Submitted:
19 August 2024
Posted:
20 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Viral Stock
2.2. Aedes aegypti Colony
2.3. Aedes aegypti Artificial Blood-Feeding
2.4. Sample Collection
2.5. RNA Isolation and qRT-PCR
2.6. Data Analyzes
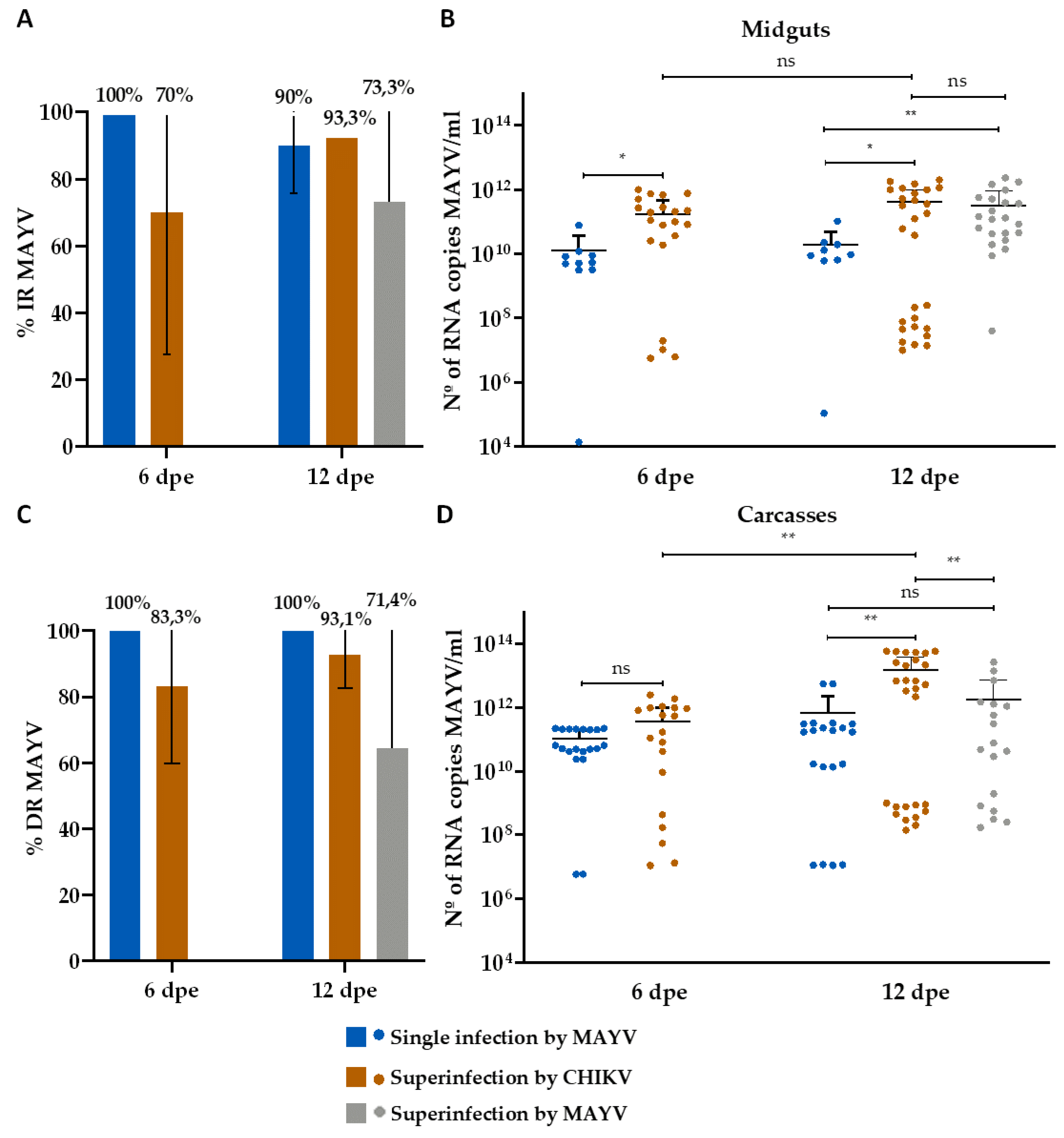
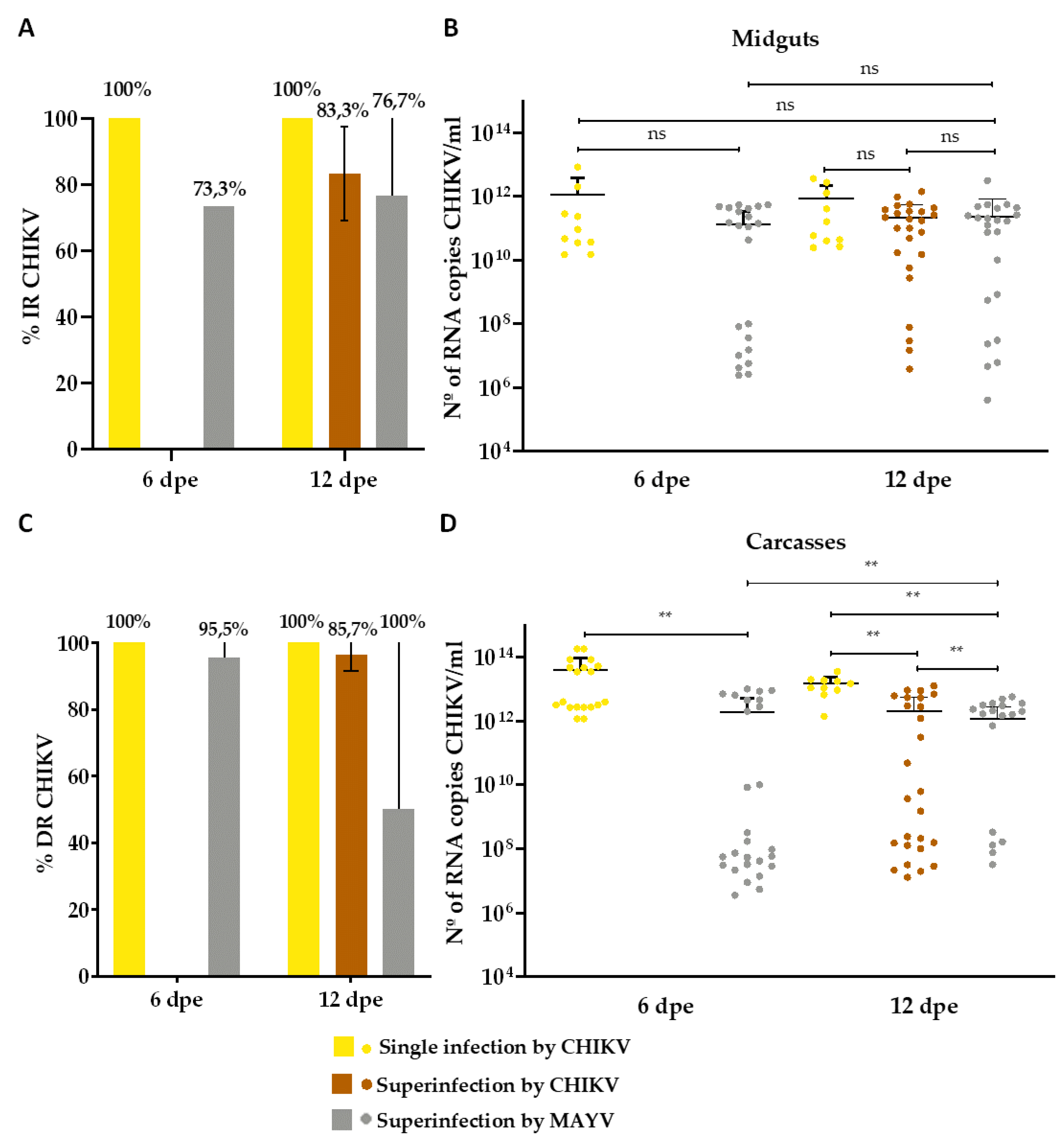
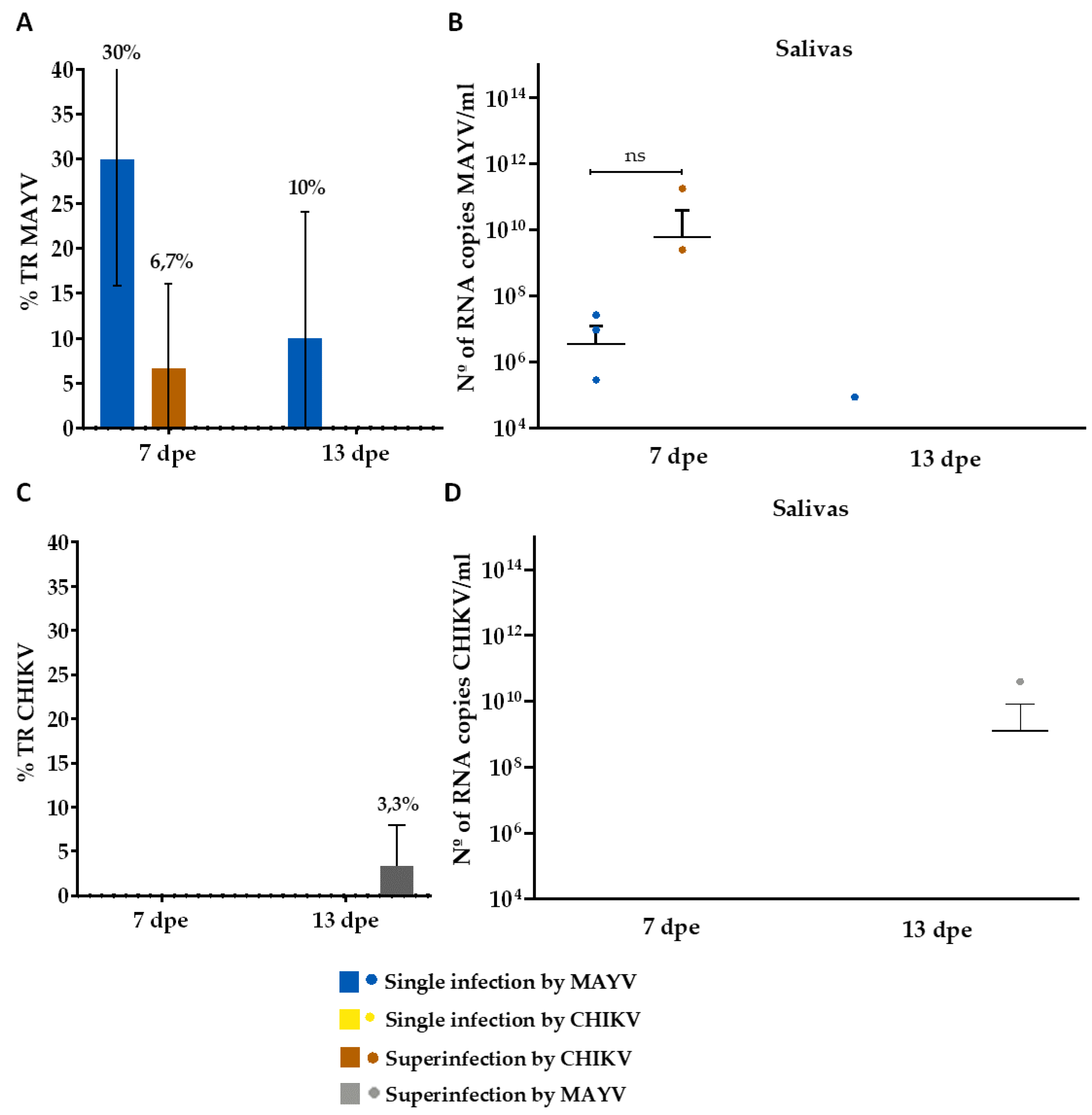
3. Results
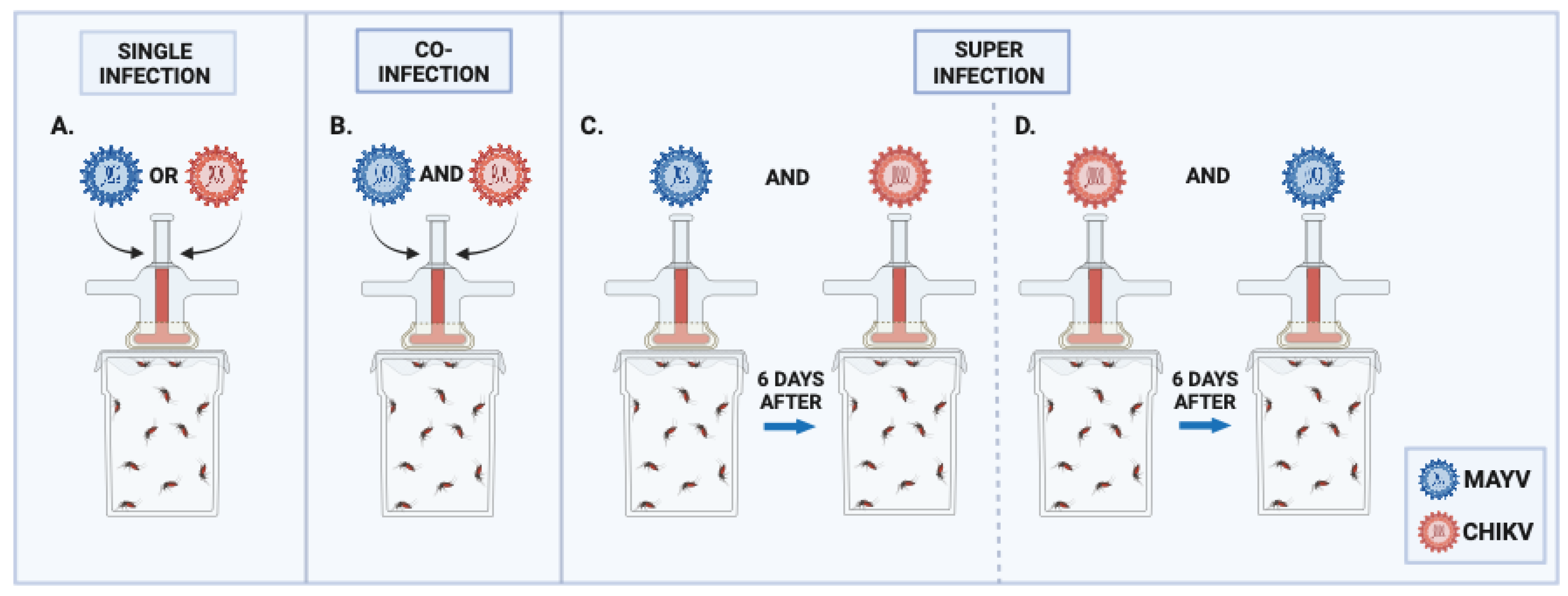
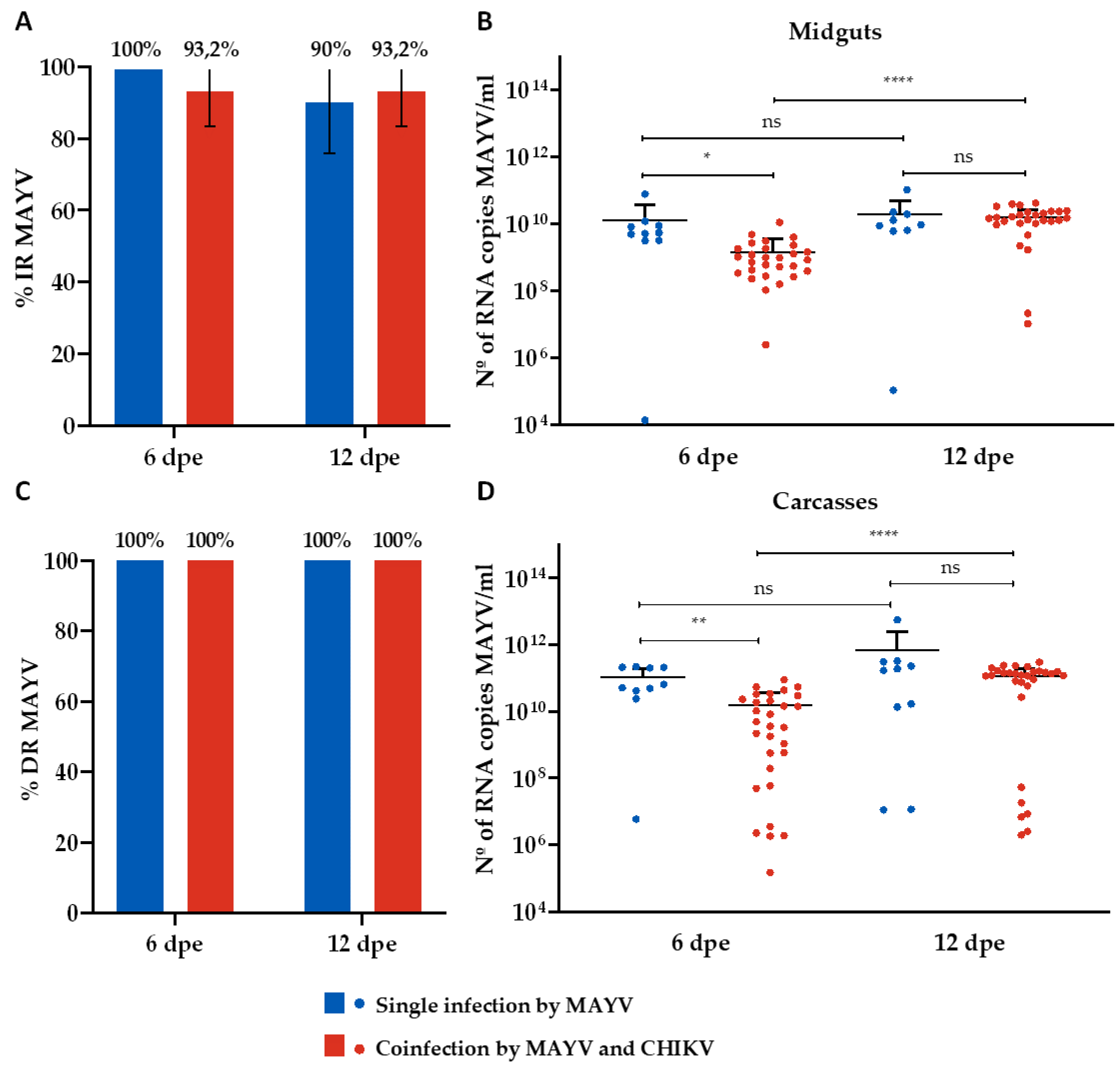
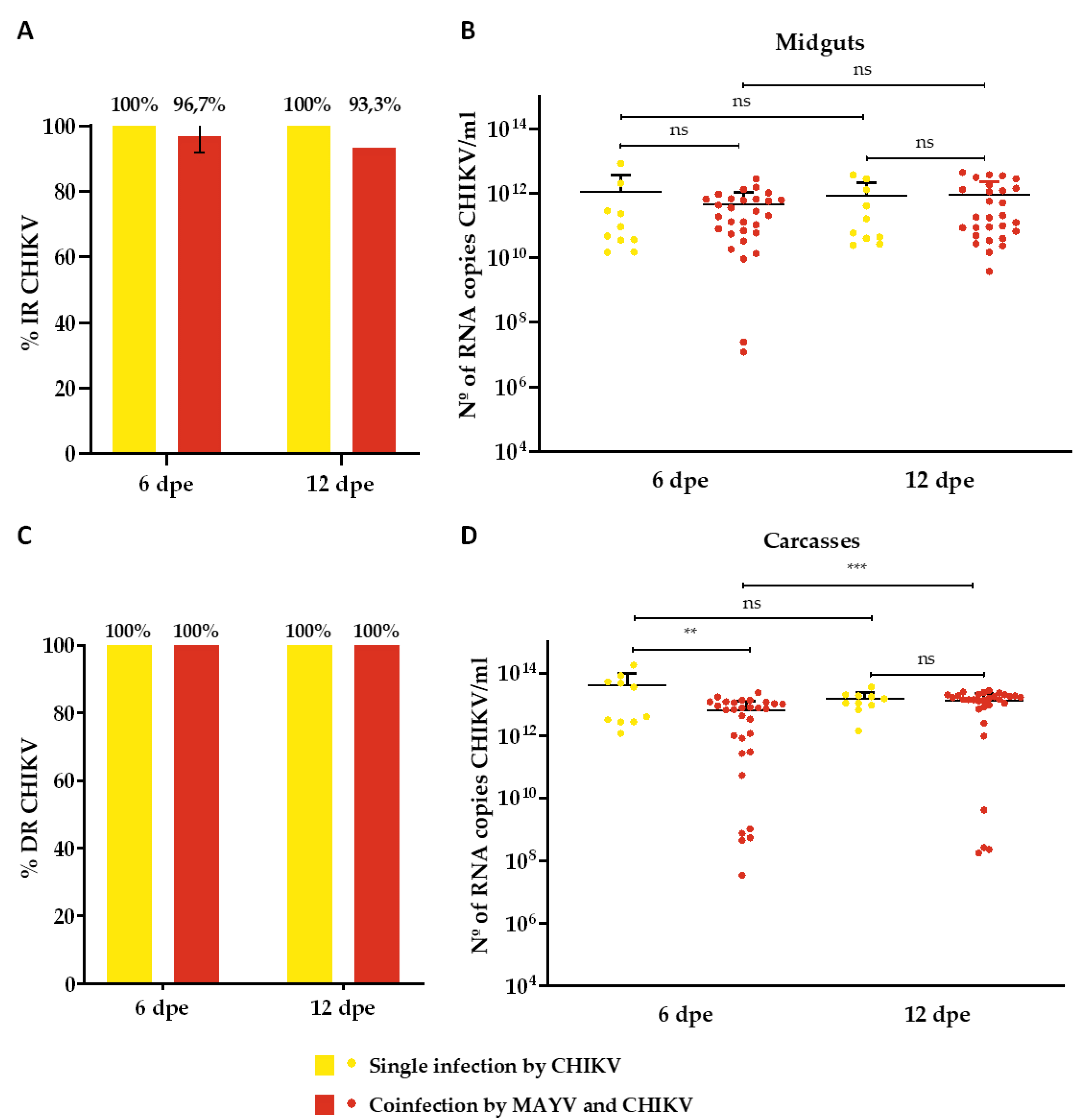
3.1. Single Infection and Coinfection Assays
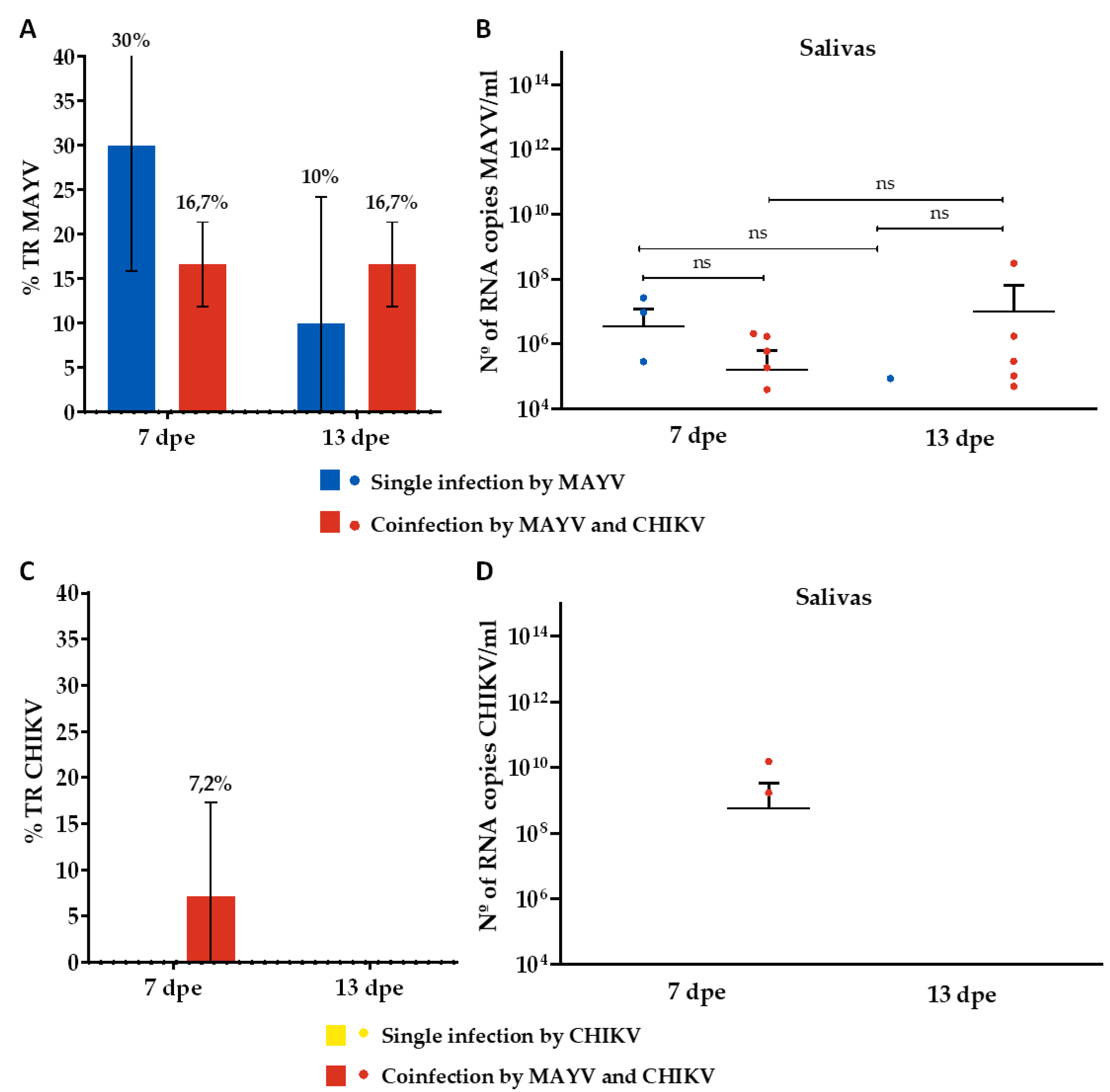
3.2. Superinfection Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, S.C.; Reisen, W.K. Present and Future Arboviral Threats. Antiviral Res. 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Rückert, C.; Cavany, S.M.; Perkins, T.A.; Ebel, G.D.; Grubaugh, N.D. Arbovirus Coinfection and Co-Transmission: A Neglected Public Health Concern? PLOS Biol. 2019, 17, e3000130. [Google Scholar] [CrossRef]
- Almeida, L.S.; Cota, A.L.S.; Rodrigues, D.F. Sanitation, Arboviruses, and Environmental Determinants of Disease: impacts on urban health. Cienc. Saude Coletiva 2020, 25, 3857–3868. [Google Scholar] [CrossRef]
- Santos, L.L.M.; de Aquino, E.C.; Fernandes, S.M.; Ternes, Y.M.F.; Feres, V.C. de R. Dengue, Chikungunya, and Zika Virus Infections in Latin America and the Caribbean: A Systematic Review. Rev. Panam. Salud Pública 2023, 47, e34. [Google Scholar] [CrossRef]
- Whiteman, A.; Loaiza, J.R.; Yee, D.A.; Poh, K.C.; Watkins, A.S.; Lucas, K.J.; Rapp, T.J.; Kline, L.; Ahmed, A.; Chen, S.; et al. Do Socioeconomic Factors Drive Aedes Mosquito Vectors and Their Arboviral Diseases? A Systematic Review of Dengue, Chikungunya, Yellow Fever, and Zika Virus. One Health Amst. Neth. 2020, 11, 100188. [Google Scholar] [CrossRef]
