Submitted:
25 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
Keywords:
1.0. Introduction
2.0. Pathophysiology of APS
3.0. Clinical Features of APS
4.0. Current Treatments of APS
5.0. Involvement of Microorganisms in APS
6.0. Gut Microbiome
6.1. Overview
6.2. Gut Microbiome - Metabolic and Protective Role
6.3. Microbiota and the Gut-Brain Axis
6.4. Gut Microbiome in Health and Diseases
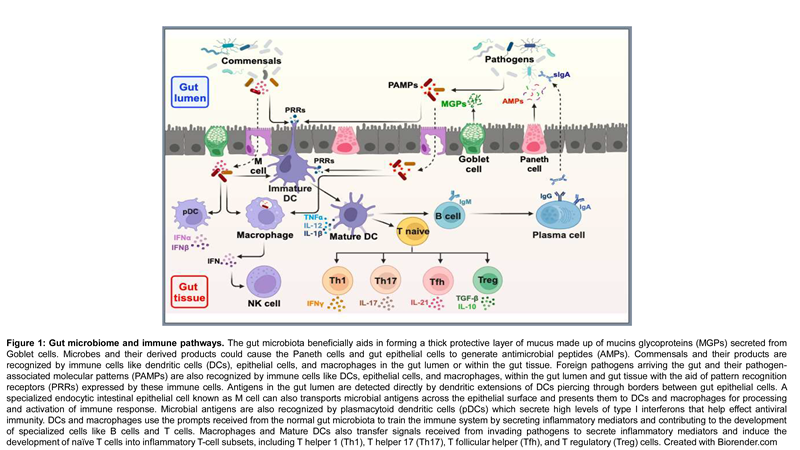 |
6.5. Gut Microbiome and Age
6.6. Gut microbiome and Environmental influences – Focus on APS
7.0. Recommendations and Perspectives: A Need for a Holistic Treatment Approach
Author Contributions
Funding
Ethics Approval and Consent to Participate
Conflicts of Interest
References
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.; Cervera, R. Antiphospholipid syndrome. Arthritis Research & Therapy 2008, 10, 230. [Google Scholar]
- Riancho-Zarrabeitia, L.; Martínez-Taboada, V.; Rúa-Figueroa, I.; Alonso, F.; Galindo-Izquierdo, M.; Ovalles, J.; Olivé-Marqués, A.; Fernández-Nebro, A.; Calvo-Alén, J.; Menor-Almagro, R.; et al. Antiphospholipid syndrome (APS) in patients with systemic lupus erythematosus (SLE) implies a more severe disease with more damage accrual and higher mortality. Lupus 2020, 29, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Torres-Jimenez, A.-R.; Ramirez-Nova, V.; Cespedes-Cruz, A.I.; Sanchez-Jara, B.; Velazquez-Cruz, A.; Bekker-Méndez, V.C.; Guerra-Castillo, F.X. Primary antiphospholipid syndrome in pediatrics: beyond thrombosis. Report of 32 cases and review of the evidence. Pediatr. Rheumatol. 2022, 20, 1–9. [Google Scholar] [CrossRef]
- Harris, E.N. Syndrome of the black swan. British Journal of Rheumatology 1987, 26, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Eby, C. Antiphospholipid Syndrome Review. Clin. Lab. Med. 2009, 29, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Corban, M.T.; Duarte-Garcia, A.; McBane, R.D.; Matteson, E.L.; Lerman, L.O.; Lerman, A. Antiphospholipid syndrome: role of vascular endothelial cells and implications for risk stratification and targeted therapeutics. Journal of the American College of Cardiology 2017. [Google Scholar] [CrossRef]
- Cervera, R.; Bucciarelli, S.; Plasín, M.A.; Gómez-Puerta, J.A.; Plaza, J.; Pons-Estel, G.; Shoenfeld, Y.; Ingelmo, M.; Espinos, G. Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of a series of 280 patients from the “CAPS Registry”. J. Autoimmun. 2009, 32, 240–245. [Google Scholar] [CrossRef]
- Arreola-Diaz, R.; Majluf-Cruz, A.; Sanchez-Torres, L.; Hernandez-Juarez, J. The Pathophysiology of The Antiphospholipid Syndrome: A Perspective From The Blood Coagulation System. Clin. Appl. Thromb. 2022, 28. [Google Scholar] [CrossRef]
- Zuo, Y.; Shi, H.; Li, C.; Knight, J.S. Antiphospholipid syndrome: a clinical perspective. Chin. Med J. 2020, 133, 929–940. [Google Scholar] [CrossRef]
- Zhang, J.; McCrae, K.R. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood 2005. [Google Scholar]
- Sorice, M.; Longo, A.; Capozzi, A.; Garofalo, T.; Misasi, R.; Alessandri, C.; Valesini, G. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis & Rheumatism 2007, 56, 2687–2697. [Google Scholar]
