Submitted:
07 August 2024
Posted:
08 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
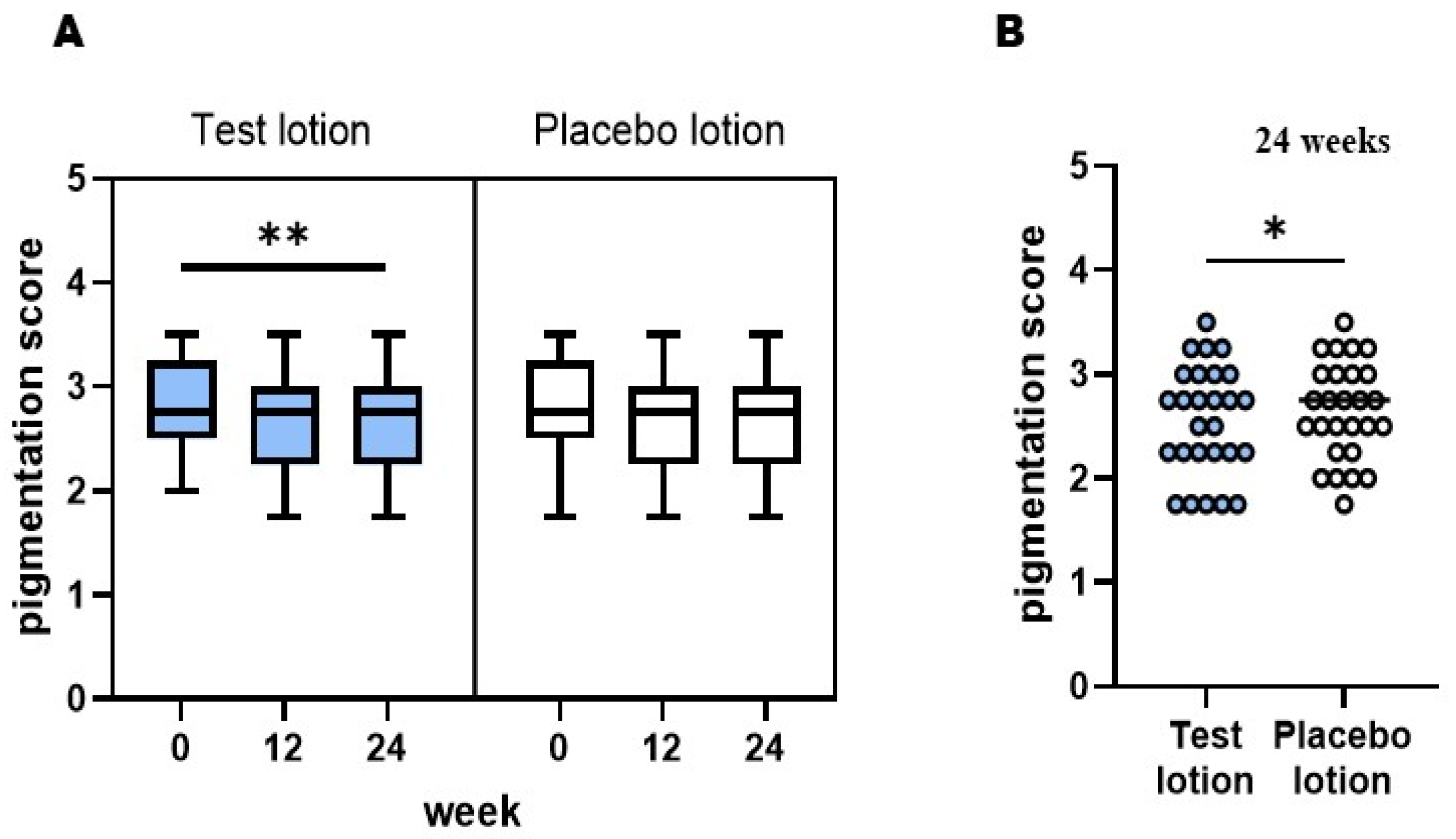
2.1. Visible pigmentation level of SLs
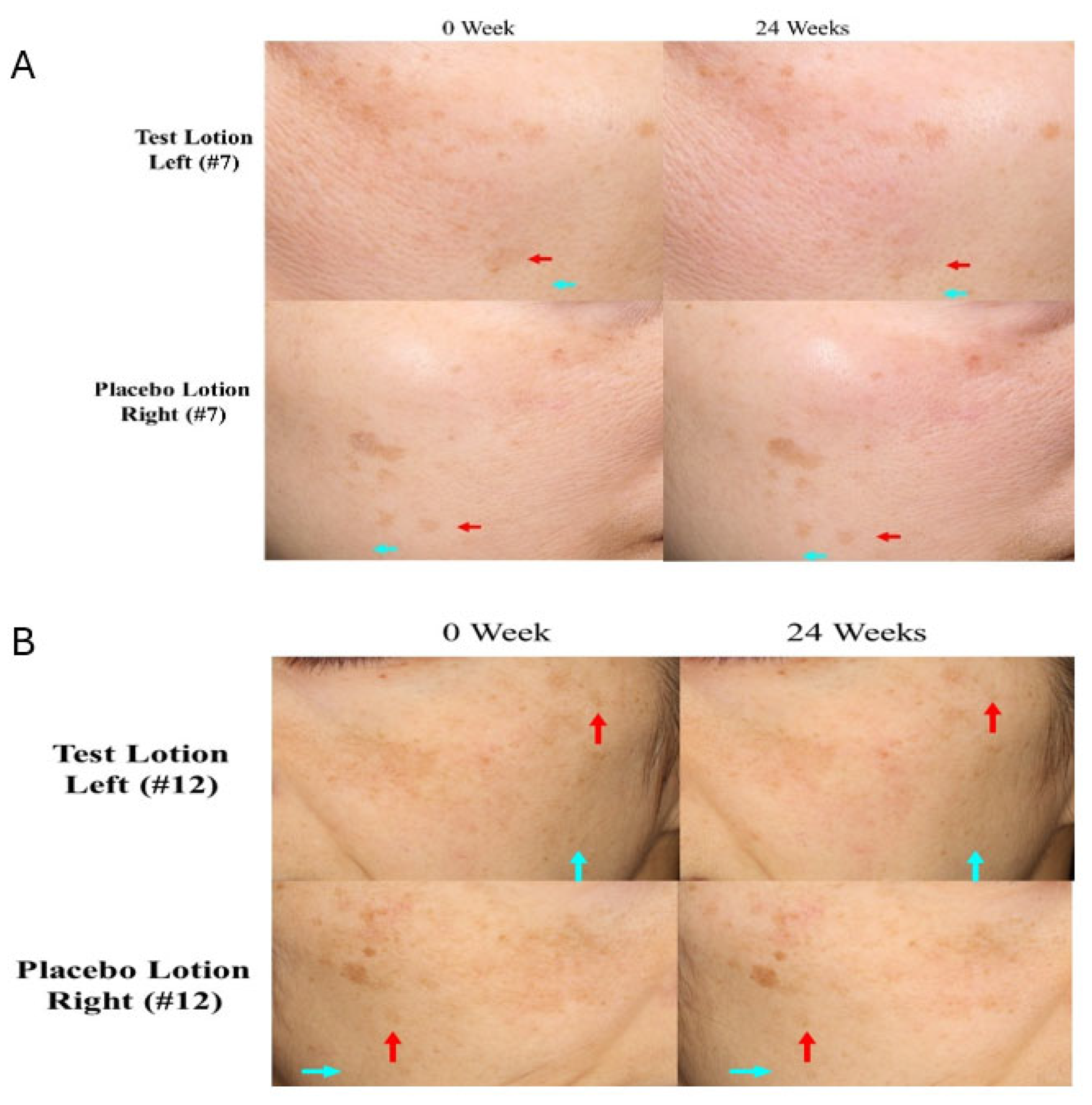
2.2. Clinical photographs of SLs and NLS
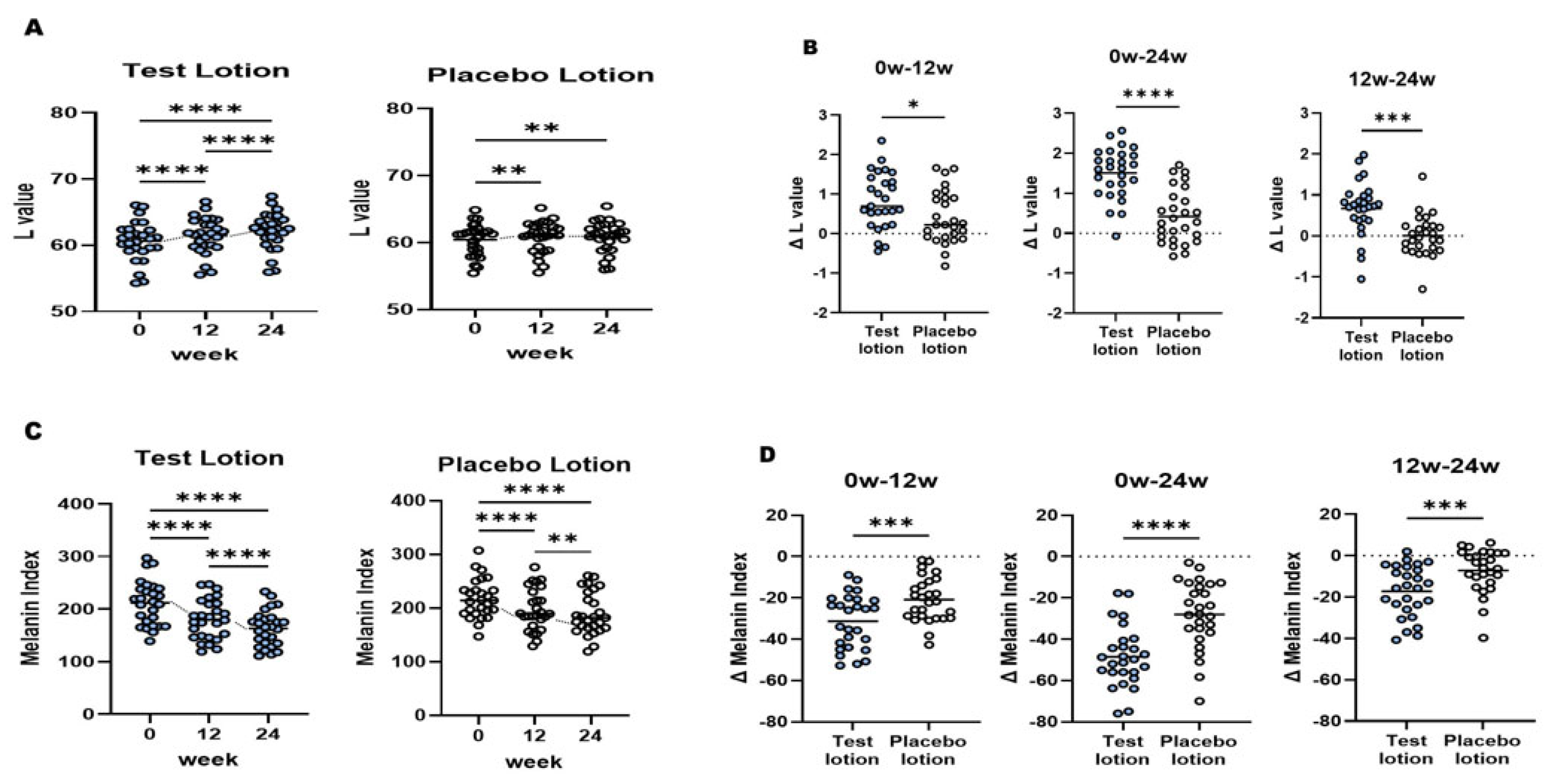
2.3. L and MI values of SLs
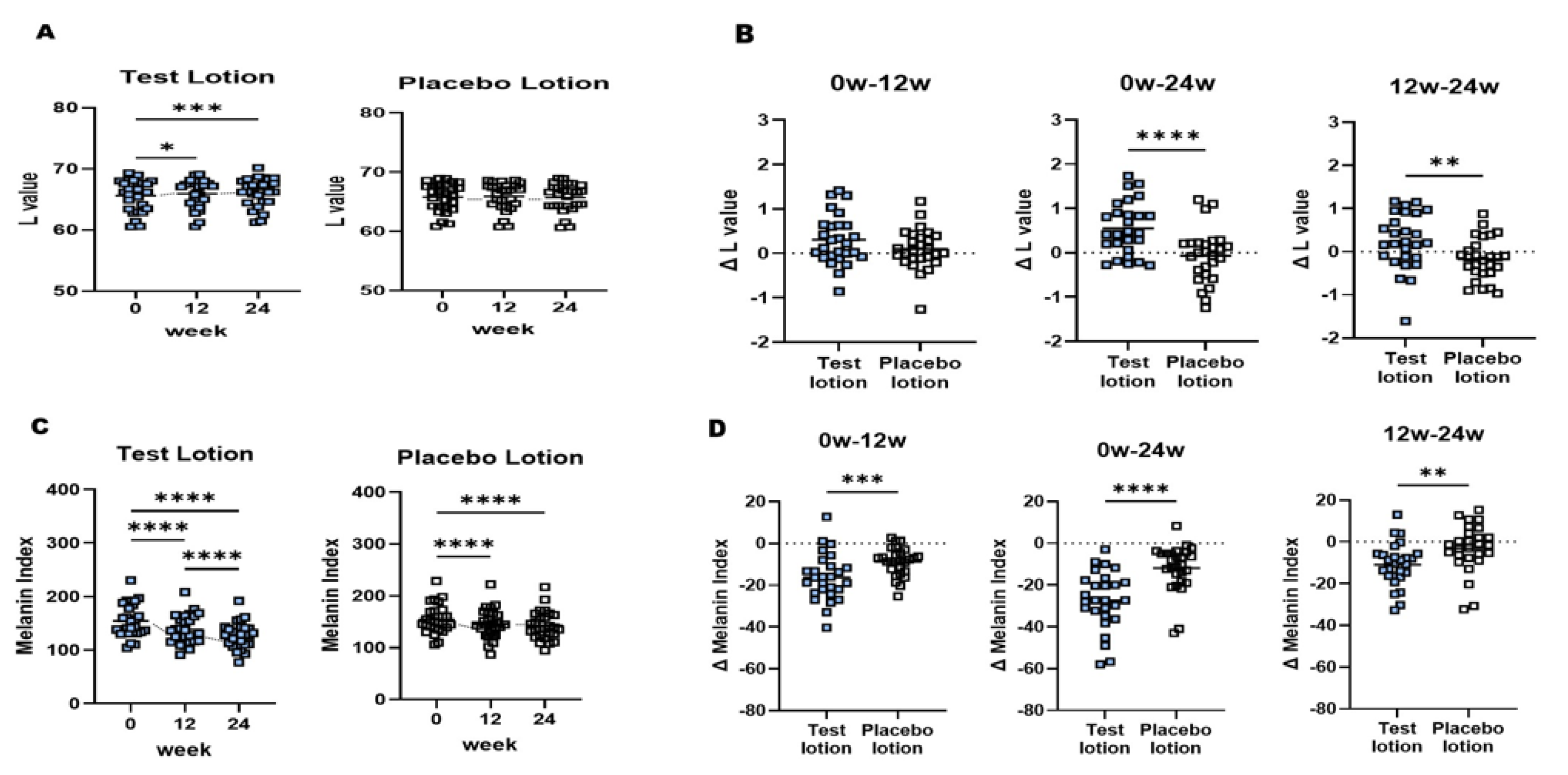
2.4. L and MI values of NLS
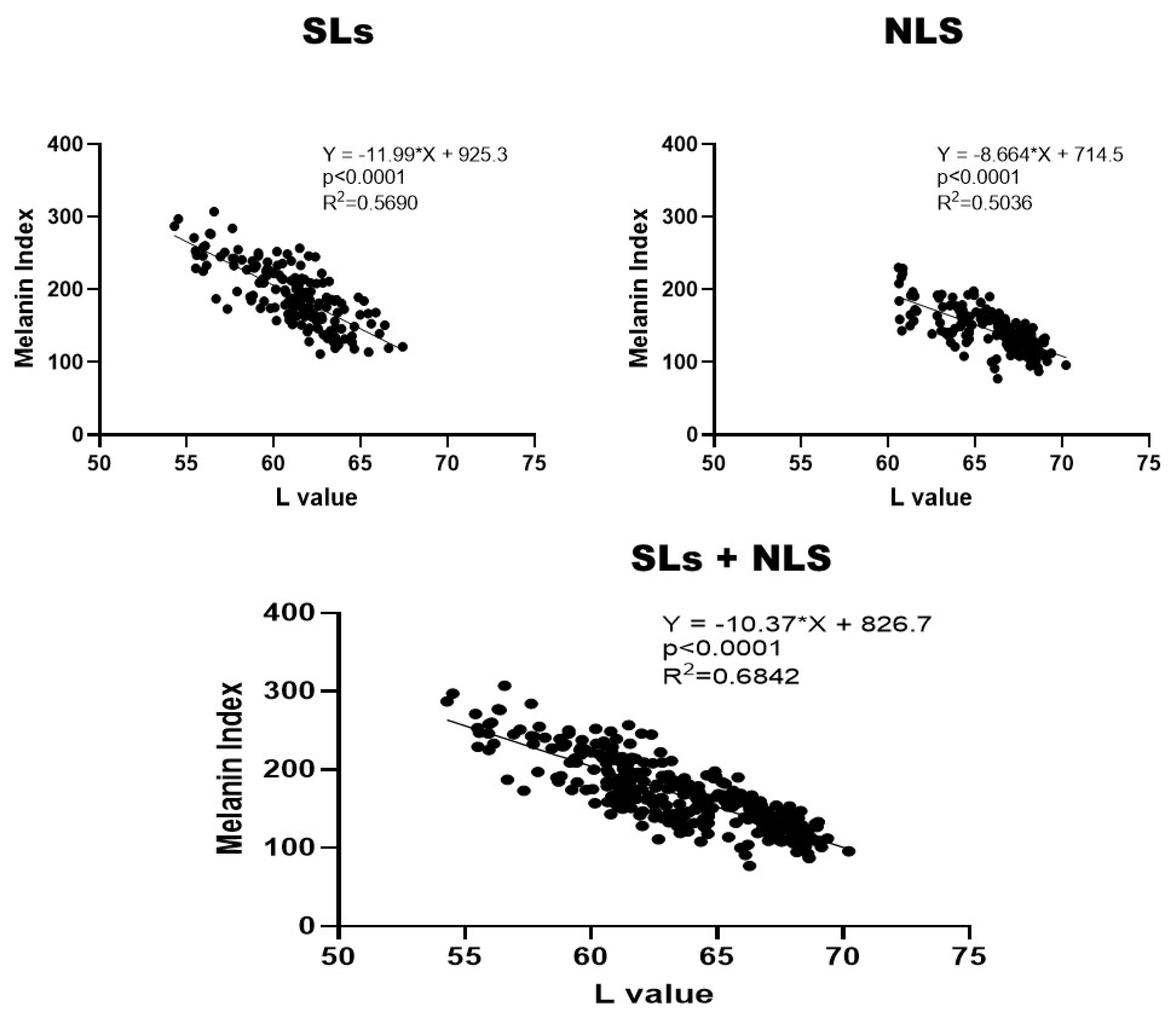
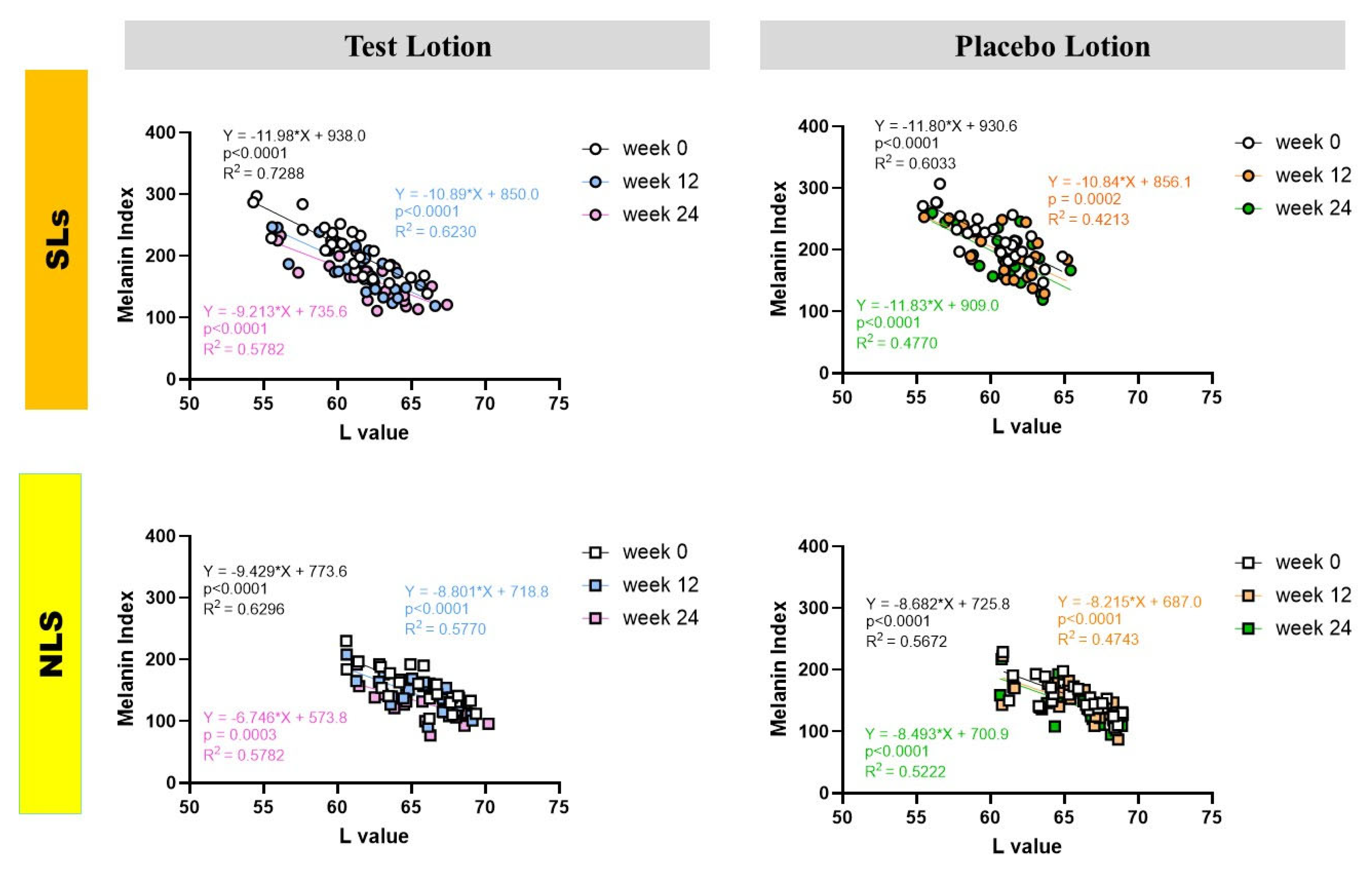
2.5. Correlations between L and MI values in SLs and NLS
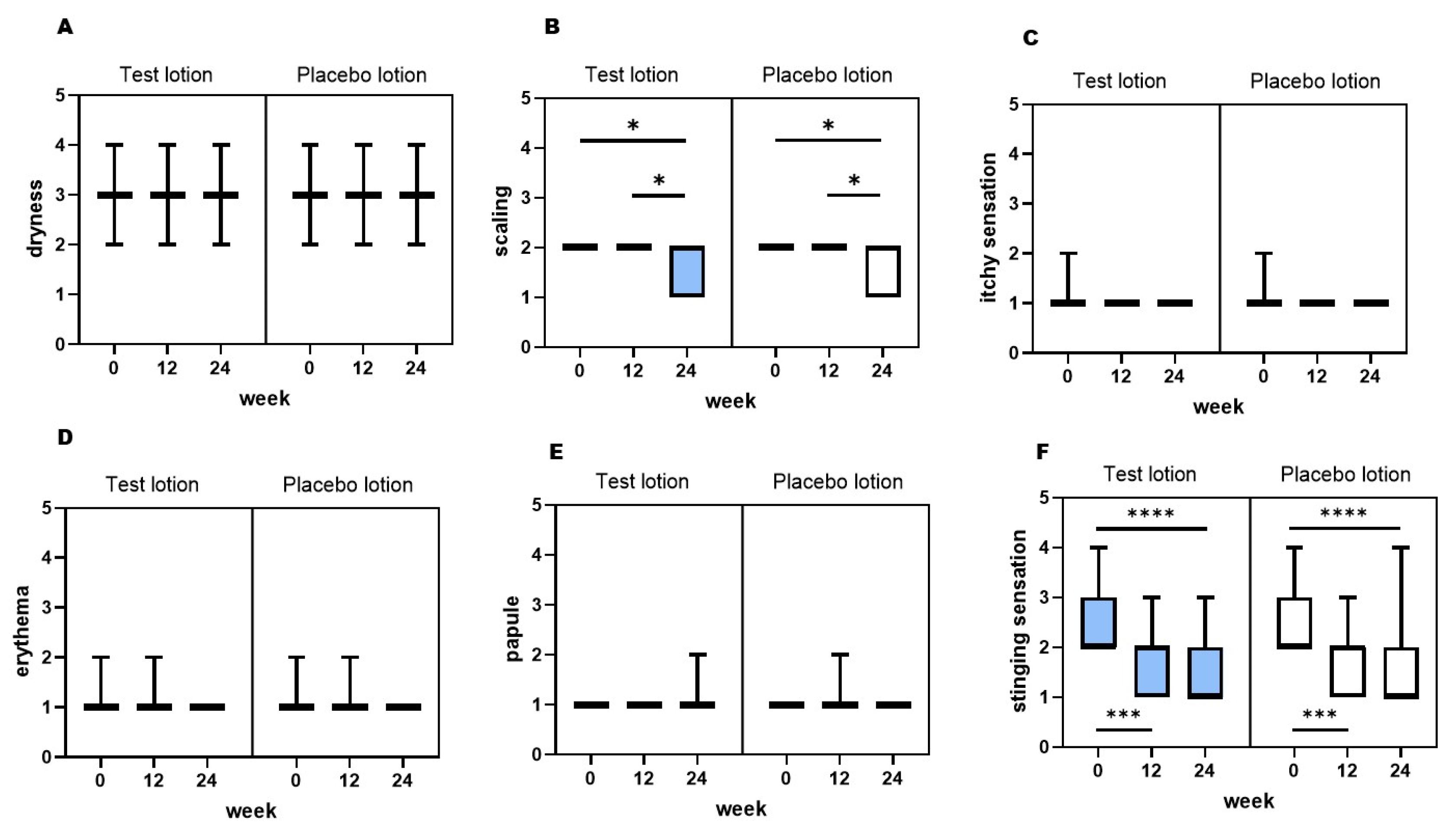
2.6. General clinical evaluation
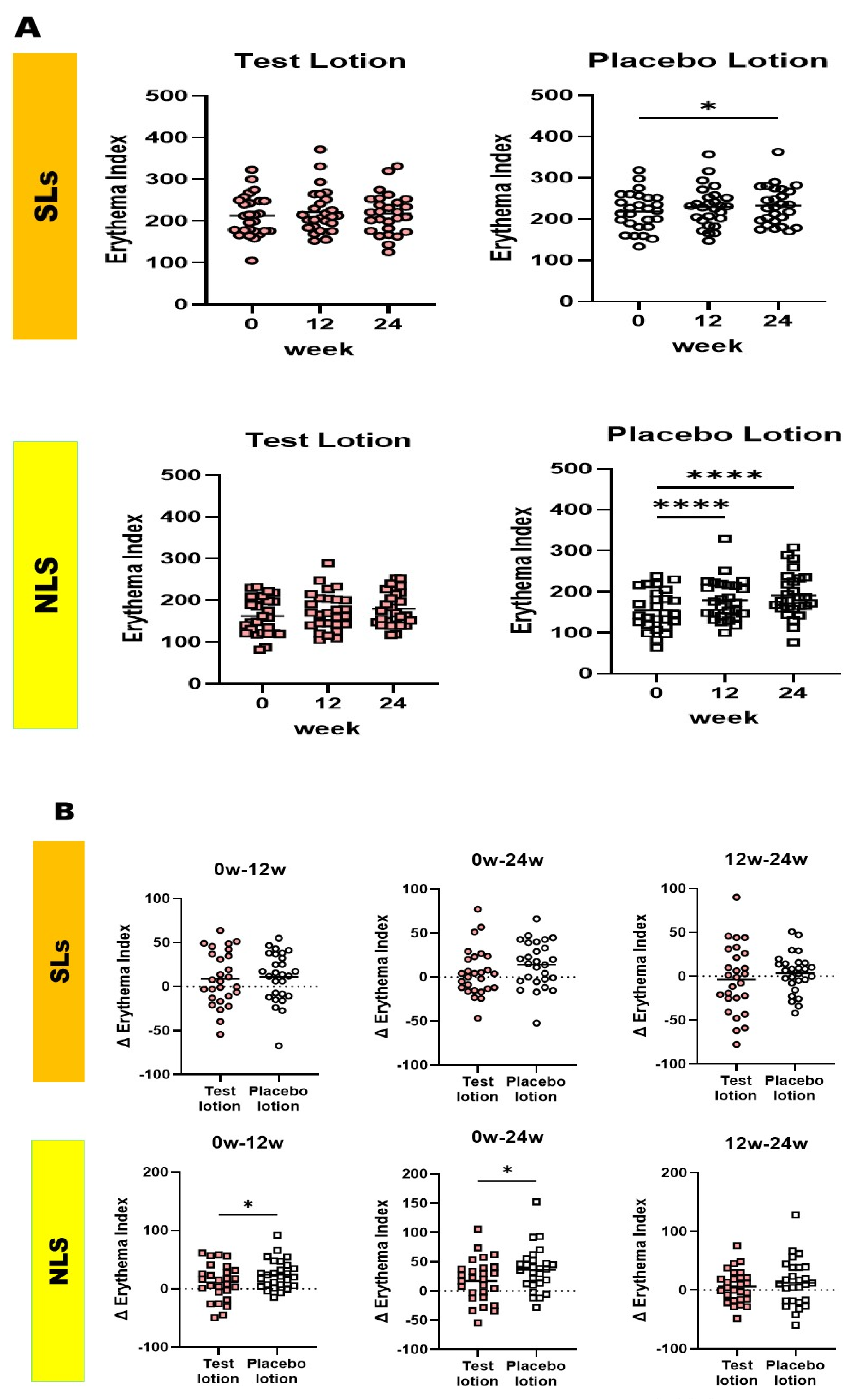
2.7. Clinical evaluation of skin redness based on erythema index values measured using a Mexameter MX18
2.8. Skin Safety
3. Discussion
4. Materials and Methods
4.1. Test materials
4.2. Study design
4.3. Compliance with ethical standards
4.4. Evaluation of pigmentation level
4.5. General clinical evaluation
4.6. Evaluation of skin redness level
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imokawa, G. , Melanocyte Activation Mechanisms and Rational Therapeutic Treatments of Solar Lentigos. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, A.; Kobayashi, A.; Yoshida, Y.; Kitahara, T.; Takema, Y.; Imokawa, G. , Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am J Pathol 2004, 165, 2099–109. [Google Scholar] [CrossRef] [PubMed]
- Yada, Y.; Higuchi, K.; Imokawa, G. , Effects of endothelins on signal transduction and proliferation in human melanocytes. J Biol Chem 1991, 266, 18352–7. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. , Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res 2004, 17, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Yada, Y.; Miyagishi, M. , Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem 1992, 267, 24675–80. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Miyagishi, M.; Yada, Y. , Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol 1995, 105, 32–7. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Kawai, M.; Mishima, Y.; Motegi, I. , Differential analysis of experimental hypermelanosis induced by UVB, PUVA, and allergic contact dermatitis using a brownish guinea pig model. Arch Dermatol Res 1986, 278, 352–62. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Kobayasi, T.; Miyagishi, M. , Intracellular signaling mechanisms leading to synergistic effects of endothelin-1 and stem cell factor on proliferation of cultured human melanocytes. Cross-talk via trans-activation of the tyrosine kinase c-kit receptor. J Biol Chem 2000, 275, 33321–8. [Google Scholar] [CrossRef]
- Imokawa, G.; Yada, Y.; Kimura, M. , Signalling mechanisms of endothelin-induced mitogenesis and melanogenesis in human melanocytes. Biochem J 1996, 314 ( Pt 1) Pt 1, 305–312. [Google Scholar] [CrossRef]
- Hachiya, A.; Kobayashi, T.; Takema, Y.; Imokawa, G. , Biochemical characterization of endothelin-converting enzyme-1alpha in cultured skin-derived cells and its postulated role in the stimulation of melanogenesis in human epidermis. J Biol Chem 2002, 277, 5395–403. [Google Scholar] [CrossRef]
- Hachiya, A.; Kobayashi, A.; Ohuchi, A.; Takema, Y.; Imokawa, G. , The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol 2001, 116, 578–86. [Google Scholar] [CrossRef] [PubMed]
- Sato-Jin, K.; Nishimura, E. K.; Akasaka, E.; Huber, W.; Nakano, H.; Miller, A.; Du, J.; Wu, M.; Hanada, K.; Sawamura, D.; Fisher, D. E.; Imokawa, G. , Epistatic connections between microphthalmia-associated transcription factor and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. FASEB J 2008, 22, 1155–68. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Kawashima, M.; Ichikawa, Y.; Imokawa, G. , The epidermal stem cell factor is over-expressed in lentigo senilis: implication for the mechanism of hyperpigmentation. J Invest Dermatol 2004, 122, 1256–65. [Google Scholar] [CrossRef] [PubMed]
- Kadono, S.; Manaka, I.; Kawashima, M.; Kobayashi, T.; Imokawa, G. , The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J Invest Dermatol 2001, 116, 571–7. [Google Scholar] [CrossRef] [PubMed]
- Rajaratnam, R.; Halpern, J.; Salim, A.; Emmett, C. , Interventions for melasma. Cochrane Database Syst Rev 2010, 7, Cd003583. [Google Scholar] [CrossRef] [PubMed]
