Submitted:
21 August 2024
Posted:
21 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Extraction and Isolation of Plant Material
2.3. Total Phytochemical Contents
2.4. Antioxidant Assay
2.5. Anti-Helicobacter pylori Assay
2.6. Enzyme Inhibitory Assay
2.7. Biofilm Formation Inhibitory Assay
2.8. Scanning Electron Microscopy
2.9. Membrane Permeability Assay
2.10. Cell line Cultures and Cytotoxicity Assay
2.11. Statistical Analysis
3. Results
3.1. Total Phytochemical Contents
3.2. Antioxidant Activity
3.3. Anti-H. pylori Activity
3.4. Enzyme Inhibitory Activity
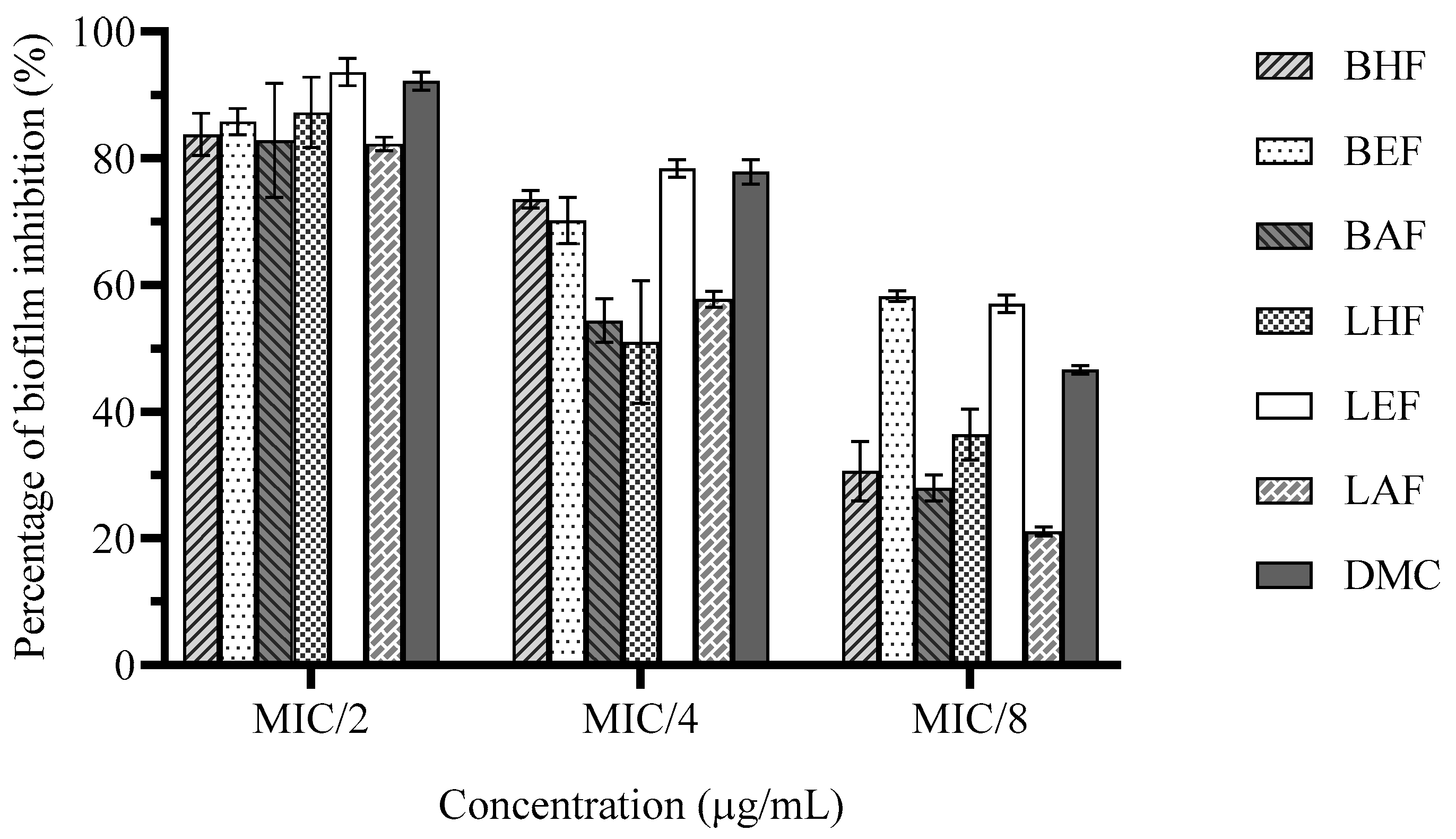
3.5. Effect on Biofilm Formation of H. pylori
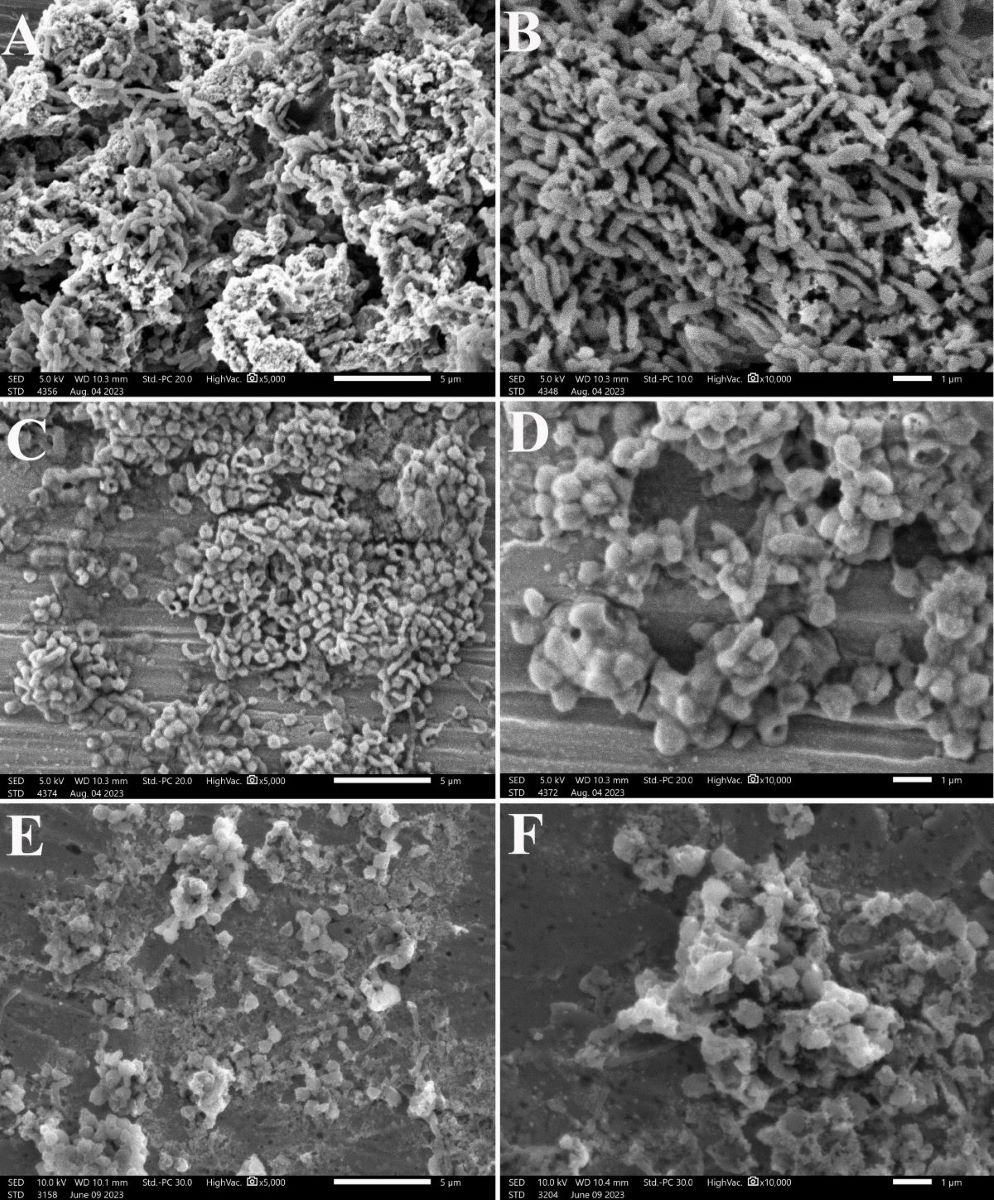
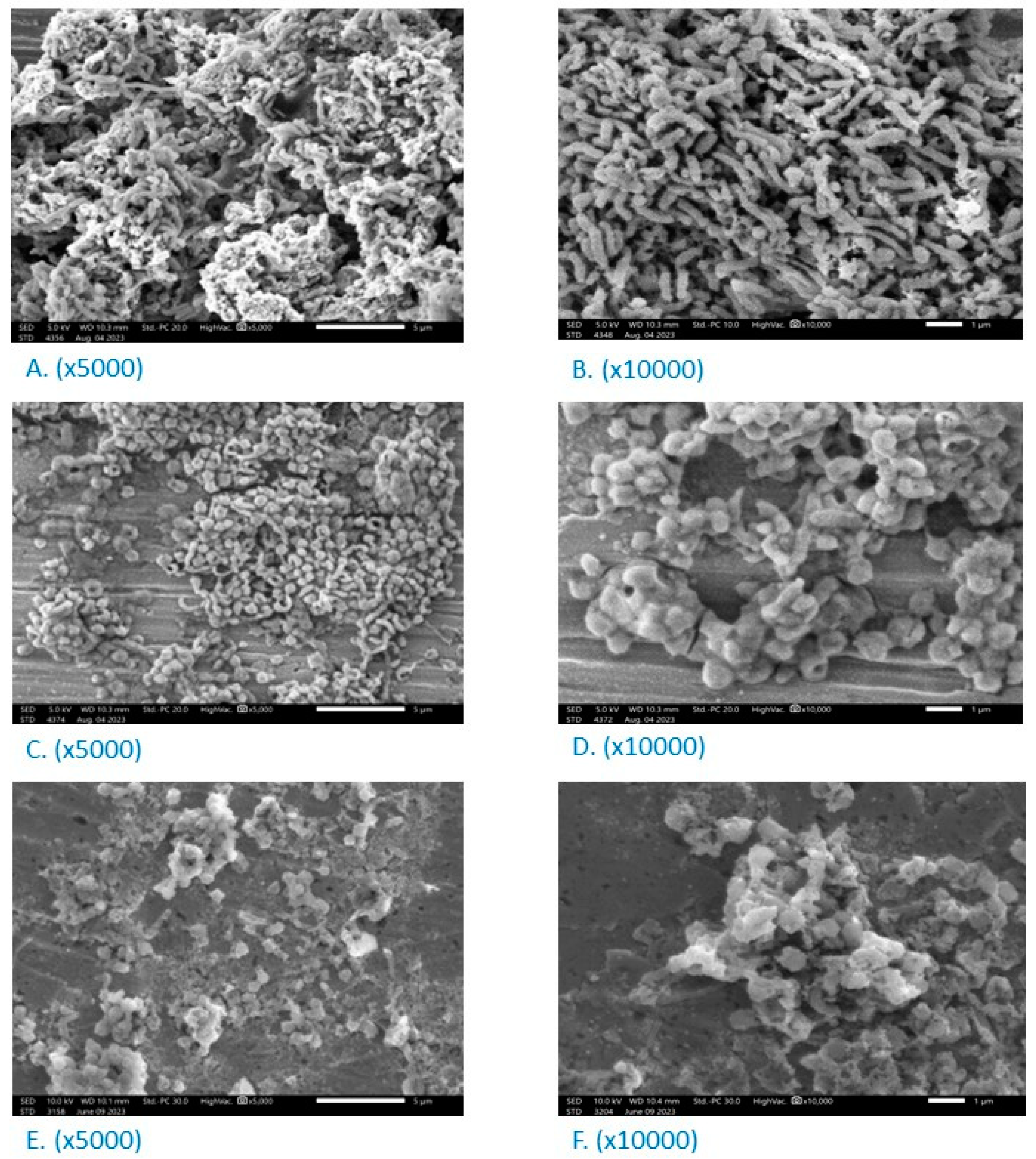
3.6. Effect on the Morphology of H. pylori
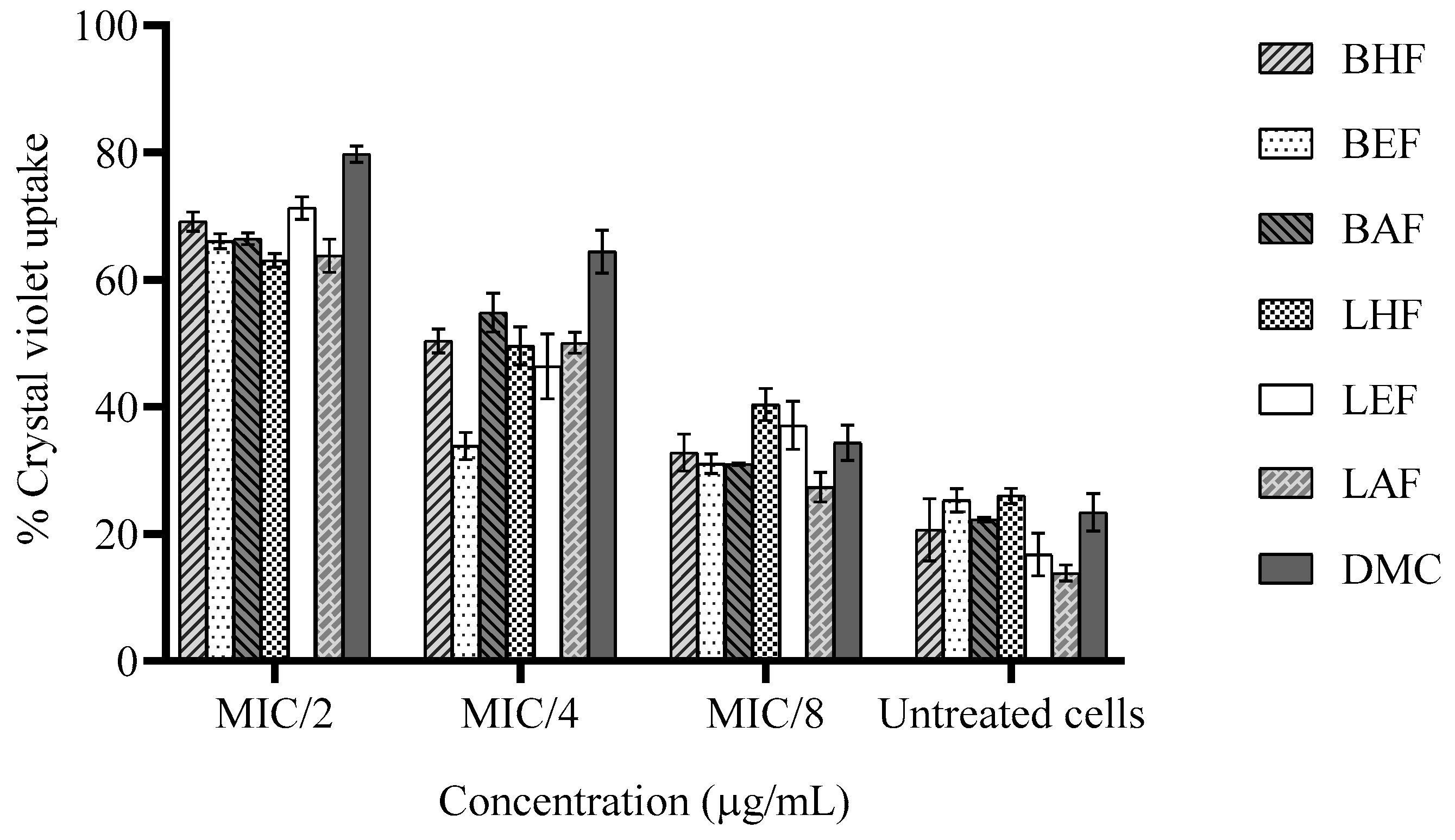
3.7. Effect on Membrane Permeability
3.8. Cytotoxicity Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, C.L.; Lu, Y.H.; Li, X.D.; Wei, D.Z. HPLC analysis of a bioactive chalcone and triterpene in the buds of Cleistocalyx operculatus. S. Afr. J. Bot. 2005; 71, 312–315. [Google Scholar] [CrossRef]
- Ye, C.L.; Liu, X.G.; Huang, Q. Antioxidant activity and protection of human umbilical vein endothelial cells from hydrogen peroxide-induced injury by DMC, a chalcone from buds of Cleistocalyx operculatus. S. Afr. J. Bot. 2013, 86, 36–40. [Google Scholar] [CrossRef]