- Albuquerque, M. de F.; Souza, W.; Araújo, T.; Braga, C.; Filho, D.; Ximenes, R.; Filho, D.; Brito, C.; Valongueiro, S.; Melo, A.; et al. Epidemia de Microcefalia e Vírus Zika: A Construção Do Conhecimento Em Epidemiologia. Cad. Saúde Pública 2018, 34. [Google Scholar] [CrossRef] [PubMed]
- Souza, W.V. de; Albuquerque, M. de F.P.M. de; Vazquez, E.; Bezerra, L.C.A.; Mendes, A. da C.G.; Lyra, T.M.; Araujo, T.V.B. de; Oliveira, A.L.S. de; Braga, M.C.; Ximenes, R.A. de A.; et al. Microcephaly Epidemic Related to the Zika Virus and Living Conditions in Recife, Northeast Brazil. BMC Public Health 2018, 18, 130. [Google Scholar] [CrossRef]
- Weaver, S.C. Prediction and Prevention of Urban Arbovirus Epidemics: A Challenge for the Global Virology Community. Antiviral Res. 2018, 156, 80–84. [Google Scholar] [CrossRef]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rodríguez, Y.; Pacheco, Y.; Anaya, J.-M.; Ramírez-Santana, C. Mayaro: An Emerging Viral Threat? Emerg. Microbes Infect. 2018, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Luis, M.A.; del Valle-Mendoza, J.; Sandoval, I.; Silva-Caso, W.; Mazulis, F.; Carrillo-Ng, H.; Tarazona-Castro, Y.; Martins-Luna, J.; Aquino-Ortega, R.; Peña-Tuesta, I.; et al. A Silent Public Health Threat: Emergence of Mayaro Virus and Co-Infection with Dengue in Peru. BMC Res. Notes 2021, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, E.-Y.; Charniga, K.; Rueda, A.; Dorigatti, I.; Mendez, Y.; Hamlet, A.; Carrera, J.-P.; Cucunubá, Z.M. The Epidemiology of Mayaro Virus in the Americas: A Systematic Review and Key Parameter Estimates for Outbreak Modelling. PLoS Negl. Trop. Dis. 2021, 15, e0009418. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Pereira, R.S.; da Cruz Nizer, W.S.; Brito, J.C.M.; Godói, I.P.; Cardoso, V.N.; Fernandes, S.O.A.; Ferreira, J.M.S. Rate of Exposure to Mayaro Virus (MAYV) in Brazil between 1955 and 2018: A Systematic Review and Meta-Analysis. Arch. Virol. 2021, 166, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Parida, M.; Dash, P.K. Impact of Transmission Cycles and Vector Competence on Global Expansion and Emergence of Arboviruses. Rev. Med. Virol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kain, M.P.; Skinner, E.B.; Athni, T.S.; Ramirez, A.L.; Mordecai, E.A.; van den Hurk, A.F. Not All Mosquitoes Are Created Equal: Incriminating Mosquitoes as Vectors of Arboviruses; Infectious Diseases, 2022.
- Krokovsky, L.; Lins, C.R.B.; Guedes, D.R.D.; Wallau, G. da L.; Ayres, C.F.J.; Paiva, M.H.S. Dynamic of Mayaro Virus Transmission in Aedes Aegypti, Culex Quinquefasciatus Mosquitoes, and a Mice Model. Viruses 2023, 15, 799. [Google Scholar] [CrossRef]
- Serra, O.P.; Cardoso, B.F.; Ribeiro, A.L.M.; Santos, F.A.L. dos; Slhessarenko, R.D. Mayaro Virus and Dengue Virus 1 and 4 Natural Infection in Culicids from Cuiabá, State of Mato Grosso, Brazil. Mem Inst Oswaldo Cruz 2016, 20–29. [Google Scholar] [CrossRef]
- de Curcio, J.S.; Salem-Izacc, S.M.; Pereira Neto, L.M.; Nunes, E.B.; Anunciação, C.E.; Silveira-Lacerda, E. de P. Detection of Mayaro Virus in Aedes Aegypti Mosquitoes Circulating in Goiânia-Goiás-Brazil. Microbes Infect. 2022, 24, 104948. [Google Scholar] [CrossRef]
- Esposito, D.L.A.; Fonseca, B.A.L. da. Will Mayaro Virus Be Responsible for the next Outbreak of an Arthropod-Borne Virus in Brazil? Braz. J. Infect. Dis. 2017, 21, 540–544. [Google Scholar] [CrossRef]
- Miralles, R.; Ferrer, R.; Solé, R.V.; Moya, A.; Elena, S.F. Multiple Infection Dynamics Has Pronounced Effects on the Fitness of RNA Viruses. J. Evol. Biol. 2001, 14, 654–662. [Google Scholar] [CrossRef]
