Submitted:
23 August 2024
Posted:
23 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Solvents
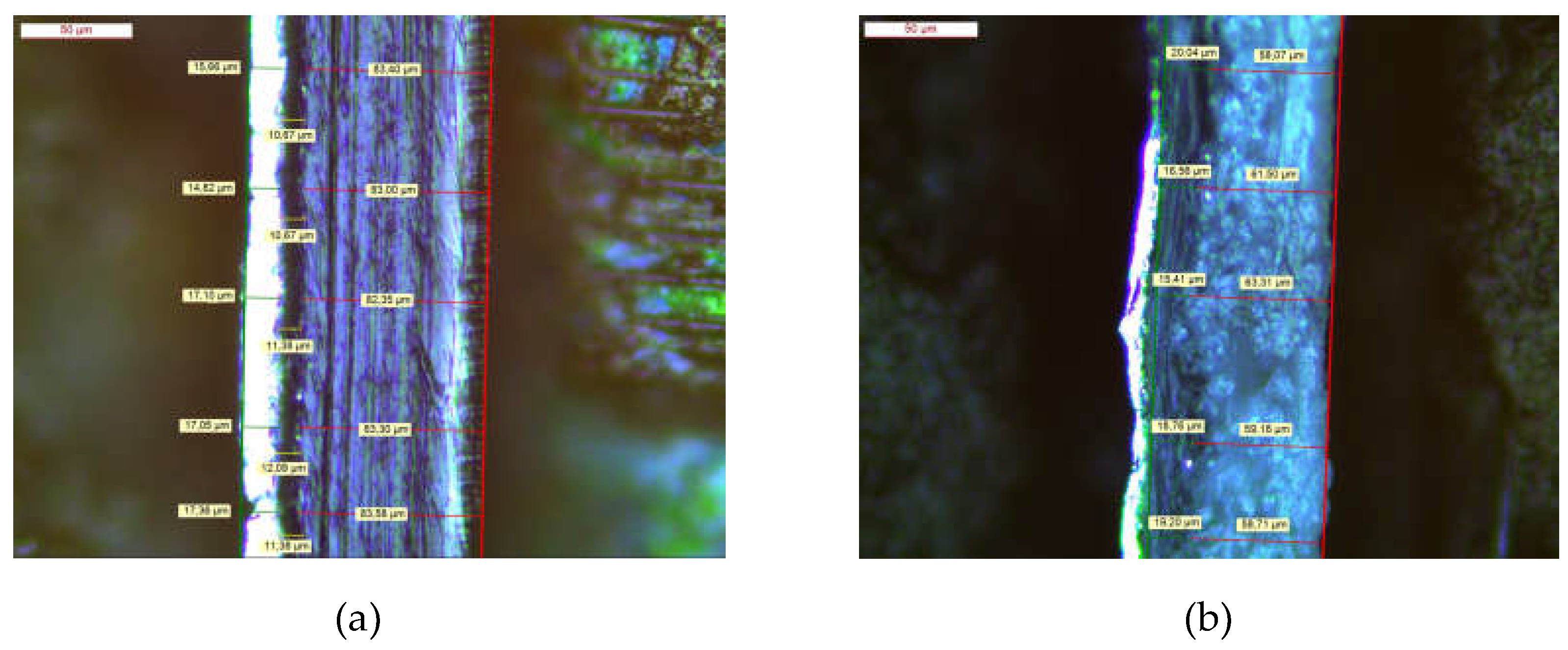
2.2. Packaging Films and Blister Packaging
2.3. Delamination
2.3.1. Screening Tests
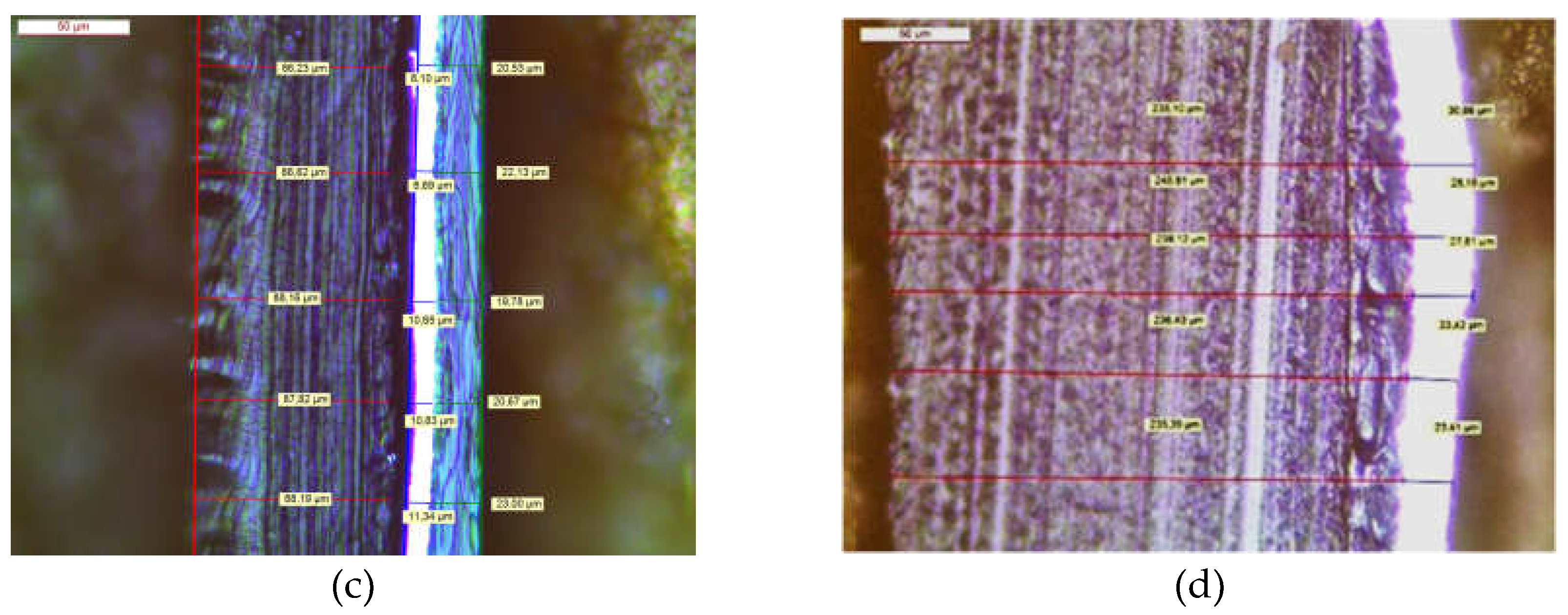
2.3.2. Design of Experiments
2.4. DSC
2.5. FTIR
3. Results and Discussion
3.1. Delamination
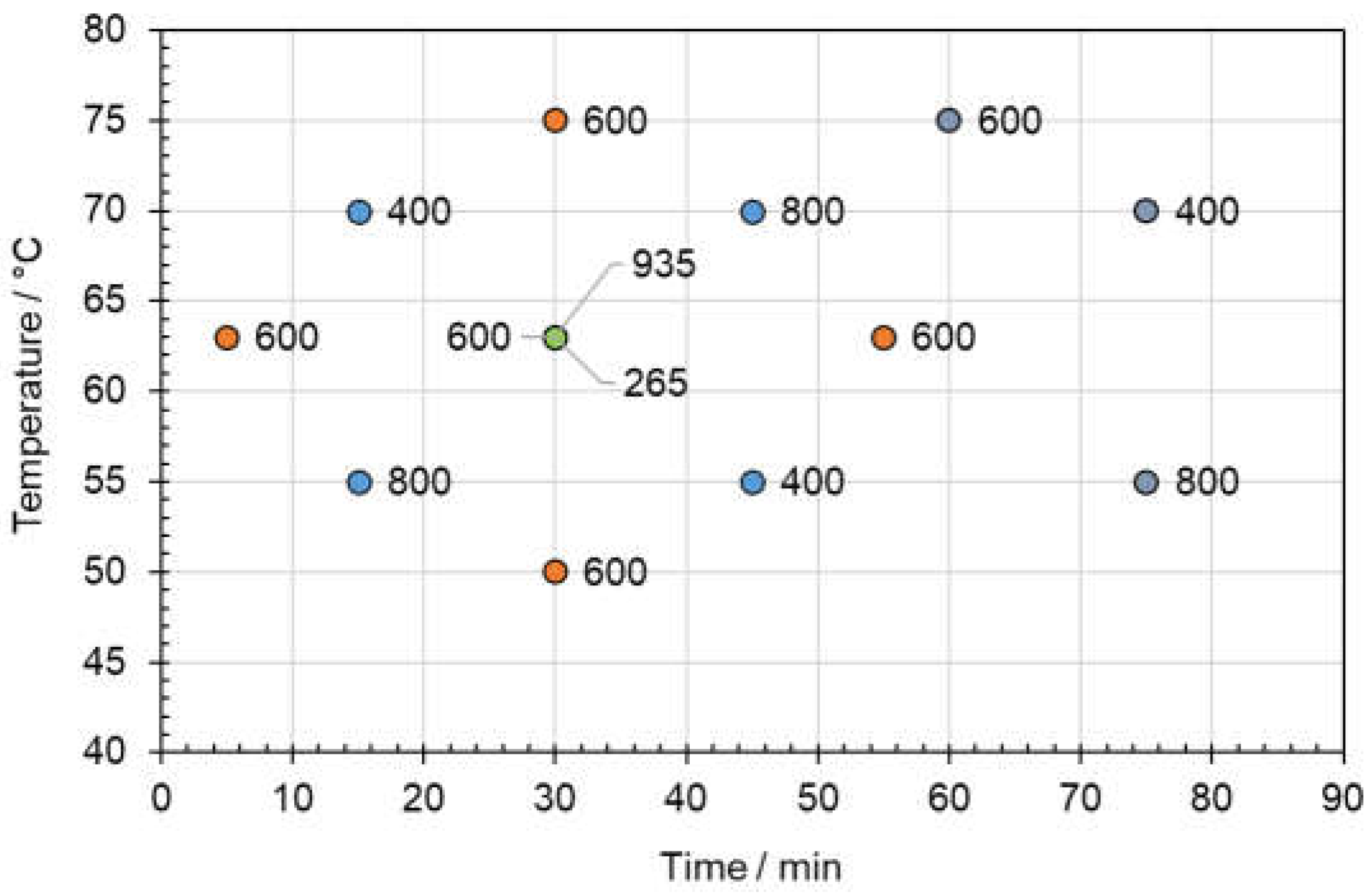
3.1.1. Screening Experiments
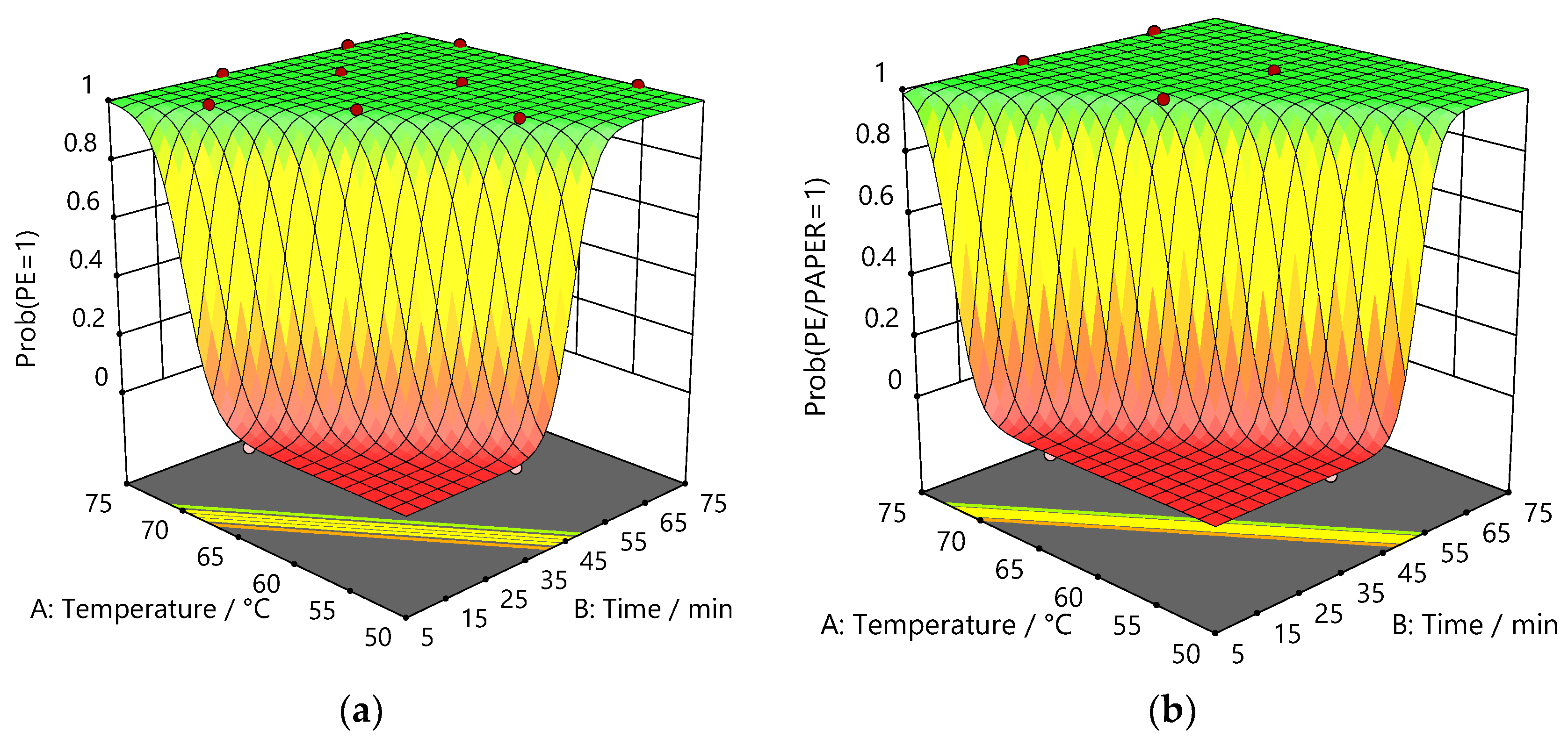
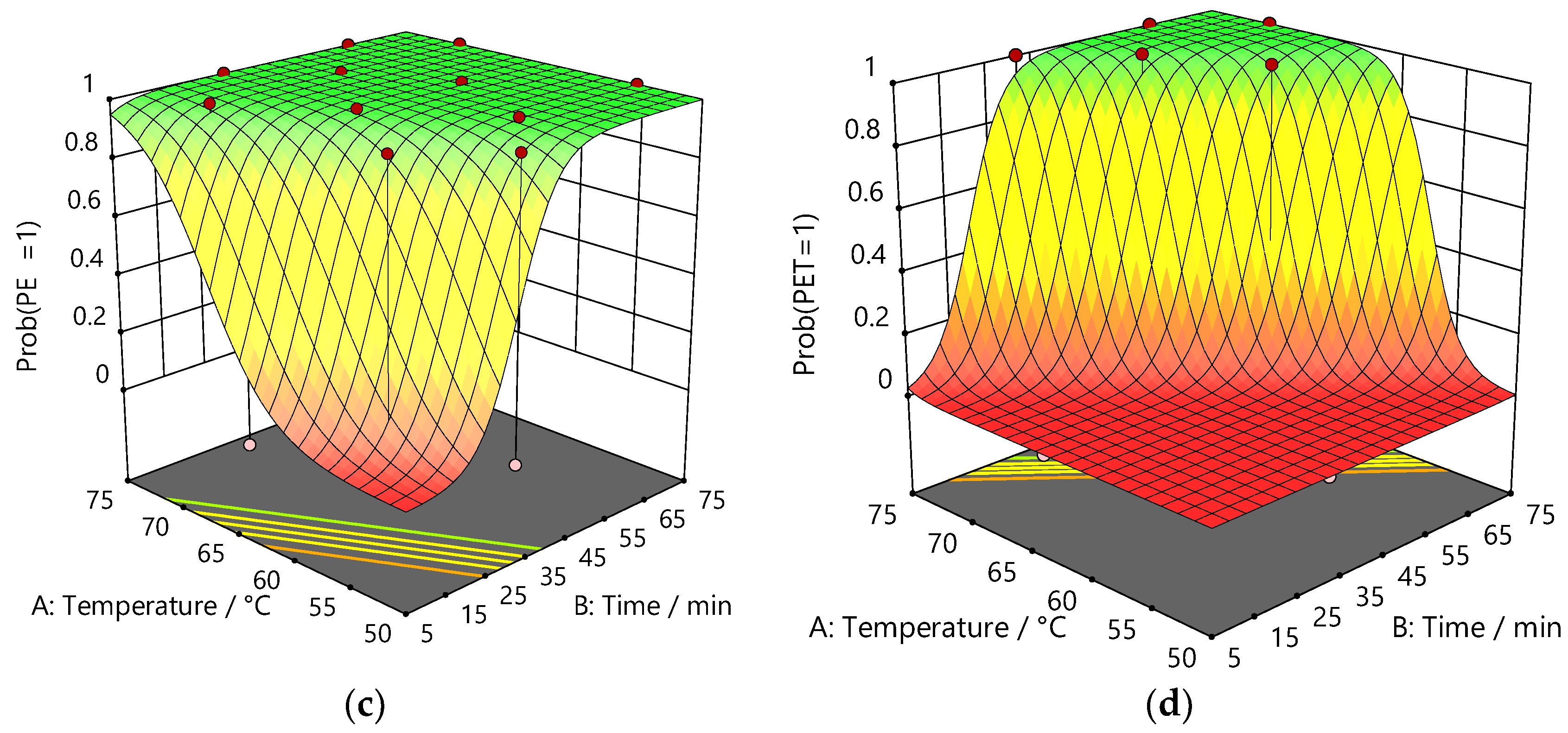
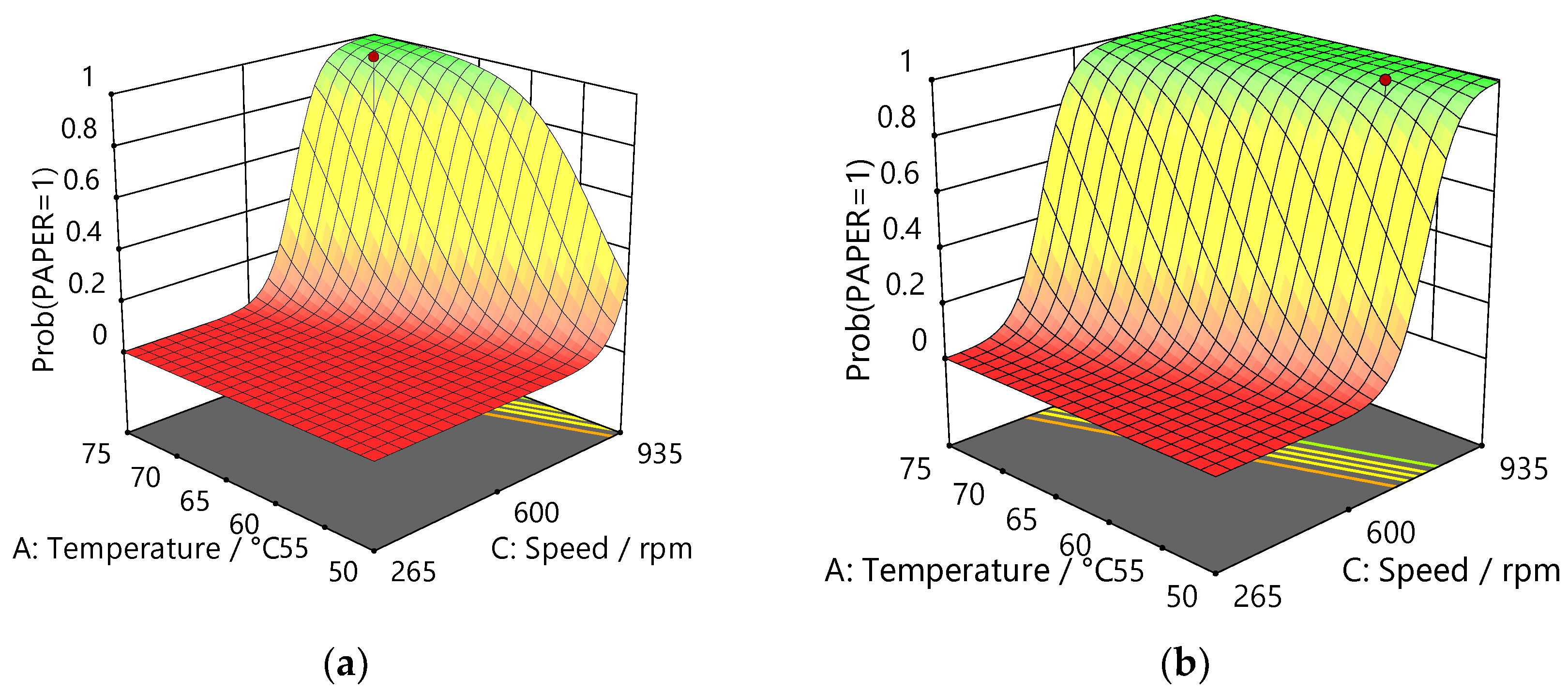
3.1.2. Design of Experiments
3.1.3. Thymol:Acetic Acid
3.1.4. Betaine: Acetic Acid
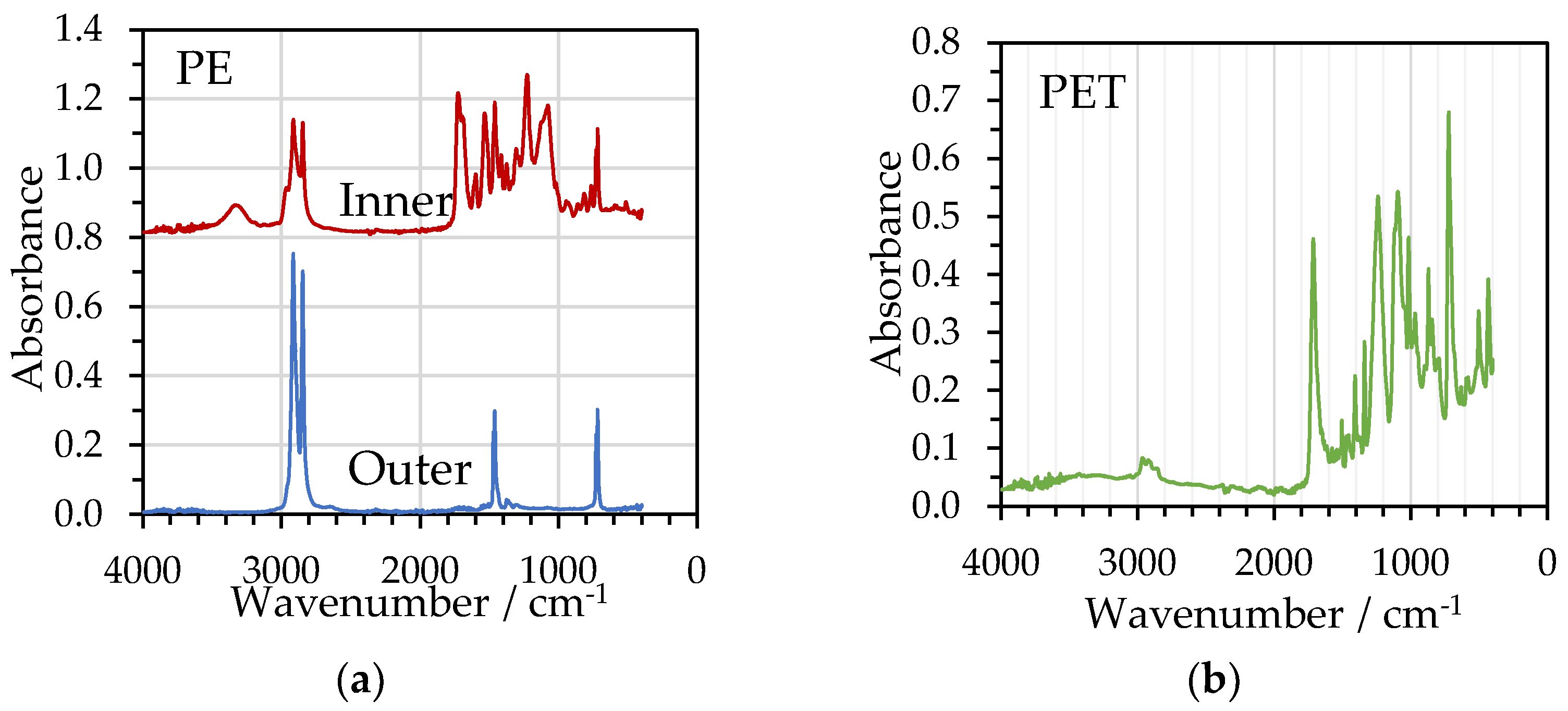
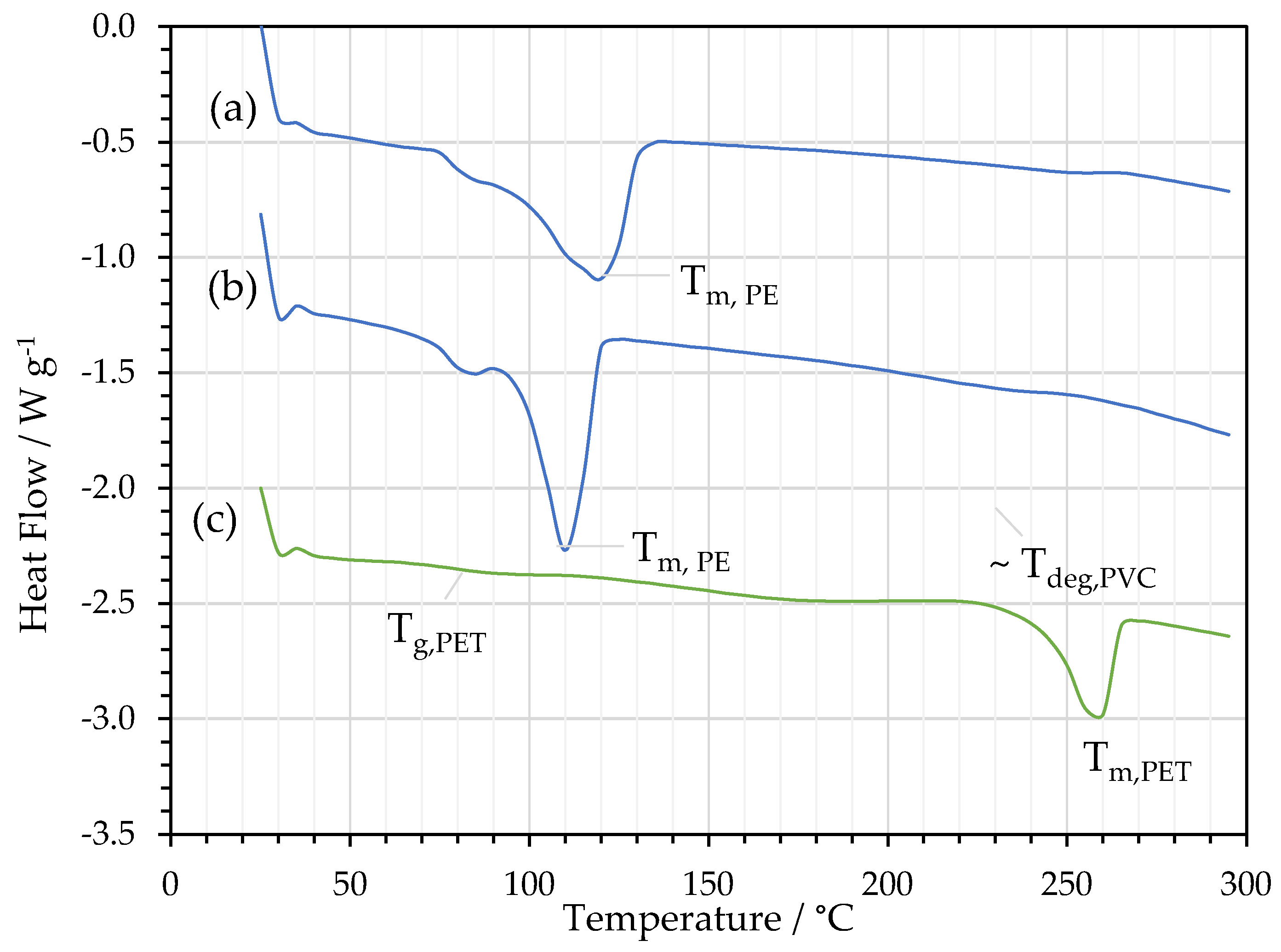
3.2. FTIR and DSC Analysis of Delaminated Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, J.; Grau, L.; Auer, M.; Maletz, R.; Woidasky, J. Multilayer Packaging in a Circular Economy. Polymers 2022, 14, 1825. [Google Scholar] [CrossRef] [PubMed]
- Ügdüler, S., De Somer, T., Van Geem, K.M, De Wilde, J., Roosen, M., Deprez, B., De Meester, S., Analysis of the kinetics, energy balance and carbon footprint of the delamination of multilayer flexible packaging films via carboxylic acids, Resources, Conservation and Recycling 2022, 181, 106256. [CrossRef]
