YC, Thacker MM. Infection Mimics: Differential Diagnoses of Musculoskeletal Infections. In Pediatric Musculoskeletal Infections: Principles & Practice; Belthur, M.V., Ranade, A.S., Herman, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar]
- Kang HJ, Choi HY, Park JS, Park SY, Jin W, Ryu KN Lesions that mimic musculoskeletal infection: A pictorial essay. Korean J Radiol 2018, 78, 200. [CrossRef]
- Lim W, Barras CD, Zadow S. Radiologic Mimics of Osteomyelitis and Septic Arthritis: A Pictorial Essay. Radiol Res Pract 2021, 2021, 9912257. [Google Scholar]
- Crombé A, Fadli D, Clinca R, Reverchon G, Cevolani L, Girolami M, Hauger O, Matcuk GR, Spinnato P. Imaging of Spondylodiscitis: A Comprehensive Updated Review-Multimodality Imaging Findings, Differential Diagnosis, and Specific Microorganisms Detection. Microorganisms 2024. [CrossRef]
- Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166, 193–199. [CrossRef]
- Burke JG, Watson RWG, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br 2002, 84, 196–201. [CrossRef]
- Albert HB, Lambert P, Rollason J, et al Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J 2013, 22, 690–696. [CrossRef]
- Teichtahl AJ, Urquhart DM, Wang Y et al. Modic changes in the lumbar spine and their association with body composition, fat distribution and intervertebral disc height – a 3.0 T-MRI study. BMC Musculoskelet Disord 2016, 17, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Schwarz-Nemec U, Friedrich KM, Stihsen C, Schwarz FK, Trattnig S, Weber M, Grohs JG, Nemec SF. Vertebral Bone Marrow and Endplate Assessment on MR Imaging for the Differentiation of Modic Type 1 Endplate Changes and Infectious Spondylodiscitis. J Clin Med Res. 2020. [CrossRef]
- Oztekin O, Calli C, Kitis O, Adibelli ZH, Eren CS, Apaydin M, Zileli M, Yurtseven T Reliability of diffusion weighted MR imaging in differentiating degenerative and infectious end plate changes. Radiol Oncol 2010, 44, 97–102.
- Andersson O. Rontgenbilden vid spondylarthritis ankylopoetica. 1937.
- Hunter T The spinal complications of ankylosing spondylitis. Semin Arthritis Rheum 1989, 19, 172–182. [CrossRef] [PubMed]
- Kim S-K, Shin K, Song Y, Lee S, Kim T-H Andersson lesions of whole spine magnetic resonance imaging compared with plain radiography in ankylosing spondylitis. Rheumatol Int 2016, 36, 1663–1670. [CrossRef]
- Bennett AN, Rehman A, Hensor EMA, Marzo-Ortega H, Emery P, McGonagle D Evaluation of the diagnostic utility of spinal magnetic resonance imaging in axial spondylarthritis. Arthritis Rheum 2009, 60, 1331–1341. [CrossRef] [PubMed]
- Kabasakal Y, Garrett SL, Calin A The epidemiology of spondylodiscitis in ankylosing spondylitis--a controlled study. Br J Rheumatol 1996, 35, 660–663. [CrossRef] [PubMed]
- Madsen KB, Jurik AG MRI grading method for active and chronic spinal changes in spondyloarthritis. Clin Radiol 2010, 65, 6–14. [CrossRef]
- Park Y-S, Kim J-H, Ryu J-A, Kim T-H The Andersson lesion in ankylosing spondylitis: distinguishing between the inflammatory and traumatic subtypes. J Bone Joint Surg Br 2011, 93, 961–966.
- Assessment of SpondyloArthritis International Society. The Assessment of SpondyloArthritis International Society (ASAS) Handbook: A Guide to Assess Spondyloarthritis. BMJ Publ. Group 2009.
- Benhamou CL, Chamot AM, Kahn MF Synovitis-acne-pustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol 1988, 6, 109–112. [Google Scholar]
- Kuntz D, Naveau B, Bardin T, Drueke T, Treves R, Dryll A Destructive spondylarthropathy in hemodialyzed patients. A new syndrome. Arthritis Rheum 1984, 27, 369–375. [Google Scholar]
- Theodorou DJ, Theodorou SJ, Resnick D Imaging in dialysis spondyloarthropathy. Semin Dial 2002, 15, 290–296.