- Dung, N.T.; Kim, J.M.; Kang, S.C. Chemical composition, antimicrobial and antioxidant activities of the essential oil and the ethanol extract of Cleistocalyx operculatus (Roxb.) Merr and Perry buds. Food Chem. Toxicol. 2018, 46, 3632–3639. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.M.; Schultze, N.; Boger, C.; Alresley, Z.; Bolhuis, A.; Lindequist, U. Anticaries and antimicrobial activities of methanolic extract from leaves of Cleistocalyx operculatus L. Asian Pac. J. Trop. Biomed. 2017, 7(1), 43–48. [Google Scholar] [CrossRef]
- Woo, A.Y.H.; Waye, M.M.Y.; Kwan, H.S.; Chan, M.C.Y.; Chau, C.F.; Cheng, C.H.K. Inhibition of ATPases by Cleistocalyx operculatus. A possible mechanism for the cardiotonic actions of the herb. Vasc. Pharmacol. 2002, 38(3), 163–8. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Y. Inhibitory activities of extracts from Cleistocalyx operculatus flower buds on pancreatic lipase and α-amylase. Eur. Food Res. Technol. 2012, 235(6), 1133–1139. [Google Scholar] [CrossRef]
- Ye, C.L.; Lu, Y.H.; Wei, D.Z. Flavonoids from Cleistocalyx operculatus. Phytochem. 2004, 65(4), 445–447. [Google Scholar] [CrossRef]
- Ngan, L.T.M.; Dung, P.P.; Nhi, N.V.T.Y.; Hoang, N.V.M.; Hieu, T.T. Antibacterial activity of ethanolic extracts of some Vietnamese medicinal plants against Helicobacter pylori. AIP Conf. Proc. 2017, 1878, 020030. [Google Scholar] [CrossRef]
