Submitted:
24 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.3. Procedure
2.4. BMI
2.5. Body Composition Analysis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Group
3.2. Anthropometric Indicators
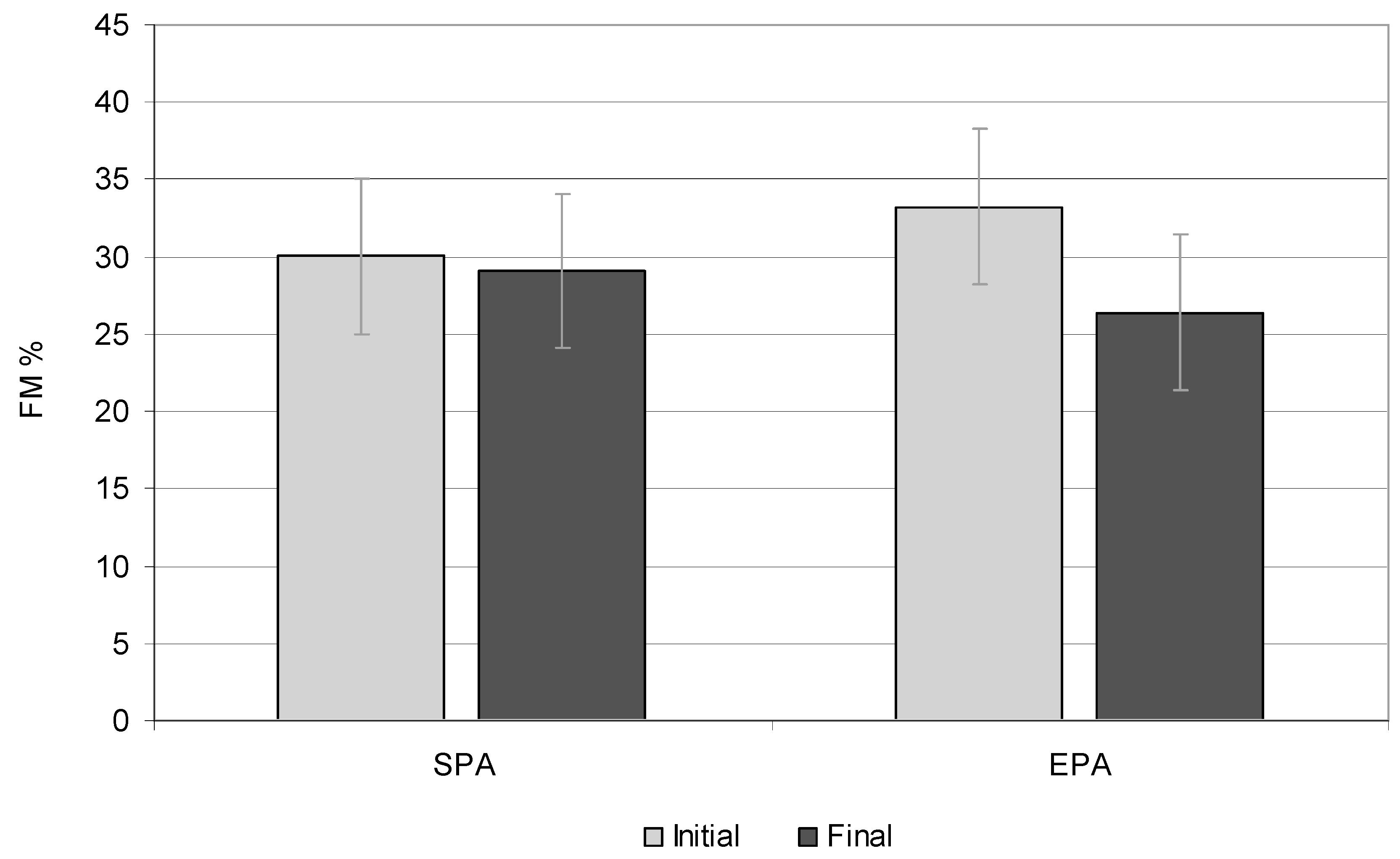
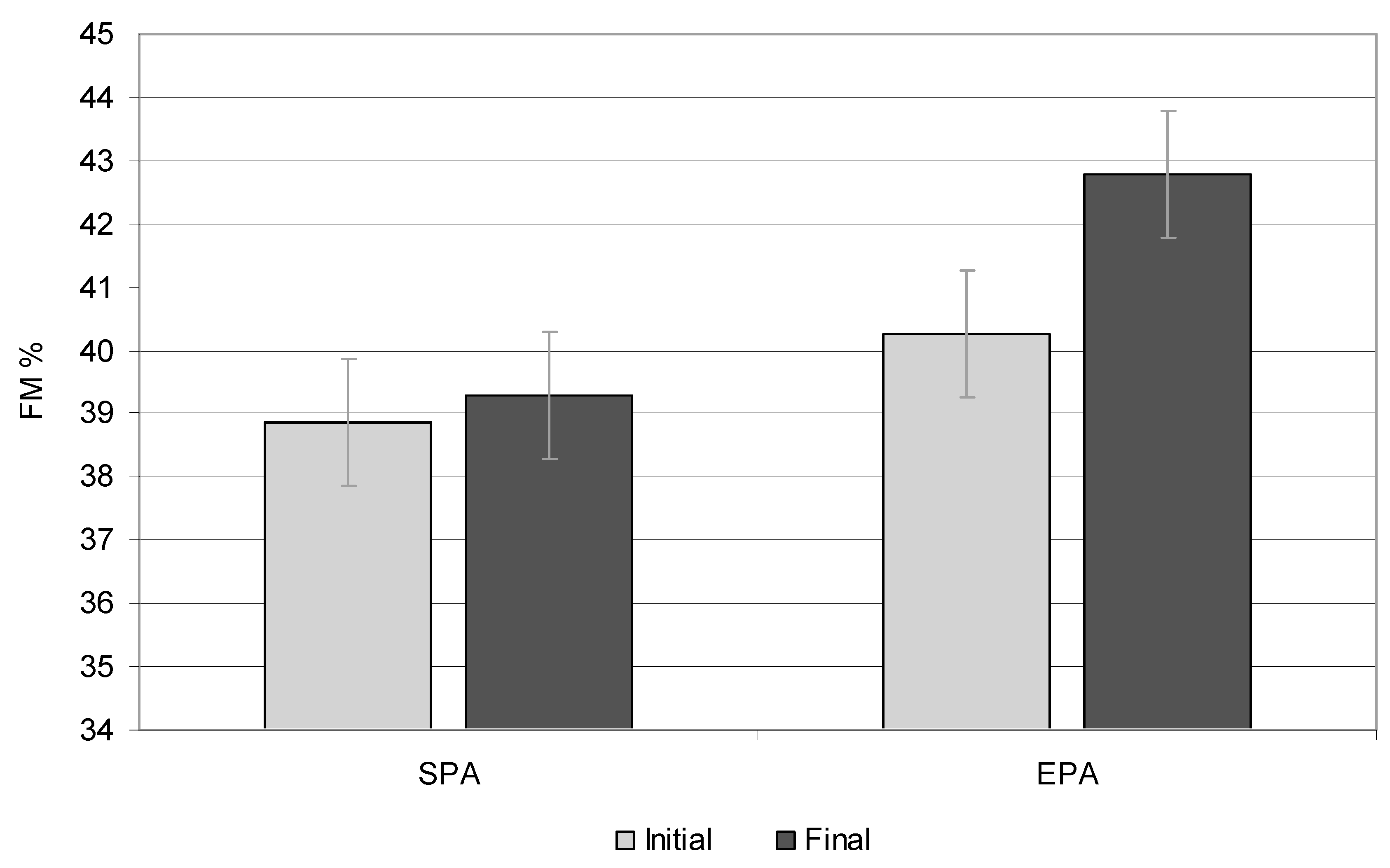
3.3. Fat Tissue
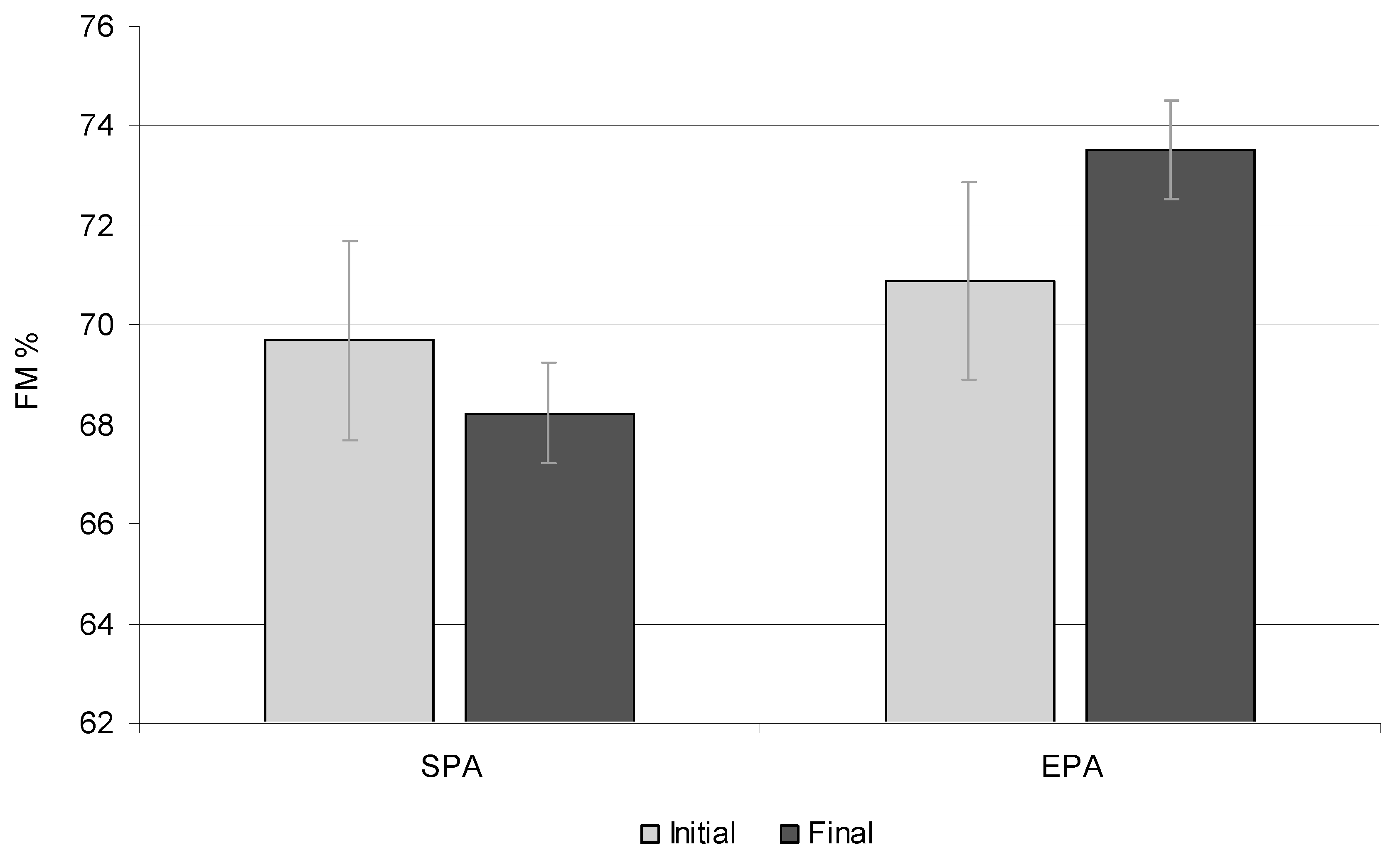
3.4. Fat-Free Mass
3.5. Skeletal Muscle Mass
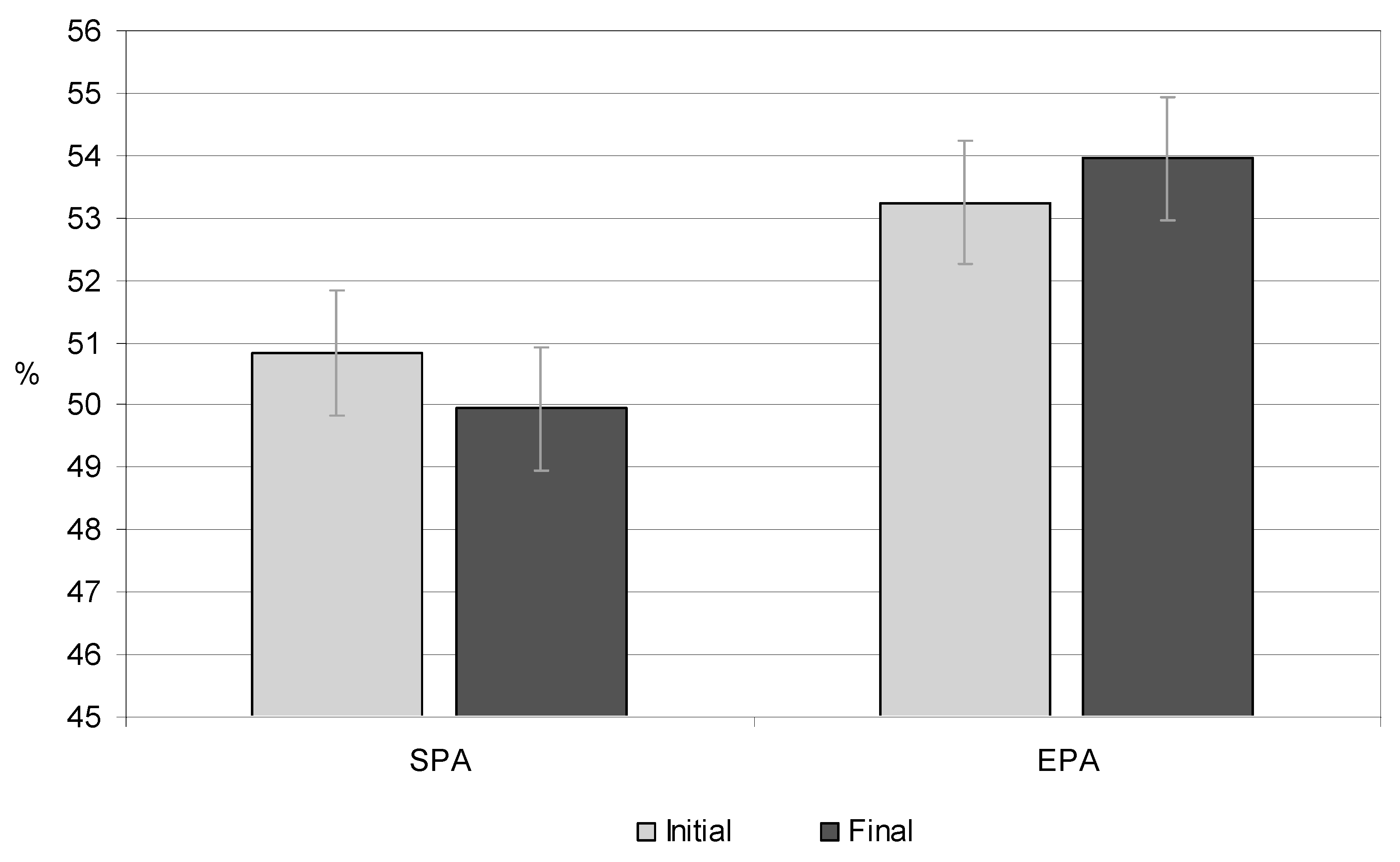
3.6. Total Body Water
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-641sheets/detail/obesity-and-overweight (accessed on 4 May 2023).
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-641 sheets/detail/ obesity-and-overweight (accessed on 4 July 2023).
