Submitted:
06 August 2024
Posted:
07 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Method

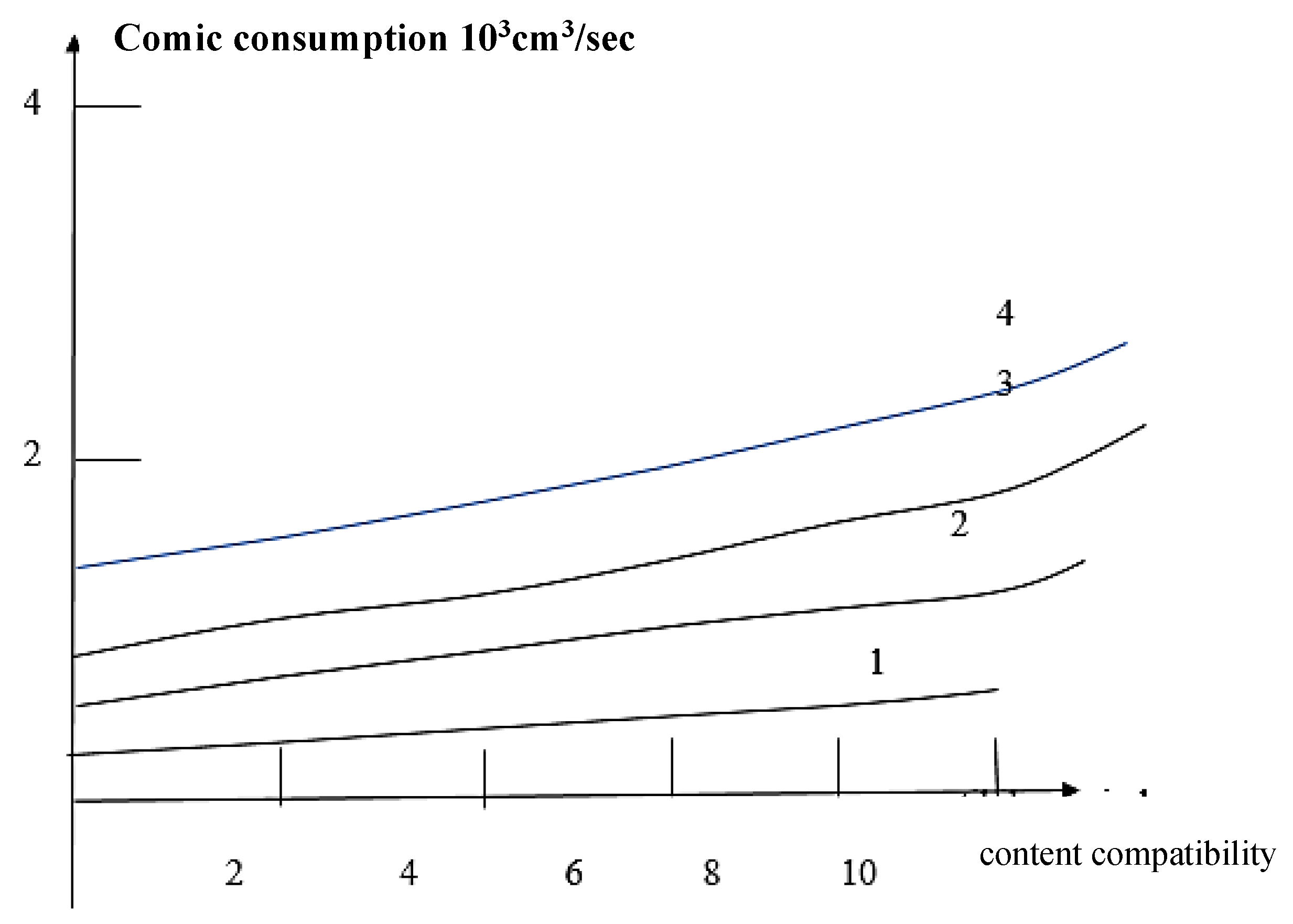
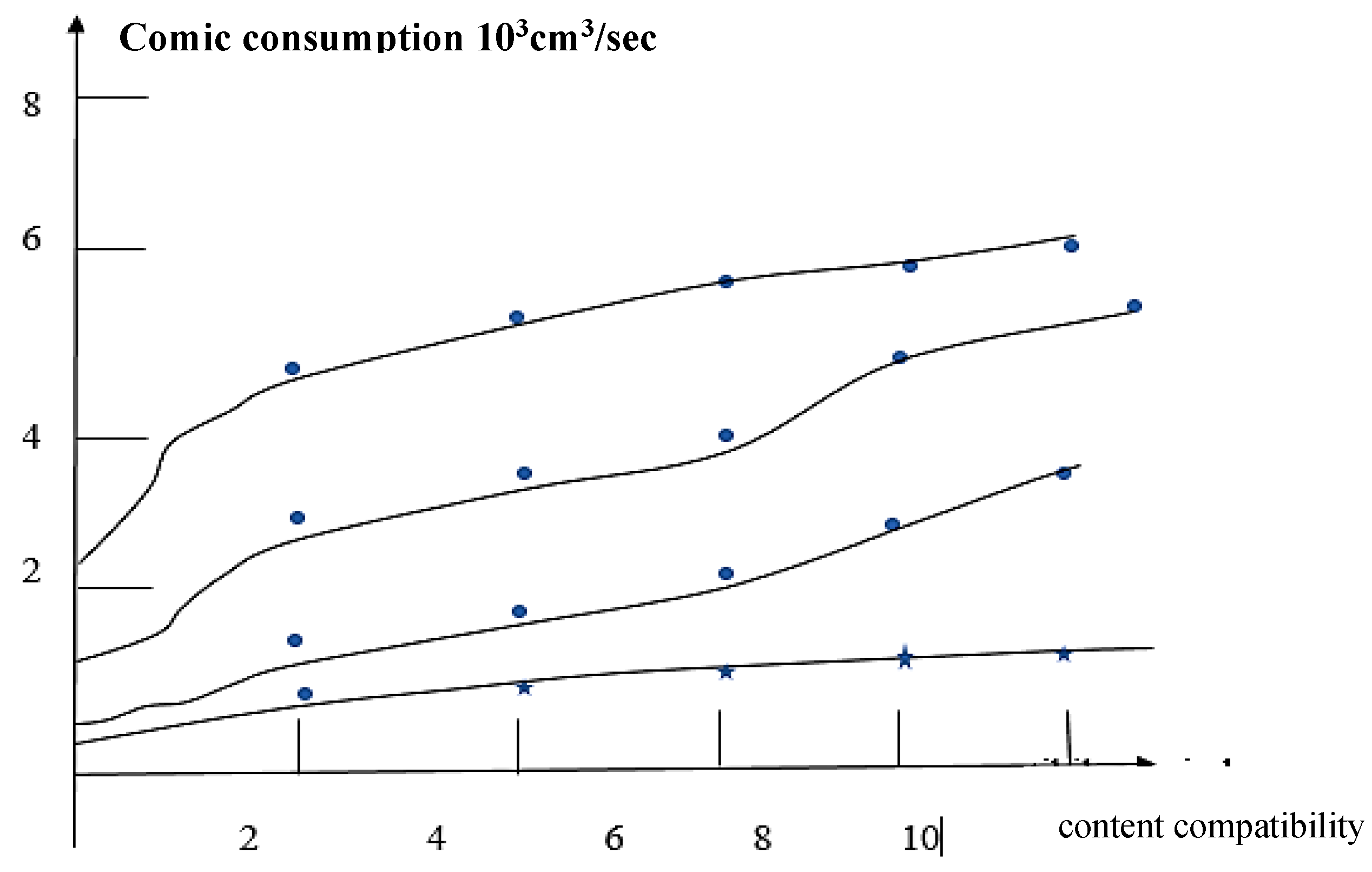
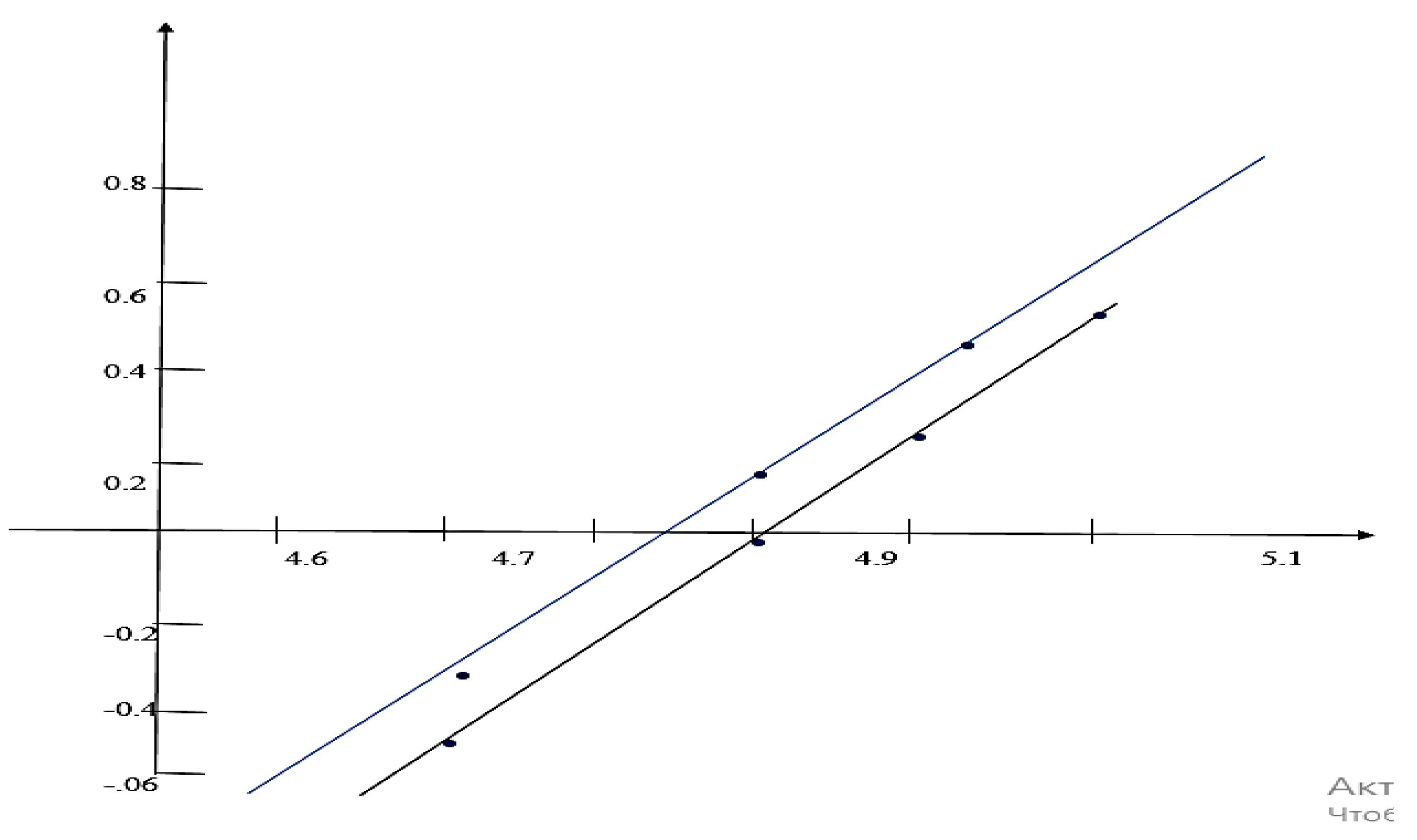
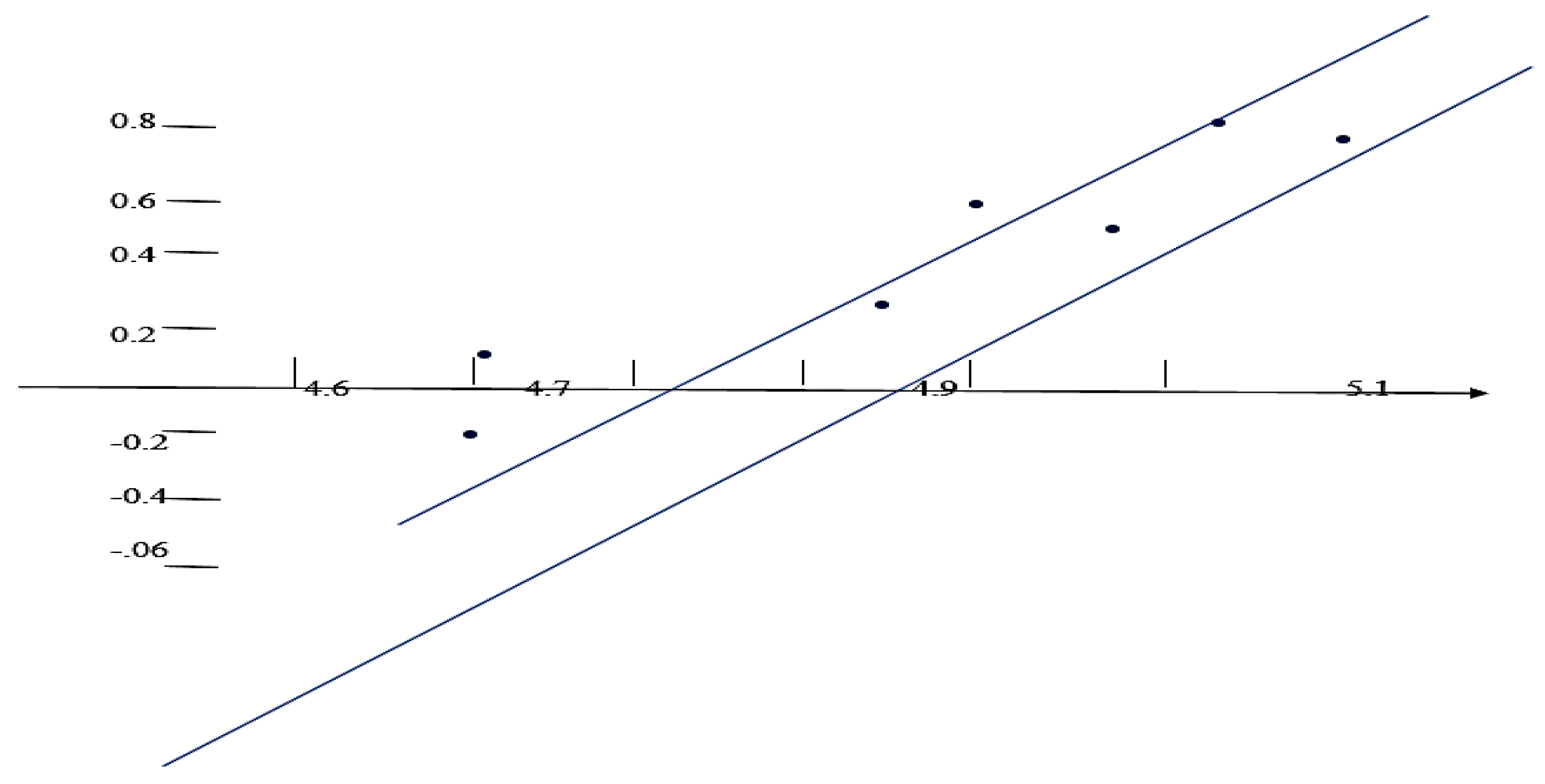
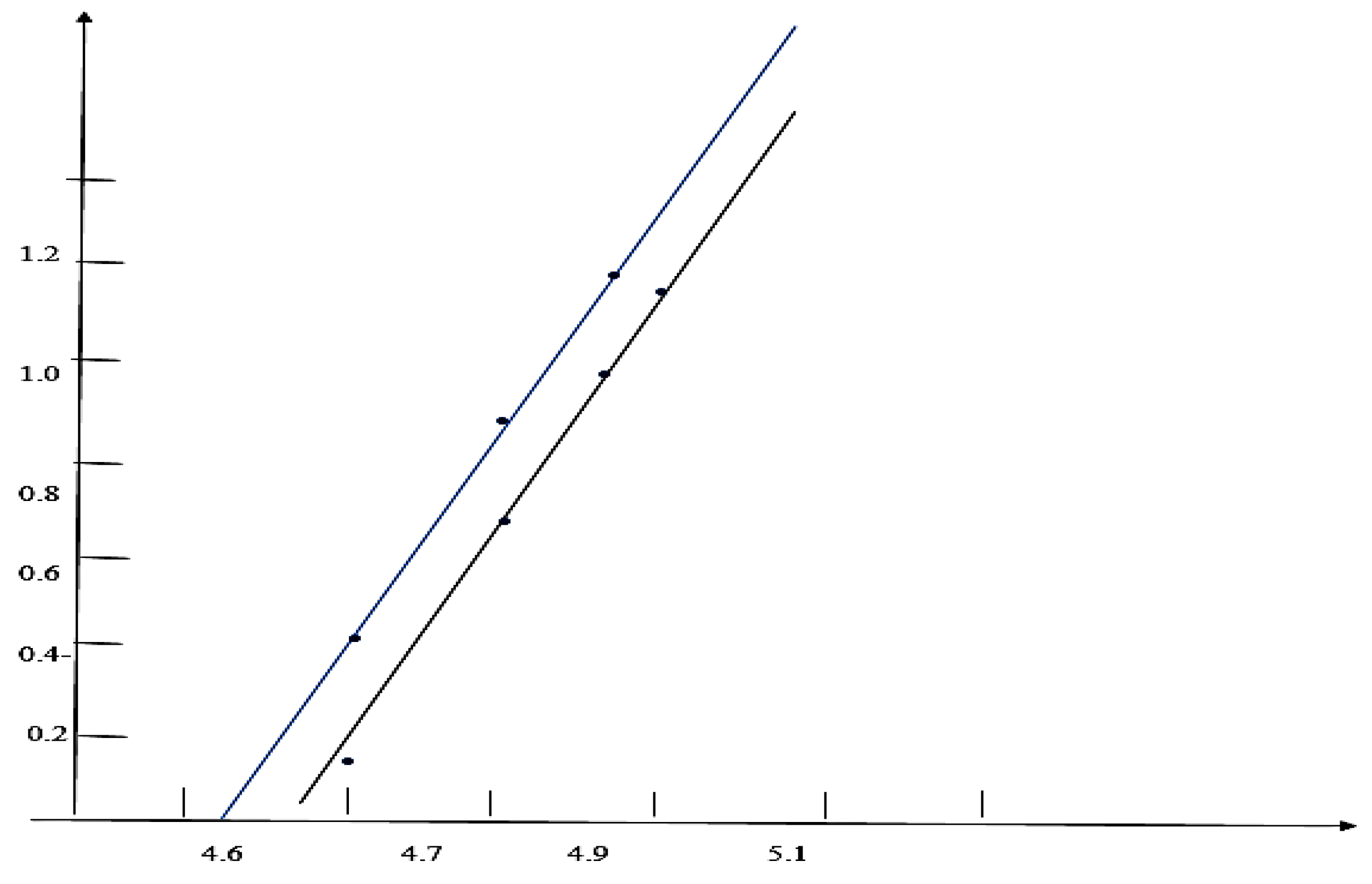
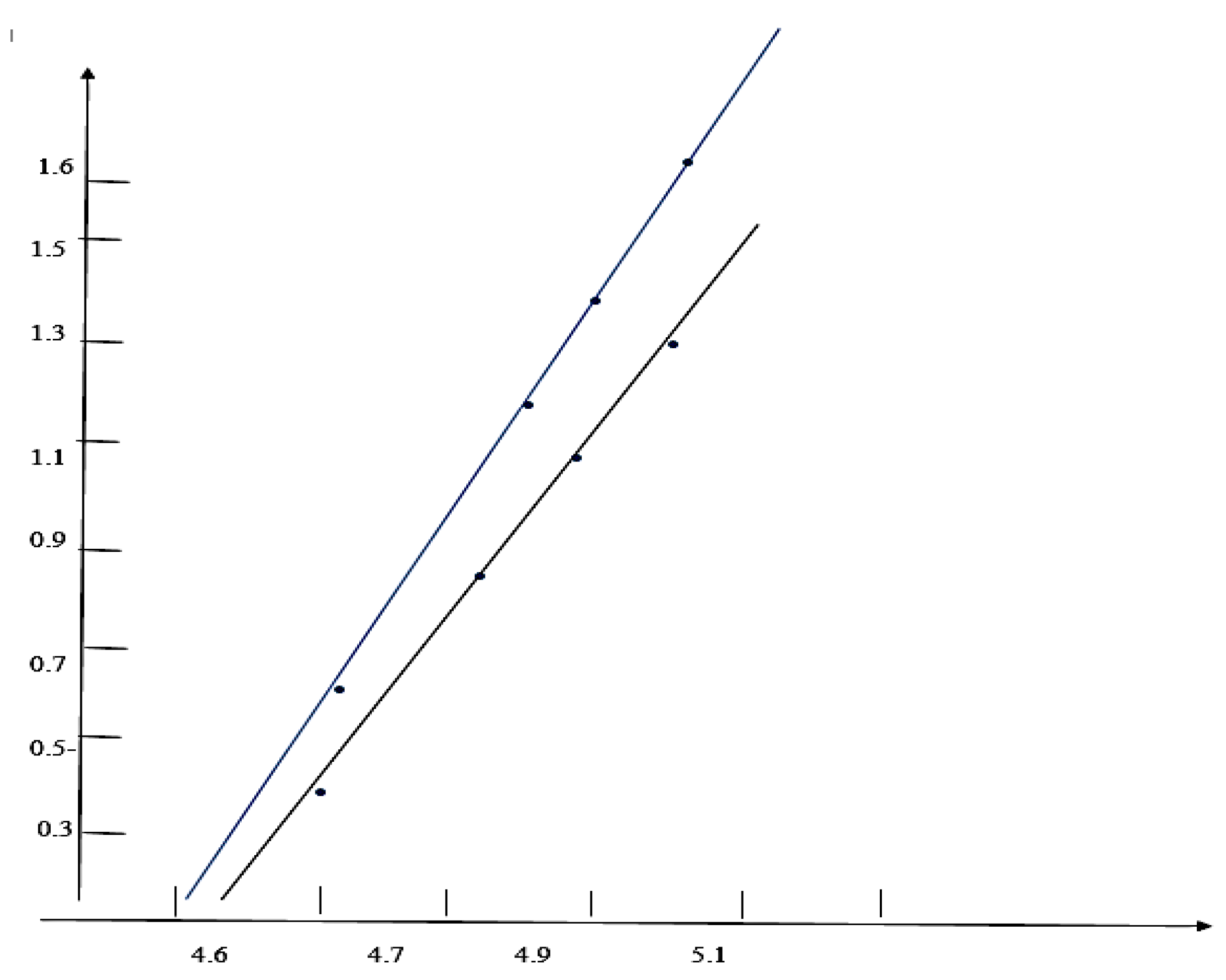
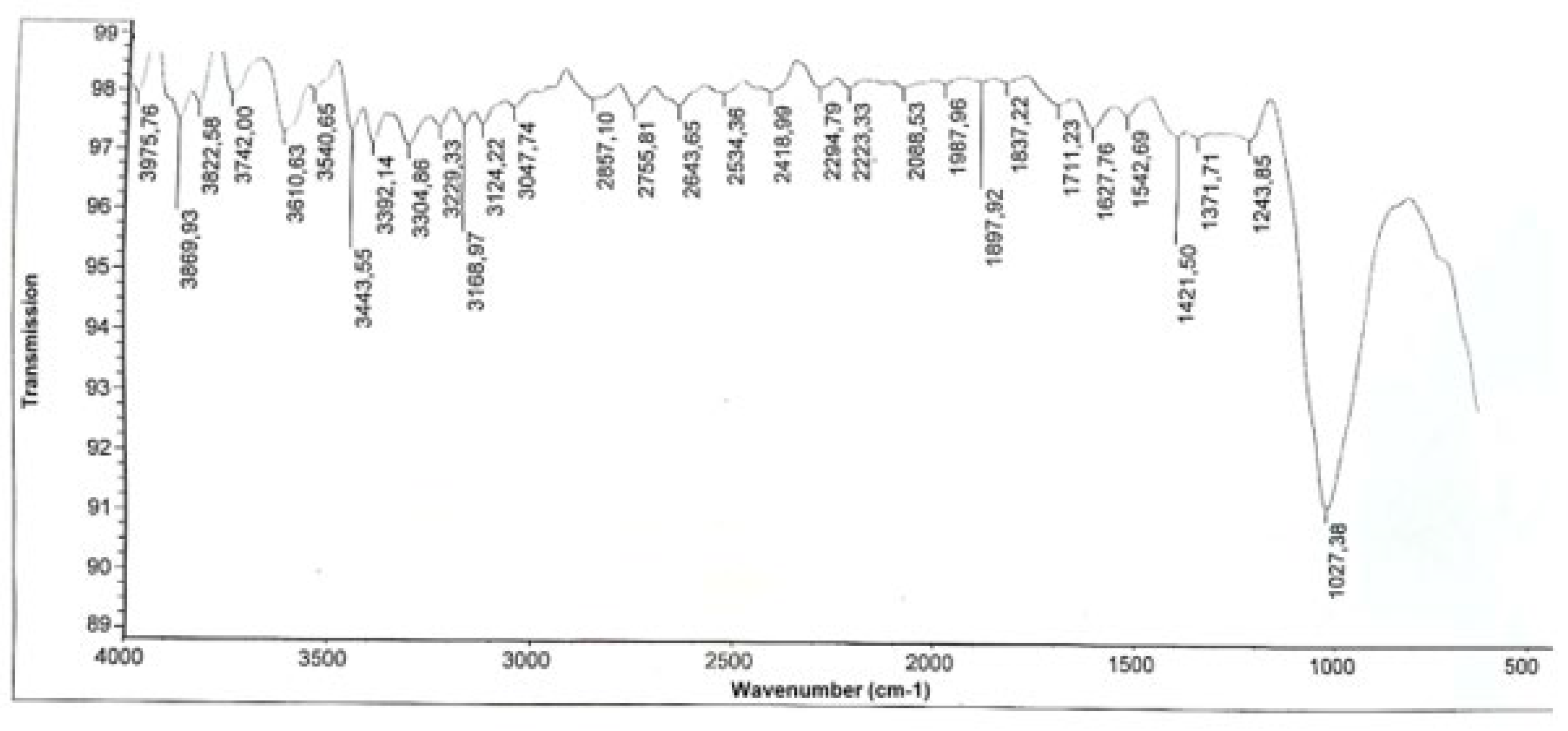
Results and Discussions Results of Experemental Research
Study of Rheological Properties
Rubber Mixture Based on BTFR and Astragalus
Results
References
