Submitted:
23 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Physiological Processes during Fruit Drop
2.1. Physiology of Abscission
2.1.1. Role of Seeds in Regulation of Abscission.
2.1.2. Role of Leaves in Regulation of Abscission
2.2. Phytohormones and their Role in Fruit-Drop Process
2.3. Competition for Carbohydrates in Generative Organs
3. Genetic Regulation of Fruit Drop
3.1. Ethylene Pathway Genes Participating in Abscission
3.2. Abscission Hormone Abscisic Acid
3.3. Auxin Pathway Genes in Abscission
3.4. Transcription Factors in Abscission Regulation
3.5. Abscission Zone Cell Remodelling Genetics
4. Interplay of Phytohormones and Gene Expression in Abscission
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Juhnevica-Radenkova, K.; Radenkovs, V.; Seglina, D. Effect of storage technology on structure and physical attributes of apples (Malus × domestica Borkh.). Am -Eurasian. J. Sustain. Agric., 2017, 11, 8–22. [Google Scholar]
- FAOSTAT, 2024. Food and Agriculture Organization of the United Nations Statistics. https://www.fao.org/faostat/en/#compare (archived on 24.06.2024).
- Greene, D.W.; Krupa, J.; Autio, W. Factors influencing preharvest drop of apples. Proc. XI. Th. IS Plant Bioreg. Fruit Prod. Acta Hortic. 2014, 1042, 231–236. [Google Scholar] [CrossRef]
- Arseneault, M. H.; Cline, J.A. A review of apple preharvest fruit drop and practices for horticultural management. Sci. Hort. 2016, 211, 40–52. [Google Scholar] [CrossRef]
- Orlova, Y.; Linker, R.; Spektor, B. Forecasting the potential of apple fruitlet drop by in-situ vis-nir spectroscopy. Comp. and Electron. in Agricult. 2020, 169, 105225. [Google Scholar] [CrossRef]
- Kviklienė, N. The influence of chemical bud thinning on the quality of 'Lodel' apple fruits (in Lithuanian). Sodininkystė ir daržininkystė 2008, 2, 22–28. [Google Scholar]
- Din, S.; Wani, R.A.; Wani, A.W.; Nisar, F.; Farwah, S.; Rizvi, S.; Wani, T.F.; Nisar, S. Fruit set and development: Pre-requisites and enhancement in temperate fruit crops. Int. J. Pharmacogn. Phytochem. Res. 2019, 8, 1203–1216. [Google Scholar]
- Kon, T.M.; Schupp, J. R. Apple Crop Load Management with Special Focus on Early Thinning Strategies. Hort. Rev. 2018, 46, 255–298. [Google Scholar]
- Fioravanco, J.; Czermainski, A.B. Biennial bearing in apple cultivars. Revista Ceres. 2018, 65, 144–149. [Google Scholar] [CrossRef]
- Campbell, T.; Kalcsits, L. Strategies to overcome biennial bearing in apple – A review. Eur J Agron 2024, 158, 127231. [Google Scholar] [CrossRef]
- Smith, H.M; Samach, A. Constraints to obtaining consistent annual yields in perennial tree crops. I: Heavy fruit load dominates over vegetative growth. Plant Sci. 2013, 207, 158–167. [Google Scholar] [CrossRef]
- Starkus, A.; Frercks, B.; Gelvonauskiene, D.; Mazeikiene, I.; Rugienius, R.; Bendokas, V.; Stanys, V. Potential Markers for Selecting Self-Eliminating Apple Genotypes. Plants 2021, 10, 1612. [Google Scholar] [CrossRef]
- Elsysy, M. A.; Mickelbart, M. V.; Hirst, P. M. Effect of Fruiting and Biennial Bearing Potential on Spur Quality and Leaf Gas Exchange in Apple. J. Amer. Soc. Hort. Sci. 2019, 144, 31–37. [Google Scholar] [CrossRef]
- Milyaev, A.; Tandron-Moya, Y.A.; von Wirén, N.; Neuwald, D.; Flachowsky, H.; Wünsche, J.N. What else don’t we know about biennial bearing? Phytohormone profile of seeds and seed number per fruit differ between a biennial and a non-biennial apple cultivar. Acta Hortic. 2022, 1342, 7–14. [Google Scholar] [CrossRef]
- Jarvis, M.C.; Briggs, S.P.H.; Knox, J.P. Intercellular adhesion and cell separation in plants. Plant Cell Environ. 2003, 26, 977–989. [Google Scholar] [CrossRef]
- Sundaresan, S.; Riov, S. H.; Belausov, E.; Kochanek, B.; Tucker, M.L; Meir, S. Abscission of flowers and floral organs is closely associated with alkalization of the cytosol in abscission zone cells. J. Exp. Bot. 2015, 66, 1355–1368. [Google Scholar] [CrossRef]
- Verjans, W.; Schoofs, H.; Deckers, T.; Bylemans, D.; Remy, S. Early induction of pear drop using ethephon. Acta Hortic. 2021, 1303, 243–250. [Google Scholar] [CrossRef]
- Hapuarachchi, N.S.; Kämper, W.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Wallace, H.M.; Trueman, S.J. Selective Retention of Cross-Fertilised Fruitlets during Premature Fruit Drop of Hass Avocado. Horticult. 2024, 10, 591. [Google Scholar] [CrossRef]
- Racskó, J.; Nagy, J.; Soltész, M.; Nyéki, J.; Szabó, Z. Fruit drop: I. Biological background of flower and fruit drop. Int. J. Hortic. Sci. 2006, 12, 103–108. [Google Scholar] [CrossRef]
- Starkus, A.; Gelvonauskienė, D.; Frercks, B.; Bendokas, V.; Sasnauskas, A.; Stanys, V. Relation between apple-tree yield self-regulation and meteorological conditions during fruit set. Proc. of 8th Internal Sci. Conf., Rural Development., 2017, 128 – 13.