- Shi, T.; Giannakopoulos, B.; Yan, X.; Yu, P.; Berndt, M.C.; Andrews, R.K.; Rivard, G.E. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis & Rheumatism 2006, 54, 2558–2567. [Google Scholar]
- Pierangeli, S.S.; Girardi, G.; Vega-Ostertag, M.; Liu, X.; Espinola, R.G.; Salmon, J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody–mediated thrombophilia. Arthritis Rheum. 2005, 52, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, F.; Durigutto, P.; Pellis, V.; Debeus, A.; Macor, P.; Bulla, R.; Tedesco, F. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood 2005, 106, 2340–2346. [Google Scholar] [CrossRef]
- Girardi, G.; Redecha, P.; E Salmon, J. Heparin prevents antiphospholipid antibody–induced fetal loss by inhibiting complement activation. Nat. Med. 2004, 10, 1222–1226. [Google Scholar] [CrossRef]
- Gómez-Puerta, J.A.; Espinosa, G.; Cervera, R. Antiphospholipid Antibodies: From General Concepts to Its Relation with Malignancies. Antibodies 2016, 5, 18. [Google Scholar] [CrossRef]
- Vassalo, J.; Spector, N.; de Meis, E.; Rabello, L.S.; Rosolem, M.M.; Brasil, P.E.D.; Salluh, J.I.; Soares, M. Antiphospholipid antibodies in critically ill patients with cancer: A prospective cohort study. J. Crit. Care 2014, 29, 533–538. [Google Scholar] [CrossRef]
- Sciascia, S.; Radin, M.; Bazzan, M.; Roccatello, D. Novel diagnostic and therapeutic frontiers in thrombotic anti-phospholipid syndrome. Intern. Emerg. Med. 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Virachith, S.; Saito, M.; Watanabe, Y.; Inoue, K.; Hoshi, O.; Kubota, T. Anti-beta2-glycoprotein I antibody with DNA binding activity enters living monocytes via cell surface DNA and induces tissue factor expression. Clinical and Experimental Immunology 2019, 195, 167–178. [Google Scholar] [CrossRef]
- Islam, M.A. Antiphospholipid antibodies and antiphospholipid syndrome in cancer: Uninvited guests in troubled times. Seminars in Cancer Biology 2020, 64, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.; Sciascia, S.; de Groot, P.G.; Devreese, K.; Jacobsen, S.; Ruiz-Irastorza, G.; Hunt, B.J. Antiphospholipid syndrome. Nature Reviews Disease Primers 2018, 4, 17103. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Tapias, P.; Blank, M.; Anaya, J.-M.; Shoenfeld, Y. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr. Opin. Rheumatol. 2012, 24, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.N.; Asherson, R.A.; Hughes, G.R. Antiphospholipid antibodies–autoantibodies with a difference. Annual Review of Medicine 1988, 39, 261–271. [Google Scholar] [CrossRef]
- Gharavi, A.; Pierangeli, S. Origin of antiphospholipid antibodies: Induction of aPL by viral peptides. Lupus 1998, 7, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Sherer, Y.; Blank, M.; Shoenfeld, Y. Antiphospholipid syndrome (APS): where does it come from? Best Practice & Research Clinical Rheumatology 2007, 21, 1071–1078. [Google Scholar]
- Movva, S.; Belilos, E.; Carsons, S. (2022). Antiphospholipid Syndrome. In: StatPearls.
- Bustamante, J.G.; Goyal, A.; Singhal, M. (2023). Antiphospholipid Syndrome. [Updated 7th February 2023].
