Submitted:
22 May 2023
Posted:
23 May 2023
You are already at the latest version
Abstract
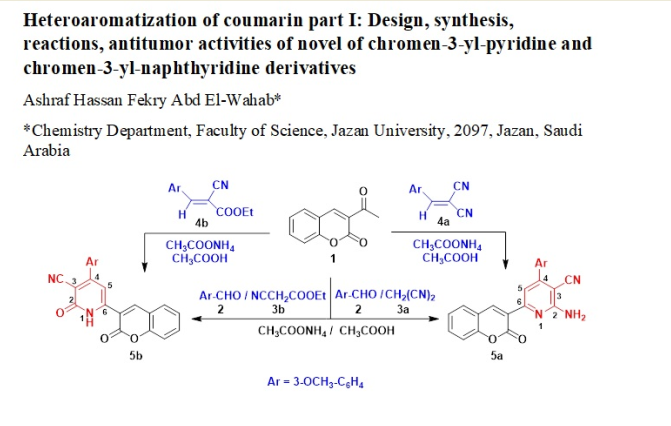
Keywords:
Introduction
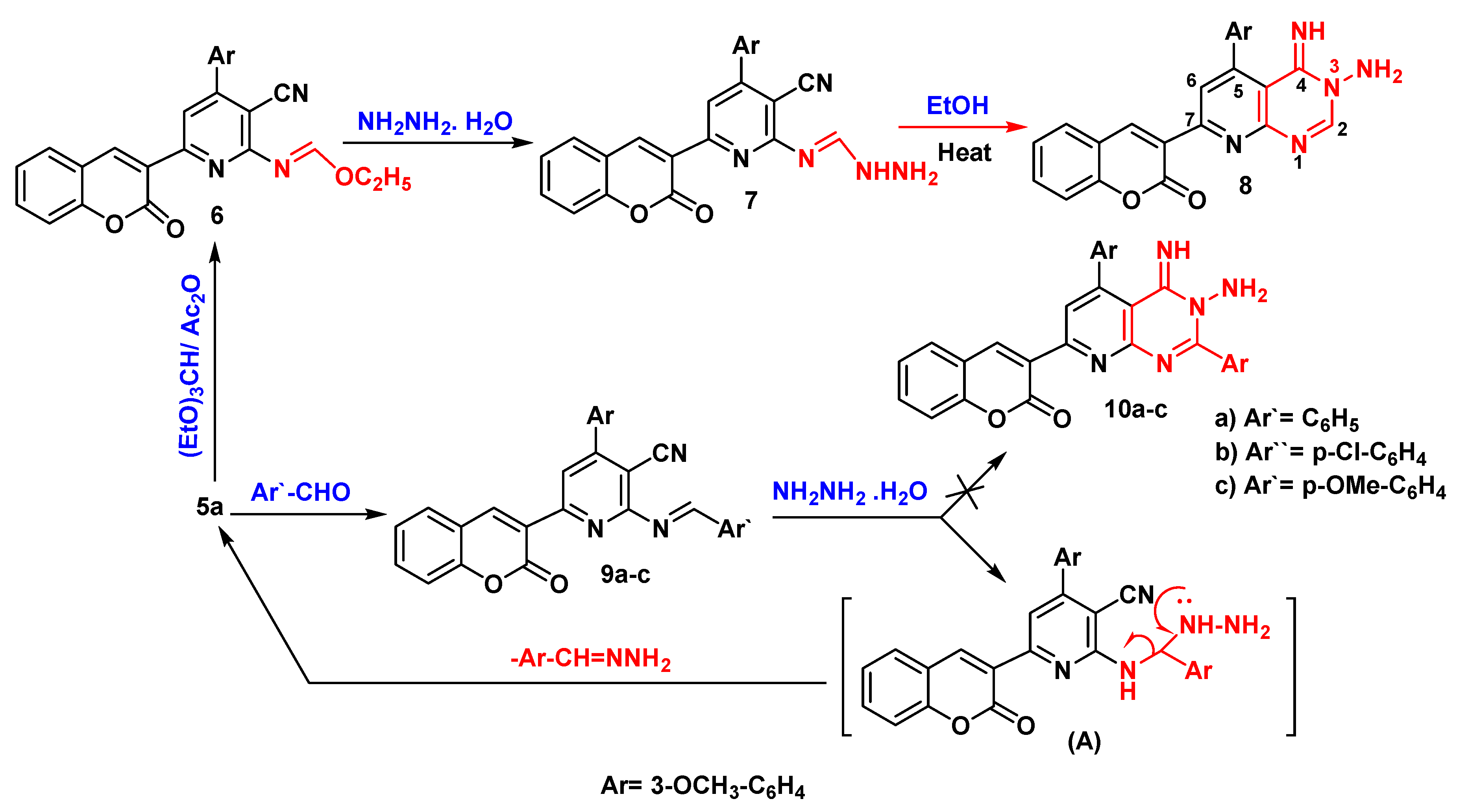
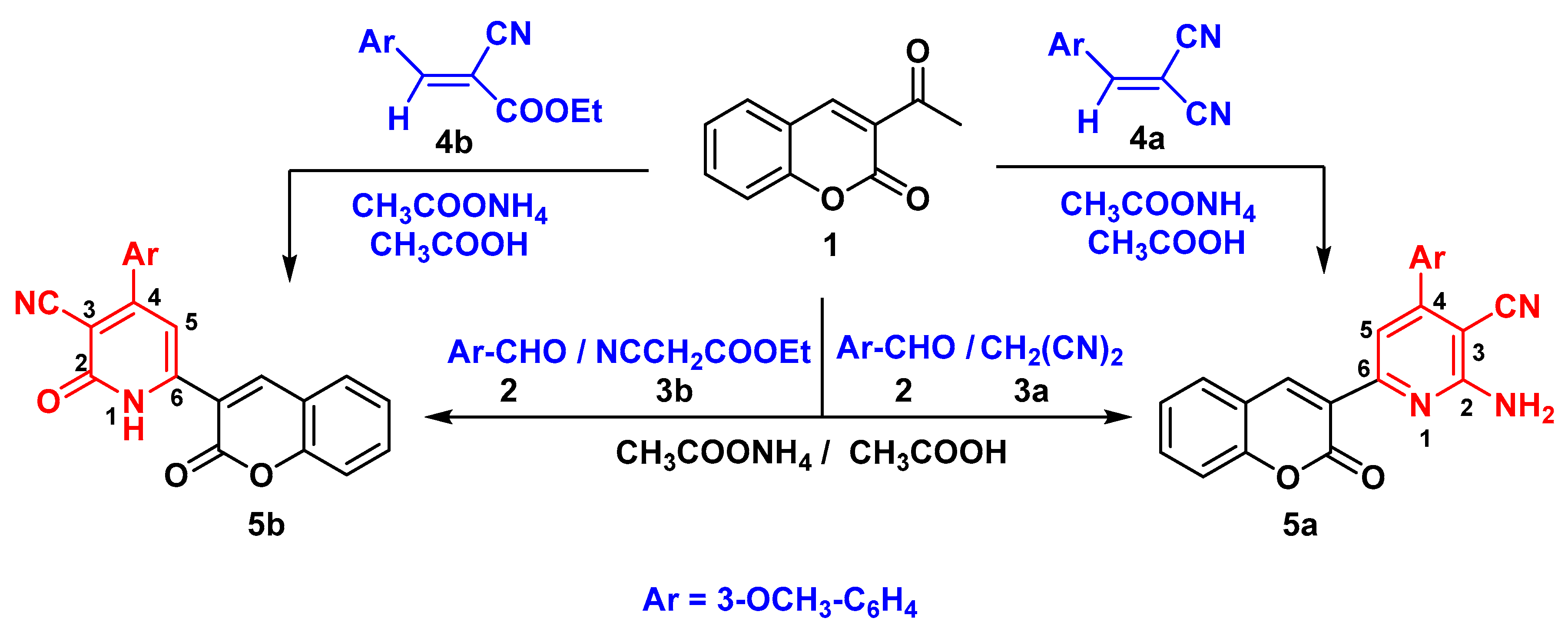
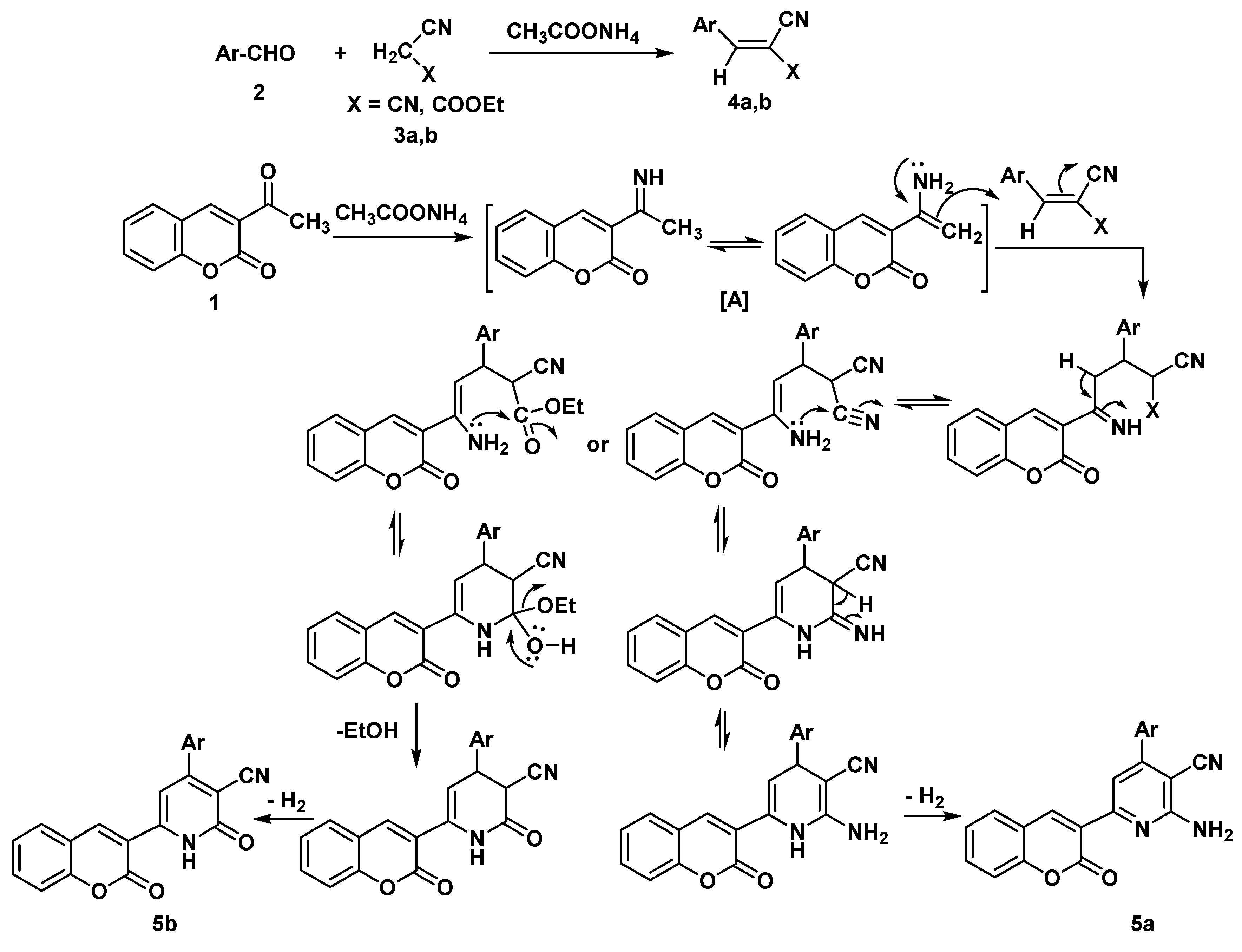
Results and Discussion
Experimental Section
3.1. Materials and Equipment
Synthesis
General Procedure: Synthesis of chromen-3-yl pyridine Derivatives 5a,b
2-amino-4-(3-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl) pyridine-3-carbonitrile (5a)
4-(3-methoxyphenyl)-2-oxo-6-(2-oxo-2H-chromen-3-yl)-1,2-dihydropyridine-3-carbonitrile (5b)
Ethyl (E)-N-(3-cyano-4-(3-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl)pyridin-2-yl)formimidate (6)
(E)-N''-(3-cyano-4-(3-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl)pyridin-2-yl)formimidohydrazide (7)
3-(3-amino-4-imino-5-(3-methoxyphenyl)-3,4-dihydropyrido[2,3-d]pyrimidin-7-yl)-2H-chromen-2-one (8)
3.2.8. Synthesis of Benzylideneamino Derivatives (9a-c)
(E)-2-(benzylideneamino)-4-(3-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl) pyridine-3-carbonitrile (9a)
(E)-2-((4-chlorobenzylidene)amino)-4-(3-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl) pyridine-3-carbonitrile (9b)
(E)-2-((4-methoxybenzylidene)amino)-4-(3-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl) pyridine-3-carbonitrile (9c)