- Ackerman, M.; Samach, A. Daubts regarding carbohydrates shortage as a trigger toward abscission af specific Apple (Malus domestica) freuitlets. New Negatives. Plant Sci. 2015, 1–2, 46–52.
- Celton, J.M.; Kelner, J.J.; Martinez, S.; Bechti, A.; Touhami, A.K.; James, M.J.; Durel, C.E.; Laurens, F.; Costes, E. Fruit Self-Thinning: A Trait to Consider for Genetic Improvement of Apple Tree. Plos one 2013. [CrossRef]
- Untiedt, R.; Blanke, M. Effects of fruit thinning agents on apple tree canopy photosynthesis and dark respiration. Plant Growth Regul., 2001, 35, 1–9. [Google Scholar] [CrossRef]
- Racskó, J.; Leite, G.B.; Petri, J.L; Zhongfu, S.; Wang, Y.; Szabó, Z.; Soltész, M.; Nyéki, J. Fruit drop: The role of inner agents and environmental factors in the drop of flowers and fruits. Hort Sci. 2007, 13, 13–23. [Google Scholar] [CrossRef]
- Tromp, J.; Webster, A.D.; Wertheim, S.J. Fundamentals of temperate zone fruit tree production. Bachuys Publishers, Leiden, 2005, P. 400.
- Kolarič, J. Abscission of young apple fruits (Malus domestica Borkh.). Agricult. (Maribor), 2010, 7, 31–36. [Google Scholar]
- Lauri, P.E.; Terouanne, E.; Lespinasse, J.M. Quantitative analysis of relationships between inflorescence size, bearing-axis size and fruit-set. An apple tree case study. Ann. of Bot. 1996, 77, 277–286. [Google Scholar] [CrossRef]
- Costa, G.; Blanke, M.M.; Widmer, A. Principles of thinning in fruit tree crops - needs and novelties. Acta. Hortic. 2013, 998, 17–26. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Defraeye, T.; Ketelaere, B.; Herremans, E.; Hertog, M.L.; Saeys, W.; Torricelli, A.; Vandendriessche, T.; Verboven, P. Nondestructive measurement of fruit and vegetable quality. Annu. Rev. Food Sci. Technol. 2014, 5, 285–312. [Google Scholar] [CrossRef] [PubMed]
- Win, N.M.; Song, Y.Y.; Nam, J.C.; Yoo, J.; Kang, I.K.; Cho, Y.S.; Yang, S.J.; Park, J. Influence of Mechanical Flower Thinning on Fruit Set and Quality of ‘Arisoo’ and ‘Fuji’ Apples. Int. J. Plant Biol. 2023, 14, 503–511. [Google Scholar] [CrossRef]
- United States environmental protection agency https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-099801_1-Apr-95.pdf. (accessed on 27 July 2024).
- United States environmental protection agency https://www.epa.gov/sites/default/files/2014-09/documents/health_effects_support_document_for_terbacil.pdf. (accessed on 27 July 2024).
