Submitted:
23 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Subject Demographics
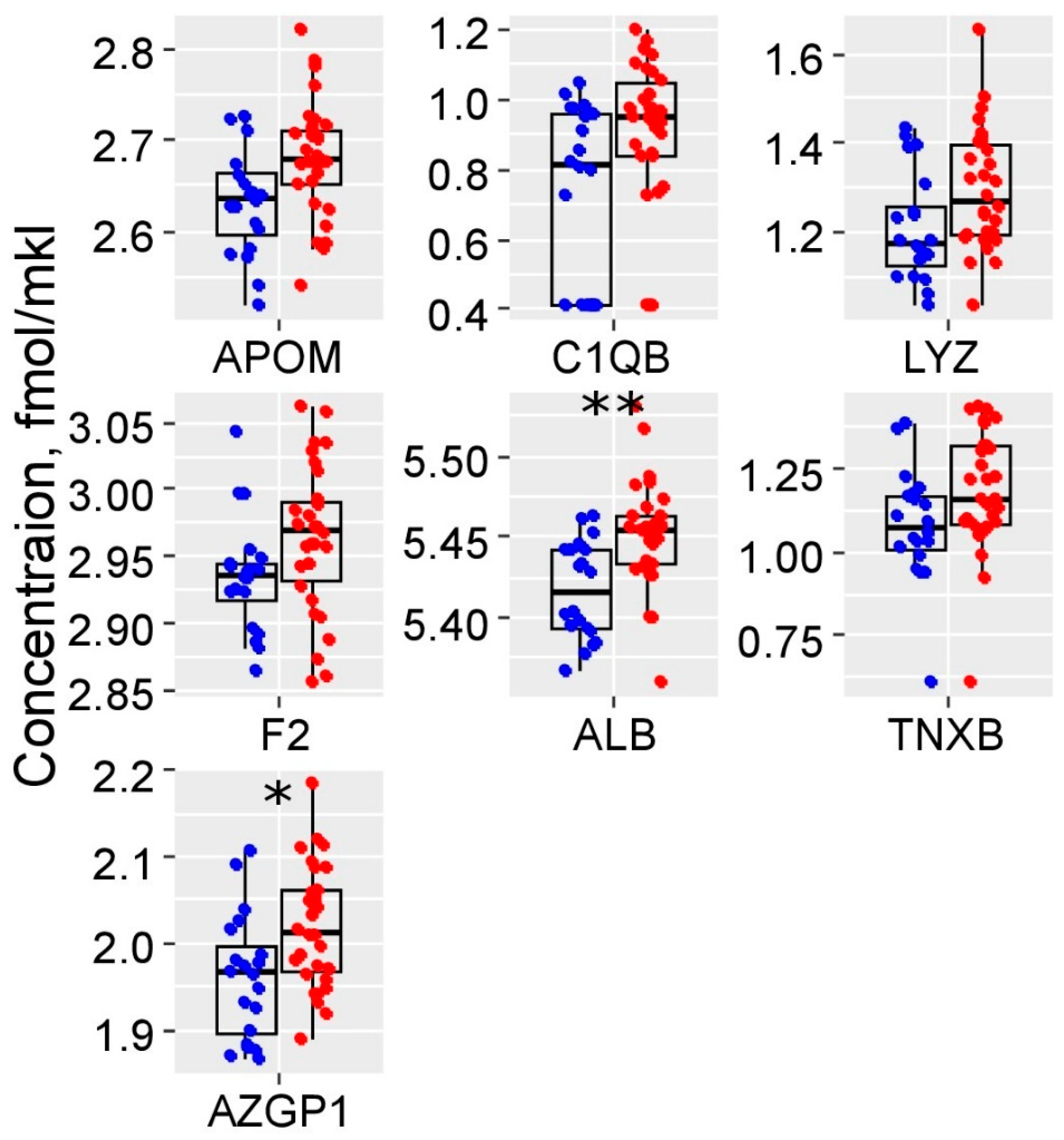
2.2. Maternal Serum Quantitative Proteomics (LC-MRM-MS)
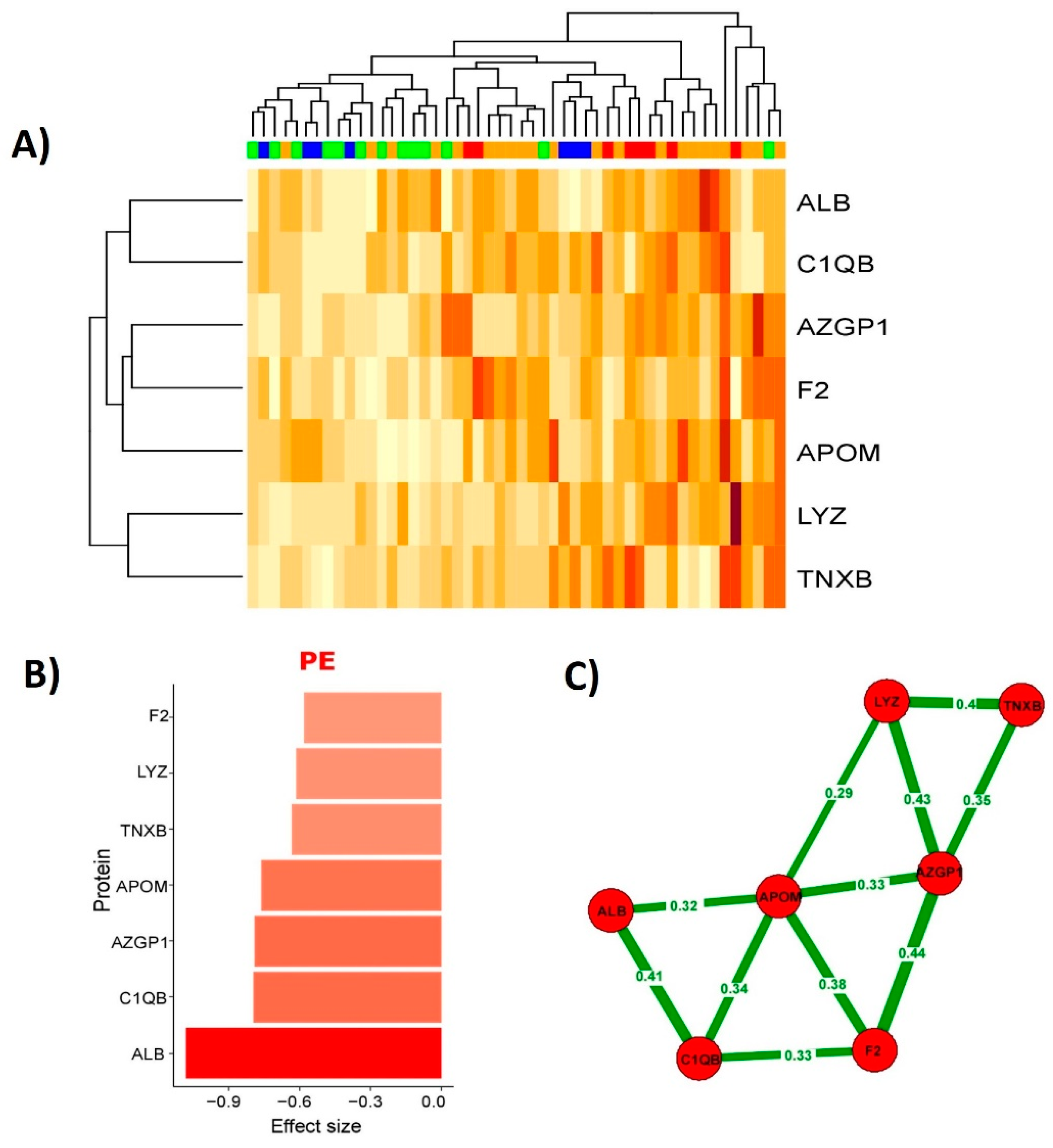
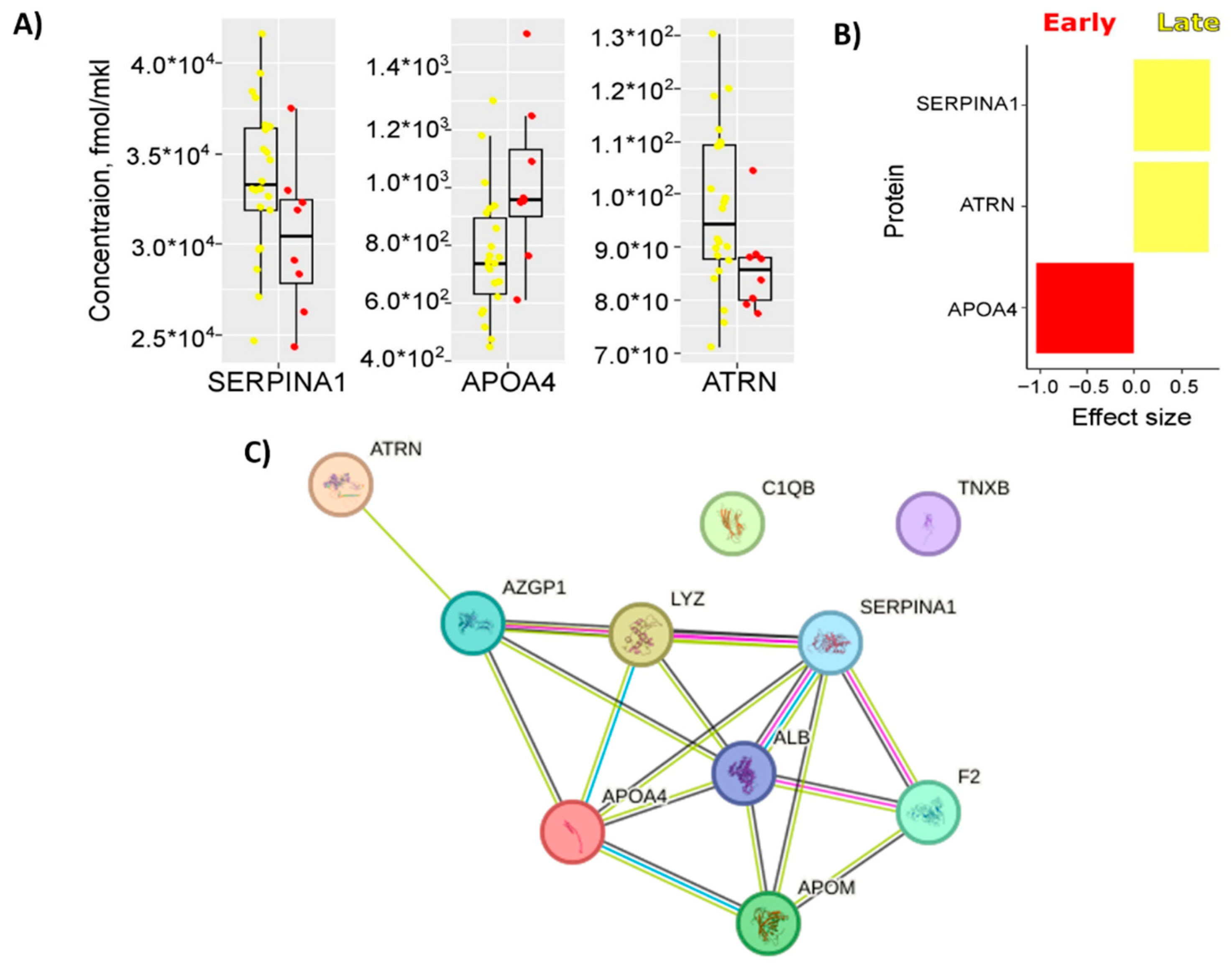
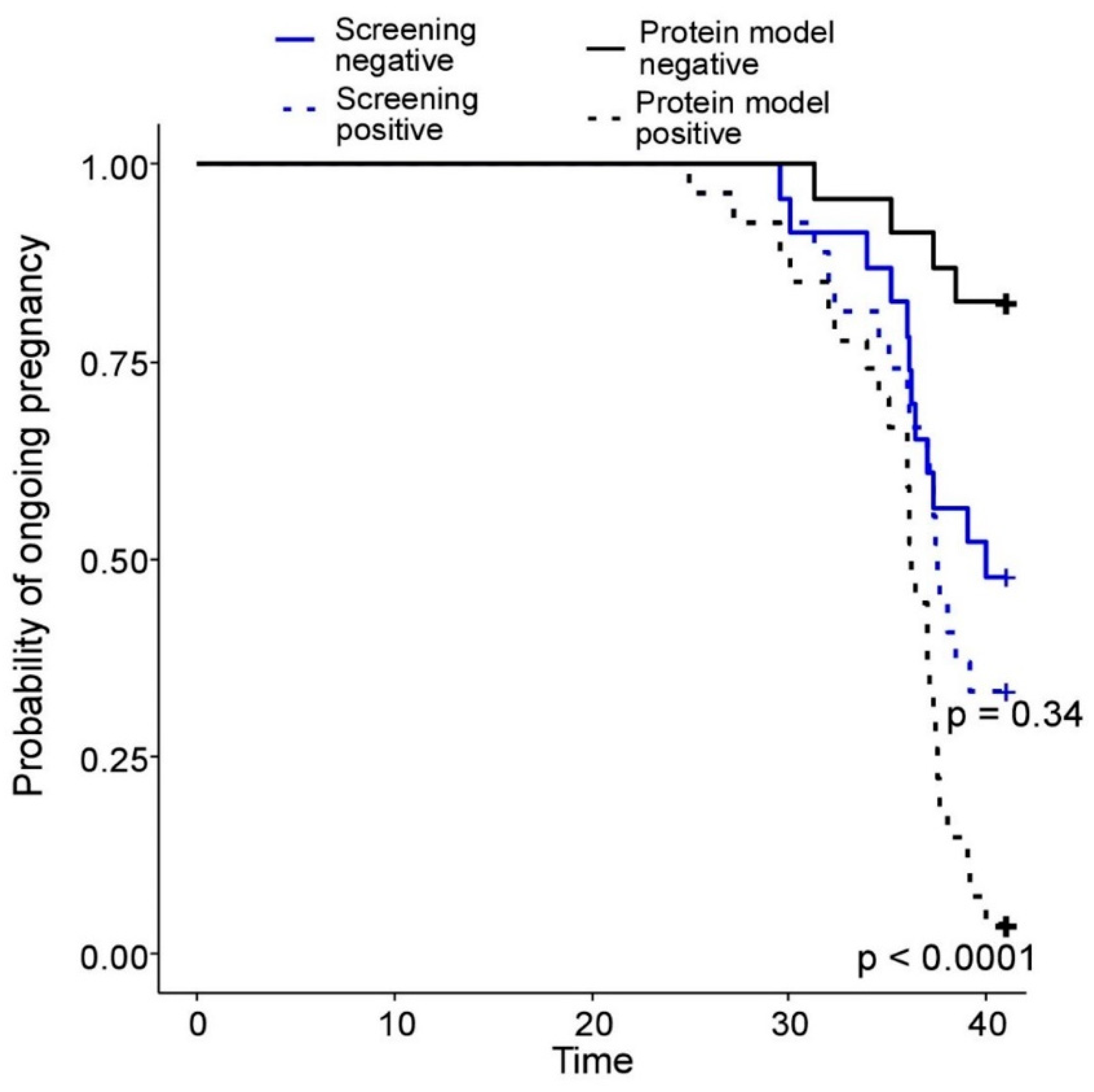
2.3. Building of PE Prediction Model Based on the First Trimester Maternal Serum Proteome
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Serum Preparation for Quantative Proteomics
4.3. Quantitive Analysis of 125 Serum Proteins (LC- MRM-MS)
4.4. Data Statistical Processing
4.5. SVM Model Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moe, T.G. Pre-Eclampsia, Placental Factors, and Offspring Congenital Heart Disease. JACC. Adv. 2024, 3, 101010. [Google Scholar] [CrossRef]
- Kuklina, E. V; Ayala, C.; Callaghan, W.M. Hypertensive Disorders and Severe Obstetric Morbidity in the United States. Obstet. Gynecol. 2009, 113, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Karumanchi, S.A. Two Decades of Advances in Preeclampsia Research: Molecular Mechanisms and Translational Studies. J. Clin. Invest. 2024, 134. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P. V; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-Eclampsia. Nat. Rev. Dis. Prim. 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Erez, O.; Romero, R.; Jung, E.; Chaemsaithong, P.; Bosco, M.; Suksai, M.; Gallo, D.M.; Gotsch, F. Preeclampsia and Eclampsia: The Conceptual Evolution of a Syndrome. Am. J. Obstet. Gynecol. 2022, 226, S786–S803. [Google Scholar] [CrossRef]
- Lisonkova, S.; Joseph, K.S. Incidence of Preeclampsia: Risk Factors and Outcomes Associated with Early- versus Late-Onset Disease. Am. J. Obstet. Gynecol. 2013, 209, 544.e1–544.e12. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.S.; Agrawal, A.K.; Singhal, D.; Bawiskar, D.; Shedge, S.S. Pregnancy-Induced Hypertension Pathophysiology and Contemporary Management Strategies: A Narrative Review. Cureus 2024, 16, e63961. [Google Scholar] [CrossRef] [PubMed]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy Classification, Diagnosis & Management Recommendations for International Practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Navajas, R.; Corrales, F.; Paradela, A. Quantitative Proteomics-Based Analyses Performed on Pre-Eclampsia Samples in the 2004-2020 Period: A Systematic Review. Clin. Proteomics 2021, 18, 6. [Google Scholar] [CrossRef]