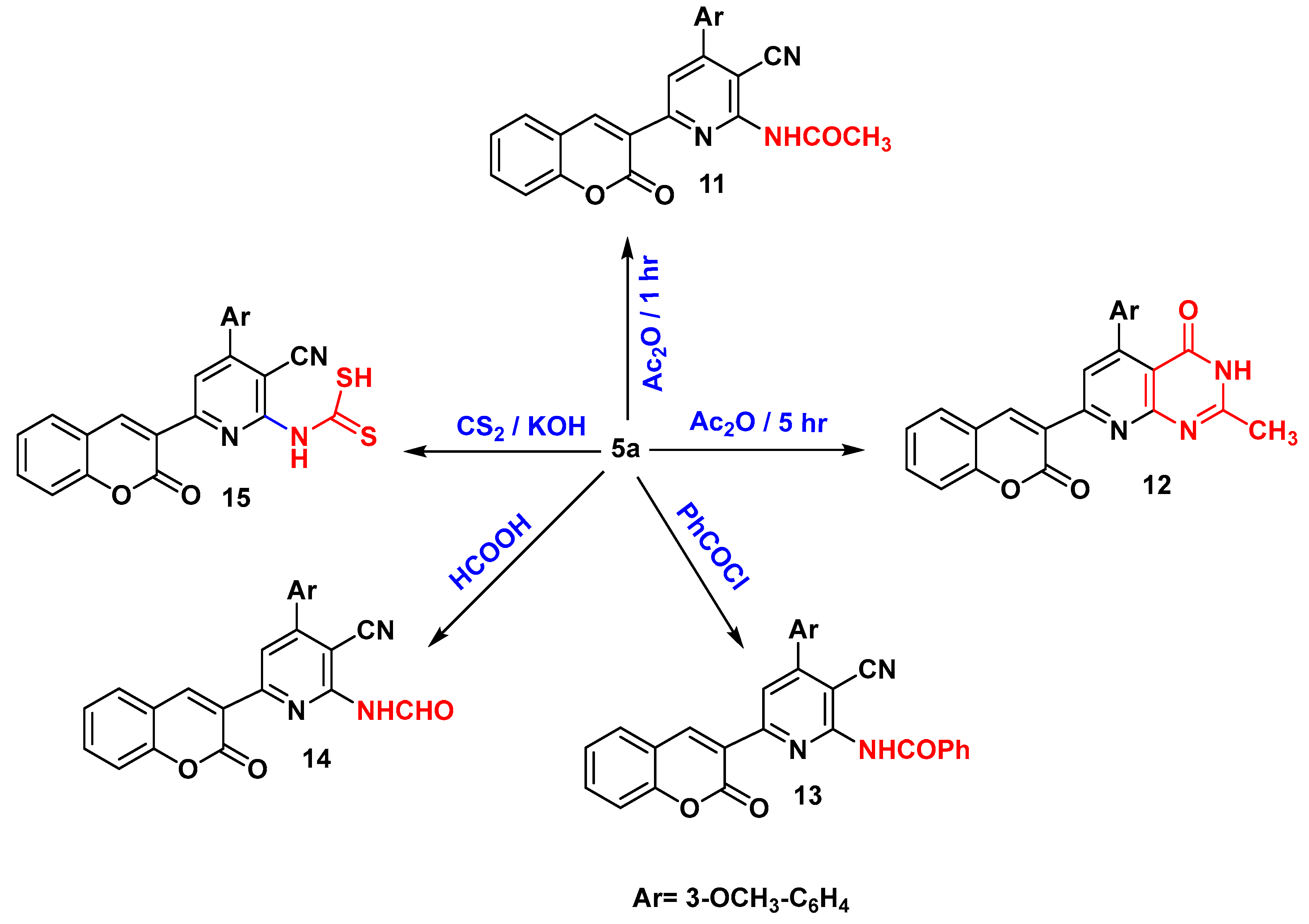
N-(3-cyano-4-(3-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl)pyridin-2-yl)acetamide (11)
5-(3-methoxyphenyl)-2-methyl-7-(2-oxo-2H-chromen-3-yl)pyrido[2,3-d]pyrimidin-4(3H)-one (12)
N-(3-cyano-4-(3-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl)pyridin-2-yl)benzamide (13)
N-(3-cyano-4-(3-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl)pyridin-2-yl)formamide (14)
(3-cyano-4-(3-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl)pyridin-2-yl)carbamodithioic acid (15)
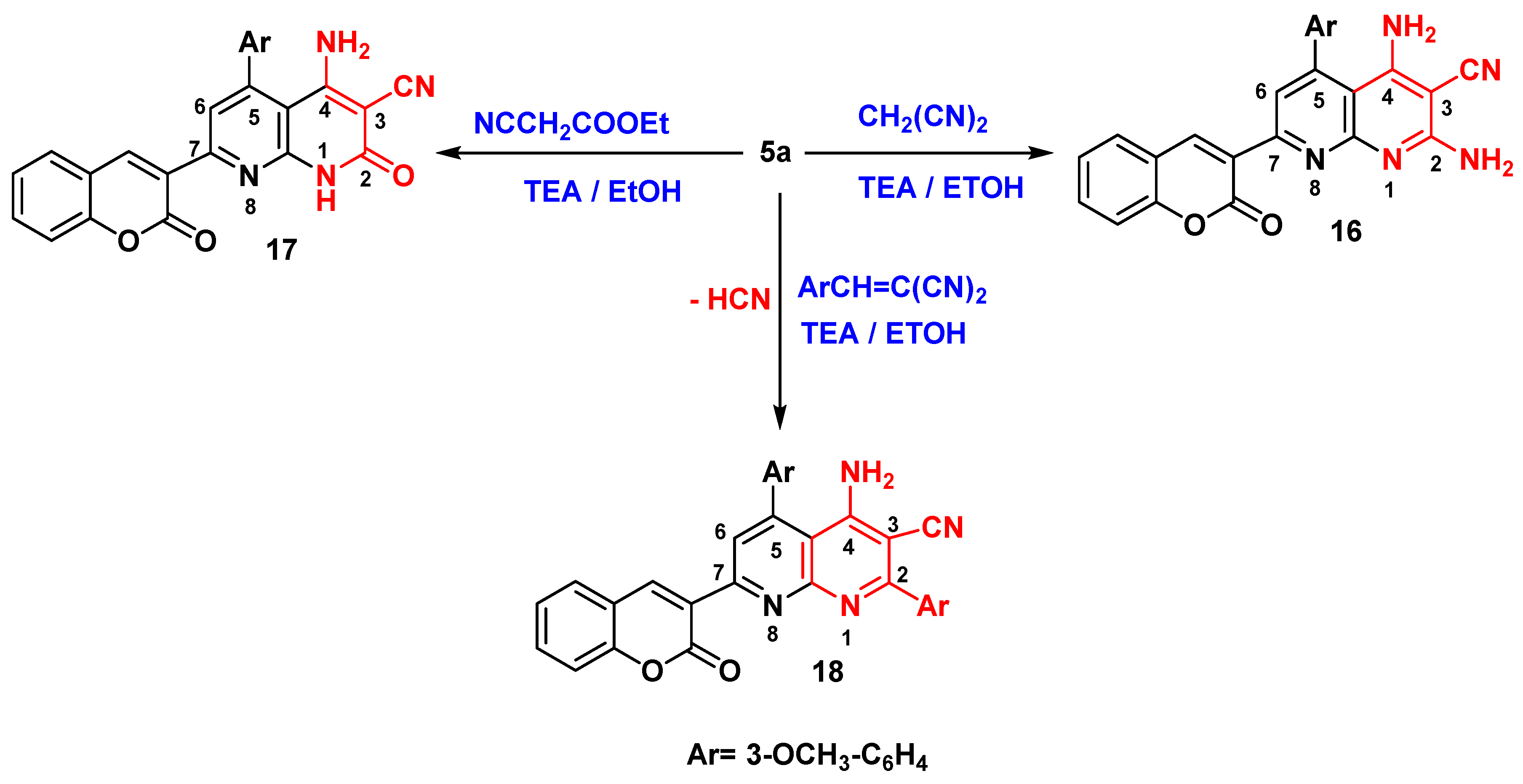
Synthesis of 1,8-Naphthyridine-3-carbonitrile Derivatives (16-18)
2,4-diamino-5-(3-methoxyphenyl)-7-(2-oxo-2H-chromen-3-yl)-1,8-naphthyridine-3-carbonitrile (16)
4-amino-5-(3-methoxyphenyl)-2-oxo-7-(2-oxo-2H-chromen-3-yl)-1,2-dihydro-1,8-naphthyridine-3-carbonitrile (17)
4-amino-2,5-bis(3-methoxyphenyl)-7-(2-oxo-2H-chromen-3-yl)-1,8-naphthyridine-3-carbonitrile (18)
Cytotoxicity Activities
2.3. Methodology. Cell culture
Sulforhodamine-B cytotoxicity assay
CONCLUSION
Acknowledgments
References