- Bauer, A.-S.; Tacker, M.; Uysal-Unalan, I.; Cruz, R.M.S.; Varzakas, T.; Krauter, V. Recyclability and Redesign Challenges in Multilayer Flexible Food Packaging—A Review. Foods 2021, 10, 2702. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2018, 3, 1. [Google Scholar] [CrossRef]
- Z. O. G. Schyns, M. P. Shaver, Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [CrossRef] [PubMed]
- Ragaert, K., Delva, L., Van Geem, K., Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [CrossRef] [PubMed]
- Giorgia Faraca, G., Thomas Astrup, T, Plastic waste from recycling centres: Characterisation and evaluation of plastic recyclability, Waste Management, 2019, 95, 388-398. [CrossRef]
- Ügdüler, S., De Somer, T., Van Geem, K.M., Roosen, M., Kulawig, A., Leineweber, R., De Meester, S., Towards a Better Understanding of Delamination of Multilayer Flexible Packaging Films by Carboxylic Acids. ChemSusChem. 2021, 14(19):4198-4213. [CrossRef]
- Šleiniūtė, A.; Denafas, G.; Mumladze, T. Analysis of the Delamination Process with Nitric Acid in Multilayer Composite Food Packaging. Appl. Sci. 2023, 13, 5669. [Google Scholar] [CrossRef]