- Salmon, J.E.; Girardi, G. Antiphospholipid antibodies and pregnancy loss: a disorder of inflammation. J. Reprod. Immunol. 2007, 77, 51–56. [Google Scholar] [CrossRef]
- Prima, F.A.F.D.; Valenti, O.; Hyseni, E.; Giorgio, E.; Faraci, M.; Renda, E.; De Domenico, R.; Monte, S. Antiphospholipid Syndrome during pregnancy: the state of the art. J. Prenat. Med. 2011, 5, 41–53. [Google Scholar]
- Cervera, R. Lessons from the ‘Euro-Phospholipid’ project. Autoimmunity Reviews 2008, 7, 174–178. [Google Scholar] [CrossRef]
- Martínez-Cordero, E.; García, B.E.R.; León, D.E.A. Anticardiolipin antibodies in serum and cerebrospinal fluid from patients with systemic lupus erythematosus. J. Investig. Allergol. Clin. Immunol. 1998, 7, 596–601. [Google Scholar]
- Ruiz-Irastorza, G.; Crowther, M.; Branch, W.; Khamashta, M.A. Antiphospholipid syndrome. The Lancet 2010, 376, 1498–1509. [Google Scholar] [CrossRef]
- Espinosa, G.; Cervera, R. Current treatment of antiphospholipid syndrome: lights and shadows. Nat. Rev. Rheumatol. 2015, 11, 586–596. [Google Scholar] [CrossRef]
- Rumsey, D.G.; Myones, B.; Massicotte, P. Diagnosis and treatment of antiphospholipid syndrome in childhood: A review. Blood Cells, Mol. Dis. 2017, 67, 34–40. [Google Scholar] [CrossRef]
- Zuo, Y.; Shi, H.; Li, C.; Knight, J.S. Antiphospholipid syndrome: a clinical perspective. Chin. Med J. 2020, 133, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Hubben, A.; McCrae, K.R. Emerging Therapies in Antiphospholipid Syndrome. Transfus. Med. Rev. 2022, 36, 195–203. [Google Scholar] [CrossRef]
- American College of Rheumatology. (2023). Antiphospholipid Syndrome.
- Knight, J.S.; Branch, D.W.; Ortel, T.L. Antiphospholipid syndrome: advances in diagnosis, pathogenesis, and management. BMJ 2023, 380, e069717. [Google Scholar] [CrossRef]
- Tumian, N.R.; Hunt, B.J. Clinical Management of Thrombotic Antiphospholipid Syndrome. J. Clin. Med. 2022, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Erkan, D. Diagnosis and Management of the Antiphospholipid Syndrome. N. Engl. J. Med. 2018, 378, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Misasi, R.; Longo, A.; Recalchi, S.; Caissutti, D.; Riitano, G.; Manganelli, V.; Garofalo, T.; Sorice, M.; Capozzi, A. Molecular Mechanisms of “Antiphospholipid Antibodies” and Their Paradoxical Role in the Pathogenesis of “Seronegative APS”. Int. J. Mol. Sci. 2020, 21, 8411. [Google Scholar] [CrossRef]
- Hughes, G. Thrombosis, abortion, cerebral disease, and the lupus anticoagulant. British Medical Journal (Clinical research ed.) 1983, 287, 1088. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Pinto, C.; García-Carrasco, M.; Cervera, R. (2023). Microorganisms in the Pathogenesis and Management of Anti-phospholipid Syndrome (Hughes Syndrome) Role of Microorganisms in Pathogenesis and Management of Autoimmune Diseases: Volume II: Kidney, Central Nervous System, Eye, Blood, Blood Vessels & Bowel (pp. 341-357): Springer.