- Ring D, Vaccaro AR, Scuderi G, Pathria MN, Garfin SR Acute calcific retropharyngeal tendinitis. Clinical presentation and pathological characterization. J Bone Joint Surg Am 1994, 76, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Offiah CE, Hall E Acute calcific tendinitis of the longus colli muscle: spectrum of CT appearances and anatomical correlation. Br J Radiol 2009, 82, e117–e121. [CrossRef]
- Rosenthal AK, Ryan LM Calcium Pyrophosphate Deposition Disease. N Engl J Med 2016, 374, 2575–2584. [CrossRef]
- Bartlett CS 3rd, Casden AM, Abdelwahab IF Calcium pyrophosphate deposition disease mimicking infection in the lumbar spine. Orthopedics 1999, 22, 79–81. [CrossRef]
- Bouvet JP, le Parc JM, Michalski B, Benlahrache C, Auquier L Acute neck pain due to calcifications surrounding the odontoid process: the crowned dens syndrome. Arthritis Rheum 1985, 28, 1417–1420. [CrossRef] [PubMed]
- Ye C, Shi M, Xie D, Wu H, Chen Q, Yang L A rare case of intervertebral disc calcification combined with ossification of the posterior longitudinal ligament in a child: a case report and literature review. BMC Musculoskelet Disord 2024, 25, 118.
- Van Goethem JW, Van de Kelft E, Biltjes IG, van Hasselt BA, van den Hauwe L, Parizel PM, De Schepper AM. MRI after successful lumbar discectomy. Neuroradiology 1996, 38 (Suppl. 1), S90–S96. [Google Scholar] [CrossRef]
- Shafaie FF, Bundschuh C, Jinkins JR. The posttherapeutic lumbosacral spine. Posttherapeutic neurodiagnostic imaging Lippincott-Raven, Philadelphia. 1997.
- Pawar AY, Biswas SK Postoperative spine infections. Asian Spine J 2016, 10, 176–183. [CrossRef]
- Kumar Y, Gupta N, Chhabra A, Fukuda T, Soni N, Hayashi D Magnetic resonance imaging of bacterial and tuberculous spondylodiscitis with associated complications and non-infectious spinal pathology mimicking infections: a pictorial review. BMC Musculoskelet Disord 2017, 18, 244.
- Kumaran SP, Thippeswamy PB, Reddy BN, Neelakantan S, Viswamitra S An Institutional review of tuberculosis spine mimics on MR imaging: Cases of Mistaken Identity. Neurol India 2019, 67, 1408–1418. [CrossRef]
- Joaquim AF, Appenzeller S Cervical spine involvement in rheumatoid arthritis — A systematic review. Autoimmun Rev 2014, 13, 1195–1202. [CrossRef]
- Interligator S, Le Bozec A, Cluzel G, et al. Infectious sacroiliitis: MRI- and CT-based assessment of disease extent, complications, and anatomic correlation. Skeletal Radiol. 2023. [CrossRef]
- Kang Y, Hong SH, Kim JY, Yoo HJ, Choi J-Y, Yi M, Kang HS Unilateral sacroiliitis: Differential diagnosis between infectious sacroiliitis and spondyloarthritis based on MRI findings. AJR Am J Roentgenol 2015, 205, 1048–1055. [CrossRef]
- Mulligan ME The “gray cortex”: an early sign of stress fracture. Skeletal Radiol 1995, 24, 201–203.
- Berger FH, de Jonge MC, Maas M Stress fractures in the lower extremity. The importance of increasing awareness amongst radiologists. Eur J Radiol 2007, 62, 16–26. [Google Scholar]
- Wright AA, Hegedus EJ, Lenchik L, Kuhn KJ, Santiago L, Smoliga JM Diagnostic Accuracy of Various Imaging Modalities for Suspected Lower Extremity Stress Fractures: A Systematic Review With Evidence-Based Recommendations for Clinical Practice. Am J Sports Med 2016, 44, 255–263. [CrossRef] [PubMed]
- Lee YJ, Sadigh S, Mankad K, Kapse N, Rajeswaran G The imaging of osteomyelitis. Quant Imaging Med Surg 2016, 6, 184–198.