- Bhattacharyya, S.S.; Mandal, S.K.; Biswas, R.; Paul, S.; Pathak, S.; Boujedaini, N.; Belon, P.; Khuda-Bukhsh, A.R. A synthetic coumarin (4-methyl-7-hydroxy coumarin) has anti-cancer potentials against DMBA-induced skin cancer in mice. Eur. J. Pharmacol. 2009, 614, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.K.; Sharma, P.K.; Dudhe, R.; Chaudhary, A.; Verma, P.K. Synthesis, analgesic and ulcerogenic activity of novel pyrimidine derivative of coumarin moiety. Anal. Univ. Bucuresti-Chim. 2010, 19, 9–21. [Google Scholar]
- Pavurala, S.; Vedula, R.R. An efficient, multicomponent synthesis of pyrazolyl-triazolothiadiazinyl chromen-2-ones. J. Heterocycl. Chem. 2015, 52, 306–309. [Google Scholar] [CrossRef]
- Abdelhafez, O.M.; Amin, K.M.; Batran, R.Z.; Maher, T.J.; Nada, S.A.; Sethumadhavan, S. Synthesis, anticoagulant and PIVKA-II induced by new 4-hydroxycoumarin derivatives. Bioorg. Med. Chem. 2010, 18, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.Z.; Watanabe, T.; Kawamoto, M.; Nishiyama, K.; Yamashita, H.; Ishii, M.; Iwamura, M.; Furuta, T. Coumarin-4-ylmethoxycarbonyls as photo-triggers for alcohols and phenols. Org. Lett. 2003, 5, 4867–4870. [Google Scholar] [CrossRef] [PubMed]
- Sunthitikawinsakul, A.; Kongkathip, N.; Kongkathip, B.; Phonnakhu, S.; Daly, J.W.; Spande, T.F.; Nimit, Y.; Rochanaruangrai, S. Coumarins and carbazoles from Clausena excavate exhibited antimycobacterial and antifungal activities. Planta Med. 2003, 69, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Weigt, S.; Huebler, N.; Strecker, R.; Braunbeck, T.; Broschard, T.H. Developmental effects of coumarin and the anticoagulant coumarin derivative warfarin on zebrafish (Danio rerio) embryos. Reprod. Toxicol. 2012, 33, 133–141. [Google Scholar] [CrossRef]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.; Rahman, T. In-vitro antiproliferative activity of benzopyranone derivatives in comparison with standard chemotherapeutic drugs. Arch. Pharm. 2011, 344, 102–110. [Google Scholar] [CrossRef]
- Musa, M.A.; Badisa, V.L.D.; Latinwo, L.M.; Waryoba, C.; Ugochukwu, N. In vitro cytotoxicity of benzopyranone derivatives with basic side chain against human lung cell lines. Anticancer Res. 2010, 30, 4613–4617. [Google Scholar]
- Musa, M.A.; Khan, M.O.F.; Cooperwood, J.S. Synthesis and antiproliferative activity of coumarin-estrogen conjugates against breast cancer cell lines. Lett. Drug Des. Discov. 2009, 6, 133–138. [Google Scholar] [CrossRef]
- Williamson, R.C.N.; Lyndon, P.T.; Tudway, A.J.C. Effects of anticoagulation and ileal resection on the development and spread of experimental intestinal carcinomas. Br. J. Cancer 1980, 42, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.J.; Ketcham, A.S.; Wexler, H. Warfarin Therapy as an Adjunct to the Surgical Treatment of Malignant Tumors in Mice. Cancer Res. 1969, 29, 2191–2194. [Google Scholar]