- World Obesity Federation, World Obesity Atlas 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 4 June 2023).
- Jebeile, H.; Kelly, A.S.; O‘Malley, G.; Baur, L.A. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Gomula, A.; Nowak-Szczepańska, N.; Danel, D.P.; Koziel, S. Overweight trends among Polish schoolchildren before and after the transition from communism to capitalism. Econ Hum Biol. 2015, 19, 246–257. [Google Scholar] [CrossRef]
- Oblacińska, A. Rozwój fizyczny i samoocena masy ciała. In: Mazur J, Małkowska-Szkutnik, A. Pupils’ health in 2018 against the new HBSC research model. Warsaw, Instytut Matki i Dziecka. 2018, 70-81.
- Jarosz, M.; Charzewska, J.; Wolnicka, W.; et al. Nutritional status of children and adolescents - preliminary results the programme KIK/34 “Preventing overweight and obesity” in Swiss-Polish Cooperation Programme. Polish J Human Nutr. 2016, 43, 231–238. [Google Scholar]
- Fijałkowska, A.; Oblacińska, A.; Stalmach, M.; Nadwaga i otyłość u polskich 8-latków w świetle uwarunkowań biologicznych, behawioralnych i społecznych. Raport z międzynarodowych badań WHO European Childhood Obesity Surveillance Initiative (COSI). Warszawa, 2017. Available online: http://www.imid. med.pl/. (accessed on 4 July 2023).
