Submitted:
23 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Obesity: A Global Pandemic
1.2. Need for Plant Extracts in Curbing Obesity
2. Salvia: Nature’s Therapeutic Blessing
3. Diving into Diversity: A Comprehensive Exploration of Salvia Species and their Phytochemical Marvels
3.1. Salvia Officinalis
| Salvia species | Experimental model, dose, interval period | Experimental outcome | Reference |
|---|---|---|---|
| S. tribola | Male Albino rats N= 20 Methanol extract, 750 mg/kg Gastric intubation |
↓ Plasma triglyceride levels ↑ inhibition of Pancreatic lipase |
[166] |
| S. officinalis | Adult female Wistar rats N= 40 Aqueous extract,10 mg/kg/day Orally 6 weeks |
Group with (OVX + SOE + OR) Body weight & visceral fat ↓ Triglyceride(mg/ml) ↓ TC (mg/ml) ↓ HDL (mg/ml) ↑ LDL (mg/ml) ↓ Glucose levels(mg/ml) ↓ Adiponectin(ng/ml) ↓ Leptin levels(ng/ml) ↓ Pancreatic lipase(ng/ml) ↓ AST(U/ml) ↓ ALT (U/ml) ↓ MDA (nmol/mg protein) ↓ GSH (mmol/mg protein) ↑ TAC (mmol/mg protein) ↑ |
[167] |
| S. hispanica L | C57BL/6J male mice, N= 6 organic solvents: hexane, dichloromethane, ethyl acetate, and ethanol (50 mg/kg/day) Orally 12 weeks |
Loss in Body weight (HFD-BE)- 1.8 ± 0.5 GOT(U/ml) ↓ GPT (U/ml) ↓ ALP (U/ml) ↓ ↓ fasting glucose ↓ fasting insulin ↓ in lipid droplets ↓ in lipid peroxidation degree |
[168] |
| S. officinalis & S. sclarea | Adult white outbred male rats N= 24 5% dry shredded shoots of Salvia officinalis & Salvia sclarea 30 days |
Body weight ↓ ↓ urea concentration ↓ total bilirubin ↓ triglycerides ↑ total protein in blood ↑ alkaline phosphatase activity ↑ Hemoglobin ↑ Erythrocytes ↑ Erythrocyte sedimentation rate (ESR), ↑ WBC, |
[25] |
| S. officinalis L. | Male Wistar rats N=30 Sage Essential oil (2.5 μL/rat) Oral gavage 30 days |
inhibited α-amylase and lipase activities ↓ glycemia ↑ glycogen storage ↓ the ALT ↓ AST ↓ LDH ↓ Creatinine ↓ Uric acid |
[169] |
| S. lavandulifolia | Wistar rats and albino mice Aqueous extract (400 mg/kg) orally |
↓ post-prandial hyperglycemia AUC glucose levels ↓ |
[170] |
| NCT Number | Study Title | Interventions/ Treatment | Sponsor and Collaborators | Phase | Enrollment | Study type |
|---|---|---|---|---|---|---|
| NCT03233906 | The Effects of Chia on Overweight/Obese Women |
Dietary Supplement: Chia Seeds- Salvia Hispanica |
California State Polytechnic University, Pomona | NA | Female: 60 | Interventional |
| NCT01403571 |
Effectiveness and Safety of Salba on Weight Loss in Overweight Individuals With Type 2 Diabetes (LOSS) |
Dietary Supplement: Salba (Salvia hispanica L.) |
Unity Health Toronto [171] |
Phase II | Male/ Female: 77 |
Interventional |
| NCT02298426 | Body Constitution Classification Based Comprehensive Health Management Intervention on Obese Population |
Dietary Supplement: SCHSANDRA PLUS, YI RUI CAPSULE, Gest Aid Plus YI RUI CAPSULE health product made of Salvia, hawthorn, alisma, perilla oil microcapsules, panax powder, ginkgo biloba and other plant materials |
Hai-Jun Wang, Peking University | Completed | Male/ Female: 1452 | Interventional |
| NCT05068557 | EPICO: (Study for the Pro-resolution of Chronic Inflammation in Obesity. Original Acronym From Spanish) |
Dietary Supplement: Dietary plan along with chia/linseed oil capsules |
University of Guadalajara | NA | Male/ Female: 80 |
Interventional |
| NCT04208308 | Comparison of Effect of Animal and Plant Sources of Omega-3 PUFA in Prevention of Cardiovascular and Metabolic Diseases | Chia seeds supplementation | Pavol Jozef Safarik University | Early phase I | Male/ Female: 300 (Estimated) |
Interventional |
3.1.1. Experimental Evidence of Salvia Officinalis Against Obesity and Metabolic Disorders
3.2. Salvia hispanica
3.2.1. Experimental Evidence of Salvia hispanica Against Obesity and Metabolic Disorders
3.3. Salvia miltiorrhiza
3.3.1. Experimental Evidence of Salvia miltiorrhiza Against Obesity and Metabolic Disorders
3.4. Salvia libanotica
3.4.1. Experimental Evidence of Salvia libanotica Against Obesity and Metabolic Disorders
3.5. Salvia plebeia
3.5.1. Experimental Evidence of Salvia plebeia Against Obesity and Metabolic Disorders
4. Unveiling the Role of Gut Microbiota in Obesity
5. Exploring the Mechanisms by which Gut Microbiota Influence Obesity
5.1. Gut Microbiota Interactions with Adipose Tissue
5.2. Microbial Influence on Bile-Acid and Cholesterol Metabolism in the Gut
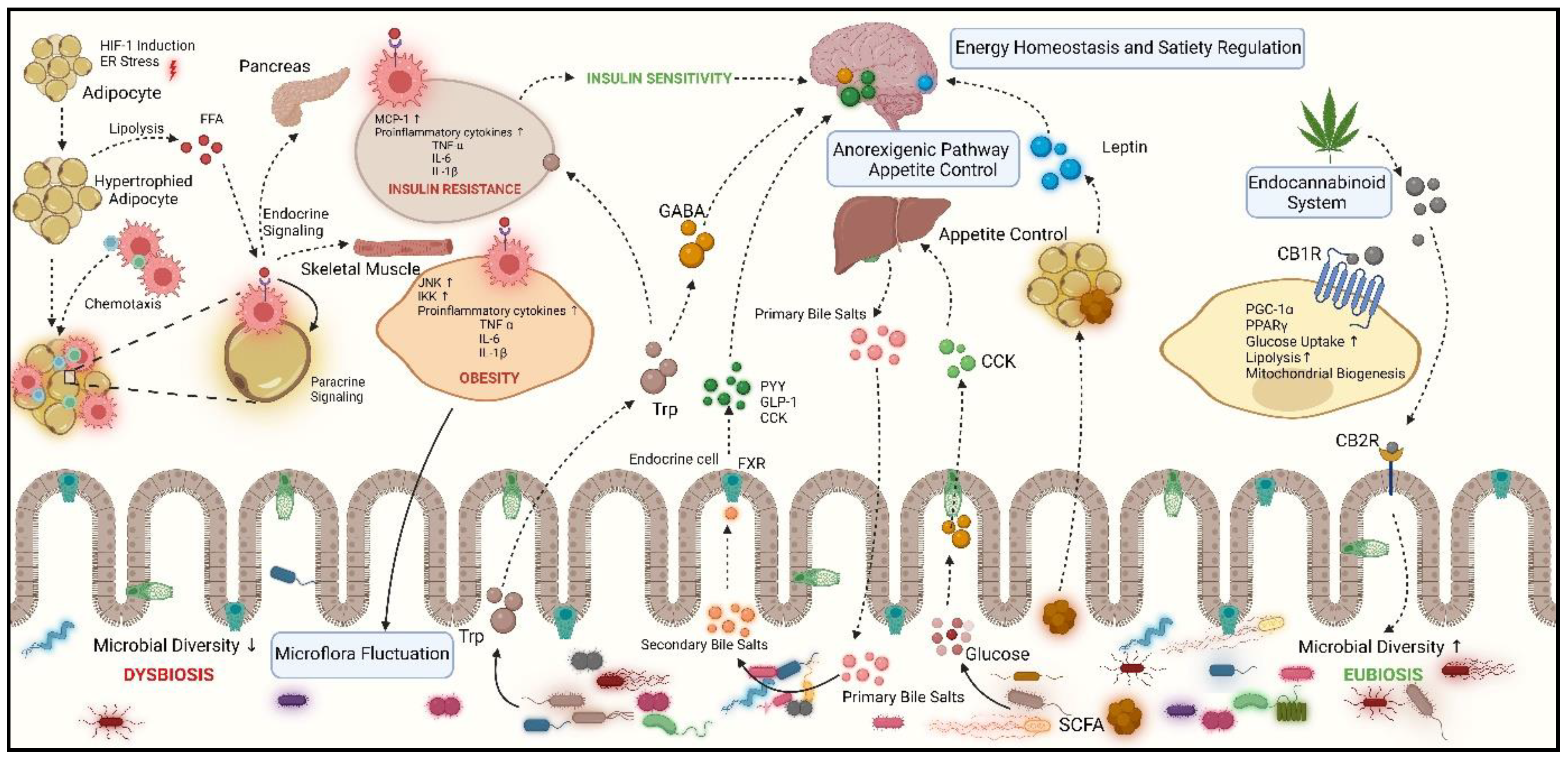
5.3. Influence of Gut Microbiota on Energy Homeostasis and Satiety Regulation
5.4. Endocannabinoid System
6. Conclusion and Future Perspective
Data availability
Acknowledgments
Conflict of Interests
References
- A. Alice, M. Yadav, R. Verma, M. Kumari, S. Arora, Effect of obesity on balance: A literature review, Ijhs (2022) 3261–3279. [CrossRef]
- WHO, World Health Organization. Obesity and Overweight., (2023). https://www.who.int/news-room/fact-sheets/detail/ obesity-and-overweight.