- Muturi, E.J.; Bara, J. Sindbis Virus Interferes with Dengue 4 Virus Replication and Its Potential Transmission by Aedes Albopictus. Parasit. Vectors 2015, 8, 65. [Google Scholar] [CrossRef]
- Brustolin, M.; Pujhari, S.; Terradas, G.; Werling, K.; Asad, S.; Metz, H.C.; Henderson, C.A.; Kim, D.; Rasgon, J.L. In Vitro and In Vivo Coinfection and Superinfection Dynamics of Mayaro and Zika Viruses in Mosquito and Vertebrate Backgrounds. J. Virol. 2023, 0, e01778–22. [Google Scholar] [CrossRef]
- Kantor, A.M.; Lin, J.; Wang, A.; Thompson, D.C.; Franz, A.W.E. Infection Pattern of Mayaro Virus in Aedes Aegypti (Diptera: Culicidae) and Transmission Potential of the Virus in Mixed Infections With Chikungunya Virus. J. Med. Entomol. 2019, 56, 832–843. [Google Scholar] [CrossRef]
- Leroy, E.M.; Nkoghe, D.; Ollomo, B.; Nze-Nkogue, C.; Becquart, P.; Grard, G.; Pourrut, X.; Charrel, R.; Moureau, G.; Ndjoyi-Mbiguino, A.; et al. Concurrent Chikungunya and Dengue Virus Infections during Simultaneous Outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009, 15, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Blitvich, B.J.; Firth, A.E. Insect-Specific Flaviviruses: A Systematic Review of Their Discovery, Host Range, Mode of Transmission, Superinfection Exclusion Potential and Genomic Organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.E.; Wan, K.; Bose, H.R. Homologous Interference Induced by Sindbis Virus. J. Virol. 1974, 14, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Abrao, E.P.; Lopes da Fonseca, B.A. Infection of Mosquito Cells (C6/36) by Dengue-2 Virus Interferes with Subsequent Infection by Yellow Fever Virus. Vector-Borne Zoonotic Diseases. 