- Meroni, P.L.; Borghi, M.O.; Grossi, C.; Chighizola, C.B.; Durigutto, P.; Tedesco, F. Obstetric and vascular antiphospholipid syndrome: same antibodies but different diseases? Nature Reviews Rheumatology 2018, 14, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Van Os, G.; Meijers, J.; Agar, C.; Seron, M.; Marquart, J.; Åkesson, P. . Mörgelin, M. Induction of anti-β2-glycoprotein I autoantibodies in mice by protein H of Streptococcus pyogenes. Journal of Thrombosis and Haemostasis 2011, 9, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- Albert, L.J.; Inman, R.D. Molecular Mimicry and Autoimmunity. New Engl. J. Med. 1999, 341, 2068–2074. [Google Scholar] [CrossRef]
- Oldstone, M.B. Molecular Mimicry: Its Evolution from Concept to Mechanism as a Cause of Autoimmune Diseases. Monoclon. Antibodies Immunodiagn. Immunother. 2014, 33, 158–165. [Google Scholar] [CrossRef]
- Blank, M.; Krause, I.; Magrini, L.; Spina, G.; Kalil, J.; Jacobsen, S.; Thiesen, H.J.; Cunningham, M.W.; Guilherme, L.; Shoenfeld, Y. Overlapping humoral autoimmunity links rheumatic fever and the antiphospholipid syndrome. Rheumatology 2006, 45, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, A.; Aminov, R.; Manukyan, G. Environmental Triggers of Autoreactive Responses: Induction of Antiphospholipid Antibody Formation. Front. Immunol. 2019, 10, 1609. [Google Scholar] [CrossRef]
- Blank, M.; Krause, I.; Fridkin, M.; Keller, N.; Kopolovic, J.; Goldberg, I. . Shoenfeld, Y. Bacterial induction of autoantibodies to β2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. The Journal of clinical investigation 2002, 109, 797–804. [Google Scholar] [CrossRef]
- Ruff, W.E.; Dehner, C.; Kim, W.J.; Pagovich, O.; Aguiar, C.L.; Yu, A.T.; Roth, A.S.; Vieira, S.M.; Kriegel, C.; Adeniyi, O.; et al. Pathogenic Autoreactive T and B Cells Cross-React with Mimotopes Expressed by a Common Human Gut Commensal to Trigger Autoimmunity. Cell Host Microbe 2019, 26, 100–113. [Google Scholar] [CrossRef]
- Ruff, W.E.; Vieira, S.M.; Kriegel, M.A. The Role of the Gut Microbiota in the Pathogenesis of Antiphospholipid Syndrome. Curr. Rheumatol. Rep. 2014, 17, 1–10. [Google Scholar] [CrossRef]
- van Mourik, D.J.M.; Salet, D.M.; Middeldorp, S.; Nieuwdorp, M.; van Mens, T.E. The role of the intestinal microbiome in antiphospholipid syndrome. Front. Immunol. 2022, 13, 954764. [Google Scholar] [CrossRef]
- Vieira, S.M.; Yu, A.; Pagovich, O.E.; Tiniakou, E.; Sterpka, J.; Kriegel, M.A. (2013). Depletion Of The Gut Microbiota Prevents beta (2)-Glycoprotein I Antibody Production and Mortality In a Model Of Antiphospholipid Syndrome. Paper presented at the ARTHRITIS AND RHEUMATISM.
- Abdel-Wahab, N.; Talathi, S.; A Lopez-Olivo, M.; E Suarez-Almazor, M. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus 2017, 27, 572–583. [Google Scholar] [CrossRef]
- Mendoza-Pinto, C.; García-Carrasco, M.; Cervera, R. Role of Infectious Diseases in the Antiphospholipid Syndrome (Including Its Catastrophic Variant). Curr. Rheumatol. Rep. 2018, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Palomo, I.; Alarcón, M.; Sepulveda, C.; Pereira, J.; Espinola, R.; Pierangeli, S. Prevalence of antiphospholipid and antiplatelet antibodies in human immunodeficiency virus (HIV)-infected Chilean patients. J. Clin. Lab. Anal. 2003, 17, 209–215. [Google Scholar] [CrossRef]
- Bowles, L.; Platton, S.; Yartey, N.; Dave, M.; Lee, K.; Hart, D.P.; MacDonald, V.; Green, L.; Sivapalaratnam, S.; Pasi, K.J.; et al. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19. New Engl. J. Med. 2020, 383, 288–290. [Google Scholar] [CrossRef]