- Nieminen, J., Anugwom, I., Kallioinen, M., Mänttäri, M. Green solvents in recovery of aluminium and plastic from waste pharmaceutical blister packaging. Waste Manag. 2020, 107:20-27. [CrossRef]
- Fávaro, S.L., Freitas, A.R., Ganzerli, T.A., Pereira, A.G.B., Cardozo, A.L., Baron, O., Muniz, E.C., Girotto, E.M., Radovanovic, E., PET and aluminum recycling from multilayer food packaging using supercritical ethanol, The Journal of Supercritical Fluids, 2013, 75, 138-143. [CrossRef]
- O'Rourke, G., Houbrechts, M., Nees, M., Roosen, M., De Meester, S., De Vos, D., Delamination of polyamide/polyolefin multilayer films by selective glycolysis of polyurethane adhesive, Green Chem., 2022, 24, 6867-6878. [CrossRef]
- Hamilton, J.A., Transport of fatty acids across membranes by the diffusion mechanism, Prostaglandins, Leukotrienes and Essential Fatty Acids, 1999, 60, 291-297. [CrossRef]
- Zainal-Abidin, M.H., Hayyan, M., Wong, W.F., Hydrophobic deep eutectic solvents: Current progress and future directions. Journal of Industrial and Engineering Chemistry 2021, 97, 142-162. [CrossRef]
- Abbott, A.P., David Boothby, Glen Capper, David L. Davies, and Raymond K. Rasheed, Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids, Journal of the American Chemical Society 2004, 126, 9142-9147. [CrossRef]