- WHO, Prevalence of obesity. https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity, (2021). https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity.
- World Obesity Atlas. https://www.indiaenvironmentportal.org.in/content/474617/world-obesity-atlas-2023/, (2023). http://www.indiaenvironmentportal.org.in/content/474617/world-obesity-atlas-2023/.
- N. Hamjane, F. Benyahya, N.G. Nourouti, M.B. Mechita, A. Barakat, Cardiovascular diseases and metabolic abnormalities associated with obesity: What is the role of inflammatory responses? A systematic review, Microvascular Research 131 (2020) 104023. [CrossRef]
- A. De Lorenzo, S. Gratteri, P. Gualtieri, A. Cammarano, P. Bertucci, L. Di Renzo, Why primary obesity is a disease?, J Transl Med 17 (2019) 169. [CrossRef]
- A. Gasmi, P.K. Mujawdiya, A. Nehaoua, M. Shanaida, Y. Semenova, S. Piscopo, A. Menzel, V. Voloshyn, O. Voloshyn, V. Shanaida, G. Bjørklund, Pharmacological Treatments and Natural Biocompounds in Weight Management, Pharmaceuticals 16 (2023) 212. [CrossRef]
- M. Mervić, M. Bival Štefan, M. Kindl, B. Blažeković, M. Marijan, S. Vladimir-Knežević, Comparative Antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase Activities of Mediterranean Salvia Species, Plants 11 (2022) 625. [CrossRef]
- I. Marchioni, B. Najar, B. Ruffoni, A. Copetta, L. Pistelli, L. Pistelli, Bioactive Compounds and Aroma Profile of Some Lamiaceae Edible Flowers, Plants 9 (2020) 691. [CrossRef]
- M.-D. Mot, S. Gavrilaș, A.I. Lupitu, C. Moisa, D. Chambre, D.M. Tit, M.A. Bogdan, A.-M. Bodescu, L. Copolovici, D.M. Copolovici, S.G. Bungau, Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients, Antioxidants 11 (2022) 808. [CrossRef]
- M. Sharifi-Rad, B. Ozcelik, G. Altın, C. Daşkaya-Dikmen, M. Martorell, K. Ramírez-Alarcón, P. Alarcón-Zapata, M.F.B. Morais-Braga, J.N.P. Carneiro, A.L. Alves Borges Leal, H.D.M. Coutinho, R. Gyawali, R. Tahergorabi, S.A. Ibrahim, R. Sahrifi-Rad, F. Sharopov, B. Salehi, M. Del Mar Contreras, A. Segura-Carretero, S. Sen, K. Acharya, J. Sharifi-Rad, Salvia spp. plants-from farm to food applications and phytopharmacotherapy, Trends in Food Science & Technology 80 (2018) 242–263. [CrossRef]
- M. Hamidpour, R. Hamidpour, S. Hamidpour, M. Shahlari, Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer, Journal of Traditional and Complementary Medicine 4 (2014) 82–88. [CrossRef]
- A. Soltanbei̇Gi̇, E. Samadpourrigani, PHENOLOGICAL CYCLE AND DIURNAL VARIATION EFFECTS ON THE VOLATILE OIL CHARACTERISTICS OF SAGE (Salvia officinalis L.), Trakya University Journal of Natural Sciences 22 (2021) 59–65. [CrossRef]
- A. Ghorbani, M. Esmaeilizadeh, Pharmacological properties of Salvia officinalis and its components, Journal of Traditional and Complementary Medicine 7 (2017) 433–440. [CrossRef]
- H.-Y. Li, D.-D. Zhou, R.-Y. Gan, S.-Y. Huang, C.-N. Zhao, A. Shang, X.-Y. Xu, H.-B. Li, Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review, Nutrients 13 (2021) 3211. [CrossRef]
- P. Badiee, A.R. Nasirzadeh, M. Motaffaf, Comparison of Salvia officinalis L. essential oil and antifungal agents against candida species, J Pharm Technol Drug Res 1 (2012) 7. [CrossRef]
- M. Couladis, A. Koutsaviti, Chemical composition of the essential oils of Salvia officinalis, S. fruticosa, Melissa officinalis, and their infusions, Ratar i Povrt 54 (2017) 36–41. [CrossRef]
- M. Grdiša, Dalmatian Sage (Salvia officinalis L.): A Review of Biochemical Contents, Medical Properties and Genetic Diversity, (n.d.).
- C.F. Lima, P.C.R. Valentao, P.B. Andrade, R.M. Seabra, M. Fernandes-Ferreira, C. Pereira-Wilson, Water and methanolic extracts of Salvia officinalis protect HepG2 cells from t-BHP induced oxidative damage, Chemico-Biological Interactions 167 (2007) 107–115. [CrossRef]
- A.F. Afonso, O.R. Pereira, Â. Fernandes, R.C. Calhelha, A.M.S. Silva, I.C.F.R. Ferreira, S.M. Cardoso, Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts, Molecules 24 (2019) 4327. [CrossRef]
- B.N. Silva, V. Cadavez, C. Caleja, E. Pereira, R.C. Calhelha, M. Añibarro-Ortega, T. Finimundy, M. Kostić, M. Soković, J.A. Teixeira, L. Barros, U. Gonzales-Barron, Phytochemical Composition and Bioactive Potential of Melissa officinalis L., Salvia officinalis L. and Mentha spicata L. Extracts, Foods 12 (2023) 947. [CrossRef]
- H.M. Rashwan, H.E. Mohammed, A.A. El-Nekeety, Z.K. Hamza, S.H. Abdel-Aziem, N.S. Hassan, M.A. Abdel-Wahhab, Bioactive phytochemicals from Salvia officinalis attenuate cadmium-induced oxidative damage and genotoxicity in rats, Environ Sci Pollut Res 28 (2021) 68498–68512. [CrossRef]
- A.R. Jassbi, S. Zare, O. Firuzi, J. Xiao, Bioactive phytochemicals from shoots and roots of Salvia species, Phytochem Rev 15 (2016) 829–867. [CrossRef]
- M.R. Ben Khedher, M. Hammami, J.R.S. Arch, D.C. Hislop, D. Eze, E.T. Wargent, M.A. Kępczyńska, M.S. Zaibi, Preventive effects of Salvia officinalis leaf extract on insulin resistance and inflammation in a model of high fat diet-induced obesity in mice that responds to rosiglitazone, PeerJ 6 (2018) e4166. [CrossRef]
- M.A. Lieshchova, A.A. Bohomaz, V.V. Brygadyrenko, Effect of Salvia officinalis and S. sclarea on rats with a high-fat hypercaloric diet, Regul. Mech. Biosyst. 12 (2021) 554–563. [CrossRef]
- M.S. Othman, A.M. Khaled, G.M. Aleid, M.A. Fareid, R.A. Hameed, M.S. Abdelfattah, D.E. Aldin, A.E.A. Moneim, Evaluation of antiobesity and hepatorenal protective activities of Salvia officinalis extracts pre-treatment in high-fat diet-induced obese rats, Environ Sci Pollut Res 29 (2022) 75043–75056. [CrossRef]
- L. Abdulhussein, A. Allaithi, W. Matrood, W.M. Kadhem, STUDY OF THE EFFECT OF SALVIA OFFICINALIS LEAVES EXTRACT AND XENICAL DRUG ON SOME OF THE BIOCHEMICAL AND HISTOLOGICAL PARAMETERS IN THE RATS INDUCED WITH HYPERLIPIDEMIA, Plant Archives 19 (2019) 1111–1112.