- Noakes, D.; Evans, K.; Pathansali, R. The return of a former foe: syphilis with antiphospholipid syndrome as a cause of acute stroke. JRSM Open 2017, 8. [Google Scholar] [CrossRef]
- Miko, E.; Csaszar, A.; Bodis, J.; Kovacs, K. The Maternal–Fetal Gut Microbiota Axis: Physiological Changes, Dietary Influence, and Modulation Possibilities. Life 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Iacob, S.; Iacob, D.G.; Luminos, L.M. Intestinal Microbiota as a Host Defense Mechanism to Infectious Threats. Front. Microbiol. 2019, 9, 3328. [Google Scholar] [CrossRef]
- Hou, K.; Wu, X.; Chen, Y.; Wang, Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; Chen, S. Microbiota in health and diseases. Signal Transduction and Targeted Therapy 2022, 7. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology: Quarterly Publication of the Hellenic Society of Gastroenterology 2015, 28, 203–209. [Google Scholar]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Bendriss, G.; MacDonald, R.; McVeigh, C. Microbial Reprogramming in Obsessive–Compulsive Disorders: A Review of Gut–Brain Communication and Emerging Evidence. Int. J. Mol. Sci. 2023, 24, 11978. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: a review. Antonie van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Shapira, M. Gut Microbiotas and Host Evolution: Scaling Up Symbiosis. Trends Ecol. Evol. 2016, 31, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Jethwani, P.; Grover, K. Gut microbiota in health and diseases—a review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1586–1599. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.-Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Martín, R.; Jiménez, E.; Heilig, H.; Fernández, L.; Marín, M.L.; Zoetendal, E.G.; Rodríguez, J.M. Isolation of Bifidobacteria from Breast Milk and Assessment of the Bifidobacterial Population by PCR-Denaturing Gradient Gel Electrophoresis and Quantitative Real-Time PCR. Appl. Environ. Microbiol. 2009, 75, 965–969. [Google Scholar] [CrossRef]
- German, J.B.; Freeman, S.L.; Lebrilla, C.B.; Mills, D.A. Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program. 2008, 62, 205–222. [Google Scholar]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Ouwehand, A.; Isolauri, E.; Salminen, S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur. J. Nutr. 2002, 41, 1–1. [Google Scholar] [CrossRef]
- Zwielehner, J.; Liszt, K.; Handschur, M.; Lassl, C.; Lapin, A.; Haslberger, A.G. Combined PCR-DGGE fingerprinting and quantitative PCR indicates shifts in fecal population sizes and diversity of Bacteroides, Bifidobacteria and Clostridium cluster IV in institutionalized elderly. Exp Gerontol. 2009, 44, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z.; Cervera, R.; Costedoat-Chalumeau, N.; Cuadrado, M.J.; Dörner, T.; Ferrer-Oliveras, R.; Hambly, K.; et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuarine, Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Campana, A.M.; Laue, H.E.; Shen, Y.; Shrubsole, M.J.; Baccarelli, A.A. Assessing the role of the gut microbiome at the interface between environmental chemical exposures and human health: Current knowledge and challenges. Environ. Pollut. 2022, 315, 120380–120380. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.L.; Little, M.; Cui, J.Y. Gut microbiome: An intermediary to neurotoxicity. NeuroToxicology 2019, 75, 41–69. [Google Scholar] [CrossRef]
- Deng, Yongfeng, Zehua Yan, Ruqin Shen, Meng Wang, Yichao Huang, Hongqiang Ren, Yan Zhang, and Bernardo Lemos. 2020.
- Cause Aggravated Adverse Effects in the Mouse Gut.” Environment International 143: 105916.