- August, G.P.; Caprio, S.; Fennoy, I.; et al. Prevention and treatment of pediatric obesity: An endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008, 93, 4576–4599. [Google Scholar] [CrossRef]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef]
- Brener, A.; Waksman, Y.; Rosenfeld, T.; Levy, S.; Peleg, I.; Raviv, A.; Interator, H.; Lebenthal, Y. The heritability of body composition. BMC Pediatr. 2021, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Verney, J.; Metz, L.; Chaplais, E.; Cardenoux, C.; Pereira, B.; Thivel, D. Bioelectrical impedance is an accurate method to assess body composition in obese but not severely obese adolescents. Nutr Res. 2016, 36, 663–670. [Google Scholar] [CrossRef]
- Laskey, M.A. Dual-energy X-ray absorptiometry and body composition. Nutrition. 1996, 12, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.G.; Kim, J.H.; Kim, Y.; Lim, H.; Ju, Y.S.; Kang, M.J.; Lee, K.; Lee, H.J.; Jang, H.B.; Park, S.I.; et al. Validation of body composition using bioelectrical impedance analysis in children according to the degree of obesity. Scand J Med Sci Sports. 2018, 28, 2207–2215. [Google Scholar] [CrossRef]
- McCarthy, H.D.; Samani–Radia, D.; Jebb, S.A.; Prentice, A.M. Skeletal muscle mass reference curves for children and adolescents. Pediatr Obes. 2014, 9, 249–259. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.D.; Cole, T.J.; Fry, T.; Jebb, S.A.; Prentice, A.M. Body fat reference curves for children. Int J Obes. 2006, 30, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B. Advanced body composition assessment: from body mass index to body composition profiling. J Investig Med. 2018, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dettlaff-Dunowska, M.; Brzeziński, M.; Zagierska, A.; Borkowska, A.; Zagierski, M.; Szlagatys-Sidorkiewicz, A. Changes in Body Composition and Physical Performance in Children with Excessive Body Weight Participating in an Integrated Weight-Loss Programme. Nutrients. 2022, 14, 3647. [Google Scholar] [CrossRef]
- Wojtyniak, B.; Goryński, P. (red.). Sytuacja zdrowotna ludności Polskiej i jej uwarunkowania. Narodowy Instytut Zdrowia Publicznego - Państwowy Zakład Higieny. Warsaw, 2020.
- Ługowska, K.; Kolanowski, W.; Trafialek, J. The Impact of Physical Activity at School on Children‘s Body Mass during 2 Years of Observation. Int J Environ Res Public Health. 2022, 19, 3287. [Google Scholar] [CrossRef]
- Nowaczyk, M.; Cieślik, K.; Waszak, M. Assessment of the Impact of Increased Physical Activity on Body Mass and Adipose Tissue Reduction in Overweight and Obese Children. Children 2023, 10, 764. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Lisinskiene, A.; Lochbaum, M. The Coach-Athlete-Parent Relationship: The Importance of the Sex, Sport Type, and Family Composition. Int J Environ Res Public Health 2022, 19, 4821. [Google Scholar] [CrossRef]
- Kułaga, Z.; Grajda, A.; Gurzkowska, B.; Góźdź, M.; Wojtyło, M.; Świąder, A.; Różdżyńska-Świątkowska, A.; Litwin, M. Polish 2012 growth references for preschool children. Eur J Pediatr. 2013, 172, 753–761. [Google Scholar] [CrossRef]
- Kułaga, Z.; Różdżyńska, A.; Palczewska, I. Percentile charts of height, body mass and body mass index in children and adolescents in Poland—results of the OLAF study. Stand Med Pediatr. 2010, 7, 690–700. [Google Scholar]
- Ługowska, K.; Kolanowski, W. The Impact of Physical Activity at School on Body Fat Content in School-Aged Children. Int J Environ Res Public Health. 2022, 19, 12514. [Google Scholar] [CrossRef]
- Declaration of Helsinki World Medical Association Declaration of Helsinki. Bull World Health Organ. 2013, 79.
- Farbo, D.J.; Rhea, D.J. A Pilot Study Examining Body Composition Classification Differences Between Body Mass Index and Bioelectrical Impedance Analysis in Children With High Levels of Physical Activity. Front Pediatr. 2021, 9, 724053. [Google Scholar] [CrossRef]
- Orsso, C.E.; Gonzalez, M.C.; Maisch, M.J.; Haqq, A.M.; Prado, C.M. Using bioelectrical impedance analysis in children and adolescents: Pressing issues. Eur J Clin Nutr. 2022, 76, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Marfell-Jones, M.; Old, T.; Steward, A.; Carter, J.E.L. International Standards for Anthropometric Assessment. ISAK: Palmerston North, New Zeland, 2012.
- Tanita SC-240MAInstruction Manual Tanita User Manual SC 240, M.A. Available online: https://www.manualslib.com/manual/1065295/Tanita-Sc-240ma.html (accessed on 12 October 2020).