- S. Samah, Phytochemical Screening and Anti-Obesity Activity of Salvia Officinalis L, International Journal of Academic Scientific Research 6 (2018) 33–45.
- D.A. ALsherif, M.A. Hussein, S.S. Abuelkasem, S.A. Hassan, M. Wink, Salvia Officinalis Improves Glucose Uptake and Suppresses Ectopic Lipid Deposition in Obese Rats with Metabolic Syndrome, CPB 24 (2023). [CrossRef]
- A.M. Jamshidi, M. Amato, A. Ahmadi, R. Bochicchio, R. Rossi, Chia (Salvia hispanica L.) as a novel forage and feed source: A review, Ital J Agronomy 14 (2019) 1–18. [CrossRef]
- D. Orona-Tamayo, O. Paredes-López, Chia—The New Golden Seed for the 21st Century: Nutraceutical Properties and Technological Uses, in: Sustainable Protein Sources, Elsevier, 2024: pp. 443–470. [CrossRef]
- R.M. Bodoira, M.C. Penci, P.D. Ribotta, M.L. Martínez, Chia (Salvia hispanica L.) oil stability: Study of the effect of natural antioxidants, LWT 75 (2017) 107–113. [CrossRef]
- B. Kulczyński, J. Kobus-Cisowska, M. Taczanowski, D. Kmiecik, A. Gramza-Michałowska, The Chemical Composition and Nutritional Value of Chia Seeds-Current State of Knowledge, Nutrients 11 (2019) 1242. [CrossRef]
- R.D.S. Marineli, S.A. Lenquiste, É.A. Moraes, M.R. Maróstica, Antioxidant potential of dietary chia seed and oil ( Salvia hispanica L.) in diet-induced obese rats, Food Research International 76 (2015) 666–674. [CrossRef]
- M. Knez Hrnčič, M. Ivanovski, D. Cör, Ž. Knez, Chia Seeds (Salvia Hispanica L.): An Overview—Phytochemical Profile, Isolation Methods, and Application, Molecules 25 (2019) 11. [CrossRef]
- N. Mohd Ali, S.K. Yeap, W.Y. Ho, B.K. Beh, S.W. Tan, S.G. Tan, The Promising Future of Chia, Salvia hispanica L., Journal of Biomedicine and Biotechnology 2012 (2012) 1–9. [CrossRef]
- B. De Falco, M. Amato, V. Lanzotti, Chia seeds products: an overview, Phytochem Rev 16 (2017) 745–760. [CrossRef]
- Md.J. Rahman, A.C. De Camargo, F. Shahidi, Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities, Journal of Functional Foods 35 (2017) 622–634. [CrossRef]
- Md.J. Rahman, A.C. De Camargo, F. Shahidi, Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities, Journal of Functional Foods 35 (2017) 622–634. [CrossRef]
- B. De Falco, A. Fiore, R. Rossi, M. Amato, V. Lanzotti, Metabolomics driven analysis by UAEGC-MS and antioxidant activity of chia (Salvia hispanica L.) commercial and mutant seeds, Food Chemistry 254 (2018) 137–143. [CrossRef]
- M. Grancieri, H.S.D. Martino, E. Gonzalez De Mejia, Chia Seed ( Salvia hispanica L.) as a Source of Proteins and Bioactive Peptides with Health Benefits: A Review, Comp Rev Food Sci Food Safe 18 (2019) 480–499. [CrossRef]
- M. Amato, M.C. Caruso, F. Guzzo, F. Galgano, M. Commisso, R. Bochicchio, R. Labella, F. Favati, Nutritional quality of seeds and leaf metabolites of Chia (Salvia hispanica L.) from Southern Italy, Eur Food Res Technol 241 (2015) 615–625. [CrossRef]
- H.S. Elshafie, L. Aliberti, M. Amato, V. De Feo, I. Camele, Chemical composition and antimicrobial activity of chia (Salvia hispanica L.) essential oil, Eur Food Res Technol 244 (2018) 1675–1682. [CrossRef]
- T. Fonte-Faria, M. Citelli, G.C. Atella, H.F. Raposo, L. Zago, T. De Souza, S.V. Da Silva, C. Barja-Fidalgo, Chia oil supplementation changes body composition and activates insulin signaling cascade in skeletal muscle tissue of obese animals, Nutrition 58 (2019) 167–174. [CrossRef]
- K. G Singh, A. Kour, A. Mohanty, In-vitro and Ex-vivo studies on Antiobesity Leptin Activation Effects of Saliva hispanica, Ocimum basilicum and Coriandrum sativum Extracts, IJPSRR 65 (2020) 114–120. [CrossRef]
- H. Martino, M. Grancieri, R. Toledo, T. Veridiano, C.T. Sant’Ana, N. Costa, E.G. De Mejia, Chia Seed (Salvia hispanica L.) Digested Total Protein Prevented Adipose Tissue Inflammation and Reduce Obesity Complications in Mice Fed a High-Fat Diet, Current Developments in Nutrition 4 (2020) nzaa045_069. [CrossRef]
- S. Rubavathi, G. Ayyappadasan, N. Sangeetha, T. Harini, D. Saranya, P. Harshapradha, Studies on Antioxidant and Anti-obesity Activity of Salvia hispanica (Chia) Seeds Extracts, J. Drug Delivery Ther. 10 (2020) 98–106. [CrossRef]
- H. Dib, M. Seladji, F.Z. Bencheikh, M. Faradji, C. Benammar, M. Belarbi, Phytochemical Screening and Antioxidant Activity of Salvia hispanica, JPRI (2021) 167–174. [CrossRef]
- H.A. Mutar, J.F.K. Alsadooni, ANTIOXIDANT AND ANTI-CANCER ACTIVITY OF CHIA SEED EXTRACT IN BREAST CANCER CELL LINE, ATMPH 22 (2019) 173–181. [CrossRef]
- A. Batista, F.T. Quitete, T.C. Peixoto, A. Almo, E.B. Monteiro, P. Trindade, L. Zago, M. Citelli, J.B. Daleprane, Chia (Salvia hispanica L.) oil supplementation ameliorates liver oxidative stress in high-fat diet-fed mice through PPAR-γ and Nrf2 upregulation, Journal of Functional Foods 102 (2023) 105462. [CrossRef]
- Z. Jiang, W. Gao, L. Huang, Tanshinones, Critical Pharmacological Components in Salvia miltiorrhiza, Front. Pharmacol. 10 (2019) 202. [CrossRef]
- H. Pang, L. Wu, Y. Tang, G. Zhou, C. Qu, J. Duan, Chemical Analysis of the Herbal Medicine Salviae miltiorrhizae Radix et Rhizoma (Danshen), Molecules 21 (2016) 51. [CrossRef]
- Y. Guo, Y. Li, L. Xue, R.P. Severino, S. Gao, J. Niu, L.-P. Qin, D. Zhang, D. Brömme, Salvia miltiorrhiza: An ancient Chinese herbal medicine as a source for anti-osteoporotic drugs, Journal of Ethnopharmacology 155 (2014) 1401–1416. [CrossRef]
- Q. Jin, X. Hu, Y. Deng, J. Hou, M. Lei, H. Ji, J. Zhou, H. Qu, W. Wu, D. Guo, Four New Depsides Isolated from Salvia miltiorrhiza and Their Significant Nerve-Protective Activities, Molecules 23 (2018) 3274. [CrossRef]
- G.-X. Zhong, P. Li, L.-J. Zeng, J. Guan, D.-Q. Li, S.-P. Li, Chemical Characteristics of Salvia miltiorrhiza (Danshen) Collected from Different Locations in China, J. Agric. Food Chem. 57 (2009) 6879–6887. [CrossRef]
- Y.-I. Li, G. Elmer, R.C. LeBoeuf, Tanshinone IIA reduces macrophage death induced by hydrogen peroxide by upregulating glutathione peroxidase, Life Sciences 83 (2008) 557–562. [CrossRef]
- L. Zou, D. Liu, H. Yang, C. Zhou, S. Deng, N. Xu, X. He, Y. Liu, M. Shao, L. Yu, J. Liu, Salvianolic acids from Salvia miltiorrhiza Bunge and their anti-inflammatory effects through the activation of α7nAchR signaling, Journal of Ethnopharmacology 317 (2023) 116743. [CrossRef]
- H.G. Choi, P.T. Tran, J.-H. Lee, B.S. Min, J.A. Kim, Anti-inflammatory activity of caffeic acid derivatives isolated from the roots of Salvia miltiorrhiza Bunge, Arch. Pharm. Res. 41 (2018) 64–70. [CrossRef]
- M. Luo, D.-D. Zhou, A. Shang, R.-Y. Gan, H.-B. Li, Influences of food contaminants and additives on gut microbiota as well as protective effects of dietary bioactive compounds, Trends in Food Science & Technology 113 (2021) 180–192. [CrossRef]
- D.Y. Jung, J.-H. Kim, M.H. Jung, Anti-Obesity Effects of Tanshinone I from Salvia miltiorrhiza Bunge in Mice Fed a High-Fat Diet through Inhibition of Early Adipogenesis, Nutrients 12 (2020) 1242. [CrossRef]
- T. An, J. Zhang, B. Lv, Y. Liu, J. Huang, J. Lian, Y. Wu, S. Gao, G. Jiang, Salvianolic acid B plays an anti-obesity role in high fat diet-induced obese mice by regulating the expression of mRNA, circRNA, and lncRNA, PeerJ 7 (2019) e6506. [CrossRef]