- Thanh, D.T.; Oanh, V.K.; Nguyen, H.C.; Ngan, L.T.M.; Hieu, T.T. Phytochemical composition, antioxidant, antibacterial, and enzyme inhibitory activities of organic extracts from flower buds of Cleistocalyx operculatus (Roxb.) Merr. et Perry. BioTechnologia. 2024; 105, 137–147. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Abalkhail, A.; Anagreyyah, S.; Anajirih, N.; Almuzaini, A.M.; Rawway, M.; Alfadhel, A.; Draz, A.; Abu-Okail, A. Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics. 2023, 12(2), 191. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23(4), 713–739. [Google Scholar] [CrossRef]
- Tsugane, S.; Sasazuki, S. Diet and the risk of gastric cancer: Review of epidemiological evidence. Gastric Cancer. 2007, 10(2), 75–83. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Zagari, R.M.; Bazzoli, F. Epidemiology of Helicobacter pylori Infection. In Helicobacter. Blackwell Publishing Ltd. 2014, 19, 1–5. [Google Scholar] [CrossRef]
- Kusters, J.G.; Van Vliet, A.H.M.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006, 19(3), 449–490. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers. 2023, 9(1), 19. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, H.; Osaki, T.; Kamiya, S. Biofilm formation by Helicobacter pylori and its involvement for antibiotic resistance. BioMed Res. Int. 2015, 2015, 914791. [Google Scholar] [CrossRef]
- Gaddy, J.A.; Radin, J.N.; Cullen, T.W.; Chazin, W.J.; Skaar, E.P.; Trent, M.S.; Algood, H.M.S. Helicobacter pylori resists the antimicrobial activity of calprotectin via lipid a modification and associated biofilm formation. mBio. 2015, 6(6), e01349-15. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.N.T.; Huynh, T.D.H. Selective cytotoxicity of a Vietnamese traditional formula, Nam Dia long, against MCF-7 cells by synergistic effects. BMC Complement. Altern. Med. 2016, 16(1), 220. [Google Scholar] [CrossRef]