- Gonzalez, L.; Torres, E., Àvila, G. et al. Effect of thinning with metamitron, NAA, BA and naphthenic acids on apple (Malus domestica) trees. Plant Growth Regul., 2024,102, 39–50. [CrossRef]
- Rahul, O.; Patharkar, J.; Walker, C. Advances in abscission signalling. J. of Exp. Bot., 2018, 69, 733–740. [Google Scholar]
- Roberts, J.A.; Elliot, K. A, Carranza, G. Abscission, dehiscence and other cell separation processes. Annu. Rev. Plant. Biol., 2002, 53, 131–58. [Google Scholar] [CrossRef]
- Pickersgill, B. Domestication of Plants in the Americas: insights from Mendelian and Molecular Genetics. Ann. Bot. 2007, 100, 925–940. [Google Scholar] [CrossRef]
- Addicott, F.T. Abscission. University of California Press, Oakland, CA. 1982. 369 pp.
- Addicott, F.T. Environmental factors in the physiology of abscission. Plant Physiol., 1968, 43, 1471–1479. [Google Scholar]
- Faeth, S.H.; Connor, E.F.; Simberloff, D. Early leaf abscission: a neglected source of mortality for folivores. The Amer. Natural., 1981, 117, 409–415. [Google Scholar] [CrossRef]
- Van Nocker, S. Development of the abscission zone. Stewart Postharvest Review, 2009. 5, 1–6.
- Arteca, R.N. Seed Germination and Seedling Growth. In: Plant Growth Substances. Springer, Boston, 1996. pp 104–126.
- Lobato, P.M.; Jimenez, G. Polyamine-induced modulation of genes involved in ethylene biosynthesis and signalling pathways and nitric oxide production during olive mature fruit abscission, J. of Exp. Bot., 2011, 62, 4447–4465. [Google Scholar] [CrossRef]
- Yu, Y. L.P.; Tavares, R. L.; Kellogg, E.A. The anatomy of abscission zones is diverse among grass species. Am. J. Bot., 2020, 107, 549–561. [Google Scholar] [CrossRef]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol., 2001, 151, 323–339. [Google Scholar] [CrossRef]
- Lewis, M.W.; Leslie, M.E.; Liljegren, S.J. Plant separation: 50 ways to leave your mother. Curr. Opin. Plant Biology., 2006, 9, 59–65. [Google Scholar] [CrossRef]
- Cho, S.K.; Larue, C.T.; Chevalier, D.; Wang, H.C.; Jinn, T.L.; Zhang, S.Q.; Walker, J.C. Regulation of floral organ abscission in Arabidopsis thaliana. Proc. of Nat. Acad. of Sci of USA., 2008, 105, 15629–15634. [Google Scholar] [CrossRef]
- McKim, S.M.; Stenvik, G.E.; Butenko, M.A.; Kristiansen, W.; Cho, S.K; Hepworth, S.R.; Aalen, R.B.; Haughn, G.W. The blade-on-petiole genes are essential for abscission zone formation in Arabidopsis. Developments, 2008, 135, 1537–1546. [Google Scholar]
- Cai, S.Q.; Lashbrook, C.C. Stamen abscission zone transcriptome profiling reveals new candidates for abscission control: enhanced retention of floral organs in transgenic plants overexpressing Arabidopsis ZINC FINGER PROTEIN2. Plant Physiol., 2008, 146, 1305–1321. [Google Scholar] [CrossRef]
- Agustí, J.; Gimeno, J.; Merelo, P.; Serrano, R.; Cercos, M.; Conesa, A.; Talon, M.; Tadeo, F.R. Early gene expression events in the laminar abscission zone of abscission-promoted citrus leaves after a cycle of water stress/rehydration: Involvement of CitbHLH1. J. Exp. Bot., 2012, 63, 6079–6091. [CrossRef]
- Meir, S.; Hunter, D.A.; Chen, J.; Halaly, V.; Reid, M.S. Molecular changes occurring during acquisition of abscission competence following auxin depletion in Mirabilis jalapa. Plant Physiol., 2006, 141, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.B.; Sexton, R.; Tucker, M.L. Analysis of gene promoters for two tomato polygalacturonases expressed in abscission zones and the stigma. Plant Physiol., 2000, 123, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Leslie, M.E.; Lewis, M.W.; Liljegren, S.J. Organ abscission. In Plant Cell Separation and Adhesion. Plant Cell & Environ., 2007, 26, 977–989. [Google Scholar]
- Estornell, L.H.; Agusti, J.; Merelo, P.; Talon, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci., 2013, 199–200, 48–60. [Google Scholar] [CrossRef]
- Janssen, B.J.; Thodey, K.; Schaffer, R.J.; Alba, R.; Balakrishnan, L.; Bishop, R. Global gene expression analysis of apple fruit development from the floral bud to ripe fruit. BMC Plant Biol., 2008, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.T.; Hall, A.J.; Gandar, P.W.; Warrington, I.J.; Fulton, T.A.; Alligan, E. A. A compartment model of the effect of early-season temperatures in potential size and growth of ‘Delicious’ apple fruits. Ann. Bot., 1999, 83, 129–143. [Google Scholar] [CrossRef]
- Al-hinai, Y. K.; Roper, T. R. Rootstock effects on growth, cell number, and cell size of ‘Gala’ apples. J. Amer. Soc. Hort. Sci., 2004, 129, 37–41. [Google Scholar] [CrossRef]
- Gillaspy, G.; David, B.H.; Gruissemet, W. 1993. Fruits: A developmental perspective. Plant Cell, 1993, 5, 1439–1451. [Google Scholar] [CrossRef]
- Buccheri, M.; Di Vaio, C. Relationship Among Seed Number, Quality, and Calcium Content in Apple Fruits. J. Plant Nutr., 27, 1735–1746.