- I. Jung, H. Kim, S. Moon, H. Lee, B. Kim, Overview of Salvia miltiorrhiza as a Potential Therapeutic Agent for Various Diseases: An Update on Efficacy and Mechanisms of Action, Antioxidants 9 (2020) 857. [CrossRef]
- Z.-L. Ai, X. Zhang, W. Ge, Y.-B. Zhong, H.-Y. Wang, Z.-Y. Zuo, D.-Y. Liu, Salvia miltiorrhiza extract may exert an anti-obesity effect in rats with high-fat diet-induced obesity by modulating gut microbiome and lipid metabolism, World J Gastroenterol 28 (2022) 6131–6156. [CrossRef]
- Y.-L. Wu, H. Lin, H.-F. Li, M.-J. Don, P.-C. King, H.-H. Chen, Salvia miltiorrhiza Extract and Individual Synthesized Component Derivatives Induce Activating-Transcription-Factor-3-Mediated Anti-Obesity Effects and Attenuate Obesity-Induced Metabolic Disorder by Suppressing C/EBPα in High-Fat-Induced Obese Mice, Cells 11 (2022) 1022. [CrossRef]
- Y.-B. Wu, Z.-Y. Ni, Q.-W. Shi, M. Dong, H. Kiyota, Y.-C. Gu, B. Cong, Constituents from Salvia Species and Their Biological Activities, Chem. Rev. 112 (2012) 5967–6026. [CrossRef]
- M.D. Gkioni, K. Zeliou, V.D. Dimaki, P. Trigas, F.N. Lamari, GC-MS and LC-DAD-MS Phytochemical Profiling for Characterization of Three Native Salvia Taxa from Eastern Mediterranean with Antiglycation Properties, Molecules 28 (2022) 93. [CrossRef]
- M. Dawra, J. Bouajila, M. El Beyrouthy, A. Abi Rizk, P. Taillandier, N. Nehme, Y. El Rayess, Chemical Characterization and Antioxidant, Antibacterial, Antiacetylcholinesterase and Antiproliferation Properties of Salvia fruticosa Miller Extracts, Molecules 28 (2023) 2429. [CrossRef]
- R. Boukhary, M. Aboul-Ela, O. Al-Hanbali, A. El-Lakany, Chemical Constituents from Salvia fruticosa libanotica, PJ 10 (2017) 45–48. [CrossRef]
- M. Villalva, L. Jaime, E. Aguado, J.A. Nieto, G. Reglero, S. Santoyo, Anti-Inflammatory and Antioxidant Activities from the Basolateral Fraction of Caco-2 Cells Exposed to a Rosmarinic Acid Enriched Extract, J. Agric. Food Chem. 66 (2018) 1167–1174. [CrossRef]
- S.B. Subramanya, B. Venkataraman, S. Almarzooqi, V. Raj, V.S. Subramanian, B.A. Bhongade, 1,8-Cineole, a bioactive monoterpenoid, mitigates colon inflammation by stimulating colon PPAR-γ transcription factor, The FASEB Journal 36 (2022) fasebj.2022.36.S1.R3110. [CrossRef]
- M. Hijazi, K. Hijazi, K. Bouhadir, Z. Fatfat, M. Aboul-Ela, H. Gali-Muhtasib, A. El-Lakany, Anticancer activity of abietane diterpenoids from Salvia libanoticum grown in Lebanon, Phcog Mag 17 (2021) 127. [CrossRef]
- A. Alqudah, E.Y. Qnais, O. Gammoh, Y. Bseiso, M. Wedyan, Potential anti-inflammatory activity of the Salvia fruticosa Mill. essential oil, J Pharm Pharmacogn Res 12 (2024) 14–26. [CrossRef]
- M. Dawra, J. Bouajila, M. El Beyrouthy, A. Abi Rizk, P. Taillandier, N. Nehme, Y. El Rayess, Chemical Characterization and Antioxidant, Antibacterial, Antiacetylcholinesterase and Antiproliferation Properties of Salvia fruticosa Miller Extracts, Molecules 28 (2023) 2429. [CrossRef]
- S. Kyriakou, V. Tragkola, M. Plioukas, I. Anestopoulos, P.S. Chatzopoulou, E. Sarrou, D.T. Trafalis, M.V. Deligiorgi, R. Franco, A. Pappa, M.I. Panayiotidis, Chemical and Biological Characterization of the Anticancer Potency of Salvia fruticosa in a Model of Human Malignant Melanoma, Plants (Basel) 10 (2021) 2472. [CrossRef]
- M. Bassil, C.F. Daher, M. Mroueh, N. Zeeni, Salvia libanotica improves glycemia and serum lipid profile in rats fed a high fat diet, BMC Complement Altern Med 15 (2015) 384. [CrossRef]
- M. Dawra, N. Nehme, Y.E. Rayess, M.E. Beyrouthy, P. Taillandier, J. Bouajila, Folk medicinal applications, phytochemical composition and biological activities of some Lebanese endemic plants, South African Journal of Botany 150 (2022) 511–527. [CrossRef]
- P. Ercan, S.N. El, Bioaccessibility and inhibitory effects on digestive enzymes of carnosic acid in sage and rosemary, International Journal of Biological Macromolecules 115 (2018) 933–939. [CrossRef]
- P. Drikvandi, S. Bahramikia, M. Alirezaei, Modulation of the antioxidant defense system in liver, kidney, and pancreas tissues of alloxan-induced diabetic rats by camphor, J. Food Biochem. 44 (2020). [CrossRef]
- Y. Liang, X. Wan, F. Niu, S. Xie, H. Guo, Y. Yang, L. Guo, C. Zhou, Salvia plebeia R. Br. : an overview about its traditional uses, chemical constituents, pharmacology and modern applications, Biomedicine & Pharmacotherapy 121 (2020) 109589. [CrossRef]
- H. Kim, J.-Y. Choi, M. Hong, H.S. Suh, Traditional medicine for the treatment of common cold in Korean adults: A nationwide population-based study, Integr Med Res 10 (2021) 100458. [CrossRef]
- S. Bae, Y.-H. Lee, J. Lee, J. Park, W. Jun, Salvia plebeia R. Br. Water Extract Ameliorates Hepatic Steatosis in a Non-Alcoholic Fatty Liver Disease Model by Regulating the AMPK Pathway, Nutrients 14 (2022) 5379. [CrossRef]
- E.M. Zahran, U.R. Abdelmohsen, H.E. Khalil, S.Y. Desoukey, M.A. Fouad, M.S. Kamel, Diversity, phytochemical and medicinal potential of the genus Ocimum L. (Lamiaceae), Phytochem Rev 19 (2020) 907–953. [CrossRef]
- Y. Liang, X. Wan, F. Niu, S. Xie, H. Guo, Y. Yang, L. Guo, C. Zhou, Salvia plebeia R. Br. : an overview about its traditional uses, chemical constituents, pharmacology and modern applications, Biomedicine & Pharmacotherapy 121 (2020) 109589. [CrossRef]
- Y. Dai, Z. Ye, H. Liu, R. Zhu, L. Sun, S. Li, G. Xie, Y. Zhu, Y. Zhao, M. Qin, The chemical profiling of Salvia plebeia during different growth periods and the biosynthesis of its main flavonoids ingredients, Front. Plant Sci. 14 (2023) 1228356. [CrossRef]
- K. Patel, D.K. Patel, Medicinal importance, pharmacological activities, and analytical aspects of hispidulin: A concise report, J Tradit Complement Med 7 (2017) 360–366. [CrossRef]
- L. Fan, X. Zhang, Y. Huang, B. Zhang, W. Li, Q. Shi, Y. Lin, F. Wu, Homoplantaginin attenuates high glucose-induced vascular endothelial cell apoptosis through promoting autophagy via the AMPK / TFEB pathway, Phytotherapy Research 37 (2023) 3025–3041. [CrossRef]
- S.-I. Choi, I.-H. Cho, S.H. Han, Y.-J. Jeon, J.-G. Choi, J.-S. Kim, J.-H. Lee, Antiobesity Effects of Salvia plebeia R. Br. Extract in High-Fat Diet-Induced Obese Mice, Journal of Medicinal Food 19 (2016) 1048–1056. [CrossRef]
- H.-H. Jang, S.-Y. Cho, M.-J. Kim, J.-B. Kim, S.-H. Lee, M.-Y. Lee, Y.-M. Lee, Anti-inflammatory effects of Salvia plebeia R. Br extract in vitro and in ovalbumin-induced mouse model, Biol Res 49 (2016) 41. [CrossRef]
- H.R. Won, Effect of Salvia plebeia Water Extract on Antioxidant Activity and Lipid Composition of Rats Fed a High Fat-High Cholesterol Diet, KJCLS 27 (2016) 233–243. [CrossRef]
- O. Pereira, M. Catarino, A. Afonso, A. Silva, S. Cardoso, Salvia elegans, Salvia greggii and Salvia officinalis Decoctions: Antioxidant Activities and Inhibition of Carbohydrate and Lipid Metabolic Enzymes, Molecules 23 (2018) 3169. [CrossRef]
- H.-J. Eom, Y.Y. Jeong, N.R. Kwon, K.H. Kim, E. Yeon, H.-S. Yoon, Y. Ryu, I.J. Kim, Nutritional Components and Physiological Activity of 4 Wild Vegetables (Salvia plebeia R. Br, Angelica acutiloba, Gynura procumbens and Saururus chinensis Baill) Cultivated in Chungbuk Province, The Korean Journal of Food And Nutrition 34 (2021) 398–406. [CrossRef]
- M.A. Senchukova, Microbiota of the gastrointestinal tract: Friend or foe?, World J Gastroenterol 29 (2023) 19–42. [CrossRef]
- A. Andoh, A. Nishida, Alteration of the Gut Microbiome in Inflammatory Bowel Disease, Digestion 104 (2023) 16–23. [CrossRef]