- Choommongkol, V.; Punturee, K.; Klumphu, P.; Rattanaburi, P.; Meepowpan, P.; Suttiarporn, P. Microwave-Assisted Extraction of Anticancer Flavonoid, 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethyl Chalcone (DMC), Rich Extract from Syzygium nervosum Fruits. Molecules 2022, 27(4), 1397. [Google Scholar] [CrossRef]
- Temesgen, S.; Sasikumar, J.M.; Egigu, M.C. Effect of Extraction Solvents on Total Polyphenolic Content and Antioxidant Capacity of Syzygium Aromaticum L. Flower Bud from Ethiopia. Biomed Res. Int. 2022, 2022, 4568944. [Google Scholar] [CrossRef]
- Rebaya, A.; Belghith, S.I.; Baghdikian, B.; Leddet, V.M.; Mabrouki, F.; Olivier, E.; Cherif, J.K.; Ayadi, M.T. Total Phenolic, Total Flavonoid, Tannin Content, and Antioxidant Capacity of Halimium halimifolium (Cistaceae). J. Appl. Pharm. Sci. 2015, 5(1), 052–057. [Google Scholar] [CrossRef]
- Ncube, B.; Nair, J.J.; Rárová, L.; Strnad, M.; Finnie, J.F.; Van Staden, J. Seasonal pharmacological properties and alkaloid content in Cyrtanthus contractus N.E. Br. S. Afr. J. Bot. 2015, 97, 69–76. [Google Scholar] [CrossRef]
- Benyong, H.; Ying, C.; Ying, R.; Chaoyin, C. Content determination of total saponins from Opuntia. BTAIJ. 2014, 10(18), 2014. [https://www.tsijournals.com/articles/content-determination-of-total-saponins-from-opuntia.pdf].