- Arndt, K. A.; Fitzpatrick, T. B. , Topical use of hydroquinone as a depigmenting agent. Jama 1965, 194, 965–7. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T. B.; Arndt, K. A.; el-Mofty, A. M.; Pathak, M. A. , Hydroquinone and psoralens in the therapy of hypermelanosis and vitiligo. Arch Dermatol 1966, 93, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A. M.; Willis, I. , A new formula for depigmenting human skin. Arch Dermatol 1975, 111, 40–8. [Google Scholar] [CrossRef] [PubMed]
- Heilgemeir, G. P.; Balda, B. R. , [Irreversible toxic depigmentation. Observations following use of hydroquinonemonobenzylether-containing skin bleaching preparations]. MMW Munch Med Wochenschr 1981, 123, 47–8. [Google Scholar]
- Halder, R. M.; Richards, G. M. , Topical agents used in the management of hyperpigmentation. Skin Therapy Lett 2004, 9, 1–3. [Google Scholar]
- Cheong, K. A.; Kim, H. J.; Kim, J. Y.; Kim, C. H.; Lim, W. S.; Noh, M.; Lee, A. Y. , Retinoic acid and hydroquinone induce inverse expression patterns on cornified envelope-associated proteins: implication in skin irritation. J Dermatol Sci 2014, 76, 112–9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Du, X.; Zhang, L.; Jia, T.; Zhang, H.; Peng, B.; Hao, Y.; Tong, Z.; Che, D.; Geng, S. , Hydroquinone-induced skin irritant reaction could be achieved by activating mast cells via mas-related G protein-coupled receptor X2. Exp Dermatol 2023, 32, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, G. H.; Cheong, K. A.; Lee, A. Y. , Increased Skin Irritation by Hydroquinone and Rsetinoic Acid Used in Combination. Ann Dermatol 2017, 29, 715–721. [Google Scholar] [CrossRef] [PubMed]
- McKesey, J.; Tovar-Garza, A.; Pandya, A. G. , Melasma Treatment: An Evidence-Based Review. Am J Clin Dermatol 2020, 21, 173–225. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Wang, C.; Wang, Z.; Liang, Y.; Du, J.; He, D.; Fan, X.; Jordt, S. E.; Liu, B. , Involvement of Transient Receptor Potential Cation Channel Member A1 activation in the irritation and pain response elicited by skin-lightening reagent hydroquinone. Sci Rep 2017, 7, 7532. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Al-Niaimi, F.; Ali, F. R. , Hydroquinone: myths and reality. Clin Exp Dermatol 2021, 46, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, J.; Chazarra, S.; Garcia-Carmona, F. , Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J Pharm Pharmacol 1994, 46, 982–5. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K. J.; Vani, M. G.; Wang, S. Y.; Liao, J. W.; Hsu, L. S.; Yang, H. L.; Hseu, Y. C. , In vitro and in vivo studies disclosed the depigmenting effects of gallic acid: a novel skin lightening agent for hyperpigmentary skin diseases. Biofactors 2013, 39, 259–70. [Google Scholar] [CrossRef] [PubMed]
- Gonçalez, M. L.; Corrêa, M. A.; Chorilli, M. , Skin delivery of kojic acid-loaded nanotechnology-based drug delivery systems for the treatment of skin aging. Biomed Res Int 2013, 2013, 271276. [Google Scholar] [CrossRef] [PubMed]
- Ki, D. H.; Jung, H. C.; Noh, Y. W.; Thanigaimalai, P.; Kim, B. H.; Shin, S. C.; Jung, S. H.; Cho, C. W. , Preformulation and formulation of newly synthesized QNT3-18 for development of a skin whitening agent. Drug Dev Ind Pharm 2013, 39, 526–33. [Google Scholar] [CrossRef]
- Breathnach, A. C.; Nazzaro-Porro, M.; Passi, S.; Zina, G. , Azelaic acid therapy in disorders of pigmentation. Clin Dermatol 1989, 7, 106–19. [Google Scholar] [CrossRef] [PubMed]
- Verallo-Rowell, V. M.; Verallo, V.; Graupe, K.; Lopez-Villafuerte, L.; Garcia-Lopez, M. , Double-blind comparison of azelaic acid and hydroquinone in the treatment of melasma. Acta Derm Venereol Suppl (Stockh) 1989, 143, 58–61. [Google Scholar] [PubMed]
- Huang, C. H.; Sung, H. C.; Hsiao, C. Y.; Hu, S.; Ko, Y. S. , Transdermal delivery of three vitamin C derivatives by Er:YAG and carbon dioxide laser pretreatment. Lasers Med Sci 2013, 28, 807–14. [Google Scholar] [PubMed]
- Won, Y. K.; Loy, C. J.; Randhawa, M.; Southall, M. D. , Clinical efficacy and safety of 4-hexyl-1,3-phenylenediol for improving skin hyperpigmentation. Arch Dermatol Res 2014, 306, 455–65. [Google Scholar] [CrossRef] [PubMed]