- S. Fujisaka, Y. Watanabe, K. Tobe, The gut microbiome: a core regulator of metabolism, Journal of Endocrinology 256 (2023) e220111. [CrossRef]
- P. Portincasa, L. Bonfrate, M. Vacca, M. De Angelis, I. Farella, E. Lanza, M. Khalil, D.Q.-H. Wang, M. Sperandio, A. Di Ciaula, Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis, IJMS 23 (2022) 1105. [CrossRef]
- B. Madhogaria, P. Bhowmik, A. Kundu, Correlation between human gut microbiome and diseases, Infectious Medicine 1 (2022) 180–191. [CrossRef]
- S.H. Zyoud, M. Shakhshir, A.S. Abushanab, A. Koni, M. Shahwan, A.A. Jairoun, S.W. Al-Jabi, Global research trends on the links between insulin resistance and obesity: a visualization analysis, Transl Med Commun 7 (2022) 18. [CrossRef]
- N. Redondo-Useros, E. Nova, N. González-Zancada, L.E. Díaz, S. Gómez-Martínez, A. Marcos, Microbiota and Lifestyle: A Special Focus on Diet, Nutrients 12 (2020) 1776. [CrossRef]
- T. Hrncir, Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options, Microorganisms 10 (2022) 578. [CrossRef]
- M. De Siena, P. Raoul, L. Costantini, E. Scarpellini, M. Cintoni, A. Gasbarrini, E. Rinninella, M.C. Mele, Food Emulsifiers and Metabolic Syndrome: The Role of the Gut Microbiota, Foods 11 (2022) 2205. [CrossRef]
- B.S. Almugadam, Y. Liu, S.-M. Chen, C.-H. Wang, C.-Y. Shao, B.-W. Ren, L. Tang, Alterations of Gut Microbiota in Type 2 Diabetes Individuals and the Confounding Effect of Antidiabetic Agents, J Diabetes Res 2020 (2020) 7253978. [CrossRef]
- D.K. Wiredu Ocansey, S. Hang, X. Yuan, H. Qian, M. Zhou, C. Valerie Olovo, X. Zhang, F. Mao, The diagnostic and prognostic potential of gut bacteria in inflammatory bowel disease, Gut Microbes 15 (2023) 2176118. [CrossRef]
- V. Liakina, S. Strainiene, I. Stundiene, V. Maksimaityte, E. Kazenaite, Gut microbiota contribution to hepatocellular carcinoma manifestation in non-alcoholic steatohepatitis, World J Hepatol 14 (2022) 1277–1290. [CrossRef]
- L. Gao, W. Peng, H. Xue, Y. Wu, H. Zhou, P. Jia, Y. Wang, Spatial–temporal trends in global childhood overweight and obesity from 1975 to 2030: a weight mean center and projection analysis of 191 countries, Global Health 19 (2023) 53. [CrossRef]
- K.W. Tham, R. Abdul Ghani, S.C. Cua, C. Deerochanawong, M. Fojas, S. Hocking, J. Lee, T.Q. Nam, F. Pathan, B. Saboo, S. Soegondo, N. Somasundaram, A.M.L. Yong, J. Ashkenas, N. Webster, B. Oldfield, Obesity in South and Southeast Asia—A new consensus on care and management, Obesity Reviews 24 (2023) e13520. [CrossRef]
- R. Williams, M. Periasamy, Genetic and Environmental Factors Contributing to Visceral Adiposity in Asian Populations, Endocrinol Metab 35 (2020) 681–695. [CrossRef]
- J. Tokarek, J. Gadzinowska, E. Młynarska, B. Franczyk, J. Rysz, What Is the Role of Gut Microbiota in Obesity Prevalence? A Few Words about Gut Microbiota and Its Association with Obesity and Related Diseases, Microorganisms 10 (2021) 52. [CrossRef]
- Z. Xu, W. Jiang, W. Huang, Y. Lin, F.K.L. Chan, S.C. Ng, Gut microbiota in patients with obesity and metabolic disorders - a systematic review, Genes Nutr 17 (2022) 2. [CrossRef]
- T.V. Kirichenko, Y.V. Markina, A.I. Bogatyreva, T.V. Tolstik, Y.R. Varaeva, A.V. Starodubova, The Role of Adipokines in Inflammatory Mechanisms of Obesity, Int J Mol Sci 23 (2022) 14982. [CrossRef]
- M. Patel, D. Fowler, J. Sizer, C. Walton, Faecal volatile biomarkers of Clostridium difficile infection, PLoS ONE 14 (2019) e0215256. [CrossRef]
- K.A. Krautkramer, J. Fan, F. Bäckhed, Gut microbial metabolites as multi-kingdom intermediates, Nat Rev Microbiol 19 (2021) 77–94. [CrossRef]
- Hernández, Canfora, Jocken, Blaak, The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity, Nutrients 11 (2019) 1943. [CrossRef]
- Z.C. Holmes, J.D. Silverman, H.K. Dressman, Z. Wei, E.P. Dallow, S.C. Armstrong, P.C. Seed, J.F. Rawls, L.A. David, Short-Chain Fatty Acid Production by Gut Microbiota from Children with Obesity Differs According to Prebiotic Choice and Bacterial Community Composition, mBio 11 (2020) e00914-20. [CrossRef]
- O. Lange, M. Proczko-Stepaniak, A. Mika, Short-Chain Fatty Acids—A Product of the Microbiome and Its Participation in Two-Way Communication on the Microbiome-Host Mammal Line, Curr Obes Rep 12 (2023) 108–126. [CrossRef]
- Y. Wu, H. Xu, X. Tu, Z. Gao, The Role of Short-Chain Fatty Acids of Gut Microbiota Origin in Hypertension, Front. Microbiol. 12 (2021) 730809. [CrossRef]
- S. Heinonen, R. Jokinen, A. Rissanen, K.H. Pietiläinen, White adipose tissue mitochondrial metabolism in health and in obesity, Obesity Reviews 21 (2020) e12958. [CrossRef]
- D. Wu, H. Wang, L. Xie, F. Hu, Cross-Talk Between Gut Microbiota and Adipose Tissues in Obesity and Related Metabolic Diseases, Front. Endocrinol. 13 (2022) 908868. [CrossRef]
- S.-H. Cho, Y.-J. Cho, J.-H. Park, The human symbiont Bacteroides thetaiotaomicron promotes diet-induced obesity by regulating host lipid metabolism, J Microbiol. 60 (2022) 118–127. [CrossRef]
- M. Kumar, R. Nagpal, R. Kumar, R. Hemalatha, V. Verma, A. Kumar, C. Chakraborty, B. Singh, F. Marotta, S. Jain, H. Yadav, Corrigendum to “Cholesterol-Lowering Probiotics as Potential Biotherapeutics for Metabolic Diseases,” Journal of Diabetes Research 2022 (2022) 1–14. [CrossRef]
- A. Kriaa, M. Bourgin, A. Potiron, H. Mkaouar, A. Jablaoui, P. Gérard, E. Maguin, M. Rhimi, Microbial impact on cholesterol and bile acid metabolism: current status and future prospects, J Lipid Res 60 (2019) 323–332. [CrossRef]
- L.V. Gromova, S.O. Fetissov, A.A. Gruzdkov, Mechanisms of Glucose Absorption in the Small Intestine in Health and Metabolic Diseases and Their Role in Appetite Regulation, Nutrients 13 (2021) 2474. [CrossRef]
- P. Strandwitz, Neurotransmitter modulation by the gut microbiota, Brain Research 1693 (2018) 128–133. [CrossRef]
- O. Contreras-Rodriguez, M. Solanas, R.M. Escorihuela, Dissecting ultra-processed foods and drinks: Do they have a potential to impact the brain?, Rev Endocr Metab Disord 23 (2022) 697–717. [CrossRef]
- B. Van Der Hee, J.M. Wells, Microbial Regulation of Host Physiology by Short-chain Fatty Acids, Trends in Microbiology 29 (2021) 700–712. [CrossRef]
- C.B. Christiansen, S. Veedfald, B. Hartmann, A.M. Gauguin, S. Møller, T. Moritz, S. Madsbad, J.J. Holst, Colonic Lactulose Fermentation Has No Impact on Glucagon-like Peptide-1 and Peptide-YY Secretion in Healthy Young Men, The Journal of Clinical Endocrinology & Metabolism 107 (2022) 77–87. [CrossRef]
- N. Forte, A.C. Fernández-Rilo, L. Palomba, V. Di Marzo, L. Cristino, Obesity Affects the Microbiota–Gut–Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators, IJMS 21 (2020) 1554. [CrossRef]
- A. Alharthi, S. Alhazmi, N. Alburae, A. Bahieldin, The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder, IJMS 23 (2022) 1363. [CrossRef]
- J. Jansma, F. Brinkman, S. Van Hemert, S. El Aidy, Targeting the endocannabinoid system with microbial interventions to improve gut integrity, Progress in Neuro-Psychopharmacology and Biological Psychiatry 106 (2021) 110169. [CrossRef]
- A.B. Bansal, Y. Al Khalili, Orlistat, in: StatPearls, StatPearls Publishing, Treasure Island (FL), 2024. http://www.ncbi.nlm.nih.gov/books/NBK542202/ (accessed January 23, 2024).