- Elouafy, Y.; El Yadini, A.; Mortada, S.; Hnini, M.; Harhar, H.; Khalid, A.; Abdalla, A.; Bouyahya, A.; Goh, K.; Ming, L.; Faouzi, M.; Tabyaoui, M. Antioxidant, antimicrobial, and α-glucosidase inhibitory activities of saponin extracts from walnut (Juglans regia L.) leaves. Asian Pac. J. Trop. Biomed. 2023, 13, 60–69. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M.; Typek, R. Transformation of phenolic acids during radical neutralization. J. Food Sci. Technol. 2024, 61(4), 790–797. [Google Scholar] [CrossRef]
- Ngan, L.T.M.; Tan, M.T.; Hoang, N.V.M.; Thanh, D.T.; Linh, N.T.T.; Hoa, T.T.H.; Nuong, N.T.M.; Hieu, T.T. Antibacterial activity of Hibiscus rosa-sinensis l. red flower against antibiotic-resistant strains of Helicobacter pylori and identification of the flower constituents. Braz. J. Med. Biol. Res. 2021, 54, e10889. [Google Scholar] [CrossRef] [PubMed]
- Ngan, L.T.M.; Moon, J.K.; Kim, J.H.; Shibamoto, T.; Ahn, Y.J. Growth-inhibiting effects of Paeonia lactiflora root steam distillate constituents and structurally related compounds on human intestinal bacteria. World J. Microbiol. Biotechnol. 2012, 28(4), 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Shai, L.J.; Magano, S.R.; Lebelo, S.L.; Mogale, A.M. Inhibitory effects of five medicinal plants on rat alpha-glucosidase: Comparison with their effects on yeast alpha-glucosidase. J. Med. Plant. Res. 2011, 18(1), 2863–2867. [http://www.academicjournals.org/JMPR].