- Eccher, G.; Ferrero, S.; Populin, F.; Colombo, L.; Botton, A. (2014). Apple (Malus domestica l. borkh) as an emerging model for fruit development. Plant Biosys., 2014, 148, 157–168. [CrossRef]
- Luckwill, L.C.; Weaver, P.; MacMillan, J. Gibberellins and other growth hormones in apple seeds. J. Hort. Sci., 1969, 44, 413–124. [Google Scholar] [CrossRef]
- Guo, L.; Luo, X.; Li, M. Mechanism of fertilization-induced auxin synthesis in the endosperm for seed and fruit development. Nat. Commun., 2022, 13, 3985. [Google Scholar] [CrossRef] [PubMed]
- Luckwill, L. C. The Hormone Content of the Seed in Relation to Endosperm Development and Fruit Drop in the Apple. J. Hort. Sci., 1948, 24, 32–44. [Google Scholar] [CrossRef]
- Dražeta, L.; Lang, A.; Hall, A.; Volz, R.; Jameson, P. Modelling the influence of seed set on fruit shape in apple. Journal of Hort. Sci. and Biotech., 2004, 79, 241–245. [Google Scholar] [CrossRef]
- Stösser, R. "Zur Befruchtungsbiologie der Zwetschensorte ‘Valjevka’. " Erwerbsobstbau. 2002, 44, 71–75. [Google Scholar]
- Ward, D. L.; Marini, R.P.; Byers, R. E. Relationships Among Day of Year of Drop, Seed Number, and Weight of Mature Apple Fruit. Hort. Sci. 2001, 36, 45–48. [Google Scholar] [CrossRef]
- Teskey, B.J.; Shoemaker, S. Apples. Springer, US, 1978, 1–126.
- Yao, J.L.; Dong, Y.H.; Kvarnheden, A.; Morris, B. Seven MADS-box genes in apple are expressed in different parts of the fruit. Amer. Soc. Hort. Sci. 1999, 124, 8–13. [Google Scholar] [CrossRef]
- Sung, S.K.; Yu, G.H.; Nam, J.; Jeong, D.H.; An, G. Developmentally regulated expression of two MADS-box genes, MdMADS3 and MdMADS4, in the morphogenesis of flower buds and fruits in apple. Planta., 2000, 210, 519–528. [Google Scholar] [CrossRef]
- Nosarzewski, M.; Archbold, D.D. Tissue-specific expression of SORBITOL DEHYDROGENASE in apple fruit during early development. J. Exp. Bot., 2007, 58, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, F.; Cheng, L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE, 2012. 7, 33055.
- Hassan, I.; Wani, Ab.W.; Dar, S. , Wani, Q.; Sofi, J.A.; Baba, T.R.; Parray, E.; Rasool, A. Physiology of Fruit Set and Development in Apple under Temperate conditions: A Review. Int. J. Curr. Microbiol. App. Sci., 2020, 9, 618–638. [Google Scholar] [CrossRef]
- Bangerth, F. Abscission and thinning of young fruit and their regulation by plant hormones and bioregulators. Plant Growth Reg., 2000, 31, 43–59. [Google Scholar] [CrossRef]
- Papp, J.; Porpáczy, A. Life cycle of agricultural plants (in Hungarn). Mezôgazdsola Kiadó, 1999 pp. 246.