2016, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Melo-Santos, M. a. V.; Varjal-Melo, J.J.M.; Araújo, A.P.; Gomes, T.C.S.; Paiva, M.H.S.; Regis, L.N.; Furtado, A.F.; Magalhaes, T.; Macoris, M.L.G.; Andrighetti, M.T.M.; et al. Resistance to the Organophosphate Temephos: Mechanisms, Evolution and Reversion in an Aedes Aegypti Laboratory Strain from Brazil. Acta Trop. 2010, 113, 180–189. [Google Scholar] [CrossRef]
- Guedes, D.R.; Paiva, M.H.; Donato, M.M.; Barbosa, P.P.; Krokovsky, L.; Rocha, S.W. dos S.; Saraiva, K.L.; Crespo, M.M.; Rezende, T.M.; Wallau, G.L.; et al. Zika Virus Replication in the Mosquito Culex Quinquefasciatus in Brazil. Emerg. Microbes Infect. 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Gloria-Soria, A.; Payne, A.F.; Bialosuknia, S.M.; Stout, J.; Mathias, N.; Eastwood, G.; Ciota, A.T.; Kramer, L.D.; Armstrong, P.M. Vector Competence of Aedes Albopictus Populations from the Northeastern United States for Chikungunya, Dengue, and Zika Viruses. Am. J. Trop. Med. Hyg. 2021, 104, 1123–1130. [Google Scholar] [CrossRef]
- Barbosa, P.P.; Guedes, D.R.D.; Melo-Santos, M. a. V.; Cordeiro, M.T.; Acioli, R.V.; Batista, C. a. V.; Gonçalves, L.S.M.; Souza, M.F.M.; Araújo, Y.V.; Magalhães, F.J.R.; et al. Vector Surveillance for Dengue Virus Detection in the Archipelago of Fernando de Noronha, Brazil. J. Med. Entomol. 2016, 53, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.; Kosoy, O.; Laven, J.; Panella, A.J.; Velez, J.; Lambert, A.; Campbell, G. Chikungunya Virus in US Travelers Returning from India, 2006. Emerg. Infect. Dis. 2007. [Google Scholar] [CrossRef]
- Naveca, F.G.; do Nascimento, V.A.; de Souza, V.C.; Nunes, B.T.D.; Rodrigues, D.S.G.; Vasconcelos, P.F. da C. Multiplexed Reverse Transcription Real-Time Polymerase Chain Reaction for Simultaneous Detection of Mayaro, Oropouche, and Oropouche-like Viruses. Mem. Inst. Oswaldo Cruz 2017, 112, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Krokovsky, L.; Paiva, M.H.S.; Guedes, D.R.D.; Barbosa, R.M.R.; de Oliveira, A.L.S.; Anastácio, D.B.; Pontes, C.R.; Ayres, C.F.J. Arbovirus Surveillance in Field-Collected Mosquitoes From Pernambuco-Brazil, During the Triple Dengue, Zika and Chikungunya Outbreak of 2015-2017. Front. Trop. Dis. 2022, 3. [Google Scholar] [CrossRef]
- Ciota, A.T. The Role of Co-Infection and Swarm Dynamics in Arbovirus Transmission. Virus Res. 2019, 265, 88–93. [Google Scholar] [CrossRef]
- Fujita, D.M.; Salvador, F.S.; da Silva Nali, L.H.; de Andrade Júnior, H.F. Oropouche in Brazil in 2024. J. Travel Med. 2024, taae075. [Google Scholar] [CrossRef]
- Nuckols, J.T.; Huang, Y.-J.S.; Higgs, S.; Miller, A.L.; Pyles, R.B.; Spratt, H.M.; Horne, K.M.; Vanlandingham, D.L. Evaluation of Simultaneous Transmission of Chikungunya Virus and Dengue Virus Type 2 in Infected Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 447–451. [Google Scholar] [CrossRef]
- Rückert, C.; Weger-Lucarelli, J.; Garcia-Luna, S.M.; Young, M.C.; Byas, A.D.; Murrieta, R.A.; Fauver, J.R.; Ebel, G.D. Impact of Simultaneous Exposure to Arboviruses on Infection and Transmission by Aedes Aegypti Mosquitoes. Nat. Commun. 2017, 8, 15412. [Google Scholar] [CrossRef] [PubMed]
- Göertz, G.P.; Vogels, C.B.F.; Geertsema, C.; Koenraadt, C.J.M.; Pijlman, G.P. Mosquito Co-Infection with Zika and Chikungunya Virus Allows Simultaneous Transmission without Affecting Vector Competence of Aedes Aegypti. PLoS Negl. Trop. Dis. 2017, 11, e0005654. [Google Scholar] [CrossRef]
- Viglietta, M.; Bellone, R.; Blisnick, A.A.; Failloux, A.-B. Vector Specificity of Arbovirus Transmission. Front. Microbiol. 2021, 12, 773211. [Google Scholar] [CrossRef]