- Ganbarov D.SH., İbrahimov A.Sh., Nabiyeva F. Geograpical areal types of Astracantha and Astragalus species spread in Nakhchivan Autonomus Republic // Kafkas Üniversitesi Fen bilimleri Enstitüsü Dergisi, 2011, pp.58-64.
- Ganbarov D.SH. Floristic analysis of the species of Astracantha and Astragalus spreadin the area of the Nakhchivan Autonomous Republic // European Academic Resears , 2013, İmpact Factor 0,485: p. 2586-2593.
- Ganbarov D.SH. Spreading of Astracantha and Astragalus species on the highland zones of the Nakhchivan autonomous republic // European Academic Research, 2014, İmpact Factor 3,1: p.-4153-4159 /.
- Ganbarov D.SH. Rear Astragalus species that spread in the NakhchivanAutonomous Republic territory // International Multidisciplinary Research Journal. Indian Streams Research Journal. 2014. P.1-4.
- Ganbarov D.SH. Astragalus Species and their Bioecological Features Spread in the Arajig Mountain of Julfa Region // European Academic Research, 2014, İmpact Factor 3,1: p. 3258-3265.
- Ganbarov D.SH. Systematic analysis of Astracantha species spread in the flora of Nakhchivan Autonomus Republic // International Journal of Scientific and Research Publications, 2014. p.1-2 17. 