- Szalay, L. Comparison of flower bud development in almond, apricot and peach genotypes. Int. J. of Hort. Sci., 2006, 12, 93. [Google Scholar] [CrossRef]
- Ropert, T.R.; Loescher, W.H.; Keller, J.; Rom, C.R. Source of photosynthate for fruit growth in `Bing` sweet cherry. Soc. of Hort. Sci., 1987, 112, 808–812. [Google Scholar] [CrossRef]
- Nyéki, J.; Soltész, M. The variation of seed content of fruits in pear varieties, also as different condition of fertilization, as open pollination, natural autogamy and allogamy the variation of seed content of fruits in pear varieties, also as function of different conditions of fertilization, as open pollination, natural autogamy and allogamy. Acta Hort., 1998, 475, 237–250. [Google Scholar]
- Casero, T.; Benavides, A.; Puy, J.; Recasens, I. Relationships Between Leaf and Fruit Nutrients and Fruit Quality Attributes in Golden Smoothee Apples Using Multivariate Regression Techniques. J. of Plant Nutri., 2004, 27, 313–324. [CrossRef]
- Feucht, W. (Das Obstgehölz – Anatomie und Physiologie des sprobsystem. Eugen Ulmer, Stuttgart. 1982, p 256.
- Soltész, M. Integrated fruit production. Kiadó, Budapest. 2002, p 843.
- Davis, T.D.; Curry, E.A. Chemical regulation of vegetative growth. Critic Rev. Plant Sci., 1991, 10, 151–188. [Google Scholar] [CrossRef]
- Goren, R.; Goldschmidt, E.E. Regulation systems in the developing citrus fruit. The hormonal balance in orange fruit tissues. Physiol. Planta., 1970, 23, 937–947. [Google Scholar] [CrossRef]
- Agusti, J.; Gimeno, J.; Merelo, P.; Serrano, R.; Cercos, M.; Conesa, A.; Talon, M.; Tadeo, F.R. Early gene expression events in the laminar abscission zone of abscission-promoted citrus leaves after a cycle of water stress/rehydration: Involvement of CitbHLH1. J. Exp. Bot., 2012, 63, 6079–6091. [CrossRef]
- Vob, U.; Bishopp, A.; Farcot, E.; Bennett, M. J. Modelling hormonal response and development. Trends Plant Sci., 2014, 19, 311–319. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, Y.; Li, C.; Wu, Q.; He, Z.; Li, J.; Zhao. M. Brassinosteroids suppress ethylene-induced fruitlet abscission through LcBZR1/2-mediated transcriptional repression of LcACS1/4 and LcACO2/3 in litchi. Hort. Res., 2021, 8, 105.
- Vriezen, W.H.; Feron, R.; Maretto, F.; Keijman, J.; Mariani, C. Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol., 2008 177, 60–76. [CrossRef]
- Basu, M.M.; Carranza, G.Z.H.; Ali, A.S.; Tang, S.; Shahid, A.A.; Roberts, J.A. The manipulation of auxin in the abscission zone cells of Arabidopsis flowers reveals that indoleacetic acid signalling is a prerequisite for organ shedding. Plant Physiol., 2013, 162, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.L.; Yunche, C.; Guangzhen, Z.; Meng, L. Phytohormones and candidate genes synergistically regulate fruitlet abscission in Areca catechu. BMC Plant Biol., 2023, 23, 537. [Google Scholar]
- Stutte, G.W.; Gage, J.W. Gibberellin inhibits fruit abscission following seed abortion in peach. J. Am. Soc. Hortic. Sci., 1990, 115, 107–110. [Google Scholar] [CrossRef]
- Moualem, B.D.; Gusev, L.; Dvir, O.; Pesis, E.; Meir, S.; Lichter, A. The effects of ethylene, methyl jasmonate and 1-MCP on abscission of cherry tomatoes from the bunch and expression of endo-1,4-β-glucanases. Plant Sci., 2004, 167, 499–507.
- Cin, V.D.; Boschetti, A.; Dorigoni, A.; Ramina, A. Benzyl aminopurine application on two different apple cultivars (Malus domestica) displays new and unexpected fruitlet abscission features. Ann. Bot., 2007, 99,1195–202.
- Eccher, G.; Begheldo, M.; Boschetti, A.; Ruperti, B.; Botton, A. Roles of ethylene production and ethylene receptor expression in regulating apple fruitlet abscission. Plant Physiol., 2015, 169, 125–137. [CrossRef]
- Klee, H. J; Ethylene signal transduction. moving beyond Arabidopsis. PlantPhysiol., 2004, 135, 660–667. [Google Scholar] [CrossRef]
- Meir, S.; Hadas, S.; Sundaresan, S.; Selvaraj, K.S.; Burd, S.; Ophir, R.; Kochanek, B.; Reid, M.S.; Jiang, C.Z.; Lers, A. Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiol., 2010, 154, 1929–1956. [CrossRef]
- Li, J.; Zhu, H.; Yuan, R. Profiling the Expression of Genes Related to Ethylene Biosynthesis, Ethylene Perception, and Cell Wall Degradation during Fruit Abscission and Fruit Ripening in Apple. J. Amer. Soc. Hort. Sci., 2010, 135, 391–401. [Google Scholar] [CrossRef]
- Harley, M. S.; Samach, A. Constraints to obtaining consistent annual yields in perennial tree crops. I: Heavy fruit load dominates over vegetative growth. Plant Sci., 2013 207, 158-167.