- Ogunyemi, O.M.; Gyebi, G.A.; Saheed, A.; Paul, J.; Nwaneri-Chidozie, V.; Olorundare, O.; Adebayo, J.; Koketsu, M.; Aljarba, N.; Alkahtani, S.; Batiha, G.E.S.; Olaiya, C.O. Inhibition mechanism of alpha-amylase, a diabetes target, by a steroidal pregnane and pregnane glycosides derived from Gongronema latifolium Benth. Front. Mol. Biosci. 2022, 9, 866719. [Google Scholar] [CrossRef] [PubMed]
- Hieu, T.T.; Truong, T.H.H.; Nguyen, T.T.L.; Nguyen, V.M.H.; Nguyen, T.M.N.; Luong, T.M.N. Growth-inhibiting, bactericidal, antibiofilm, and urease inhibitory activities of Hibiscus rosa sinensis L. flower constituents toward antibiotic sensitive- and resistant-strains of Helicobacter pylori. ACS Omega. 2020, 5, 20080–20089. [Google Scholar] [CrossRef]
- Guzman, J.; Téné, N.; Touchard, A.; Castillo, D.; Belkhelfa, H.; Haddioui-Hbabi, L.; Treilhou, M.; Sauvain, M. Anti-Helicobacter pylori properties of the ant-venom peptide bicarinalin. Toxins (Basel). 2018, 10(1), 21. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130(1), 107–115. [Google Scholar] [CrossRef]
- Ngan, L.T.M.; Vi, N.T.; Tham, D.T.M.; Loan, L.T.T.; Ho, P.T.; Hieu, T.T. Antioxidant and anti-Helicobacter pylori activities of Hericium erinaceus mycelium and culture filtrate. Biomed. Res. Ther. 2021, 8(3), 4266–4275. [Google Scholar] [CrossRef]
- Schwarz, S.; Böttner, A.; Goosens, L.; Hafez, H.M.; Hartmann, K.; Kaske, M.; Kehrenberg, C.; Kietzmann, M.; Klarmann, D.; Klein, G.; Krabisch, P.; Luhofer, G.; Richter, A.; Schulz, B.; Sigge, C.; Waldmann, K.H.; Wallmann, J.; Werckenthin, C. A proposal of clinical breakpoints for amoxicillin applicable to porcine respiratory tract pathogens. Vet. Microbiol. 2008, 126(1), 178–188. [Google Scholar] [CrossRef]
- Mai, T.T.; Fumie, N.; Chuyen, N.V. Antioxidant activities and hypolipidemic effects of an aqueous extract from flower buds of Cleistocalyx operculatus (Roxb.) Merr. and Perry. J. Food Biochem. 2009, 33, 790–807. [Google Scholar] [CrossRef]
- Seo, J.; Lee, S.; Elam, M.L.; Johnson, S.A; Kang, J.; Arjmandi, B.H. Study to find the best extraction solvent for use with guava leaves (Psidium guajava L.) for high antioxidant efficacy. Food Sci. Nutr. 2014, 2, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, M.M.J.O.; Bhat, R.; Karim, A.A. Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior Jack.) inflorescence. J. Food Compost. Anal. 2011, 24, 615–619. [Google Scholar] [CrossRef]
- Minh, T.T. L.; Kieu, L.T.B.; Mai, S.T.T.; Ngoc, D.L.B.; Thuy, L.T.B.; Quyen, N.T.; Anh, T.T.; Huy, L. V.; Phong, N.V.; Duyen, C.T.M.; Minh, N.H.; Eric, G. Addition of Mentha arvensis in infusions of Cleistocalyx operculatus improves the Hedonic Score and retains the high antioxidant and anti-lipid-peroxidation effects. Appl. Sci. 2023, 13(5), 2873. [Google Scholar] [CrossRef]
- Dung, N.X.; Van, L.H.; Khoi, T.T.; Leclercq, P.A. GC and GC/MS analysis of the leaf oil of Cleistocalyx operculatus Roxb. Merr. et Perry (Syn. Eugenia operculata Roxb.; Syzygicum mervosum DC.). J. Essent. Oil Res. 1994, 6, 661–662. [Google Scholar] [CrossRef]
- Min, B.S.; Thu, C.V.; Dat, N.T.; Nguyen, C.; Dang, H.; Jang, H.S.; Hung, T.M. Antioxidative Flavonoids from Cleistocalyx operculatus Buds. Chem. Pharm. Bull. 2008, 56(12), 1725–1728. [Google Scholar] [CrossRef]