- Son, K. H.; Heo, M. Y. , The evaluation of depigmenting efficacy in the skin for the development of new whitening agents in Korea. Int J Cosmet Sci 2013, 35, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. S.; Lee, S. M.; Lin, C. C.; Liu, C. Y.; Wu, M. C.; Shi, W. L. , Kinetic study on the tyrosinase and melanin formation inhibitory activities of carthamus yellow isolated from Carthamus tinctorius L. J Biosci Bioeng 2013, 115, 242–5. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P. W.; Chen, W. Y.; Aljuffali, I. A.; Chen, C. C.; Fang, J. Y. , Co-drug strategy for promoting skin targeting and minimizing the transdermal diffusion of hydroquinone and tranexamic acid. Curr Med Chem 2013, 20, 4080–92. [Google Scholar] [CrossRef] [PubMed]
- Tse, T. W.; Hui, E. , Tranexamic acid: an important adjuvant in the treatment of melasma. J Cosmet Dermatol 2013, 12, 57–66. [Google Scholar] [CrossRef]
- Eimpunth, S.; Wanitphadeedecha, R.; Manuskiatti, W. , A focused review on acne-induced and aesthetic procedure-related postinflammatory hyperpigmentation in Asians. J Eur Acad Dermatol Venereol 2013, 27 (Suppl. S1), 7–18. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. , Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J Enzyme Inhib Med Chem 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. , Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed Pharmacother 2019, 110, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Ayres, E.; Bak, H.; Manco, M.; Lynch, S.; Raab, S.; Du, A.; Green, D.; Skobowiat, C.; Wangari-Talbot, J.; Zheng, Q. , Effect of a Tranexamic Acid, Kojic Acid, and Niacinamide Containing Serum on Facial Dyschromia: A Clinical Evaluation. J Drugs Dermatol 2019, 18, 454–459. [Google Scholar] [PubMed]
- Thawabteh, A. M.; Jibreen, A.; Karaman, D.; Thawabteh, A.; Karaman, R. , Skin Pigmentation Types, Causes and Treatment-A Review. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Mota, S.; Rosa, G. P.; Barreto, M. C.; Garrido, J.; Sousa, E.; Cruz, M. T.; Almeida, I. F.; Quintas, C. , Comparative Studies on the Photoreactivity, Efficacy, and Safety of Depigmenting Agents. Pharmaceuticals (Basel) 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Suzuki, K.; Ito, A.; Tanemura, A.; Abe, Y.; Suzuki, T.; Yoshikawa, M.; Sumikawa, Y.; Yagami, A.; Masui, Y.; Inoue, S.; Ito, S.; Katayama, I. , Rhododendrol-induced