- Giambò, F.; Costa, C.; Teodoro, M.; Fenga, C. Role-Playing Between Environmental Pollutants and Human Gut Microbiota: A Complex Bidirectional Interaction. Front. Med. 2022, 9, 810397. [Google Scholar] [CrossRef]
- Giambò, F.; Leone, G.M.; Gattuso, G.; Rizzo, R.; Cosentino, A.; Cinà, D.; Teodoro, M.; Costa, C.; Tsatsakis, A.; Fenga, C. Genetic and Epigenetic Alterations Induced by Pesticide Exposure: Integrated Analysis of Gene Expression, MicroRNA Expression, and DNA Methylation Datasets. International Journal of Environmental Research and Public Health 2021, 18, 8697. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2019, 244, 125492. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Goemans, C.V.; Wirbel, J.; Kuhn, M.; Eberl, C.; Pruteanu, M.; Müller, P.; Garcia-Santamarina, S.; Cacace, E.; Zhang, B.; et al. Unravelling the collateral damage of antibiotics on gut bacteria. Nature 2021, 599, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.R.; Paul, S.; Dunlop, A.L.; Corwin, E.J. Maternal peripartum antibiotic exposure and the risk of postpartum depression. Res. Nurs. Heal. 2018, 41, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.F.A.; Silveira, G.G.d.O.S.; Cândido, E.d.S.; Cardoso, M.H.; Carvalho, C.M.E.; Franco, O.L. Effects of Antibiotic Treatment on Gut Microbiota and How to Overcome Its Negative Impacts on Human Health. ACS Infect. Dis. 2020, 6, 2544–2559. [Google Scholar] [CrossRef]
- Tavalire, H.F.; Christie, D.M.; Leve, L.D.; Ting, N.; Cresko, W.A.; Bohannan, B.J.M. Shared Environment and Genetics Shape the Gut Microbiome after Infant Adoption. mBio 2021, 12. [Google Scholar] [CrossRef]
- Tikka, C.; Manthari, R.K.; Ommati, M.M.; Niu, R.; Sun, Z.; Zhang, J.; Wang, J. Immune disruption occurs through altered gut microbiome and NOD2 in arsenic induced mice: Correlation with colon cancer markers. Chemosphere 2020, 246, 125791. [Google Scholar] [CrossRef]
- Verma, H.; Phian, S.; Lakra, P.; Kaur, J.; Subudhi, S.; Lal, R.; Rawat, C.D. Human Gut Microbiota and Mental Health: Advancements and Challenges in Microbe-Based Therapeutic Interventions. Indian J. Microbiol. 2020, 60, 405–419. [Google Scholar] [CrossRef]
- Lim, W.; Crowther, M.A.; Eikelboom, J.W. Management of antiphospholipid antibody syndrome: a systematic review. JAMA 2006, 295, 1050–1057. [Google Scholar] [CrossRef]
- Garabatos, N.; Santamaria, P. Gut Microbial Antigenic Mimicry in Autoimmunity. Front. Immunol. 2022, 13, 873607. [Google Scholar] [CrossRef]
- Yun, Z.; Duan, L.; Liu, X.; Cai, Q.; Li, C. An update on the biologics for the treatment of antiphospholipid syndrome. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef]
| Microorganism | aCL | anti-β2GP1 | LA | Thrombosis |
|---|---|---|---|---|
| Viral infection | ||||
| CMV | + | + | + | + |
| EBV | + | + | + | + |
| HIV | + | + | + | + |
| SARS-Cov-2 | + | + | + | + |
| Varicella zoster virus | + | + | + | + |
| Parvovirus B19 | + | + | + | + |
| Hepatitis A | + | - | - | + |
| Hepatitis B | + | + | + | - |
| Hepatitis C | + | + | + | + |
| Hepatitis D | + | + | + | - |
| Mumps | + | - | - | - |
| Rubella | + | - | - | - |
| Adenovirus | + | + | - | - |
| HTLV | + | + | - | - |
| Influenza A | + | - | - | + |
| Bacterial infections | ||||
| Mycobacterium leprae | + | + | + | + |
| Borrelia burgdorferi | + | + | + | + |
| Salmonella spp. | + | + | + | + |
| Streptococcus spp. | + | + | + | + |
| M. tuberculosis | + | + | - | + |
| Escherichia coli | + | + | - | + |
| Coxiella burnetii | + | - | + | - |
| Helicobacter pylori | + | + | - | - |
| Klebsiella spp. | + | - | - | - |
| Chlamydia | + | + | - | - |
| Mycoplasma pneumonia | + | + | - | + |
| Treponema pallidum | + | + | - | - |
| Parasitic infections | ||||
| Plasmodium malariae | + | + | - | + |
| Leishmania | + | + | + | - |
| Leptospira spp. | + | + | - | - |
| Plasmodium falciparum | + | - | - | - |
| Toxoplasmosis | + | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