- J.G. Kang, C.-Y. Park, Anti-Obesity Drugs: A Review about Their Effects and Safety, Diabetes Metab J 36 (2012) 13. [CrossRef]
- M. Chakhtoura, R. Haber, M. Ghezzawi, C. Rhayem, R. Tcheroyan, C.S. Mantzoros, Pharmacotherapy of obesity: an update on the available medications and drugs under investigation, eClinicalMedicine 58 (2023) 101882. [CrossRef]
- A. Depaoli, A. Long, G.M. Fine, M. Stewart, S. O’Rahilly, Efficacy of Metreleptin for Weight Loss in Overweight and Obese Adults with Low Leptin Levels, Diabetes 67 (2018) 296-LB. [CrossRef]
- M. Lenharo, Anti-obesity drug also protects against heart disease — what happens next?, Nature 620 (2023) 480–480. [CrossRef]
- S. Ghoneim, Tirzepatide: A New Anti-Obesity Medication, Gastroenterology 164 (2023) 159. [CrossRef]
- H.W.R. Li, K.S.L. Lam, P.C. Ho, Antiobesity drugs for obese women planning pregnancy, in: Obesity and Obstetrics, Elsevier, 2020: pp. 301–306. [CrossRef]
- C. Enright, E. Thomas, D.R. Saxon, An Updated Approach to Antiobesity Pharmacotherapy: Moving Beyond the 5% Weight Loss Goal, Journal of the Endocrine Society 7 (2023) bvac195. [CrossRef]
- E.A. Bohula, S.D. Wiviott, D.K. McGuire, S.E. Inzucchi, J. Kuder, K. Im, C.L. Fanola, A. Qamar, C. Brown, A. Budaj, A. Garcia-Castillo, M. Gupta, L.A. Leiter, N.J. Weissman, H.D. White, T. Patel, B. Francis, W. Miao, C. Perdomo, S. Dhadda, M.P. Bonaca, C.T. Ruff, A.C. Keech, S.R. Smith, M.S. Sabatine, B.M. Scirica, Cardiovascular Safety of Lorcaserin in Overweight or Obese Patients, N Engl J Med 379 (2018) 1107–1117. [CrossRef]
- I.E. Poyraz, G.A. Ciftci, N. Öztürk, Phenolic Contents, in vitro Antioxidant and Cytotoxicity Activities of Salvia aethiopis L. and S. ceratophylla L. (Lamiaceae), Records of Natural Products 11 (2017) 345–355.
- M.B. Bahadori, L. Dinparast, H. Valizadeh, M.M. Farimani, S.N. Ebrahimi, Bioactive constituents from roots of Salvia syriaca L.: Acetylcholinesterase inhibitory activity and molecular docking studies, South African Journal of Botany 106 (2016) 1–4. [CrossRef]
- J.S. Katanić Stanković, N. Srećković, D. Mišić, U. Gašić, P. Imbimbo, D.M. Monti, V. Mihailović, Bioactivity, biocompatibility and phytochemical assessment of lilac sage, Salvia verticillata L. (Lamiaceae) - A plant rich in rosmarinic acid, Industrial Crops and Products 143 (2020) 111932. [CrossRef]
- S. Stankov, H. Fidan, N. Petkova, I. Dincheva, A. Stoyanova, B.C. Senkal, H. Dogan, T. Uskutoglu, Phytochemical Composition of Salvia candidissima Vahl. ssp. occidentalis From Turkey, Journal of Essential Oil Bearing Plants 23 (2020) 710–718. [CrossRef]
- M.S. Abu-Darwish, C. Cabral, Z. Ali, M. Wang, S.I. Khan, M.R. Jacob, S.K. Jain, B.L. Tekwani, F. Zulfiqar, I.A. Khan, H. Taifour, L. Salgueiro, T. Efferth, Salvia ceratophylla L. from South of Jordan: new insights on chemical composition and biological activities, Nat. Prod. Bioprospect. 10 (2020) 307–316. [CrossRef]
- İ. Emre, M. Kurşat, S. Kirbag, P. Erecevi̇T Sönmez, M.Y. Emre, Prof.Dr.Ö. Yilmaz, Ş. Ci̇Velek, The Antioxidant and Antimicrobial Capacities of Phenolic Profiles of Some Salvia L. Seeds Grown in Turkey, International Journal of Secondary Metabolite 8 (2021) 20–30. [CrossRef]
- H. Bardakci, E. Celep, T. Gözet, I. Kurt-Celep, I. Deniz, B. Şen-Utsukarci, G. Akaydin, A comparative investigation on phenolic composition, antioxidant and antimicrobial potentials of Salvia heldreichiana Boiss. ex Bentham extracts, South African Journal of Botany 125 (2019) 72–80. [CrossRef]
- S. Guzel, M. Ulger, A. Kahraman, Chemical Composition and Some Biological Activities of Salvia longipedicellata Hedge Mericarps, Chem Nat Compd 56 (2020) 788–792. [CrossRef]
- S. Bechkri, A. Alabdul Magid, L. Voutquenne-Nazabadioko, D. Berrehal, A. Kabouche, M. Lehbili, H. Lakhal, A. Abedini, S.C. Gangloff, H. Morjani, Z. Kabouche, Triterpenes from Salvia argentea var. aurasiaca and their antibacterial and cytotoxic activities, Fitoterapia 139 (2019) 104296. [CrossRef]
- M. Doğan, N. Akıcı, M.E. Diken, S. Doğan, B. Yilmaz Kardas, T. Dirmenci, Biological activities of some Salvia species, Zeitschrift Für Naturforschung C 77 (2022) 133–143. [CrossRef]
- M. Shanaida, N. Hudz, M. Białoń, M. Kryvtsowa, L. Svydenko, A. Filipska, P. Paweł Wieczorek, Chromatographic profiles and antimicrobial activity of the essential oils obtained from some species and cultivars of the Mentheae tribe (Lamiaceae), Saudi Journal of Biological Sciences 28 (2021) 6145–6152. [CrossRef]
- M. Yılar, Y. Bayar, A. Bayar, N. Genc, Chemical composition of the essential oil of Salvia bracteata banks and the biological activity of its extracts: Antioxidant, total phenolic, total flavonoid, antifungal and allelopathic effects, Bot Serb 44 (2020) 71–79. [CrossRef]
- G. Zengin, E.J. Llorent-Martínez, M.L.F. Córdova, M.B. Bahadori, A. Mocan, M. Locatelli, A. Aktumsek, Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca, Industrial Crops and Products 111 (2018) 11–21. [CrossRef]
- M. Akdeniz, I. Yener, M.A. Yilmaz, S. Irtegun Kandemir, F. Tekin, A. Ertas, A potential species for cosmetic and pharmaceutical industries: Insight to chemical and biological investigation of naturally grown and cultivated Salvia multicaulis Vahl, Industrial Crops and Products 168 (2021) 113566. [CrossRef]
- S.M. Talebi, M. Askary, N. Khalili, A. Matsyura, M. Ghorbanpour, K. Kariman, Genetic structure and essential oil composition in wild populations of Salvia multicaulis Vahl., Biochemical Systematics and Ecology 96 (2021) 104269. [CrossRef]
- G.G. Toplan, M. Kürkçüoğlu, F. Göger, T. Taşkın, A. Civaş, G. İşcan, G. Ecevit-Genç, A. Mat, K.H.C. Başer, Phytochemical screening and biological evaluation of Salvia hydrangea DC. ex Benth. growing in eastern Anatolia, South African Journal of Botany 147 (2022) 799–807. [CrossRef]
- M. Ben Farhat, J.A. Sotomayor, M.J. Jordán, Salvia verbenaca L. essential oil: Variation of yield and composition according to collection site and phenophase, Biochemical Systematics and Ecology 82 (2019) 35–43. [CrossRef]
- F. Noorbakhsh, S. Zare, O. Firuzi, A. Sakhteman, J.N. Chandran, B. Schneider, A.R. Jassbi, Phytochemical Analysis and Biological Activity of Salvia compressa Vent., Iran J Pharm Res 21 (2022). [CrossRef]