- Yang, E.B.; Zhao, Y.N.; Zhang, K.; Mack, P. Daphnetin, one of coumarin derivatives, is a protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1999, 260, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhou, V.; Pan, S.; Liu, Y.; Hornsby, M.; McMullan, D.; Klock, H.E.; Haugan, J.; Lesley, S.A.; Gray, N.; et al. Identification of coumarin derivatives as a novel class of allosteric MEK1 inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5467–5473. [Google Scholar] [CrossRef] [PubMed]
- Eid, F.A.; Abd El-Wahab, A.H.F.; El-Hagali, G.A.M.; Khafagy, M.M. Syhthesis and antimicrobial evaluation of naphtho[2,1-b]pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1,2,4] triazolo[1,5-c]pyrimidine derivative. Acta Pharm. 2004, 54, 13–26. [Google Scholar]
- Abd El-Wahab, A.H.F. Synthesis of some new pyrano[2,3-d][1,2,4]triazolo[1,5-c]pyrimidine and pyrimido[1,6-b]triazine derivatives. Acta Pharm. 2003, 58, 701–720. [Google Scholar]
- El-Agrody, A.M.; Abd El-Latif, M.S.; El-Hady, N.A.; Fakery, A.H.; Bedair, A.H. Heteroaromatization with 4-Hyroxycoumarin. Part II: Synthesis of some new pyrano[2,3-d] [1,2,4]triazolo[1,5-c]pyrimidine and pyrimido[1,6-b]triazine derivatives. Molecules 2001, 6, 519–527. [Google Scholar] [CrossRef]