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J Grad Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Pezoa-Fuentes, P.; Cossio-Bolaños, M.; Urra-Albornoz, C.; Alvear-Vasquez, F.; Lazari, E.; Urzua-Alul, L. ’ et al. Fat-free mass and maturity status are determinants of physical fitness perform. J Pediatr. 2023, 99, 38–44. [Google Scholar] [CrossRef]
- Méndez-Hernández, L.D.; Ramírez-Moreno, E.; Barrera-Gálvez, R.; Cabrera-Morales, M.D.C.; Reynoso-Vázquez, J.; Flores-Chávez, O.R.; Morales-Castillejos, L.; Cruz-Cansino, N.D.S.; Jiménez-Sánchez, R.C.; Arias-Rico, J. Effects of Strength Training on Body Fat in Children and Adolescents with Overweight and Obesity: A Systematic Review with Meta-Analysis. Children 2022, 9, 995. [Google Scholar] [CrossRef]
- Gutin, B.; Yin, Z.; Humphries, M.C.; Hoffman, W.H.; Gower, B.; Barbeau, P. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr. 2004, 145, 737–743. [Google Scholar] [CrossRef]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes Facts 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Logan, K.; Lloyd, R.S.; Schafer-Kalkhoff, T.; Khoury, J.C.; Ehrlich, S.; Dolan, L.M.; Shah, A.S.; Myer, G.D. Youth sports participation and health status in early adulthood: A 12-year follow-up. Prev Med Rep. 2020, 19, 101107. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, H.; Chen, S.; Ma, J.; Kim, H. The Association of Body Mass Index and Fat Mass with Health-Related Physical Fitness among Chinese Schoolchildren: A Study Using a Predictive Model. Int J Environ Res Public Health 2022, 20, 355. [Google Scholar] [CrossRef]
- Tsolakis, C.; Cherouveim, E.D.; Skouras, A.Z.; Antonakis-Karamintzas, D.; Czvekus, C.; Halvatsiotis, P.; Savvidou, O.; Koulouvaris, P. The Impact of Obesity on the Fitness Performance of School-Aged Children Living in Rural Areas-The West Attica Project. Int J Environ Res Public Health 2022, 19, 11476. [Google Scholar] [CrossRef]
- Miguet, M.; Fearnbach, N.S.; Metz, L.; Khammassi, M.; Julian, V.; Cardenoux, C.; Pereira, B.; Boirie, Y.; Duclos, M.; Thivel, D. Effect of HIIT versus MICT on body composition and energy intake in dietary restrained and unrestrained adolescents with obesity. Appl Physiol Nutr Metab. 2020, 45, 437–445. [Google Scholar] [CrossRef]
- Soares, R.; Brasil, I.; Monteiro, W.; Farinatti, P. Effects of physical activity on body mass and composition of school-age children and adolescents with overweight or obesity: Systematic review focusing on intervention characteristics. J Bodywork Movement Therap. 2023, 33, 154–163. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Mata, E.; Sánchez-Sánchez, J.; Plaza-Carmona, M.; Martín-García, M.; Gallardo, L. Influence of different sports on fat mass and lean mass in growing girls. J Sport Health Sci. 2017, 6, 213–218. [Google Scholar] [CrossRef]
- Abbott, R.A.; Davies, P.S. Habitual physical activity and physical activity intensity: their relation to body composition in 5.0-10.5-y-old children. Eur J Clin Nutr. 2004, 58, 285–91. [Google Scholar] [CrossRef] [PubMed]
- Ball, E.J.; O‘Connor, J.; Abbott, R.; Steinbeck, K.S.; Davies, P.S.; Wishart, C.; Gaskin, K.J.; Baur, L.A. Total energy expenditure, body fatness, and physical activity in children aged 6-9 y. Am J Clin Nutr. 2001, 74, 524–8. [Google Scholar] [CrossRef]
- Ara, I.; Vicente-Rodríguez, G.; Jimenez-Ramirez, J.; Dorado, C.; Serrano-Sanchez, J.A.; Calbet, J.A. Regular participation in sports is associated with enhanced physical fitness and lower fat mass in prepubertal boys. Int J Obes Relat Metab Disord. 2004, 28, 1585–93. [Google Scholar] [CrossRef]
- Cvetković, N.; Stojanović, E.; Stojiljković, N.; Nikolić, D.; Scanlan, A.T.; Milanović, Z. Exercise training in overweight and obese children: Recreational football and high-intensity interval training provide similar benefits to physical fitness. Scand J Med Sci Sports. 2018, 28, 18–32. [Google Scholar] [CrossRef]