17.Ganbarov D.SH., Aliyeva S.E., Spreading of Astracantha and Astragalus species in the mountainous-cserophit plant zones of the Nakhchivan Autonomus Republic // e-Library Science Research Journal İmpact Factor 0,109: 2014. p.1-3.
- Ganbarov D.SH. Ecological groups of Astracantha and Astragalus species of Nakhchivan Autonomus Republic // European Academic Research, 2014, İmpact Factor 3,1: p.-1933-1937.
- Ganbarov D.SH. Spreading of Astracantha and Astragalus species of wild vegetation in the Nakhchivan Autonomus Republic flora // International Multidisciplinary e-Journal 2014, Scientific Journal İmpact Factor 3,5: ISRA Journal İmpact Factor 1,347, Universal İmpact Factor 1,0444 p.-50-55.
- Ganbarov D.SH., İbrahimov A.Sh. Astragalus dasyantus L. (Fabaceae) New species to the flora of Azerbaijan // International Journal of Multidisciplinary Research and Development 2015. p. 426-427.
- Seyfi, K.S.Modification of the used up polymeric materials and investigation thof e properties of the materials obtained Journal of Medical Pharmaceutical and Allied SciencesЭта ссылка oтключена., 2022, 11(2), pp 4697–4702.
- Seyfi, K.S.Development and implementation in industry technology of obtaining composite materials and products based on polyvinyl chloride Journal of Advanced Research in Dynamical and Control Systems, 2020, 12(2 Special Issue), pp 359–370.
- Seyfi, K.S.Paint and varnish materials based on epoxy novolac oligomers .Journal of Advanced Research in Dynamical and Control Systems, 2020, 12(Special Issue 2), pp 351–358.
- Maharamov TEYMUR. PHYSICO-MECHANICAL PROPERTIES OF PETROLEUM ROAD BITUMEN MODIFIED ON THE BASIS OF STYRENE-BUTADIENE BUTYL RUBBE XII international scientific conference. Philadelphia. USA. 21-22.03.2024.Pp34-40.