- Greene, D.W. Chemicals, timing, and environmental fac tors involved in thinner efficiacy on apple. Hort. Sci, 2002, 37, 77–81. [Google Scholar]
- Pethő, M. Mezögazdasági növények élettlana. Akadémiai Kiadó, Budapest, 1993; pp. 232–254.
- Bubán, T. Hormonal aspects of flower and fruit set. Akadémiai Kiadó, Budapest, 2003, pp. 3–24.
- Priestley, A.C. Carbohydrate Reserves in Deciduous Fruit Trees. Hortic. Rev., 1988, 10, 403–430. [Google Scholar]
- Kandiah, S. Turnover of carbohydrates in relation to growth in apple trees. Seasonal variation of growth and carbohydrate reserves. Ann. Bot., 1979, 44, 175–183. [Google Scholar] [CrossRef]
- Tromp, J. Nutrient reserves in roots of fruit trees, in particular carbohydrates and nitrogen. Plant Soil., 1983, 71, 401–413. [Google Scholar] [CrossRef]
- Tustin, D.S.; Lai, R. 1990. Source-sink dynamics in developing fruiting spurs of apple. XXIII Int. Hortic. Congress, Florence, Italy. 1990. pp. 611.
- Grappadelli, C.L.; Magnanini, E. A. Whole-tree sytem for gas-exchange studies. Hort. Sci., 1993, 28, 41–45. [Google Scholar]
- Archbold, D.D.; Nosarzewski, M.; Wu, B.; Vuppalapati, P. Does availability of soluble carbohydrate reserves determine apple fruit set? Acta Hortic., 2011, 903, 795–801. [Google Scholar] [CrossRef]
- Lakso, A.N.; Wünsche, J.N.; Palmer, J.W.; Grappadelli, L.C. Measurement and modeling of carbon balance of the apple tree. Hort. Sci., 1993, 4, 1040–1047. [Google Scholar] [CrossRef]
- Greene, D.W.; Krupa, J.; Vezina, M.; Lakso, A.N.; Robinson, T.L. A method to predict chemical thinner response on apples. Fruit Notes, 2005, 70, 12–17. [Google Scholar]
- Lakso, A.N.; Greene, D.W.; Palmer, J.W. Improvements on an apple carbon balance model. Acta Hortic., 2006, 707, 57–61. [Google Scholar] [CrossRef]
- Costa, G.; Botton, A.; Vizzotto, G. Fruit Thinning: Advances and trends. Hortic. Rev., 2018, 46, 185–226. [Google Scholar]
- Shi, Y.; Song, B.; Liang, Q.; Su, D.; Lu, W.; Liu, Y.; Li, Z. Molecular regulatory events of flower and fruit abscission in horticultural plants. Hortic. Plant J., 2023, 9, 867–883. [Google Scholar] [CrossRef]
- Patharkar, O.R.; Walker, J.C. Advances in abscission signalling. J. Exp. Bot., 2018, 69, 733–740. [Google Scholar] [CrossRef]
- Brummell, D.A.; Hall, B.D.; Bennett, A.B. Antisense suppression of tomato endo- 1,4-beta-glucanase Cel2 mRNA accumulation increases the force required to break fruit abscission zones but does not affect fruit softening. Plant Mol. Biol., 1999, 40 615–622.
- Sriskantharajah, K.; Kayal, E.W.; Torkamaneh, D.; Ayyanath, M.M.; Saxena, P.K.; Sullivan, A.J.; Paliyath, G.; Subramanian, J. Transcriptomics of Improved Fruit Retention by Hexanal in ‘Honeycrisp’ Reveals Hormonal Crosstalk and Reduced Cell Wall Degradation in the Fruit Abscission Zone. Int. J. Mol. Sci., 2021, 22, 8830. [Google Scholar] [CrossRef] [PubMed]
- Gil-Amado, J. A.; Gomez-Jimenez M., C. Transcriptome Analysis of Mature Fruit Abscission Control in Olive, Plant and Cell Physiol., 2013, 54, 244–269. [CrossRef]
- Pattyn, J.; Hirsch, V.J.; Mezõgazdasági, V; Poel, B. The regulation of ethylene biosynthesis: a complex multilevel control circuitry. New Phytol. 2021; 229, 770–782.