- Li, G., Row, K.H., Utilization of deep eutectic solvents in dispersive liquid-liquid micro-extraction, TrAC Trends in Analytical Chemistry, 2019, 120. [CrossRef]
- Cardellini, F., Germani, R., Cardinali, G., Corte, L., Roscini, L., Spreti, N., Tiecco, M., Room temperature deep eutectic solvents of (1S)-(+)-10-camphorsulfonic acid and sulfobetaines: hydrogen bond-based mixtures with low ionicity and structure-dependent toxicity, RSC Advances 2015, 5, 31772-31786. [CrossRef]
- Abo-Hamad, A., Hayyan, M., AlSaadi, M.A.H., Ali Hashim, M.A., Potential applications of deep eutectic solvents in nanotechnology, Chemical Engineering Journal, 2015, 273, 551-567. [CrossRef]
- Mbous, Y.P., Hayyan, M., Hayyan, A., Wong, W.F., Hashim, M.A., Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering-Promises and challenges. Biotechnol Adv. 2017, 35, 105-134. [CrossRef]
- Yang, R., R., Cao, Q., Liang, Y., Hong, S., Xia, C., Wu, Y., Li, J., Cai, L, Sonne, C., Le, Q. V., Lam, S.S., High capacity oil absorbent wood prepared through eco-friendly deep eutectic solvent delignification, Chemical Engineering Journal, 2020, 401. [CrossRef]