- Tan, M.Y.; Syngelaki, A.; Poon, L.C.; Rolnik, D.L.; O’Gorman, N.; Delgado, J.L.; Akolekar, R.; Konstantinidou, L.; Tsavdaridou, M.; Galeva, S.; et al. Screening for Pre-Eclampsia by Maternal Factors and Biomarkers at 11-13 Weeks’ Gestation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2018, 52, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Tousty, P.; Fraszczyk-Tousty, M.; Golara, A.; Zahorowska, A.; Sławiński, M.; Dzidek, S.; Jasiak-Jóźwik, H.; Nawceniak-Balczerska, M.; Kordek, A.; Kwiatkowska, E.; et al. Screening for Preeclampsia and Fetal Growth Restriction in the First Trimester in Women without Chronic Hypertension. J. Clin. Med. 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Excellence, N.I. for H. and C. Hypertension in Pregnancy : Diagnosis and Management. Am J Obs. Gynecol 2010, 77, S1–s22. [Google Scholar]
- O’Gorman, N.; Wright, D.; Syngelaki, A.; Akolekar, R.; Wright, A.; Poon, L.C.; Nicolaides, K.H. Competing Risks Model in Screening for Preeclampsia by Maternal Factors and Biomarkers at 11-13 Weeks Gestation. Am. J. Obstet. Gynecol. 2016, 214, 103.e1–103.e12. [Google Scholar] [CrossRef]
- O’Gorman, N.; Wright, D.; Poon, L.C.; Rolnik, D.L.; Syngelaki, A.; Wright, A.; Akolekar, R.; Cicero, S.; Janga, D.; Jani, J.; et al. Accuracy of Competing-Risks Model in Screening for Pre-Eclampsia by Maternal Factors and Biomarkers at 11-13 Weeks’ Gestation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2017, 49, 751–755. [Google Scholar] [CrossRef]
- O’Gorman, N.; Wright, D.; Poon, L.C.; Rolnik, D.L.; Syngelaki, A.; de Alvarado, M.; Carbone, I.F.; Dutemeyer, V.; Fiolna, M.; Frick, A.; et al. Multicenter Screening for Pre-Eclampsia by Maternal Factors and Biomarkers at 11-13 Weeks’ Gestation: Comparison with NICE Guidelines and ACOG Recommendations. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2017, 49, 756–760. [Google Scholar] [CrossRef]
- Abbatiello, S.E.; Schilling, B.; Mani, D.R.; Zimmerman, L.J.; Hall, S.C.; MacLean, B.; Albertolle, M.; Allen, S.; Burgess, M.; Cusack, M.P.; et al. Large-Scale Interlaboratory Study to Develop, Analytically Validate and Apply Highly Multiplexed, Quantitative Peptide Assays to Measure Cancer-Relevant Proteins in Plasma. Mol. Cell. Proteomics 2015, 14, 2357–2374. [Google Scholar] [CrossRef]