- Bedair, A.H.; El-Haddy, N.A.; Abd El-Latif, M.S.; Fakery, A.H.; El-Agrody, A.M. 4-Hyroxycoumarin in heterocycloic synthesis. Part III: Synthesis of some new pyrano[2,3-d] pyrimidine, 2-substituted[1,2,4]triazolo[1,5-c]pyrimidine and pyrimido[1,6-b]triazine derivatives. Farmaco 2000, 55, 708–714. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; El-Hakim, M.H.; Abd El-Latif, M.S.; Fakery, A.H.; El-Sayed, E.M.; El-Ghareab, K.A. Synthesis pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1,2,4]triazolo[2,3-c] pyrimidine derivatives with promising antibacterial activities. Acta Pharm. 2000, 50, 111–120. [Google Scholar]
- Khafagy, M.M.; AbdEl-Wahab, A.H.F.; Eid, F.A.; El-Agrody, A.M. Synthesis of halogen derivatives of benzo[h]chromene and benzo[a]anthracene with promising antimicrobial activi-ties. Il Farmaco 2002, 57, 715–722. [Google Scholar] [CrossRef]
- El-Agrody, M.A.; Abd El-Latif, S.M.; El-Hady, A.N.; Fakery, H.A.; Bedair, H.A. Heteroaromatization with 4-Hydroxycoumarin Part II: Synthesis of Some New Pyrano[2,3-d]pyrimidines, [1,2,4]triazolo[1,5-c]pyrimidines and Pyrimido[1,6-b]-[1,2,4]triazine Derivatives. Molecules 2001, 6, 519–527. [Google Scholar] [CrossRef]