- Harada, T.; Sunako, T.; Wakasa, Y.; Soejima, J.; Satoh, T.; Niizeki, M. An allele of the 1-aminocyclopropane-1-carboxylate synthase gene (Md-ACS1) accounts for the low level of ethylene production in climacteric fruits of some apple cultivars. Theor. Appl. Genet., 2000, 101, 742–746. [Google Scholar] [CrossRef]
- Kunihisa, M.; Moriya, S.; Abe, K.; Okada, K.; Haji, T.; Hayashi, T.; Kim, H.; Nishitani, C.; Terakami, S.; Yamamotom, T. Identification of QTLs for fruit quality traits in Japanese apples: QTLs for early ripening are tightly related to preharvest fruit drop. Breed Sci., 2014, 64, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Bukovac, J.M.; Forsline, P.L. Natural variation in fruit abscission-related traits in apple (Malus). Euphytica, 2009, 165, 55–67. [Google Scholar] [CrossRef]
- Wang, X.; Yi, W.; Shufang, Y.; Xuan, S.; Hongyan, L.; Beibei, C.; Xingxing, X.; Zunzheng, X; Guojun, Z. A multifaceted comparison between the fruit-abscission and fruit-retention cultivars in ornamental crabapple. Front. Plant Sci., 2022, 13.
- Li, T.; Zhang, X.; Wei, Y.; Xu, Y.; Liu, W.; Li, H.; Yang, G.; Wang, A.; Wang, X. Comparative transcriptome analysis of the climacteric of apple fruit uncovers the involvement of transcription factors affecting ethylene biosynthesis. Hort. Plant., 2023, 9, 659–669. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem., 2020, 29, 7710–7725. [Google Scholar] [CrossRef]
- Ferrero, S.; Paulet, C.L.; Mendes, M.A.; Botton, A.; Eccher, G. Transcriptomic Signatures in Seeds of Apple (Malus domestica L. Borkh) during Fruitlet. PLoS One, 2015 10, 0120503. [CrossRef]
- Cin, D.V.; Botton, D.M. Ethylene and preharvest drop: the effect of AVG and NAA on fruit abscission in apple (Malus domestica L. Borkh). Plant Growth Regul., 2008, 56, 317–325. [Google Scholar]
- Eccher, G.; Botton, A.; Dimauro, M.; Boschetti, A.; Ruperti, B.; Angelo, R. Early induction of apple fruitlet abscission is characterized by an increase of both isoprene emission and abscisic acid content. Plant Physiol., 2013, 161, 1952–1969. [Google Scholar]