- Hansen, B.B., Spittle, S., Chen, B., Poe, D., Zhang, Y., Klein, J.M., Horton, A., Adhikari, L., Zelovich, T., Doherty, B.W., Gurkan, B., Maginn, E.J., Ragauskas, A., Dadmun, M., Zawodzinski, T.A., Baker, G.A., Tuckerman, M.E., Savinell, R.F., Sangoro, J.R., Deep Eutectic Solvents: A Review of Fundamentals and Applications, Chemical Reviews, 2021, 121, 1232-1285. [CrossRef]
| Solvent | Molar ratio | Water Miscibility |
Density / g cm-3 |
Viscosity / mPa s | Crystallisation Temperature /°C |
| Acetic Acid | - | miscible | 1.049 | 2.4 ± 0.6 | |
| Betaine: Acetic acid | 1:4 | miscible | 1.118 | 41.7 ± 0.7 | Not detected |
| L-Proline: Acetic Acid | 1:3 | miscible | 1.166 | 117 ± 2 | Not detected |
| Propylene Glycol: Acetic Acid | 1:2 | miscible | 1.043 | 16.0 ± 0.5 | Not detected |
| Carvacrol: Acetic Acid | 1:1 | immiscible | 0.993 | 6.9 ± 0.5 | Not detected |
| Eugenol: Acetic Acid | 1:1 | immiscible | 1.061 | 5.0 ± 0.4 | Not detected |
| Guaiacol: Acetic Acid | 1:1 | immiscible | 1.107 | 4.0 ± 0.8 | Not detected |
| Thymol: Acetic Acid | 1:1 | immiscible | 0.991 | 7.0 ± 0.5 | Not detected |
| Material | Film Thickness /μm | ||||
| Al | PVC | PE | PET | Paper | |
| PE/Al | 16 ± 1 | - | 83 ± 1 | - | - |
| Al/PE/Paper | 10 ± 2 | - | 18 ± 2 | - | 58 ± 3 |