- Percy, A.J.; Mohammed, Y.; Yang, J.; Borchers, C.H. A Standardized Kit for Automated Quantitative Assessment of Candidate Protein Biomarkers in Human Plasma. Bioanalysis 2015, 7, 2991–3004. [Google Scholar] [CrossRef]
- Bhowmick, P.; Roome, S.; Borchers, C.H.; Goodlett, D.R.; Mohammed, Y. An Update on MRMAssayDB: A Comprehensive Resource for Targeted Proteomics Assays in the Community. J. Proteome Res. 2021, 20, 2105–2115. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Basso, O.; Rasmussen, S.; Weinberg, C.R.; Wilcox, A.J.; Irgens, L.M.; Skjaerven, R. Trends in Fetal and Infant Survival Following Preeclampsia. JAMA 2006, 296, 1357–1362. [Google Scholar] [CrossRef]
- Sinkey, R.G.; Battarbee, A.N.; Bello, N.A.; Ives, C.W.; Oparil, S.; Tita, A.T.N. Prevention, Diagnosis, and Management of Hypertensive Disorders of Pregnancy: A Comparison of International Guidelines. Curr. Hypertens. Rep. 2020, 22, 66. [Google Scholar] [CrossRef]
- Porter, T.F.; Gyamfi-Bannerman, C.; Manuck, T. Low-Dose Aspirin Use during Pregnancy. Obstet. Gynecol. 2018, 132, 44–52. [Google Scholar]
- Regnault, T.R.H.; Galan, H.L.; Parker, T.A.; Anthony, R. V Placental Development in Normal and Compromised Pregnancies-- a Review. Placenta 2002, 23 Suppl A, S119–29. [Google Scholar] [CrossRef]
- Gibbs, I.; Leavey, K.; Benton, S.J.; Grynspan, D.; Bainbridge, S.A.; Cox, B.J. Placental Transcriptional and Histologic Subtypes of Normotensive Fetal Growth Restriction Are Comparable to Preeclampsia. Am. J. Obstet. Gynecol. 2019, 220, 110–e1. [Google Scholar] [CrossRef]
- Kim, S.M.; Cho, B.-K.; Kang, M.J.; Norwitz, E.R.; Lee, S.M.; Lee, J.; Park, C.-W.; Kim, B.J.; Jun, J.K.; Park, J.S.; et al. Expression Changes of Proteins Associated with the Development of Preeclampsia in Maternal Plasma: A Case-Control Study. Proteomics 2016, 16, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Romero, R.; Györffy, D.; Posta, M.; Bhatti, G.; Done, B.; Chaemsaithong, P.; Jung, E.; Suksai, M.; Gotsch, F.; et al. Molecular Subclasses of Preeclampsia Characterized by a Longitudinal Maternal Proteomics Study: Distinct Biomarkers, Disease Pathways and Options for Prevention. J. Perinat. Med. 2023, 51, 51–68. [Google Scholar] [CrossRef]