- Kerem Shixaliyev. Discontinued High-density and Low-density polyethylene purchase of environmentally friendly road surfaces, Journal of Harbin Engineering University ISSN: 1006-7043 Vol 45 No. 1 January 2024Page № 1: 422 – 430.
- Kerem Shixaliyev. DETERMINATION OF RUBBER-CORD CONNECTION ON THE BASIS OF LATEX SYNTHESIZED ON THE BASIS OF ETHYLENE-PROPYLENE RUBBER, China Petroleum Processing and Petrochemical Technology, Catalyst Research Volume 24, Issue 1, March 2024 Pp. 117-126 ISSN: 1008-6234.
- Kerem Shixaliyev. PROPERTIES OF THE COMPOSITION BASED ON MODIFIED POLYETHYLENES, Eur. Chem. Bull. ISSN 2063-5346, 2023; Volume -12, Special Issue-5 : Page: 242-258.
- Timofeev A.G. The effect of complex modification by processing products of secondary rubber materials and polymer waste (PET) on the properties of road bitumen // Vestnik TSTU. - Tver, -2017, - issue 4.-C.7-11.
- Zolotarev V.A. About quality indicators of bitumen modified with polymers - Kiiv, -2006 r - (Zbirnik of scientific articles); - sleep. 5; -C.200-231.
- Amirov Fariz Ali., Shixaliyev Kerem Sefi., Obtaning and application of rubber mixtures based on isopeene (SRI-3) and functional grup polimers. Austrian Journal of Technical and Natural Sciences Vienna. 2017.-№3-4.- P.274.
- Shikhaliev K.S. Modification of petroleum bitumen with polymer waste. Exact science and technology journal Kemerovo.-2017., - 46 p.
- Shykhaliev K. S. Alieva Z. N., Modification of petroleum bitumen with plastic waste. Problem of modern science and education 2017. 58p.
- Shixaliyev K.S. Exelolted thermoplastics based compositions. European science review. Scientific journal- No. 5-6, - 2017. -Vienna, -C. 89-94.
- Shykhaliev Karam Sefi. Compositions and products based on polyvinyl chloride. Sat. Articles of the X International Scientific and Practical Competition. Penza. -25 .07.2017.2017.-C.19-25.
- Shykhaliev Karam Sefi., Amirov Fariz Ali., Studies of the process of obtaining coated for various purposes on the basis of oil bitumen. Innovative development of science and education. (Monograph) .MTSS, science and education, Penza, 2017.-318 p.
- Amirov Fariz Ali., Shixaliyev Kerem Sefi., Obtaning and application of rubber mixtures based on isopeene (SRI-3) and functional grup polimers. Austrian Journal of Technical and Natural Sciences.-2017. - No. 3-4 Vienna - P.27-31.
- Kisina A.M. Polymer bituminous roofing and waterproofing material L .: Stroy published .1983. - 133 p.
- Zhenjun Zhang, Lina Zhang, Yang Li, Preparation and characterization of anionically polymerized butadiene-isoprene copolymer/clay nanocomposites, Journal of Applied Polymer Science, 10.1002/app.24400, 102, 2, (1167-1172), (2006). [CrossRef]
- Makoto Kato, Azusa Tsukigase, Hiromitsu Tanaka, Arimitsu Usuki, Isamu Inai, Preparation and properties of isobutylene–isoprene rubber–clay nanocomposites, Journal of Polymer Science Part A: Polymer Chemistry, 10.1002/pola.21233, 44, 3, (1182-1188), (2005). [CrossRef]
- Mroczkowska-Szerszeń M., Orzechowski M. Infrared Spectroscopy Methods in Reservoir Rocks Analysis—Semiquantitative Approach for Carbonate Rocks. Nafta-Gaz. 2018; 74:802–812. [CrossRef]
- Jarrahian K., Seiedi O., Sheykhan M., Sefti M.V., Ayatollahi S. Wettability Alteration of Carbonate Rocks by Surfactants: A Mechanistic Study. Colloids Surf. A Physicochem. Eng. Asp. 2012; 410:1–10. [CrossRef]
- Sönmez M., Juganaru M., Ficai A., Ficai D., Oprea O., Gurau D., Alexandrescu L., Stelescu M.D., Georgescu M., Nituica M., et al. Dolomite Surface Modification with Titanium and Silicon Precursors and Its Morphostructural and Thermal Characterisation; Proceedings of the 8th International Conference on Advanced Materials and Systems 2020; Bucharest, Romania. 1–3 October 2020;
- Shahraki B.K., Mehrabi B., Dabiri R. Thermal Behavior of Zefreh Dolomite Mine (Central Iran) J. Min. Metall. Sect. B Metall. 2009;45:35–44. [CrossRef]
- Gunasekaran S., Anbalagan G., Pandi S. Raman and Infrared Spectra of Carbonates of Calcite Structure. J. Raman Spectrosc. 2006;37:892–899. [CrossRef]
- Ji J., Ge Y., Balsam W., Damuth J.E., Chen J. Rapid Identification of Dolomite Using a Fourier Transform Infrared Spectrophotometer (FTIR): A Fast Method for Identifying Heinrich Events in IODP Site U1308. Mar. Geol. 2009;258:60–68. [CrossRef]
- Chen Y., Zhang X., Wang B., Lv M., Zhu Y., Gao J. Fabrication and Characterization of Novel Shape-Stabilized Stearic Acid Composite Phase Change Materials with Tannic-Acid-Templated Mesoporous Silica Nanoparticles for Thermal Energy Storage. RSC Adv. 2017;7:15625–15631. [CrossRef]
- Syed Bakar S.S., Zakaria N.S., Wahab J.A., Salleh M.A.A.M. Batu Reput Filled Recycled Polypropylene Eco-Polymer Composites. Bdg. Indones. Adv. Environ. Biol. 2013;7:3596–3600.