- Henninger B, Glodny B, Rudisch A, Trieb T, Loizides A, Putzer D, Judmaier W, Schocke MF Ewing sarcoma versus osteomyelitis: differential diagnosis with magnetic resonance imaging. Skeletal Radiol 2013, 42, 1097–1104. [CrossRef]
- McCarville MB, Chen JY, Coleman JL, Li Y, Li X, Adderson EE, Neel MD, Gold RE, Kaufman RA Distinguishing Osteomyelitis From Ewing Sarcoma on Radiography and MRI. AJR Am J Roentgenol 2015, 205, 640–650.
- Guermazi A, Brice P, de Kerviler E E, Fermé C, Hennequin C, Meignin V, Frija J Extranodal Hodgkin disease: spectrum of disease. Radiographics 2001, 21, 161–179.
- Majeed A, Chan O, Okolo O, Shponka V, Georgescu A, Persky D Hodgkin Lymphoma Mimicking Osteomyelitis. Case Rep Oncol 2017, 10, 542–547.
- Mika J, Schleicher I, Gerlach U, Adler C-P, Uhl M, Knoeller SM Primary bone lymphomas thought to be osteomyelitis urgently demand a rapid diagnosis in bone pathology. Anticancer Res 2012, 32, 4905–4912.
- Mulligan ME, McRae GA, Murphey MD Imaging features of primary lymphoma of bone. AJR Am J Roentgenol 1999, 173, 1691–1697. [CrossRef]
- Krishnan A, Shirkhoda A, Tehranzadeh J, Armin AR, Irwin R, Les K Primary bone lymphoma: radiographic-MR imaging correlation. Radiographics 2003, 23, 1371–1383. [CrossRef]
- Mulligan ME, Kransdorf MJ Sequestra in primary lymphoma of bone: prevalence and radiologic features. AJR Am J Roentgenol 1993, 160, 1245–1248. [CrossRef] [PubMed]
- Phal PM, Myall RWT, Assael LA, Weissman JL Imaging findings of bisphosphonate-associated osteonecrosis of the jaws. AJNR Am J Neuroradiol 2007, 28, 1139–1145. [CrossRef]
- Ross JJ Septic arthritis of native joints. Infect Dis Clin North Am 2017, 31, 203–218. [CrossRef] [PubMed]
- Graif M, Schweitzer ME, Deely D, Matteucci T The septic versus nonseptic inflamed joint: MRI characteristics. Skeletal Radiol 1999, 28, 616–620. [CrossRef]
- Pierce JL, Perry MT, Wessell DE, et al ACR Appropriateness Criteria® Suspected Osteomyelitis, Septic Arthritis, or Soft Tissue Infection (Excluding Spine and Diabetic Foot): 2022 Update. J Am Coll Radiol 2022, 19, S473–S487. [CrossRef]
- Simpfendorfer CS Radiologic approach to musculoskeletal infections. Infect Dis Clin North Am 2017, 31, 299–324. [CrossRef]
- Hansford BG, Stacy GS Musculoskeletal aspiration procedures. Semin Intervent Radiol 2012, 29, 270–285. [CrossRef] [PubMed]
- Montgomery NI, Rosenfeld S Pediatric osteoarticular infection update. J Pediatr Orthop 2015, 35, 74–81. [CrossRef] [PubMed]
- Karchevsky M, Schweitzer ME, Morrison WB, Parellada JA MRI findings of septic arthritis and associated osteomyelitis in adults. AJR Am J Roentgenol 2004, 182, 119–122. [CrossRef] [PubMed]
- Hong SH, Kim SM, Ahn JM, Chung HW, Shin MJ, Kang HS Tuberculous versus pyogenic arthritis: MR imaging evaluation. Radiology 2001, 218, 848–853. [CrossRef] [PubMed]
- Colebatch AN, Edwards CJ, Østergaard M, et al EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis 2013, 72, 804–814. [CrossRef]
- Weishaupt D, Schweitzer ME MR imaging of septic arthritis and rheumatoid arthritis of the shoulder. Magn Reson Imaging Clin N Am 2004, 12, 111–124. [CrossRef]
- McQueen FM, Gao A, Ostergaard M, King A, Shalley G, Robinson E, Doyle A, Clark B, Dalbeth N High-grade MRI bone oedema is common within the surgical field in rheumatoid arthritis patients undergoing joint replacement and is associated with osteitis in subchondral bone. Ann Rheum Dis 2007, 66, 1581–1587.