- Tarca, A.L.; Romero, R.; Benshalom-Tirosh, N.; Than, N.G.; Gudicha, D.W.; Done, B.; Pacora, P.; Chaiworapongsa, T.; Panaitescu, B.; Tirosh, D.; et al. The Prediction of Early Preeclampsia: Results from a Longitudinal Proteomics Study. PLoS One 2019, 14, e0217273. [Google Scholar] [CrossRef]
- Hsu, T.-Y.; Hsieh, T.-T.; Yang, K.D.; Tsai, C.-C.; Ou, C.-Y.; Cheng, B.-H.; Wong, Y.-H.; Hung, H.-N.; Chou, A.-K.; Hsiao, C.-C.; et al. Proteomic Profiling Reveals A1-Antitrypsin, A1-Microglobulin, and Clusterin as Preeclampsia-Related Serum Proteins in Pregnant Women. Taiwan. J. Obstet. Gynecol. 2015, 54, 499–504. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, N.; Yu, H.; Chen, Y.; Liang, Y.; Deng, H.; Zhang, Z. Proteomic Analysis of Human Serum for Finding Pathogenic Factors and Potential Biomarkers in Preeclampsia. Placenta 2011, 32, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Auer, J.; Camoin, L.; Guillonneau, F.; Rigourd, V.; Chelbi, S.T.; Leduc, M.; Laparre, J.; Mignot, T.-M.; Vaiman, D. Serum Profile in Preeclampsia and Intra-Uterine Growth Restriction Revealed by ITRAQ Technology. J. Proteomics 2010, 73, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Liu, C.; Liu, Y.; Zhang, N.; Deng, H.; Zhang, Z. Serum Markers of Pre-Eclampsia Identified on Proteomics. J. Obstet. Gynaecol. Res. 2016, 42, 1111–1118. [Google Scholar] [CrossRef]
- Blankley, R.T.; Fisher, C.; Westwood, M.; North, R.; Baker, P.N.; Walker, M.J.; Williamson, A.; Whetton, A.D.; Lin, W.; McCowan, L.; et al. A Label-Free Selected Reaction Monitoring Workflow Identifies a Subset of Pregnancy Specific Glycoproteins as Potential Predictive Markers of Early-Onset Pre-Eclampsia. Mol. Cell. Proteomics 2013, 12, 3148–3159. [Google Scholar] [CrossRef] [PubMed]
- Monteith, C.; Egan, K.; O’Connor, H.; Maguire, P.; Kevane, B.; Szklanna, P.B.; Cooley, S.; Malone, F.; Áinle, F.N. Early Onset Preeclampsia Is Associated with an Elevated Mean Platelet Volume (MPV) and a Greater Rise in MPV from Time of Booking Compared with Pregnant Controls: Results of the CAPE Study. J. Perinat. Med. 2018, 46, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, M.; Hosseini, E. Platelet-Leukocyte Crosstalk: Linking Proinflammatory Responses to Procoagulant State. Thromb. Res. 2013, 131, 191–197. [Google Scholar] [CrossRef]