- A.F. Afonso, O.R. Pereira, Â.S.F. Fernandes, R.C. Calhelha, A.M.S. Silva, I.C.F.R. Ferreira, S.M. Cardoso, The Health-Benefits and Phytochemical Profile of Salvia apiana and Salvia farinacea var. Victoria Blue Decoctions, Antioxidants 8 (2019) 241. [CrossRef]
- A. Mocan, M. Babotă, A. Pop, I. Fizeșan, A. Diuzheva, M. Locatelli, S. Carradori, C. Campestre, L. Menghini, C.R. Sisea, M. Sokovic, G. Zengin, R. Păltinean, S. Bădărău, D. C. Vodnar, G. Crișan, Chemical Constituents and Biologic Activities of Sage Species: A Comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (Schur ex Griseb. & Schenk) Schur, Antioxidants 9 (2020) 480. [CrossRef]
- İ. Gülçin, A.Z. Tel, A.C. Gören, P. Taslimi, S.H. Alwasel, Sage (Salvia pilifera): determination of its polyphenol contents, anticholinergic, antidiabetic and antioxidant activities, Food Measure 13 (2019) 2062–2074. [CrossRef]
- Etsassala, Badmus, Waryo, Marnewick, Cupido, Hussein, Iwuoha, Alpha-Glucosidase and Alpha-Amylase Inhibitory Activities of Novel Abietane Diterpenes from Salvia africana-lutea, Antioxidants 8 (2019) 421. [CrossRef]
- N.G.E.R. Etsassala, J.A. Badmus, J.L. Marnewick, E.I. Iwuoha, F. Nchu, A.A. Hussein, Alpha-Glucosidase and Alpha-Amylase Inhibitory Activities, Molecular Docking, and Antioxidant Capacities of Salvia aurita Constituents, Antioxidants 9 (2020) 1149. [CrossRef]
- M.S. Kocak, C. Sarikurkcu, M. Cengiz, S. Kocak, M.C. Uren, B. Tepe, Salvia cadmica: Phenolic composition and biological activity, Industrial Crops and Products 85 (2016) 204–212. [CrossRef]
- G. Zengin, E.J. Llorent-Martínez, M.L.F. Córdova, M.B. Bahadori, A. Mocan, M. Locatelli, A. Aktumsek, Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca, Industrial Crops and Products 111 (2018) 11–21. [CrossRef]
- G. Zengin, E.J. Llorent-Martínez, M.L.F. Córdova, M.B. Bahadori, A. Mocan, M. Locatelli, A. Aktumsek, Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca, Industrial Crops and Products 111 (2018) 11–21. [CrossRef]
- O. Pereira, M. Catarino, A. Afonso, A. Silva, S. Cardoso, Salvia elegans, Salvia greggii and Salvia officinalis Decoctions: Antioxidant Activities and Inhibition of Carbohydrate and Lipid Metabolic Enzymes, Molecules 23 (2018) 3169. [CrossRef]
- M.B. Bahadori, B. Asghari, L. Dinparast, G. Zengin, C. Sarikurkcu, M. Abbas-Mohammadi, S. Bahadori, Salvia nemorosa L.: A novel source of bioactive agents with functional connections, LWT 75 (2017) 42–50. [CrossRef]
- S. Arabiyat, A. Al-Rabi’ee, H. Zalloum, M. Hudaib, M. Mohammad, Y. Bustanji, Antilipolytic and hypotriglyceridemic effects of dietary Salvia triloba Lf (Lamiaceae) in experimental rats, Trop. J. Pharm Res 15 (2016) 723. [CrossRef]
- B. Abd El-Motelp, H. Mohamed, Hanan. Abd El-Latief, M. Hassen, In Vivo Salvia officinalis Extract and Orlistat Provides a Multimechanistic Strategy for Preventing Obesity, Egyptian Academic Journal of Biological Sciences, B. Zoology 15 (2023) 83–104. [CrossRef]
- G. Maturana, J. Segovia, C. Olea-Azar, E. Uribe-Oporto, A. Espinosa, M.C. Zúñiga-López, Evaluation of the Effects of Chia (Salvia hispanica L.) Leaves Ethanolic Extracts Supplementation on Biochemical and Hepatic Markers on Diet-Induced Obese Mice, Antioxidants 12 (2023) 1108. [CrossRef]
- S. Belhadj, O. Hentati, M. Hammami, A. Ben Hadj, T. Boudawara, M. Dammak, S. Zouari, A. El Feki, Metabolic impairments and tissue disorders in alloxan-induced diabetic rats are alleviated by Salvia officinalis L. essential oil, Biomedicine & Pharmacotherapy 108 (2018) 985–995. [CrossRef]
- F. Remok, S. Saidi, A.A. Gourich, K. Zibouh, M. Maouloua, F.E. Makhoukhi, N.E. Menyiy, H. Touijer, M. Bouhrim, S. Sahpaz, A.M. Salamatullah, M. Bourhia, T. Zair, Phenolic Content, Antioxidant, Antibacterial, Antihyperglycemic, and α-Amylase Inhibitory Activities of Aqueous Extract of Salvia lavandulifolia Vahl, Pharmaceuticals 16 (2023) 395. [CrossRef]
- V. Vuksan, A.L. Jenkins, C. Brissette, L. Choleva, E. Jovanovski, A.L. Gibbs, R.P. Bazinet, F. Au-Yeung, A. Zurbau, H.V.T. Ho, L. Duvnjak, J.L. Sievenpiper, R.G. Josse, A. Hanna, Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: A double-blind randomized controlled trial, Nutrition, Metabolism and Cardiovascular Diseases 27 (2017) 138–146. [CrossRef]
| DRUG | MECHANISM OF ACTION | SIDE EFFECTS | REFERENCES |
|---|---|---|---|
|
Orlistat (Xenical, Alli) |
Inhibits fat absorption in the digestive tract: inhibiting gastric and pancreatic lipases | Steatorrhea, diarrhoea, and anal fissures, hepatotoxicity, acute kidney injury, osteoporosis, colorectal cancer |
[129] |
|
Phentermine (Qsymia) |
Appetite suppressant | Insomnia, dry mouth, dizziness, palpitation, hand tremor, and elevation in blood pressure and pulse rate | [130] |
| Setmelanotide (Imcivree) | melanocortin-4 (MC4) receptor agonist | Increased heart rate, emotional and sleep issues, memory loss, metabolic acidosis, paraesthesia, and dry mouth | [131] |
|
Metreleptin (Myalept) |
leptin analogue | Anxiety, blurred vision, body aches burning, itching, numbness, chills/fever, dizziness, increased hunger and sore throat | [131,132] |
|
Semaglutide (Wegovy) |
GLP-1 analogue | Abdominal pain, constipation, diarrhoea, vomiting, and headache | [133] |
| Tirzepatide (Mounjaro®) | GLP-1 and GIP receptor agonist | Vomiting, diarrhoea, nausea, dyspepsia, reduced appetite, and stomach discomfort. | [134] |
|
Liraglutide (Saxenda and Victoza) |
Mimics glucagon-like peptide-1 (GLP-1 agonist) | Elevated pulse rate, hypoglycemia, diarrhoea, constipation, nausea, vomiting, and headache | [145] |
| Naltrexone/Bupropion (Contrave®) | Modulates brain pathways related to appetite, reuptake inhibitor/ opioid antagonist | Nausea, headache, and constipation, insomnia, dizziness, or dry mouth | [136] |
| Lorcaserin | Activates serotonin receptors in the brain | Headache, dizziness, nausea | [137] |
| SPECIES | NATIVE AREA | EXTRACTS AND PLANT PART | MAJOR BIOACTIVE COMPONENTS | THERAPEUTIC POTENTIAL | REFERENCES |
|---|---|---|---|---|---|
| S. aethiopis | Turkey | Methanol, ethyl acetate extracts |
Rosmarinic acid |
Anti-oxidant and Cytotoxic | [138] |
|
S. syriaca L. |