- Boutry N, Morel M, Flipo R-M, Demondion X, Cotten A Early rheumatoid arthritis: a review of MRI and sonographic findings. AJR Am J Roentgenol 2007, 189, 1502–1509. [CrossRef]
- Amrami KK Imaging of the seronegative spondyloarthopathies. Radiol Clin North Am 2012, 50, 841–854. [CrossRef]
- Jones EA, Manaster BJ, May DA, Disler DG () Neuropathic osteoarthropathy: diagnostic dilemmas and differential diagnosis. Radiographics 2000, 20, S279–S293. [CrossRef] [PubMed]
- Ahmadi ME, Morrison WB, Carrino JA, Schweitzer ME, Raikin SM, Ledermann HP Neuropathic arthropathy of the foot with and without superimposed osteomyelitis: MR Imaging Characteristics. Radiology 2006, 238, 622–631. [CrossRef]
- Rosenbaum AJ, DiPreta JA Classifications in brief: Eichenholtz classification of Charcot arthropathy. Clin Orthop Relat Res 2015, 473, 1168–1171. [CrossRef]
- Schumacher HR Crystal-induced arthritis: an overview. Am J Med 1996, 100, 46S–52S. [CrossRef] [PubMed]
- Abhishek A Calcium pyrophosphate deposition disease: a review of epidemiologic findings. Curr Opin Rheumatol 2016, 28, 133–139. [CrossRef] [PubMed]
- Rosales-Alexander JL, Balsalobre Aznar J, Magro-Checa C Calcium pyrophosphate crystal deposition disease: diagnosis and treatment. Open Access Rheumatol 2014, 6, 39–47.
- Mandl P, D’Agostino MA, Navarro-Compán V, et al 2023 EULAR recommendations on imaging in diagnosis and management of crystal-induced arthropathies in clinical practice. Ann Rheum Dis 2024, 83, 752–759. [CrossRef]
- Frediani B, Filippou G, Falsetti P, Lorenzini S, Baldi F, Acciai C, Siagkri C, Marotto D, Galeazzi M, Marcolongo R Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: ultrasonographic criteria proposed. Ann Rheum Dis 2005, 64, 638–640. [CrossRef]
- Buckens CF, Terra MP, Maas M Computed Tomography and MR Imaging in crystalline-induced arthropathies. Radiol Clin North Am 2017, 55, 1023–1034. [CrossRef]
- Gersing AS, Schwaiger BJ, Heilmeier U, et al Evaluation of Chondrocalcinosis and Associated Knee Joint Degeneration Using MR Imaging: Data from the Osteoarthritis Initiative. Eur Radiol 2017, 27, 2497–2506. [CrossRef]
- Lequesne M Rapid destructive coxarthritis. Rhumatologie 1970, 22, 51–63.