- Larsen, M.N.; Nielsen, C.M.; Ørntoft, C.; Randers, M.B.; Helge, E.W.; Madsen, M.; Manniche, V.; Hansen, L.; Hansen, P.R.; Bangsbo, J.; Krustrup, P. Fitness Effects of 10-Month Frequent Low-Volume Ball Game Training or Interval Running for 8-10-Year-Old School Children. Biomed Res Int. 2017, 2017, 2719752. [Google Scholar] [CrossRef]
- Ferreira, I.; Boreham, C.A.; Twisk, J.W.; Gallagher, A.M.; Young, I.S.; Murray, L.J.; Stehouwer, C.D. Clustering of metabolic syndrome risk factors and arterial stiffness in young adults: the Northern Ireland Young Hearts Project. J Hypertens. 2007, 25, 1009–1020. [Google Scholar] [CrossRef]
- McGuigan, M.R.; Tatasciore, M.; Newton, R.U.; Pettigrew, S. Eight weeks of resistance training can significantly alter body composition in children who are overweight or obese. J Strength Cond Res. 2009, 23, 80–85. [Google Scholar] [CrossRef]
- Zouch, M.; Jaffré, C.; Thomas, T.; Frère, D.; Courteix, D.; Vico, L.; Alexandre, C. Long-term soccer practice increases bone mineral content gain in prepubescent boys. Joint Bone Spine 2008, 75, 41–49. [Google Scholar] [CrossRef]
- Larsen, M.N.; Nielsen, C.M.; Helge, E.W.; Madsen, M.; Manniche, V.; Hansen, L.; Hansen, P.R.; Bangsbo, J.; Krustrup, P. Positive effects on bone mineralisation and muscular fitness after 10 months of intense school-based physical training for children aged 8-10 years: the FIT FIRST randomised controlled trial. Br J Sports Med. 2018, 52, 254–260. [Google Scholar] [CrossRef]
- Mattoo, T.K.; Lu, H.; Ayers, E.; Thoma, R. Total body water by BIA in children and young adults with normal and excessive weight. PLoS One 2020, 15, 0239212. [Google Scholar] [CrossRef]
- Maffeisa, C.; Tommasi, M.; Tomaselli, F.; Spinelli, J. Fluid intake and hydration status in obese vs normal weight children. Eur J Clin Nutr. 2016, 70, 560–565. [Google Scholar] [CrossRef]
- Neil-Sztramko, S.E.; Caldwell, H.; Dobbins, M. School-based physical activity programs for promoting physical activity and fitness in children and adolescents aged 6 to 18. Cochrane Database System Rev. 2021, 9, CD007651. [Google Scholar]
- Juric, P.; Jurak, G.; Morrison, S.A.; Starc, G.; Soric, M. Effectiveness of a population-scaled, school-based physical activity intervention for the prevention of childhood obesity. Obesity 2023, 31, 811–822. [Google Scholar] [CrossRef]
| Measurement session |
SPA | EPA | *p | ||||
|---|---|---|---|---|---|---|---|
| Girls | Boys | Mean | Girls | Boys | Mean | ||
| Overweight | |||||||
| - Initial | 59% | 55.5% | 57.25% | 58% | 50% | 54% | 0.030 |
| - Final | 47% | 61% | 54% | 62.5% | 67% | 59.75% | 0.021 |
| Obesity | |||||||
| - Initial | 41% | 44.5% | 42.75% | 42% | 50% | 46% | 0.045 |
| - Final | 53% | 39% | 46% | 37.5% | 33% | 35.25% | 0.050 |
| A change in the BMI category from overweight to normal | |||||||
| Entire study period | 3.33% | 0 | 3.33% | 7.71% | 11.43% | 17.14% | 0.001 |
| Indicator | SPA | EPA |
Total p |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| initial | final | p | initial | final | p | |||||||
| Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | |||||
| Height (cm) | 151.25 | 145.08 | 165.41 | 159.25 | 0.001 | 144.66 | 147 | 158 | 160.62 | 0.000 | 0.000 | |
| Weight (kg) | 59.98 | 47.60 | 75.18 | 61.64 | 0.012 | 47.50 | 48.67 | 60.38 | 61.88 | 0.003 | 0.001 | |
| BMI (kg/m2) | 25.92 | 22.58 | 27.23 | 24.37 | 0.162 | 22.65 | 22.41 | 24.21 | 23.66 | 0.062 | 0.042 | |
| BMI (percentile, c) | 97 c | 90 c | 97 c | 97 c | 0.032 | 90 c | 90 c | 85 c | 90 c | 0.010 | 0.014 | |
| Indicator | SPA | EPA | ||||
|---|---|---|---|---|---|---|
| initial | final | p | initial | final | p | |
| FM (kg) | 15.02 | 20.32 | 0.001 | 15.65 | 18.87 | 0.001 |
| FM (%) | 31.62 | 32.65 | 0.025 | 32.02 | 30.02 | 0.000 |
| FM (percentile, c) | 91 c | 95 c | 0.024 | 91 c | 85 c | 0.000 |
| FFM (kg) | 32.50 | 41.17 | 0.001 | 32.07 | 43.01 | 0.001 |
| FFM (%) | 68.38 | 67.35 | 0.051 | 67.98 | 69.98 | 0.025 |
| TBW (kg) | 23.76 | 30.21 | 0.001 | 24.30 | 31.51 | 0.014 |
| TBW (%) | 50.15 | 49.62 | 0.003 | 51.95 | 52.20 | 0.000 |
| SMM (kg) | 18.47 | 23.35 | 0.001 | 18.75 | 24.12 | 0.001 |
| SMM (%) | 38.66 | 38.10 | 0.514 | 38.54 | 40.12 | 0.000 |
