Submitted:
13 August 2024
Posted:
15 August 2024
You are already at the latest version
Abstract
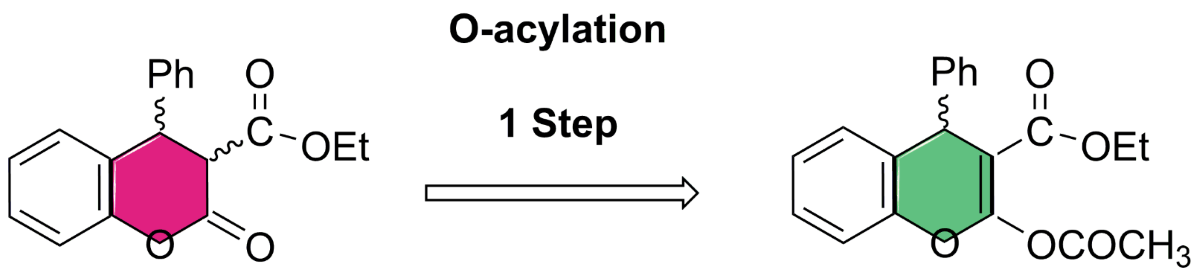
Keywords:
1. Introduction
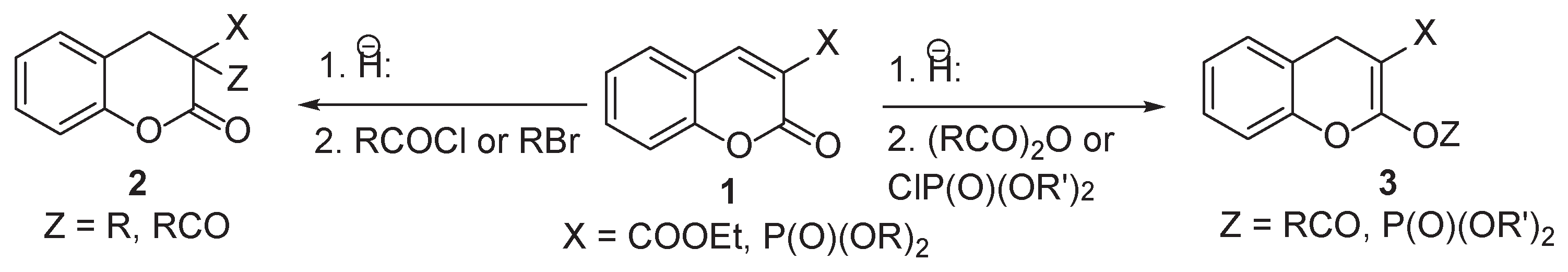
2. Results
3. Discussion
4. Materials and Methods
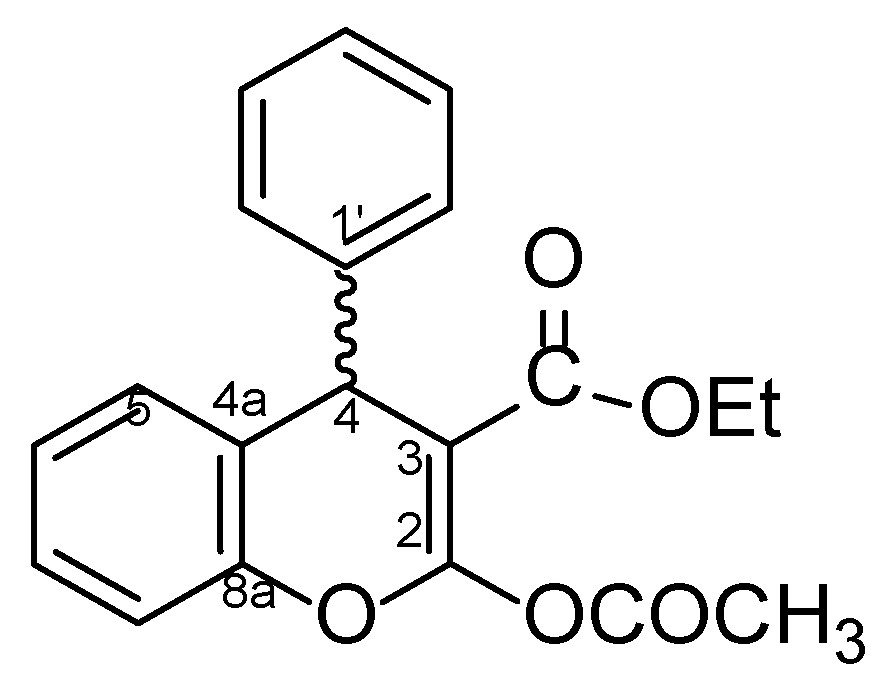
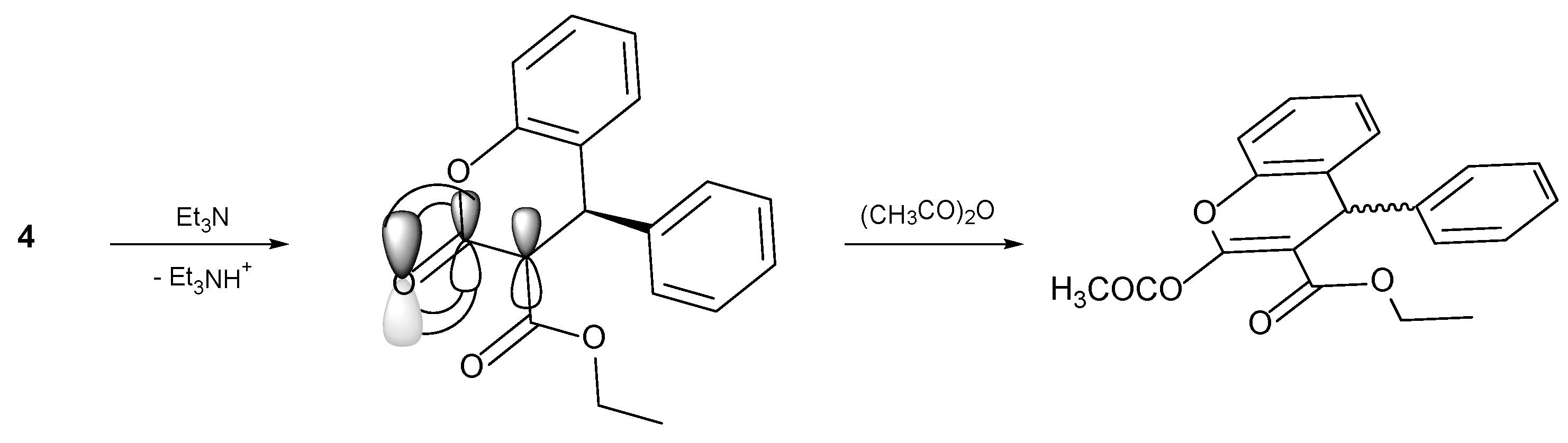
4.1. General Procedure for the Preparative of (R/S)-ethyl-2-acetoxy-4-phenyl-4H-chromene-3-carboxylate 5
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petkova-Yankova, N.I.; Nikolova, R.D. Enol phosphates from 3-diethylphosphonocoumarin: Synthesis and transformations under phase-transfer conditions. Synth. Commun. 2023, 53, 1134–1142. [Google Scholar] [CrossRef]
- Petkova-Yankova, N.I.; Nikolova, R.D. Tandem Michael-Type Reactions with 3-Substituted Coumarins: Phosphorylation Protocol. ChemistrySelect 2020, 5, 7098–7103. [Google Scholar] [CrossRef]