leukoderma update I: Clinical findings and treatment. J Dermatol 2021, 48, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Katayama, I.; Suzuki, T.; Tanemura, A.; Ito, S.; Abe, Y.; Sumikawa, Y.; Yoshikawa, M.; Suzuki, K.; Yagami, A.; Masui, Y.; Ito, A.; Matsunaga, K. , Rhododendrol-induced leukoderma update II: Pathophysiology, mechanisms, risk evaluation, and possible mechanism-based treatments in comparison with vitiligo. J Dermatol 2021, 48, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Madhogaria, S.; Ahmed, I. , Leucoderma after use of a skin-lightening cream containing kojic dipalmitate, liquorice root extract and Mitracarpus scaber extract. Clin Exp Dermatol 2010, 35, e103–e105. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kondo, M.; Sato, K.; Umeda, M.; Kawabata, K.; Takahashi, Y.; Suzuki, T.; Matsunaga, K.; Inoue, S. , Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigment Cell Melanoma Res 2014, 27, 754–63. [Google Scholar] [CrossRef]
- Elmore, A. R. , Final report of the safety assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as used in cosmetics. Int J Toxicol 2005, 24 (Suppl. S2), 51–111. [Google Scholar]
- Kameyama, K.; Sakai, C.; Kondoh, S.; Yonemoto, K.; Nishiyama, S.; Tagawa, M.; Murata, T.; Ohnuma, T.; Quigley, J.; Dorsky, A.; Bucks, D.; Blanock, K. , Inhibitory effect of magnesium L-ascorbyl-2-phosphate (VC-PMG) on melanogenesis in vitro and in vivo. J Am Acad Dermatol 1996, 34, 29–33. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takehana, M.; Itoh, S.; Ogata, E. , Protective effect of magnesium-L-ascorbyl-2 phosphate against skin damage induced by UVB irradiation. Photochem Photobiol 1996, 64, 224–8. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, F.; Bangash, A. R.; Khushdil, A.; Noor, S. M. , Efficacy of Trichloro-Acetic Acid Peel Alone Versus Combined Topical Magnesium Ascorbyl Phosphate for Epidermal Melasma. J Coll Physicians Surg Pak 2016, 26, 557–61. [Google Scholar]
- Shaikh, Z. I.; Mashood, A. A. , Treatment of refractory melasma with combination of topical 5% magnesium ascorbyl phosphate and fluorescent pulsed light in Asian patients. Int J Dermatol 2014, 53, 93–9. [Google Scholar] [CrossRef]