| Indicator | SPA | EPA | ||||
|---|---|---|---|---|---|---|
| initial | final | p | initial | final | p | |
| FM (kg) | 17.39 | 23.19 | 0.001 | 12.43 | 13.76 | 0.001 |
| FM (%) | 29 | 30.85 | 0.000 | 26.20 | 22.80 | 0.000 |
| FM (percentile, c) | 95 | 95 | 0.452 | 91 | 85 | 0.000 |
| FFM (kg) | 42.58 | 51.98 | 0.014 | 35.66 | 46.61 | 0.014 |
| FFM (%) | 71 | 69.11 | 0.001 | 73.80 | 77.20 | 0.001 |
| TBW (kg) | 30.73 | 37.77 | 0.025 | 25.64 | 33.31 | 0.000 |
| TBW (%) | 51.25 | 50.25 | 0.012 | 54.56 | 55.70 | 0.045 |
| SMM (kg) | 22.89 | 29.84 | 0.024 | 20.57 | 33.07 | 0.001 |
| SMM (%) | 39.05 | 40.46 | 0.071 | 42.01 | 45.45 | 0.001 |
| Fat Mass, % (kg) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement session |
Average Age (Years) |
Mean* Total |
SPA | EPA | p** | ESs | ||||||||||
| Mean | Median | Min. | Max. | SD | 95% CI |
Mean | Median | Min. | Max. | SD | 95% CI |
|||||
| Average | ||||||||||||||||
| I | 10.27 | 29.48** (15.11) |
30.32 (16.20) |
30.50 | 17.50 | 47.40 | 5.61 | 4.45-7.59 | 28.65 (14.03) |
30.00 | 18.80 | 37.30 | 5.00 | 3.98-6.73 | 0.018 | 0.789 |
| II | 10.90 | 29.35 (16.01) |
30.62 (17.67) |
29.05 | 17.10 | 47.90 | 5.76 | 4.67-7.51 | 28.08 (14.35) |
28.00 | 16.60 | 38.00 | 5.42 | 4.29-7.38 | 0.029 | 0.830 |
| III | 11.27 | 29.28 (17.04) |
31.18 (19.32) |
30.10 | 18.50 | 48.00 | 6.17 | 4.93-8.25 | 27.39 (14.76) |
29.00 | 17.70 | 39.00 | 5.45 | 4.29-7.48 | 0.043 | 0.904 |
| IV | 11.90 | 29.21 (18.05) |
31.44 (20.59) |
30.50 | 18.80 | 48.50 | 6.36 | 5.18-8.23 | 26.98 (15.54) |
28.50 | 17.89 | 38.90 | 6.31 | 5.04-8.44 | 0.041 | 0.744 |
| V | 12.26 | 29.08 (19.03) |
31.75 (21.75) |
29.76 | 19.10 | 48.90 | 6.43 | 5.24-8.32 | 26.41 (16.31) |
29.30 | 16.50 | 38.50 | 5.50 | 4.35-7.49 | 0.033 | 0.643 |
| Mean from all sessions | 29.28 (17.04) |
31.06 (19.11) |
27.50 (15.00) |
0.001 | 0.320 | |||||||||||
| Girls | ||||||||||||||||
| I | 10.27 | 31.77 (15.33) |
31.62 (15.02) 91 c |
31.20 | 27.00 | 34.40 | 2.28 | 1.67-3.59 | 31.93 (15.64) 91 c |
31.87 | 27.80 | 38.30 | 3.01 | 2.24-4.58 | 0.062 | 0.322 |
| II | 10.90 | 31.33 (16.08) |
31.79 (16.21) 91 c |
31.70 | 27.60 | 37.30 | 2.88 | 2.15-4.39 | 30.87 (15.95) 91 c |
31.43 | 27.30 | 38.00 | 3.61 | 2.67-5.60 | 0.027 | 0.287 |
| III | 11.27 | 31.29 (17.34) |
31.86 (17.86) 91 c |
31.00 | 28.40 | 38.10 | 2.76 | 2.00-4.44 | 30.73 (16.83) 91 c |
30.50 | 26.40 | 37.00 | 3.31 | 2.40-5.33 | 0.033 | 0.479 |
| IV | 11.90 | 31.13 (18.28) |
32.14 (19.02) 95 c |
30.00 | 28.50 | 39.20 | 2.98 | 2.25-4.40 | 30.12 (17.56) 91 c |
30.10 | 25.80 | 37.90 | 4.07 | 3.03-6.20 | 0.025 | 0.137 |
| V | 12.26 | 31.33 (19.59) |
32.65 (20.32) 95 c |
31.35 | 28.80 | 39.60 | 3.16 | 3.16-2.40 | 30.02 (18.87) 85 c |
30.05 | 25.60 | 37.50 | 3.40 | 2.51-2.27 | 0.004 | 0.571 |
| Mean from all sessions | 31.37 (17.32) |
31.93 (17.69) |
30.73 (16.97) |
0.014 | 0.351 | |||||||||||
| Boys | ||||||||||||||||
| I | 10.27 | 27.18 (14.91) |
29.00 (17.39) 95 c |
28.45 | 17.50 | 47.40 | 7.84 | 5.68-12.63 | 25.37 (12.43) 91 c |
25.40 | 18.80 | 34.30 | 4.66 | 3.34-7.70 | 0.003 | 0.128 |
| II | 10.90 | 27.57 (15.94) |
29.85 (19.13) 95 c |
27.20 | 17.60 | 47.90 | 7.37 | 5.57-10.91 | 25.30 (12.75) 91 c |
24.40 | 17.60 | 34.10 | 5.09 | 3.61-8.65 | 0.010 | 0.274 |
| III | 11.27 | 27.27 (16.74) |
30.50 (20.79) 95 c |
27.30 | 18.50 | 48.00 | 7.97 | 5.93-12.13 | 24.05 (12.69) 85 c |
23.60 | 16.70 | 33.90 | 4.53 | 3.24-7.47 | 0.008 | 0.075 |
| IV | 11.90 | 27.30 (17.85) |
30.75 (22.17) 95 c |
26.00 | 18.80 | 48.50 | 8.00 | 6.05-11.84 | 23.85 (13.53) 85 c |
24.00 | 16.89 | 32.00 | 5.45 | 3.95-8.78 | 0.020 | 0.386 |
| V | 12.26 | 26.82 (18.47) |
30.85 (23.19) 95 c |
24.50 | 19.10 | 48.90 | 7.64 | 5.73-11.45 | 22.80 (13.76) 85 c |
24.30 | 16.50 | 31.40 | 4.71 | 3.33-8.00 | 0.032 | 0.339 |
| Mean from all sessions | 27.22 (16.78) |
30.19 (20.53) |
24.27 (13.03) |
0.001 | 0.420 | |||||||||||
| Indicator | SPA | EPA | Total p |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| initial | final | p | initial | final | p | ||||||
| Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | ||||