- Forstner, D.; Guettler, J.; Gauster, M. Changes in Maternal Platelet Physiology during Gestation and Their Interaction with Trophoblasts. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Erez, O.; Romero, R.; Vaisbuch, E.; Kusanovic, J.P.; Mazaki-Tovi, S.; Chaiworapongsa, T.; Gotsch, F.; Mittal, P.; Edwin, S.S.; Nhan-Chang, C.-L.; et al. The Pattern and Magnitude of “in Vivo Thrombin Generation” Differ in Women with Preeclampsia and in Those with SGA Fetuses without Preeclampsia. J. Matern. neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2018, 31, 1671–1680. [Google Scholar] [CrossRef]
- Navajas, R.; Ramos-Fernandez, A.; Herraiz, I.; Galindo, A.; Bartha, J.L.; Corrales, F.; Paradela, A. Quantitative Proteomic Analysis of Serum-Purified Exosomes Identifies Putative Pre-Eclampsia-Associated Biomarkers. Clin. Proteomics 2022, 19, 5. [Google Scholar] [CrossRef]
- Chen, H.; Aneman, I.; Nikolic, V.; Karadzov Orlic, N.; Mikovic, Z.; Stefanovic, M.; Cakic, Z.; Jovanovic, H.; Town, S.E.L.; Padula, M.P.; et al. Maternal Plasma Proteome Profiling of Biomarkers and Pathogenic Mechanisms of Early-Onset and Late-Onset Preeclampsia. Sci. Rep. 2022, 12, 19099. [Google Scholar] [CrossRef]
- de Almeida, L.G.N.; Young, D.; Chow, L.; Nicholas, J.; Lee, A.; Poon, M.-C.; Dufour, A.; Agbani, E.O. Proteomics and Metabolomics Profiling of Platelets and Plasma Mediators of Thrombo-Inflammation in Gestational Hypertension and Preeclampsia. Cells 2022, 11. [Google Scholar] [CrossRef]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Neeser, O.; Huber, A.; Stanga, Z.; Mueller, B.; et al. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am. J. Med. 2020, 133, 713–722.e7. [Google Scholar] [CrossRef]
- Hassen, F.S.; Malik, T.; Dejenie, T.A. Evaluation of Serum Uric Acid and Liver Function Tests among Pregnant Women with and without Preeclampsia at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS One 2022, 17, e0272165. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Jiang, Y.; Lu, Z. Correlation of 24-h Urinary Protein Excretion, Serum Indicators, and Placental Growth Factor in Patients with Preeclampsia and Their Adverse Outcome. Altern. Ther. Health Med. 2024. [Google Scholar]