- Silva, G. M.; Maia Campos, P. M. , Histopathological, morphometric and stereological studies of ascorbic acid and magnesium ascorbyl phosphate in a skin care formulation. Int J Cosmet Sci 2000, 22, 169–79. [Google Scholar] [CrossRef]
- Wang, P. C.; Huang, Y. L.; Hou, S. S.; Chou, C. H.; Tsai, J. C. , Lauroyl/palmitoyl glycol chitosan gels enhance skin delivery of magnesium ascorbyl phosphate. J Cosmet Sci 2013, 64, 273–86. [Google Scholar] [PubMed]
- Yamamoto, K.; Shichiri, H.; Ishida, T.; Kaku, K.; Nishioka, T.; Kume, M.; Makimoto, H.; Nakagawa, T.; Hirano, T.; Bito, T.; Nishigori, C.; Yano, I.; Hirai, M. , Effects of Ascorbyl-2-phosphate Magnesium on Human Keratinocyte Toxicity and Pathological Changes by Sorafenib. Biol Pharm Bull 2017, 40, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Foco, A.; Gasperlin, M.; Kristl, J. , Investigation of liposomes as carriers of sodium ascorbyl phosphate for cutaneous photoprotection. Int J Pharm 2005, 291, 21–9. [Google Scholar] [CrossRef]
- Nayama, S.; Takehana, M.; Kanke, M.; Itoh, S.; Ogata, E.; Kobayashi, S. , Protective effects of sodium-L-ascorbyl-2 phosphate on the development of UVB-induced damage in cultured mouse skin. Biol Pharm Bull 1999, 22, 1301–5. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Akhtar, N.; Ali, A.; Khan, H. M. S.; Sohail, M.; Naeem, M.; Nawaz, Z. , PHYSICAL AND CHEMICAL STABILITY ANALYSIS OF COSMETIC MULTI- PLE EMULSIONS LOADED WITH ASCORBYL PALMITATE AND SODIUM ASCORBYL PHOSPHATE SALTS. Acta Pol Pharm 2016, 73, 1339–1349. [Google Scholar]
- Ishikawa, Y.; Niwano, T.; Hirano, S.; Numano, K.; Takasima, K.; Imokawa, G. , Whitening effect of L-ascorbate-2-phosphate trisodium salt on solar lentigos. Arch Dermatol Res 2019, 311, 183–191. [Google Scholar] [CrossRef]
- Feather, J. W.; Ellis, D. J.; Leslie, G. , A portable reflectometer for the rapid quantification of cutaneous haemoglobin and melanin. Phys Med Biol 1988, 33, 711–22. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Kobayashi, T.; Miyagishi, M.; Higashi, K.; Yada, Y. , The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res 1997, 10, 218–28. [Google Scholar] [CrossRef] [PubMed]