- Charoensin, S.; Taya, S.; Wongpornchai, S.; Wongpoomchai, R. Assessment of genotoxicity and antigenotoxicity of an aqueous extract of Cleistocalyx nervosum var. paniala in in vitro and in vivo models. Interdiscip. Toxicol. 2012, 5(4), 201–206. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Spósito, L.; Oda, F.B.; Vieira, J.H.; Carvalho, F.A.; dos Santos Ramos, M.A.; de Castro, R.C.; Crevelin, E.J.; Crotti, A.E.M.; Santos, A.G.; da Silva, P.B.; Chorilli, M.; Bauab, T.M. In vitro and in vivo anti-Helicobacter pylori activity of Casearia sylvestris leaf derivatives. J. Ethnopharmacol 2019, 233, 1–12. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Dung, N.T.; Suh, H.J.; Kang, S.C. Antibacterial activity of essential oil and extracts of Cleistocalyx operculatus buds against the bacteria of Xanthomonas spp. J. Am. Oil Chem. Soc. 2010, 87, 1341–1349. [Google Scholar] [CrossRef]
- Thuy, B.T.P.; Hieu, L.T.; My, T.T.A.; Hai, N.T.T.; Loan, H.T.P.; Thuy, N.T.T.; Triet, N.T.; Van Anh, T.T.; Dieu, N.T.X.; Quy, P.T.; Van Trung, N.; Quang, D.T.; Huynh, L.K.; Nhung, N.T.A. Screening for Streptococcus pyogenes antibacterial and Candida albicans antifungal bioactivities of organic compounds in natural essential oils of Piper betle L., Cleistocalyx operculatus L. and Ageratum conyzoides L. Chem. Pap. 2021, 75, 1507–1519. [Google Scholar] [CrossRef]
- Utama, K.; Khamto, N.; Meepowpan, P.; Aobchey, P.; Kantapan, J.; Sringarm, K.; Roytrakul, S.; Sangthong, P. Effects of 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone from Syzygium nervosum Seeds on Antiproliferative, DNA Damage, Cell Cycle Arrest, and Apoptosis in Human Cervical Cancer Cell Lines. Molecules. 2022, 27(4), 1154. [Google Scholar] [CrossRef]
- Nostro, A.; Cellini, L.; Di Bartolomeo, S.; Di Campli, E.; Grande, R.; Cannatelli, M.A.; Marzio, L.; Alonzo, V. Antibacterial effect of plant extracts against Helicobacter pylori. Phytother. Res. 2005, 19(3), 198–202. [Google Scholar] [CrossRef] [PubMed]
- Sufian, A.S.; Ramasamy, K.; Ahmat, N.; Zakaria, Z.A.; Yusof, M.I.M. Isolation and identification of antibacterial and cytotoxic compounds from the leaves of Muntingia calabura L. J. Ethnopharmacol. 2013, 146(1), 198–204. [Google Scholar] [CrossRef]
- Amin, M.; Anwar, F.; Naz, F.; Mehmood, T.; Saari, N. Anti-Helicobacter pylori and urease inhibition activities of some traditional medicinal plants. Molecules. 2013, 18(2), 2135–2149. [Google Scholar] [CrossRef]
- Egas, V.; Salazar-Cervantes, G.; Romero, I.; Méndez-Cuesta, C.A.; Rodríguez-Chávez, J.L.; Delgado, G. Anti-Helicobacter pylori metabolites from Heterotheca inuloides (Mexican arnica). Fitoterapia. 2018, 127, 314–321. [Google Scholar] [CrossRef]
- Chukiatsiri, S.; Wongsrangsap, N.; Ratanabunyong, S.; Choowongkomon, K. In vitro Evaluation of Antidiabetic Potential of Cleistocalyx nervosum var. paniala Fruit Extract. Plants 2023, 12(1), 112. [Google Scholar] [CrossRef]
- Wink, M. Importance of plant secondary metabolites for protection against insects and microbial infections. In Advances in Phytomedicine; Rai, M., M. C. Carpinella, M.C., Eds.; Publisher: Elsevier, Amsterdam, Netherlands, 2006; Volume 3. [Google Scholar] [CrossRef]
- Tran, T.H.; Luong, T.M.N.; Bui, V.L.; Tran, T.H. Effects of plant essential oils and their constituents on Helicobacter pylori: A Review. Plant Sci. Today. 2023, 10(2), 334–344. [Google Scholar] [CrossRef]