| BMI (kg/m2) | 26.18 | 22.34 | 27.94 | 25.16 | 0.120 | 23.12 | 22.57 | 25.12 | 24.40 | 0.002 | 0.002 |
| FM (kg) | 19.90 | 15.85 | 24.23 | 21.25 | 0.001 | 13.55 | 16.20 | 16.63 | 20.20 | 0.001 | 0.010 |
| FM (%) | 30.61 | 31.95 | 32.25 | 33.05 | 0.010 | 27.22 | 32.23 | 25.13 | 31.20 | 0.000 | 0.031 |
| FFM (kg) | 40.36 | 31.22 | 52.59 | 40.36 | 0.041 | 35.47 | 31.73 | 49.33 | 41.85 | 0.050 | 0.001 |
| FFM (%) | 67.17 | 68.13 | 66.45 | 66.58 | 0.001 | 71.95 | 65.31 | 72.90 | 67.03 | 0.002 | 0.000 |
| TBW (kg) | 29.78 | 23.16 | 38.50 | 29.61 | 0.032 | 25.81 | 24.15 | 35.00 | 30.00 | 0.000 | 0.002 |
| TBW (%) | 50.10 | 49.17 | 49.11 | 48.41 | 0.001 | 52.31 | 50.62 | 53.27 | 51.06 | 0.000 | 0.002 |
| SMM (kg) | 23.13 | 18.23 | 29.95 | 22.89 | 0.041 | 20.65 | 18.73 | 33.60 | 24.23 | 0.001 | 0.000 |
| SMM (%) | 38.75 | 38.52 | 39.95 | 37.95 | 0.050 | 41.10 | 38.37 | 42.86 | 39.25 | 0.000 | 0.001 |
| FFM, % (kg) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement session |
Average Age (Years) |
Mean* Total |
SPA | EPA |
p* |
ESs |
||||||||||
| Mean | Median | Min. | Max. | SD | 95% CI |
Mean | Median | Min. | Max. | SD | 95% CI |
|||||
| Average | ||||||||||||||||
| I | 10.27 | 69.80** (35.70) |
69.68 (37.54) |
69.50 | 60.50 | 74.00 | 4.30 | 4.20-6.14 | 69.97 (33.87) |
68.20 | 64.50 | 76.00 | 4.20 | 3.80-5.17 | 0.288 | 0.262 |
| II | 10.90 | 70.69 (38.36) |
69.53 (40.13) |
68.70 | 59.00 | 75.50 | 4.70 | 3.80-5.42 | 71.85 (36.59) |
70.60 | 64.00 | 76.50 | 3.41 | 3.60-5.90 | 0.013 | 0.258 |
| III | 11.27 | 70.66 (40.67) |
68.76 (42.66) |
66.90 | 60.20 | 74.00 | 3.90 | 4.18-6.30 | 72.57 (38.69) |
71.59 | 65.50 | 76.00 | 3.90 | 4.15-6.24 | 0.020 | 0.620 |
| IV | 11.90 | 70.54 (43.41) |
68.31 (44.73) |
67.20 | 57.00 | 76.00 | 5.20 | 4.80-6.50 | 72.78 (42.10) |
71.90 | 65.00 | 76.80 | 4.25 | 4.39-6.15 | 0.012 | 0.172 |
| V | 12.26 | 70.65 (45.69) |
67.95 (46.57) |
65.90 | 58.00 | 77.00 | 4.63 | 5.20-6.08 | 73.35 (44.81) |
72.12 | 66.50 | 78.50 | 4.60 | 5.18-6.14 | 0.014 | 0.087 |
| Mean from all sessions | 70.46 (40.76) |
68.83 (42.33) |
72.10 (39.21) |
0.001 | 0.241 | |||||||||||
| Girls | ||||||||||||||||
| I | 10.27 | 67.08 (32.28) |
68.27 (32.50) |
68.70 | 65.60 | 73.00 | 5.90 | 4.07-10.80 | 65.89 (32.07) |
64.00 | 64.50 | 70.50 | 4.20 | 3.80-5.17 | 0.092 | 0.508 |
| II | 10.90 | 68.96 (35.41) |
68.91 (35.30) |
67.20 | 62.70 | 73.70 | 4.30 | 2.74-8.40 | 69.01 (35.52) |
67.00 | 64.00 | 72.00 | 3.60 | 3.60-4.70 | 0.801 | 0.858 |
| III | 11.27 | 68.58 (37.61) |
67.97 (37.93) |
66.00 | 62.00 | 72.80 | 6.20 | 6.72-12.20 | 69.20 (37.30) |
68.00 | 65.50 | 71.00 | 4.10 | 4.02-6.18 | 0.098 | 0.528 |
| IV | 11.90 | 68.42 (40.27) |
67.43 (39.54) |
66.40 | 60.80 | 73.20 | 6.70 | 5.48-12.50 | 69.42 (41.00) |
68.50 | 65.00 | 72.50 | 5.20 | 3.50-6.14 | 0.047 | 0.860 |
| V | 12.26 | 68.14 (42.09) |
66.79 (41.17) |
65.80 | 61.00 | 73.00 | 7.12 | 5.40-11.70 | 69.50 (43.01) |
68.00 | 66.50 | 74.00 | 6.17 | 3.90-5.79 | 0.042 | 0.447 |
| Meanfrom all sessions | 68.23 (37.53) |
67.87 (37.29) |
68.60 (37.78) |
0.035 | 0.320 | |||||||||||
| Boys | ||||||||||||||||
| I | 10.27 | 72.52 (39.12) |
70.99 (42.58) |
69.70 | 60.50 | 74.00 | 6.40 | 3.90-5.70 | 74.05 (35.66) |
73.00 | 66.50 | 76.00 | 3.80 | 4.20-5.74 | 0.022 | 0.116 |
| II | 10.90 | 72.43 (44.34) |
70.16 (51.02) |
68.50 | 59.00 | 75.50 | 4.72 | 4.20-6.05 | 74.70 (37.66) |
72.50 | 65.80 | 76.50 | 4.20 | 3.75-5.34 | 0.036 | 0.172 |
| III | 11.27 | 72.72 (43.73) |
69.49 (47.39) |
67.90 | 60.20 | 74.00 | 5.14 | 4.80-6.20 | 75.95 (40.08) |
74.30 | 65.90 | 76.00 | 3.42 | 3.20-6.14 | 0.011 | 0.277 |
| IV | 11.90 | 72.67 (46.30) |
69.20 (49.93) |
68.70 | 57.00 | 76.00 | 5.80 | 4.60-5.80 | 76.14 (43.21) |
75.20 | 66.40 | 76.80 | 3.24 | 2.80-6.40 | 0.017 | 0.061 |
| V | 12.26 | 73.17 (49.29) |
69.14 (51.98) |
67.40 | 58.00 | 77.00 | 6.17 | 4.70-6.80 | 77.20 (46.61) |
75.90 | 68.90 | 78.50 | 4.20 | 3.15-5.79 | 0.030 | 0.128 |
| Mean from all sessions | 72.70 (44.55) |
69.79 (47.37) |
75.60 (40.64) |
0.020 | 0.351 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