- Niwano, T.; Terazawa, S.; Nakajima, H.; Imokawa, G. , The stem cell factor-stimulated melanogenesis in human melanocytes can be abrogated by interrupting the phosphorylation of MSK1: evidence for involvement of the p38/MSK1/CREB/MITF axis. Arch Dermatol Res 2018, 310, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Niwano, T.; Terazawa, S.; Sato, Y.; Kato, T.; Nakajima, H.; Imokawa, G. , Glucosamine abrogates the stem cell factor + endothelin-1-induced stimulation of melanogenesis via a deficiency in MITF expression due to the proteolytic degradation of CREB in human melanocytes. Arch Dermatol Res 2018, 310, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Ishida, K. , Inhibitors of intracellular signaling pathways that lead to stimulated epidermal pigmentation: perspective of anti-pigmenting agents. Int J Mol Sci 2014, 15, 8293–315. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Imokawa, G. , Hyperpigmentation mechanisms involved in UVB-melanosis and solar lentigo and clinical effects of Chamomira ET extract on the pigmentation. Mon. Book Derma. 2005, 98, 43–61. [Google Scholar]
- Imokawa, G. , Analysis of carbohydrate properties essential for melanogenesis in tyrosinases of cultured malignant melanoma cells by differential carbohydrate processing inhibition. J Invest Dermatol 1990, 95, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J. B.; Barker, D. J.; Ellis, D. J.; Grassam, E.; Cotterill, J. A.; Fisher, G. W.; Feather, J. W. , A theoretical and experimental study of light absorption and scattering by in vivo skin. Phys Med Biol 1980, 25, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Feather, J. W.; Hajizadeh-Saffar, M.; Leslie, G.; Dawson, J. B. , A portable scanning reflectance spectrophotometer using visible wavelengths for the rapid measurement of skin pigments. Phys Med Biol 1989, 34, 807–20. [Google Scholar] [CrossRef]
- Kollias, N.; Baqer, A. , Spectroscopic characteristics of human melanin in vivo. J Invest Dermatol 1985, 85, 38–42. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