- Zhu, H.; Dardick, C.D.; Beers, E.P. Transcriptomics of shading-induced and NAA-induced abscission in apple (Malus domestica) reveals a shared pathway involving reduced photosynthesis, alterations in carbohydrate transport and signaling and hormone crosstalk. BMC Plant Biol., 2011, 11, 138. [Google Scholar] [CrossRef]
- Devoghalaere, F.; Doucen, T.; Guitton, B. A genomics approach to understanding the role of auxin in apple (Malus x domestica) fruit size control. BMC Plant Biol., 2012, 12, 7. [Google Scholar] [CrossRef]
- Botton, A.; Eccher, G.; Forcato, C.; Ferrarini, C.; Begheldo, M.; Zermiani, M.; Moscatello, S.; Battistelli, A.; Velasco, R Plant.; Ruperti, B.; Ramina, A. Signaling Pathways Mediating the Induction of Apple Fruitlet Abscission. Physiol. 2011; 155, 185–208. [CrossRef]
- Celton, J.M.; Dheilly, E.; Guillou, M.C.; Simonneau, F.; Juchaux, M.; Costes, E.; Laurens, F.; Renou, J.P. Additional Amphivasal Bundles in Pedicel Pith Exacerbate Central Fruit Dominance and Induce Self-Thinning of Lateral Fruitlets in Apple. Plant Physiol., 2014, 164, 1930–1951. [Google Scholar] [CrossRef] [PubMed]
- Botton, A.; Ruperti, B. The Yes and No of the Ethylene Involvement in Abscission. Plants 2019, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, D.; Qin, Z.; Zhang, D.; Yin, L.; Wu, L.; Colasanti, J.; Li, A.; Mao, L. The SEPALLATA MADS-box protein SLMBP21 forms protein complexes with JOINTLESS and MACROCALYX as a transcription activator for development of the tomato flower abscission zone. Plant. J., 2014, 77, 284–296. [Google Scholar] [CrossRef]
- Schaffer, R.J.; Ireland, H.S.; Ross, J.J.; Ling, T.J.; David, K.M. SEPALLATA1/2-suppressed mature apples have low ethylene, high auxin and reduced transcription of ripening-related genes. AoB. Plants., 2013, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Fujisawa, M.; Shima, Y.; Ito, I. The AP2/ERF transcription factor SlERF52 functions in flower pedicel abscission in tomato. J. of Exp. Bot., 2014, 65, 3111–3119. [Google Scholar] [CrossRef]
- Heo, S.; Chung, Y.S. Validation of MADS-box genes from apple fruit pedicels during early fruit abscission by transcriptome analysis and real-time PCR. Genes Genom., 2019, 41, 1241–1251. [Google Scholar] [CrossRef]
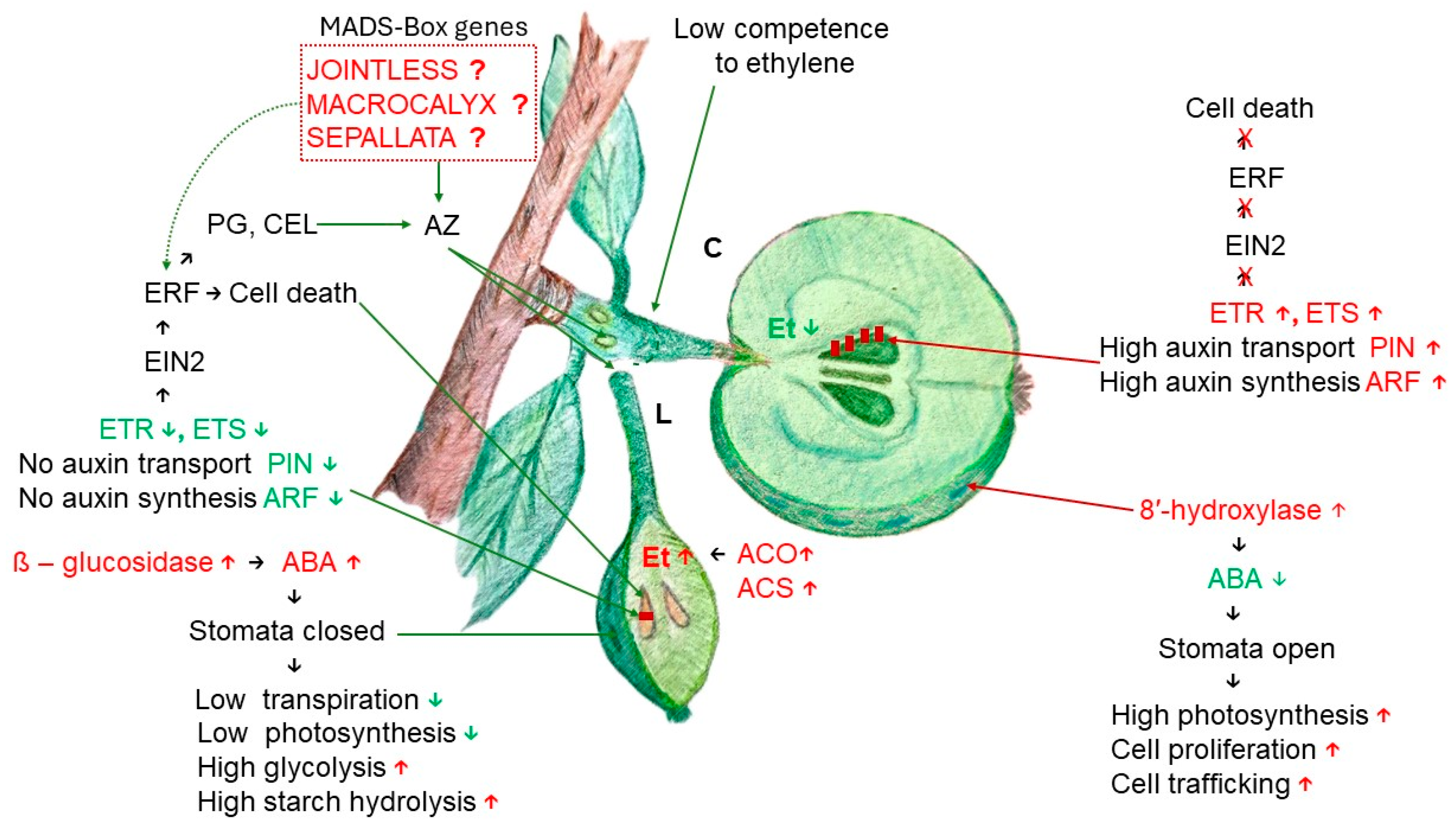
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