| PE/Al/PET | 10 ± 1 | - | 87 ± 1 | 21 ± 1 | - |
| Blister Pack | 26 ± 1 | 211 ± 1 | - | - | - |
| Solvent | Molar Ratio | PE/Al | Al/PE/Paper | PE/Al/PET | Blister Pack | ||||
| PE | PE/Paper | PE | Paper | PE | PET | PVC | Lidding Film | ||
| Acetic Acid | - | 10 | 10a | 10 | 10 | 10 | 10 | 0 | 0 |
| Betaine: Acetic Acid | 1:4 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 |
| L-Proline: Acetic Acid | 1:3 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 |
| Propylene Glycol: Acetic Acid | 1:2 | 9 | 10a | 10 | 10 | 8 | 0 | 0 | 0 |
| Carvacrol: Acetic Acid | 1:1 | 10 | 10a | 2 | 2 | 10 | 5 | 0 | 0 |
| Eugenol: Acetic Acid | 1:1 | 10 | 10 | 0 | 0 | 10 | 7 | 0 | 0 |
| Guaiacol: Acetic Acid | 1:1 | 10 | 10 | 0 | 0 | 10 | 10 | 10 | 10 |
| Thymol: Acetic Acid | 1:1 | 10 | 10 | 0 | 0 | 10 | 10 | 0 | 0 |
| # | Conditions | PE/Al | Al/PE/Paper | PE/Al/PET | |||||
|---|---|---|---|---|---|---|---|---|---|
| A: Temp. / °C |
B: Time / min |
C: Speed / rpm |
PE | PE/Paper | PE | Paper | PE | PET | |
| 1 | 55 | 45 | 400 | 10 | 0 | 0 | 0 | 10 | 0 |
| 2 | 70 | 15 | 400 | 10 | 0 | 0 | 0 | 10 | 0 |
| 3 | 55 | 15 | 800 | 0 | 0 | 0 | 0 | 5 | 0 |
| 4 | 70 | 45 | 800 | 10 | 10 | 0 | 0 | 10 | 10 |
| 5 | 63 | 30 | 600 | 10 | 10 | 0 | 0 | 10 | 0 |