Urmia, West Azarbaijan province | Acetone extract of powdered roots | ursolic acid corosolic acid β-sitosterol urs-12-en-2α,3β-diol daucosterol (1–5) β-sitosterol daucosterol |
Antioxidant, Anticholinesterase inhibition | [139] |
| S. verticillata | Western Serbia | Methanol extract from aerial parts | Rosmarinic acid, Caffeic acid, Salvianolic acid, Yunnaneic acids, Flavonoids |
Antimicrobial, Antibacterial, Antifungal, Antioxidant, Anti-inflammatory |
[140] |
|
S. candidissima subsp. occidentalis |
Turkey | Ethanol extract | Sclareol, Spathulenol, Caryophyllene oxide |
Antiviral, Cytotoxic activity, Antioxidant |
[141] |
| S. ceratophylla | South of Jordan | Methanol extract from aerial parts | Linalool, Germacrene-D, Bicyclogermacrene, Bicyclic diterpene- alcoholsclareol |
Antiviral, Cytotoxic activity, Anti-inflammatory, Antiprotozoal activitiy |
[142] |
|
S. frigida Boiss., S. candidissima subsp. candidissima Vahl., S. virgata Jacq., S. verticillata L. var. verticillate, S. russellii Benth |
Turkey | Methanol, Acetonitrile, Water extract from plant seeds |
Catechin, Rosmarinic acid, vanilic acid |
Antiviral, Cytotoxic activity, Antimicrobial, Antioxidant |
[143] |
| S. heldreichiana | Turkey | Methanol, Chloroform, ethyl acetate from Aerial parts | p-coumaric acid, caffeic acid, chlorogenic acid, rosmarinic acid, apigenin, apigenin-7-O-glucoside, vitexin, isoorientin, luteolin-7-O-glucoside, hyperoside | Antioxidant, Antimicrobial | [144] |
| S. longipedicellata | East Anatolia (Turkey) | Ethanol, Hexane extracts from mericarps |
α-linolenic acid, β-sitosterol, γ-tocopherol, potassium |
Antioxidant, Antimicrobial | [145] |
| S. argentea | Mediterranean region | Acetone extract from aerial parts | 1β,3β-dihydroxyurs-9(11)-12-diene, 3β-hydroxy-urs-9(11),12-diene, 1β,3β,15α,28-tetrahydroxyurs-12-en, 1β,3β,11α,15α-tetrahydroxy-urs-12-ene, 3β-hydroxy-olean9(11),12-diene |
Antibacterial, Cytotoxic activity | [146] |
|
S. macrochlamys S. kronenburgii, S. euphratica Montbret, S. huberi, S. kurdica |
Turkey | rutin hydrate, luteolin-7-glucoside, Vanillic acid |
Antioxidant, Antibacterial, Cytotoxic activity | [147] | |
|
S. sclarea |
Italy, Ukraine | Aerial, Fresh raw, Leaves |
Linalol, linaliyl acetate, germacrene D, α-terpineol |
Anti-inflammatory | [148] |
| S. bracteata | Province of Kırşehir, Turkey | Methanol, Ethyl acetate, Hexane extracts, Essentials oil, from flowers, shoots, leaves |
ledol, camphor, valencene |
Antioxidant, Antifungal | [149] |
| S. blepharochlaena | Central Türkiye | Dichloromethane, methanol, water extracts |
Rosmarinic acid | Antioxidant, Cytotoxic activity | [150] |
| S. multicaulis | Iran | Essential oil and ethanol extracts from leaf, roots, flower, |
α-pinene, camphene, 1,8-cineole, Camphor, Caryophllene, rosmarinic acid |
Anti-inflammatory, Antimicrobial, Antioxidant Analgesic effects | [151] [152] |
| S. hydrangea | Eastern region of Turkey | Water, n-hexane, chloroform, methanol from Aerial parts |
Camphor, 1,8-cineole, Camphene, Limonene, β-pinene, α-pinene, rosmarinic acid, luteolin glycoside |
Antioxidant, Antimicrobial, Anti-inflammatory, Antispasmodic, Aypolipidemic |
[153] |
| S. verbenaca | Tunisia | Aerial | Viridiflorol, α-pinene, β-caryophyllene, p-cymene |
Antibacterial, Anticancer, Antioxidant, Antidiabetic | [154] |
| S. compressa | Iran | Dichloromethane extract from shoot extract | Citrostadienol, β-sitosterol, two glyceride esters of linolenic, linoleic, and palmitic acids geraniol |
Cytotoxic, Antibacterial |
[155] |
|
S. apiana S. farinacea var. Victoria Blue |
Portugal | Aqueous extract from Aerial parts | Rosmanol, Hydroxycarnosic acid, derivative of sageone |
Antioxidant Anti-inflammatory Antimicrobial Cytotoxic |
[156] |
|
S. transsylvanica S. glutinosa S. officinalis |
Schenk Romania |
Ethanol extract from aerial parts | Rutin, Catechin, Epicatechin kaempferol, Apigenin, Quercetin derivatives, Caffeic acid, p-coumaric acid, naringenin |
Antimicrobial, Antifungal, Cytotoxic | [157] |
| S. pilifera | Turkey | Methanol, Water extract from aerial parts |
Salvigenin quercetagetin-3,6-dimethylether, caffeic acid, epicatechin, ferulic acid, fumaric acid |
Anticholinergic, Antidiabetic, Antioxidant | [158] |
| Salvia species | Experimental plant extracts–Extract form / Constituents, dose | Phytochemicals (PY) isolated and Experimental outcome (EO) | Reference |
|---|---|---|---|
| S. africana-lutea | MeOH extract 20 μL |
PY: Oleanolic (OlA) Ursolic acids 11,12-dehydroursolic acid lactone β-amyrin EO: Inhibition of α-glucosidases Inhibition of α-Amlyase |
[159] |
| S. aurita | MeOH extracts 20 μL |
PY: 4,7-dimethylapigenin ether 7-methoxyrosmanol 12-methoxycarnosic acid Rosmanol Acarbose EO: Inhibition of α-glucosidases Inhibition of α-Amlyase |
[160] |
| S. cadmica |
Aqueous extract (H2O) Methanol extract (MeOH) ethyl acetate extract (EA) |
Inhibition of α-glucosidases: MeOH > EA > H2O Inhibition of α-Amlyase MeOH > EA > H2O |
[161] |
| S. blepharochlaena | Aqueous extract (H2O) Dichloromethane extract (DCM), Methanol (MeOH) extract |
Inhibition of α-Amlyase: DCM > MeOH > H2O Inhibition of α-glucosidase : MeOH > H2O > DCM Inhibition of Lipase: DCM > MeOH > H2O |
[162] |
| S. euphratica var. leiocalycina | Aqueous extract (H2O) Dichloromethane extract (DCM), Methanol (MeOH) extract |
Inhibition of α-glucosidase:- H2O > MeOH > DCM Inhibition of α-Amlyase DCM > MeOH > H2O Inhibition of Lipase: DCM > MeOH |
[163] |
| S. greggii | Aqueous (H2O) extract |
PY: Luteolin-C-hexoside Apigenin -C-exoside EO: Inhibited α-glucosidase Inhibited α-Amlyase Inhibited Lipase |
[164] |
| S. nemorosa |
n-hexane extract (n-hex) Dichloromethane extract (DCM) Methanol (MeOH) extract |
PY: Caffeic acid Quercetin EO: Inhibition of α-glucosidase: n-hex > DCM > MeOH |
[165] |
| S. officinalis | Aqueous (H2O) extract |
PY: Apigenin-O-glucuronide Rosmarinic acid Scutellarein-O- glucuronide EO: Inhibited α-glucosidase Inhibited lipase |
[164] |
| S. hispanica(Chia) | organic solvent (Hexane extract, Ethyl acetate extract, Methanol extract, Water extract) | AgNPS + methanolic extract showed lipase inhibition ↓ Cholesterol levels |
[47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