- Abd El-Wahab, F.H.A. Synthesis, Reactions and Evaluation of the Antimicrobial Activity of Some 4-(p-Halophenyl)-4H-naphthopyran, Pyranopyrimidine and Pyranotriazolopyrimidine Derivatives. Pharmaceuticals (Basel) 2012, 5, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Bedair, H.A.; Emam, A.H.; El-Hady, A.N.; Ahmed, R.A.K.; El-Agrody, M.A. Synthesis and antimicrobial activities of novel naphtho[2,1-b]pyran, pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1,2,4]triazolo[2,3-c]-pyrimidine derivatives. Farmaco 2001, 56, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, M.M.; Abd El-Wahab, F.H.A.; Eid, A.F.; El-Agrody, M.A. Synthesis of halogen derivatives of benzo[h]chromene and benzo[a]anthracene with promising antimicrobial activities. Farmaco 2002, 57, 715–722. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Abd El-wahab, F.H.A. Heteroaromatization with 4-phenyldiazenyl-1-naphthol. Part I: Synthesis of some new naphthopyrans and naphthopyranopyrimidines. Eur. J. Chem. 2016, 7, 230–237. [Google Scholar]
- Ashraf, H.F.A.; Mosa, H.M.K.; Ali, H.H.A.; Mohammad, Y.M.A. Synthesis, Antimicrobial, and Antitumor Activity of Some New Chromene Compounds. Indian Journal of Heterocyclic Chemistry 2020, 30, 369–379. [Google Scholar]
- Ashraf Hassan Fekry Abdelwahab and Salma Ashraf Hassan Fekry; Anti-cancerous Properties of the Synthesized Substituted Chromene Compounds and their Pharmacological Activities. Letters in Drug Design & Discover 2023, 20, 1098–1106.