- Burwick, R.M.; Easter, S.R.; Dawood, H.Y.; Yamamoto, H.S.; Fichorova, R.N.; Feinberg, B.B. Complement Activation and Kidney Injury Molecule-1-Associated Proximal Tubule Injury in Severe Preeclampsia. Hypertens. (Dallas, Tex. 1979) 2014, 64, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Venuthurupalli, S.K.; Gobe, G.C.; Coombes, J.S.; Cooper, M.A.; Hoy, W.E. Biomarkers in Chronic Kidney Disease: A Review. Kidney Int. 2011, 80, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Karantanos, T.; DeZern, A.E. Biology and Clinical Management of Hypoplastic MDS: MDS as a Bone Marrow Failure Syndrome. Best Pract. Res. Clin. Haematol. 2021, 34, 101280. [Google Scholar] [CrossRef] [PubMed]
- Soykan Sert, Z.; Bertizlioğlu, M. Predictive Value of the HALP Score for Pre-Eclampsia with Severe Features. Postgrad. Med. 2024, 136, 468–473. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus Definition of Fetal Growth Restriction: A Delphi Procedure. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertens. (Dallas, Tex. 1979) 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Poon, L.C.Y.; Zymeri, N.A.; Zamprakou, A.; Syngelaki, A.; Nicolaides, K.H. Protocol for Measurement of Mean Arterial Pressure at 11-13 Weeks’ Gestation. Fetal Diagn. Ther. 2012, 31, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, W.; Maiz, N.; Bonino, S.; Kaihura, C.; Nicolaides, K.H. Uterine Artery Doppler at 11 + 0 to 13 + 6 Weeks in the Prediction of Pre-Eclampsia. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2007, 30, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Gaither, C.; Popp, R.; Borchers, S.P.; Skarphedinsson, K.; Eiriksson, F.F.; Thorsteinsdóttir, M.; Mohammed, Y.; Borchers, C.H. Performance Assessment of a 125 Human Plasma Peptide Mixture Stored at Room Temperature for Multiple Reaction Monitoring-Mass Spectrometry. J. Proteome Res. 2021, 20, 4292–4302. [Google Scholar] [CrossRef]