| 6 | 63 | 55 | 600 | 10 | 10a | 2 | 2 | 10 | 7 |
| 7 | 75 | 30 | 600 | 10 | 10a | 0 | 0 | 10 | 8 |
| 8 | 50 | 30 | 600 | 0 | 0 | 0 | 0 | 3 | 0 |
| 9 | 63 | 5 | 600 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 63 | 30 | 600 | 10 | 10 | 0 | 0 | 10 | 0 |
| 11 | 63 | 30 | 265 | 10 | 0 | 0 | 0 | 10 | 0 |
| 12 | 63 | 30 | 935 | 10 | 10 | 0 | 0 | 10 | 0 |
| 13 | 75 | 60 | 600 | 10 | 10 | 0 | 0 | 10 | 10 |
| 14 | 70 | 75 | 400 | 10 | 10 | 0 | 0 | 10 | 10 |
| 15 | 55 | 75 | 800 | 10 | 10a | 1 | 1 | 10 | 0 |
| Factor p-values | |||||
|---|---|---|---|---|---|
| Material | Film | Model | A: Temperature (°C) |
B: Time (min) |
C: Stirrer Speed (rpm) |
| PE/Al | PE | < 0.0001 | < 0.0001 | < 0.0001 | n/a |
| Al/PE/Paper | PE/Paper | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| PE/Al/PET | PE | < 0.0001 | < 0.0001 | < 0.0001 | 0.0006 |
| PET | < 0.0001 | < 0.0001 | < 0.0001 | 0.0192 | |
| # | Conditions | PE/Al | Al/PE/Paper | PE/Al/PET | |||||
|---|---|---|---|---|---|---|---|---|---|
| A: Temp. / °C |
B: Time / min |
C: Speed / rpm |
PE | PE/Paper | PE | Paper | PE | PET | |
| 1 | 70 | 45 | 800 | 0 | 0 | 0 | 10 | 0 | 0 |