- Kuete, V.; Krusche, B.; Youns, M.; Voukeng, I.; Fankam, A.G.; Tankeo, S.; Lacmata, S.; Efferth, T. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. Journal of Ethnopharmacology 2011, 134, 803–812. [Google Scholar] [CrossRef]
- Youns, M.; Fu, Y.J.; Zu, Y.G.; Kraner, A.; Konkimalla, V.B.; Radlwimmer, B.; Sultmann, H.; Efferth, T.N.S. Sensitivity and resistance towards isoliquiritigenin, doxorubicin and methotrexate in T cell acute lymphoblastic leukaemia cell lines by pharmacogenomics Naunyn Schmiedebergs. Arch Pharmacol 2010, 382, 221. [Google Scholar] [CrossRef]
| In vitro cytotoxicity IC50 (µg/mL) | |||
|---|---|---|---|
| Comp. | MCF-7 | HeLa | PC-3 |
| 5a | 18.32 ± 0. 41 | 20.61 ± 0. 23 | 19.64 ± 0.08 |
| 5b | 16.05 ± 0. 82 | 19.01 ± 0.03 | 15.90 ± 0.42 |
| 9c | 21.17 ± 0.50 | 26.01 ± 0.02 | 24.06 ± 0.03 |
| 11 | 20.07 ± 0.82 | 24.61 ± 0.01 | 23.63 ± 0.03 |
| 12 | 13.04 ± 0. 08 | 16.09 ± 0.21 | 12.05 ± 0.85 |
| 13 | 27.05 ± 0.22 | 28.01 ± 0.87 | 26.08 ± 0.65 |
| 14 | 25.61 ± 0.13 | 26.77 ± 0.01 | 28.60 ± 0.42 |
| 16 | 5.02 ± 0.07 | 8.30± 0.61 | 5.60 ± 0.01 |
| 17 | 8.42 ± 0.30 | 10.31 ± 0. 10 | 7.96 ± 0.01 |
| 18 | 13.04 ± 0. 08 | 16.09 ± 0.21 | 12.05 ± 0.85 |
| 5-Fluorourcail | 3.70 ± 0.50 | 6.48 ±0. 10 | 4.92 ± 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