- Singh, A.; Amod, A.; Pandey, P.; Bose, P.; Pingali, S.; Shivalkar, S.; Varadwaj, P.; Sahoo, A.; Samanta, S. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed. Mater. 2022, 17, 022003. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.M.I.I.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules. 2021, 26(23), 7177. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2014, 22(1), 132–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Wang, C.M.; Su, C.H.; Ho, H.C.; Chang, C.H.; Chou, C.H.; Hsu, Y.M. Eudesmin attenuates Helicobacter pylori-induced epithelial autophagy and apoptosis and leads to eradication of H. pylori infection. Exp. Ther. Med. 2018, 15(3), 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Lee, J.H.; Kim, K.T.; Park, Y.S.; Nah, S.Y.; Ahn, D.U.; Paik, H.D. Antimicrobial effect of 7-O-butylnaringenin, a novel flavonoid, and various natural flavonoids against Helicobacter pylori strains. Int. J. Environ. Res. Public Health. 2013, 10(11), 5459–5469. [Google Scholar] [CrossRef]
- Sharaf, M.; Arif, M.; Hamouda, H.I.; Khan, S.; Abdalla, M.; Shabana, S.; Rozan, H.E.; Khan, T.U.; Chi, Z.; Liu, C. Preparation, urease inhibition mechanisms, and anti-Helicobacter pylori activities of hesperetin-7-rhamnoglucoside. Curr. Res. Microb. Sci. 2022, 3, 100103. [Google Scholar] [CrossRef]
- Ergüden, B.; Ünver, Y. Phenolic chalcones lead to ion leakage from Gram-positive bacteria prior to cell death. Arch. Microbiol. 2021, 204(1), 3. [Google Scholar] [CrossRef]
| Fractions | TPC | TFC | TSC | TTC | TAC |
|---|---|---|---|---|---|
| BHF | 426.77c ± 1.22 | 134.77a ± 7.75 | 153.33a ± 4.69 | 42.97a ± 2.93 | 1.66d ± 0.09 |
| BEF | 280.46d ± 11.06 | 85.88b ± 2.52 | 158.10a ± 5.97 | 22.97c ± 1.73 | 1.50d ± 0.17 |
| BAF | 768.18a ± 12.20 | 11.04e ± 0.53 | 81.59b ± 1.20 | 13.53d ± 2.93 | 3.04c ± 0.20 |
| LHF | 201.63f ± 3.45 | 76.54bc ± 1.72 | 88.25b ± 9.46 | 33.17b ± 2.10 | 4.81b ± 0.09 |
| LEF | 238.47e ± 4.18 | 71.72c ± 1.74 | 75.24b ± 5.95 | 10.39d ± 1.29 | 4.73b ± 0.05 |
| LAF | 490.74b ± 7.29 | 25.56d ± 0.21 | 25.56c ± 2.44 | 4.36e ± 0.96 | 5.41a ± 0.08 |
| Samples | FRAP (mg TE/g extract) |
DPPH IC50 (µg/ml) |
ABTS IC50 (µg/ml) |
|---|---|---|---|
| BHF | 69.01e ± 1.833 | 33.99c ± 0.76 | 1.70b ± 0.09 |
| BEF | 91.95c ± 1.302 | 58.46a ± 1.370 | 1.48b ± 0.162 |
| BAF | 201.80b ± 4.502 | 24.69d ± 0.194 | 1.08c ± 0.013 |
| LHF | 55.57f ± 1.265 | 39.96b ± 0.237 | 1.06c ± 0.084 |
| LEF | 78.56d ± 1.13 | 16.05e ± 0.031 | 0.98c ± 0.007 |
| LAF | 301.82a ± 2.306 | 11.24f ±1.524 | 0.55d ± 0.004 |
| Ascorbic acid | ND | 3.34g ± 0.017 | ND |
| Trolox | ND | ND | 2.63a ± 0.05 |
| Samples | MIC (µg/ml) | |
|---|---|---|
| H. pylori ATCC 51932 | H. pylori ATCC 43504 | |
| BHF | 125 | 125 |
| BEF | 250 | 500 |
| BAF | 500 | 500 |
| LHF | 125 | 125 |
| LEF | 250 | 500 |
| LAF | 500 | 1000 |
| DMC | 25 | 50 |
| Amoxicillin | 0.01 | 0.01 |
| Samples | IC50 (µg/ml) | ||
|---|---|---|---|
| H. pylori-Urease | α-Glucosidase | α-Amylase | |
| BHF | 2.3e ± 0.13 | 1.5c ± 0.09 | 398.5d ± 5.3 |
| BEF | 4.9c ± 0.40 | 0.9c ± 0.01 | 497.2b ± 17.1 |
| BAF | 2.5de ± 0.21 | 0.8c ± 0.01 | 444.3c ±14.3 |
| LHF | 3.2de ± 0.26 | 2.6c ± 0.16 | 191.3f ± 9.5 |
| LEF | 3.6d ± 0.01 | 1.2c ± 0.06 | 292.6e ± 16.6 |
| LAF | 6.8b ± 0.07 | 0.6c ± 0.02 | 1281.7a ±23.7 |
| DMC | 3.2de ± 0.03 | 94.6a ± 2.57 | 83.80g ± 0.08 |
| Thiourea | 44.3a ± 1.12 | ND | ND |
| Acarbose | ND | 25.6b ± 0.70 | 75.0g ± 3.86 |
| Samples | CC50 (µg/ml) | |||
|---|---|---|---|---|
| MCF-7 | Jurkat | HeLa | Fibroblast | |
| BHF | 30.79d ± 0.83 | 18.51d ± 1.37 | 31.70c ± 5.74 | >100 |
| BEF | 57.74c ± 1.13 | 51.06c ± 1.42 | 92.53a ± 4.90 | >100 |
| BAF | > 100 | >100 | >100 | >100 |
| LHF | 89.00a ± 1.33 | 56.78c ± 2.62 | 86.45ab ± 4.06 | >100 |
| LEF | 85.43a ± 2.76 | 66.73b ± 1.02 | 91.72a ± 1.43 | >100 |
| LAF | >100 | 97.25a ± 1.28 | 77.07b ± 5.06 | >100 |
| DMC | 71.41b ± 2.14 | 73.82b ± 7.23 | >100 | >100 |
| Camptothecin | 0.007e ± 0.002 | 0.005e ± 0.001 | 0.89d ± 0.088 | 1.57 ± 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