- Nasar, F.; Erasmus, J.H.; Haddow, A.D.; Tesh, R.B.; Weaver, S.C. Eilat Virus Induces Both Homologous and Heterologous Interference. Virology 2015, 484, 51–58. [Google Scholar] [CrossRef]
- Kuwata, R.; Isawa, H.; Hoshino, K.; Sasaki, T.; Kobayashi, M.; Maeda, K.; Sawabe, K. Analysis of Mosquito-Borne Flavivirus Superinfection in Culex Tritaeniorhynchus (Diptera: Culicidae) Cells Persistently Infected with Culex Flavivirus (Flaviviridae). J. Med. Entomol. 2015, 52, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Kent, R.J.; Crabtree, M.B.; Miller, B.R. Transmission of West Nile Virus by Culex Quinquefasciatus Say Infected with Culex Flavivirus Izabal. PLoS Negl. Trop. Dis. 2010, 4, e671. [Google Scholar] [CrossRef]
- Goenaga, S.; Kenney, J.L.; Duggal, N.K.; Delorey, M.; Ebel, G.D.; Zhang, B.; Levis, S.C.; Enria, D.A.; Brault, A.C. Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes. Viruses 2015, 7, 5801–5812. [Google Scholar] [CrossRef]
- Long, K.; Ziegler, S.; Thangamani, S.; Hausser, N.; Kochel, T.; Higgs, S.; Tesh, R. Experimental Transmission of Mayaro Virus by Aedes Aegypti. Am. J. Trop. Med. Hyg. 2011, 85, 750–757. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, N.R.; Costa, A.R.M.; Feitosa, C.A.; Loth, T.P.; Klingelfus, A. A EVOLUÇÃO DE CASOS DE ARBOVIROSES DENGUE, CHIKUNGUNYA E ZIKA VÍRUS NO BRASIL ENTRE 2018 E 2020. Braz. J. Infect. Dis. 2022, 26, 101956. [Google Scholar] [CrossRef]
- Weaver, S.C.; Forrester, N.L.; Liu, J.; Vasilakis, N. Population Bottlenecks and Founder Effects: Implications for Mosquito-Borne Arboviral Emergence. Nat. Rev. Microbiol. 2021, 19, 184–195. [Google Scholar] [CrossRef]