- Kononikhin, A.S.; Zakharova, N. V; Semenov, S.D.; Bugrova, A.E.; Brzhozovskiy, A.G.; Indeykina, M.I.; Fedorova, Y.B.; Kolykhalov, I. V; Strelnikova, P.A.; Ikonnikova, A.Y.; et al. Prognosis of Alzheimer’s Disease Using Quantitative Mass Spectrometry of Human Blood Plasma Proteins and Machine Learning. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Starodubtseva, N.L.; Tokareva, A.O.; Rodionov, V. V; Brzhozovskiy, A.G.; Bugrova, A.E.; Chagovets, V. V; Kometova, V. V; Kukaev, E.N.; Soares, N.C.; Kovalev, G.I.; et al. Integrating Proteomics and Lipidomics for Evaluating the Risk of Breast Cancer Progression : A Pilot Study. 2023.
- Starodubtseva, N.L.; Tokareva, A.O.; Volochaeva, M. V; Kononikhin, A.S.; Brzhozovskiy, A.G.; Bugrova, A.E.; Timofeeva, A. V; Kukaev, E.N.; Tyutyunnik, V.L.; Kan, N.E.; et al. Quantitative Proteomics of Maternal Blood Plasma in Isolated Intrauterine Growth Restriction. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- MacLean, B.X.; Pratt, B.S.; Egertson, J.D.; MacCoss, M.J.; Smith, R.D.; Baker, E.S. Using Skyline to Analyze Data-Containing Liquid Chromatography, Ion Mobility Spectrometry, and Mass Spectrometry Dimensions. J. Am. Soc. Mass Spectrom. 2018, 29, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Whiteaker, J.R.; Halusa, G.N.; Hoofnagle, A.N.; Sharma, V.; MacLean, B.; Yan, P.; Wrobel, J.A.; Kennedy, J.; Mani, D.R.; Zimmerman, L.J.; et al. CPTAC Assay Portal: A Repository of Targeted Proteomic Assays. Nat. Methods 2014, 11, 703–704. [Google Scholar] [CrossRef]
- Kamburov, A.; Herwig, R. ConsensusPathDB 2022 : Molecular Interactions Update as a Resource for Network Biology. 2022, 50, 587–595.
- Yasar, S.; Yagin, F.H.; Melekoglu, R.; Ardigò, L.P. Integrating Proteomics and Explainable Artificial Intelligence: A Comprehensive Analysis of Protein Biomarkers for Endometrial Cancer Diagnosis and Prognosis. Front. Mol. Biosci. 2024, 11, 1389325. [Google Scholar] [CrossRef]
- Wu, L.; An, J.; Li, X.; Tao, Q.; Liu, Z.; Zhang, K.; Zhou, L.; Zhang, X. Comprehensive Proteomic Profiling of Aqueous Humor in Idiopathic Uveitis and Vogt − Koyanagi − Harada Syndrome. 2024. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Zhang, F.; Johnson, L.A.; Hall, J.; Petersen, M.; Oh, E.S.; Lyketsos, C.G.; Rissman, R.A. Precision Medicine for Preventing Alzheimer’s Disease: Analysis of the ADAPT Study. J. Alzheimers. Dis. 2023, 95, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Guyon, I. Gene Selection for Cancer Classification. 2002, 389–422.