- Flemming DJ, Gustas-French CN Rapidly progressive osteoarthritis: a Review of the clinical and radiologic presentation. Curr Rheumatol Rep 2017, 19, 42. [CrossRef] [PubMed]
- Boutry N, Paul C, Leroy X, Fredoux D, Migaud H, Cotten A Rapidly destructive osteoarthritis of the hip: MR imaging findings. AJR Am J Roentgenol 2002, 179, 657–663. [CrossRef] [PubMed]
- Zazgyva A, Gurzu S, Gergely I, Jung I, Roman CO, Pop TS Clinico-radiological diagnosis and grading of rapidly progressive osteoarthritis of the hip. Medicine 2017, 96, e6395. [CrossRef]
- Chai JW, Hong SH, Choi J-Y, Koh YH, Lee JW, Choi J-A, Kang HS Radiologic diagnosis of osteoid osteoma: from simple to challenging findings. Radiographics 2010, 30, 737–749. [CrossRef]
- Bedoya MA, Iwasaka-Neder J, Tsai A, Bixby SD Intra-articular osteoid osteomas: Imaging manifestations and mimics. Radiographics 2024, 44, e230208. [CrossRef]
- Bohndorf, K. Infection of the appendicular skeleton. Eur Radiol 2004, 14 (Suppl. 3), E53–E63. [Google Scholar] [CrossRef] [PubMed]
- Davies AM, Grimer R The penumbra sign in subacute osteomyelitis. Eur Radiol 2005, 15, 1268–1270. [CrossRef]
- Hayeri MR, Pouya Ziai, Shehata ML, Teytelboym OM, Huang BK Soft-Tissue infections and their imaging mimics: From cellulitis to necrotizing fasciitis. RadioGraphics 2016, 36, 1888–1910. [CrossRef] [PubMed]
- Yu JS, Habib P. MR imaging of urgent inflammatory and infectious conditions affecting the soft tissues of the musculoskeletal system. Emerg Radiol 2009, 16, 267–276. [Google Scholar] [CrossRef]
- Baumann F, Brühlmann P, Andreisek G, Michel BA, Marincek B, Weishaupt D. MRI for diagnosis and monitoring of patients with eosinophilic fasciitis. AJR Am J Roentgenol 2005, 184, 169–174. [Google Scholar] [CrossRef]
- Gaeta M, Mileto A, Musumeci O. MRI findings of neutrophilic fasciitis in a patient with acute febrile neutrophilic dermatosis (Sweet’s syndrome). Skeletal Radiol 2011, 40, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Leung LY, Shu SJ, Chan AC, Chan MK, Chan CH. Nodular fasciitis: MRI appearance and literature review. Skeletal Radiol 2002, 31, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Wang XL, De Schepper AM, Vanhoenacker F, et al. Nodular fasciitis: correlation of MRI findings and histopathology. Skeletal Radiol 2002, 31, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Tanboon J, Uruha A, Stenzel W, Nishino I. Where are we moving in the classification of idiopathic inflammatory myopathies? Curr Opin Neurol. 2020, 33, 590–603. [Google Scholar] [CrossRef]
- Bottai M, Tjärnlund A, Santoni G et al. EULAR/ACR Classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: A methodology report. RMD Open. 2017, 3, e000507. [Google Scholar] [CrossRef]
- O’Connell MJ, Powell T, Brennan D, Lynch T, McCarthy CJ, Eustace SJ. Whole-body MR imaging in the diagnosis of polymyositis. AJR Am J Roentgenol 2002, 179, 967–971. [Google Scholar] [CrossRef]
- S. Kamath, N. Venkatanarasimha, M. A. Walsh & P. M. Hughes. MRI appearance of muscle denervation. Skeletal Radiol 2008, 37, 397–404. [Google Scholar]
- Petersilge CA, Pathria MN, Gentili A, Recht MP, Resnick D. Denervation hypertrophy of muscle: MR features. J Comput Assist Tomogr 1995, 19, 596–600. [Google Scholar] [CrossRef]
- Guermazi A, Roemer F, Robinson P, Tol J, Regatte R, Crema M. Imaging of muscle injuries in sports medicine: Sports imaging series. Radiology. 2017, 282, 646–663. [Google Scholar] [CrossRef]
- Lu CH, Tsang YM, Yu CW, Wu MZ, Hsu CY, Shih TT. Rhabdomyolysis: magnetic resonance imaging and computed tomography findings. J Comput Assist Tomogr 2007, 31, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Beltran J, Simon DC, Katz W, Weis LD. Increased MR signal intensity in skeletal muscle adjacent to malignant tumors: pathologic correlation and clinical relevance. Radiology 1987, 162 Pt 1, 251–255. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