- Cunha, M.S.; Costa, P.A.G.; Correa, I.A.; de Souza, M.R.M.; Calil, P.T.; da Silva, G.P.D.; Costa, S.M.; Fonseca, V.W.P.; da Costa, L.J. Chikungunya Virus: An Emergent Arbovirus to the South American Continent and a Continuous Threat to the World. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Causey, O.R.; Maroja, O.M. Mayaro Virus: A New Human Disease Agent. III. Investigation of an Epidemic of Acute Febrile Illness on the River Guama in Pará, Brazil, and Isolation of Mayaro Virus as Causative Agent. Am. J. Trop. Med. Hyg. 1957, 6, 1017–1023. [Google Scholar] [PubMed]
- Gloria-Soria, A.; Brackney, D.E.; Armstrong, P.M. Saliva Collection via Capillary Method May Underestimate Arboviral Transmission by Mosquitoes. Parasit. Vectors 2022, 15, 103. [Google Scholar] [CrossRef]
- Franz, A.W.E.; Sanchez-Vargas, I.; Adelman, Z.N.; Blair, C.D.; Beaty, B.J.; James, A.A.; Olson, K.E. Engineering RNA Interference-Based Resistance to Dengue Virus Type 2 in Genetically Modified Aedes Aegypti. Proc. Natl. Acad. Sci. 2006, 103, 4198–4203. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Dong, S.; Dizaji, N.B.; Rutkowski, N.; Pohlenz, T.; Myles, K.; Dimopoulos, G. The Aedes Aegypti siRNA Pathway Mediates Broad-Spectrum Defense against Human Pathogenic Viruses and Modulates Antibacterial and Antifungal Defenses. PLOS Biol. 2022, 20, e3001668. [Google Scholar] [CrossRef]
- Wiggins, K.; Eastmond, B.; Alto, B.W. Transmission Potential of Mayaro Virus in Florida Aedes Aegypti and Aedes Albopictus Mosquitoes. Med. Vet. Entomol. 2018, 32, 436–442. [Google Scholar] [CrossRef]
| Group | Virus | 1st blood-meal | 2nd blood-meal |
|---|---|---|---|
| Single infection- MAYV | MAYV | 5,5 x 107 | -* |
| Single infection- CHIKV | CHIKV | 3,1 x 107 | -* |
| Coinfection MAYV + CHIKV | MAYV | 5,5 x 107 | -* |
| CHIKV | 3,1 x 107 | -* | |
| Superinfection by MAYV | CHIKV | 1,4 x 107 | - |
| MAYV | - | 3,1 x 107 | |
| Superinfection by CHIKV | MAYV | 3,5 x 107 | - |
| CHIKV | - | 8,5 x 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