| 2 | 55 | 45 | 400 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 63 | 30 | 600 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 70 | 15 | 400 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 75 | 30 | 600 | 0 | 0 | 0 | 1 | 0 | 0 |
| 6 | 63 | 55 | 600 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 55 | 75 | 800 | 0 | 0 | 0 | 10 | 0 | 0 |
| 8 | 75 | 60 | 600 | 0 | 0 | 0 | 3 | 0 | 0 |
| 9 | 70 | 75 | 400 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 55 | 15 | 800 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | 70 | 15 | 800 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 70 | 45 | 800 | 0 | 0 | 0 | 10 | 0 | 0 |
| 13 | 63 | 30 | 935 | 0 | 0 | 0 | 3 | 0 | 0 |
| 14 | 63 | 30 | 265 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | 50 | 30 | 600 | 0 | 0 | 0 | 0 | 0 | 0 |
| Factor p-values | |||||
| Material | Film | Model | A: Temperature (°C) |
B: Time (min) |
C: Stirrer Speed (rpm) |
| Al/PE/Paper | Paper | < 0.0001 | 0.0018 | < 0.0001 | < 0.0001 |
| Material | Film | DSC Parameters | |||
|---|---|---|---|---|---|
| Tg / °C |
Tm / °C |
ΔHf / J g-1 |
χc / % |
||
| PE/Al | PE | - | 118 | 103 | 35 |
| Al/PE/Paper | PE | - | 111 | 97.4 | 33 |
| PE/Al/PET | PET | 78 | 257 | 38.2 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