- Dinse, G.E.; Lagakos, S.W. Nonparametric Estimation of Lifetime and Disease Onset Distributions from Incomplete Observations. Biometrics 1982, 38, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Crawford, K.; Cavanagh, E.; da Silva Costa, F.; Kumar, S. Prediction of Preterm Birth in Growth-Restricted and Appropriate-for-Gestational-Age Infants Using Maternal PlGF and the SFlt-1/PlGF Ratio-A Prospective Study. BJOG 2024, 131, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
| Feature | Group 2 eo-PE (n=8) |
Group 2, lo-PE (n=22) |
Group 3, GAH (n=7) |
Group 4, CTR (n=13) |
p-Value |
|---|---|---|---|---|---|
| Age, years, Me[Q1;Q3] | 36.0 [34.2;38.3] |
33.5 [29.0;37.0] |
37.0 [36.5;37.5] |
34.0 [32.0;36.0] |
>0.05 |
| BMI, Me[Q1;Q3] | 25.5 [23.5;26.25] |
26.0 [23.0;29.0] |
29.0 [29.0;33.5] | 28.0 [25.0;30.0] |
p13=0.006 |
| Previous PE/IUGR n (%) |
2 (25.0%) | 2 (19%) | 1 (14.3%) | 0 | >0.05 |
| HAG, Me[Q1;Q3] | 2 (25.0%) | 5 (22.7%) | 0 | 0 | >0.05 |
| Nulliparous, n(%) | 6 (75.0%) | 15 (68.2%) | 4 (57.2%) | 8 (61.5%) | >0.05 |
| High rick of PE (1st trimester prenatal screening), n(%) | 5 (62.5%) | 13 (59.0%) | 3 (42.9%) | 6 (46.2%) | >0.05 |
| Max. SBP, Me[Q1;Q3] | 160 [150,165] |
150 [140;160] |
145 [143;150] |
120 [115;127] |
>0.05 |
| Max. DBP, Me[Q1;Q3] | 110 [100;110] |
95 [90;100] |
90 [90;95] |
80 [75;85] |
p12=0.04 p14<0.001 |
| Proteinuria, g/l, Me[Q1;Q3] | 2.2 [1.3;2.8] |
1.2 [0.4;2.3] |
0 [0;0.05] |
0 [0;0] |
p13=0.001 p14<0.001 |
| Platelet count, Me[Q1;Q3] | 196 [153;224] |
216 [146;253] |
270 [239;307] |
247 [226;286] |
p13=0.04 |
| ALT, Me[Q1;Q3] | 38.8 [14.5;66.5] |
16.3 [12.5;21.1] |
16.2 [13.4; 18.5] |
24.0 [14.6;27.4] |
>0.05 |
| AST, Me[Q1;Q3] | 27.4 [21.0;54.6] |
21.4 [15.7;24.7] |
14.9 [14.2;18.6] |
17.3 [7.8;19.7] |
p13=0.01 p14=0.01 |
| LDH, Me[Q1;Q3] | 452 [366;565] |
424 [372;445] |
368 [342;397] |
269 [134;347] |
p14<0.001 |
| sFlt-1/PlGF, Me[Q1;Q3] | 423.9 [342.3;525.5] |
128.7 [100.4;213.0] |
35.8 [27.9;50.8] |
28.5 [21.8;45.0] |
p12=0.003 p13=0.001 p14<0.001 |
| HELLP syndrome, Me[Q1;Q3] | 1(12.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | >0.05 |
| IUGR, Me[Q1;Q3] | 7 (87.5%) | 4 (18.2%) | 0 (0.0%) | 0 (0.0%) |
p12=0.002 p13=0.004 p14<0.001 |
| Premature birth, n(%) | 8 (100%) | 6 (27.3%) | 0 (0.0%) | 0 (0.0%) |
p12=0.002 p13<0.001 p14<0.001 |
| Gestational age at birth, wks, Me[Q1;Q3] | 31.3 [30.2;32.2] |
37.5 [36.7;38.3] |
39.0 [38.7;39;3] |
38.4 [38.2;39.0] |
p12<0.001 p13<0.001 p14<0.001 |
| Emergency caesarean section, n(%) | 8 (100%) | 11 (50.0%) | 0 (0.0%) | 2 (15.3%) |
p12=0.04 p13<0.001 p14<0.001 |
| Newborn mass, g, Me[Q1;Q3] | 1215 [1123;1385] |
2937 [2565;3224] |
3410 [3251;3550] | 3290 [3042;3612] |
p12<0.001 p13<0.001 p14<0.001 |
| Apgar, 5 min , scores, value: n (%) | 8: 4(50.0%) 7: 4(50.0%) |
9: 16(72.7%) 8: 6(17.3%) |
9: 7(100%) | 9: 13(100%) |
p12<0.001 p13=0.009 p14<0.001 |
| Model | Predicted outcome | Clinical group | |||
| Control (n = 13) |
GAH (n = 7) |
Late PE (n = 22) |
Early PE (n = 8) |
||
| Routine screening | not PE | 7 (54%) | 4 (57%) | 9 (41%) | 3 (38%) |
| PE | 6 (46%) | 3 (43%) | 13 (59%) | 5 (62%) | |
| SVM-model | not PE | 12 (92%) | 7 (100%) | 3 (14%) | 1 (13%) |
| PE | 1 (8%) | 0 (0%) | 19 (86%) | 7 (87%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