- Petkova, N.I.; Nikolova, R.D.; Bojilova, A.G.; Rodios, N.A.; Raptopoulou, C.P. Hydrogenation/Regioselective C-Acylation Reaction of Diethyl Coumarin-3-phosphonate With NaBH4 /Acid Anhydrides: A New One-Pot Tandem Reaction. Synth. Commun. 2006, 36, 509–524. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W. The Chemistry and Properties of Enol Phosphates Chem. Rev. 1961, 61, 6–607. [Google Scholar] [CrossRef]
- Savignac, P.; Bogdan, I. Modern Phosphonate Chemistry; CRC Press LLC: Boca Raton, FL, USA, 2003. [Google Scholar]
- Holmberg, G.A. The action of phenylmagnesium bromide on ethyl 3-coumarincarboxylate. Acta. Chem. Scand., 1961, 15, 1255–1258. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Bojilova, A.; Ivanov, C. Two new routes to esters of 2-oxochroman-4-acetic acid. Synthesis 1976, 267–268. [Google Scholar] [CrossRef]
- Ivanov, C.; Bojilova, A. On the reaction of 3-phenylcoumarin with organomagnesium compounds. Synthesis 1974, 708–709. [Google Scholar] [CrossRef]
- Koleva, A.I.; Petkova, N.I.; Nikolova, R.D. Ultrasound-assisted Conjugate Addition of Organometallic Reagents to 3-Diethylphosphono-coumarin. Synlett 2016, 27, 2676–2680. [Google Scholar] [CrossRef]
- Gustafsson, B.; Wennstrom, U.; Holmberg, G.A. Acta Acad. Abo 1975, 29, 273–274. [CrossRef]
- Ilieva, E.D.; Petkova, N.I.; Nikolova, R.D. Ring Opening Reactions of 3-Phosphonocoumarin Under Michael Reaction Conditions. Phosphorus, Sulfur, Silicon Relat. Elem. 2012, 187, 39–50. [Google Scholar] [CrossRef]
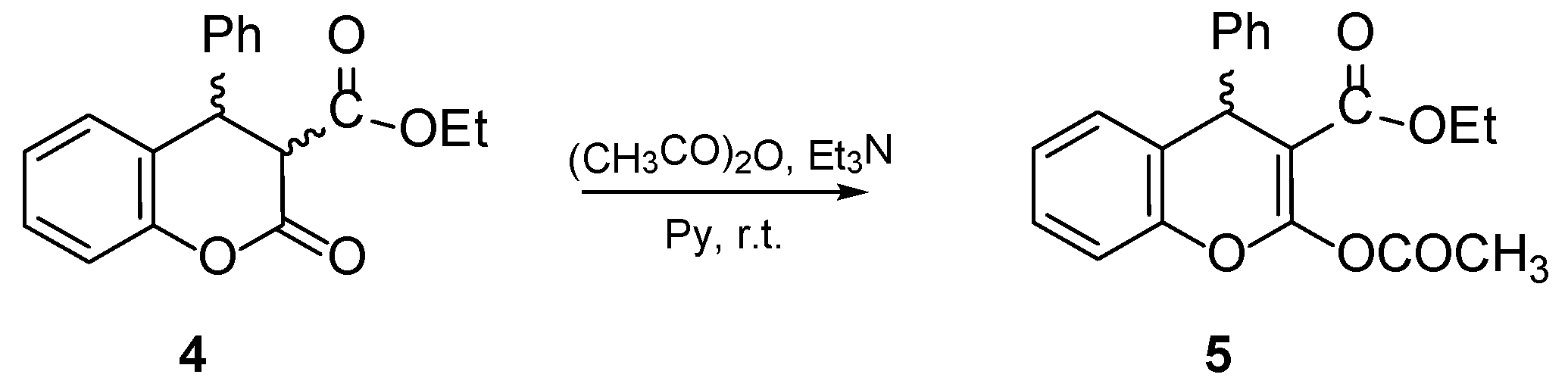
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