- Cepuritis R., Wigum B.J., Garboczi E.J., Mørtsell E., Jacobsen S. Filler from crushed aggregate for concrete: Pore structure, specific surface, particle shape and size distribution. Cem. Concr. Compos. 2014; 54:2–16. [CrossRef]
- Ganbarov D.SH. Ecological groups of Astracantha and Astragalus species of Nakhchivan Autonomous Republic // European Academic Research, 2014, Impact Factor 3.1: p.-1933-1937.
- Ganbarov D.SH. Spreading of Astracantha and Astragalus species of wild vegetation in the Nakhchivan Autonomous Republic flora // International Multidisciplinary e-Journal 2014, Scientific Journal Impact Factor 3.5: ISRA Journal Impact Factor 1.347, Universal Impact Factor 1.0444 p.-50-55.
- Ganbarov D.Sh. Bioecological features of species of the genus 35 Astragantha podlech in the Nakhchivan Autonomous Republic of Azerbaijan // Herald of the Altai State Agrarian University, Barnaul 2014. p.64-67.
- D.S. Qanbarov Distribution of Astracantha and Astragalus species in semi-desert vegetation in the territory of Nakhchivan Autonomous Republic // Nakhchivan State University Nakhchivan, 2014, No. 8, pp. 24-27.
- D.S. Qanbarov Distribution of Astragalus species in the middle mountainous zone in Nakhchivan Autonomous Republic // Nakhchivan State University Nakhchivan, 2014 #4, p. 57-61.
- Ganbarov D.SH., Ibrahimov A.Sh. Astragalus dasyantus L. (Fabaceae) New species to the flora of Azerbaijan // International Journal of Multidisciplinary Research and Development 2015. p. 426-427.
- Ganbarov D.SH., Ibrahimov A.Sh., New species and their bioecological features of Astragalus spread in the area of Nakhchivan Autonomous Republic // International Journal of Multidisciplinary Research and Development 2015. p. 696-697.
- Ganbarov D.SH. Spreading of Astracantha and Astragalus species in Nakhchivan AR subalpine and Alpine flora // European Academic Research, 2015, p. 15375-15379.
- Ganbarov D.SH., Gasumov H.Z. Useful features and usage methods of the herbs included in the Astragalus species // European Academic Research, 2015, p.-14306-14309.
- Ganbarov D.SH., Evaluation and productivity of the populations of Astracantha microcephala species // International Journal of Multidisciplinary Research and Development 2015. p. 104-107.
- Ganbarov D.Ş. Distribution of Astracantha and Astragalus species in the flora of Nakhchivan Autonomous Republic by altitude zones, phytocenological characteristics and significance // Nakhchivan State University, Gayret-2015 No. 4, pp. 44-53.
- Ganbarov D.S. Dye properties of some species belonging to the genera Astracantha and Astragalus distributed in the territory of Nakhchivan Autonomous Republic // Actual problems of modern biology and chemistry. Ganja State University, 2015, p. 32-36.
- Vagizova, R.R., Effect of the composition of rubbers based on radiation-induced butyl rubber reclaim on the properties / R.R. Vagizova, Yu.N. Khakimullin, P.A. Stepanov // Collection of scientific papers of young scientists and specialists Cheboksary, Chuvashia University - 2006 - pp. 185-190.
- Vagizova, R.R., Possibilities of using radiation-induced butyl rubber reclaim in roofing and waterproofing materials / R.R. Vagizova, Yu.N. Khakimullin, A. Stepanov // Construction materials - 2007 - No. 6 - pp. 2-4.
- Vagizova, R.R., Influence of the nature of cross-linking on radiation destruction of butyl rubber vulcanizates // Proceedings of the XIII International Conference of Students, Postgraduates and Young Scientists “Lomonosov” T IV M Publishing House of Moscow State University -2006 -pp406-407.
- Kharlov V.A., Radiation devulcanizate R-5 as a full-fledged substitute for butyl rubber in compositions / V. A. Kharlov, V. Yu. Saburov, R. R. Vagiyuva, P. A. Stepanov, Yu. N. Khakimullin // Proceedings of the XI All-Russian scientific and practical conference “Rubber industry raw materials, materials, technologies”, Moscow -2005 - pp. 213.
- Vagizova, R. R., Radiation destruction of rubbers based on radiation reclaimed butyl rubber / R. R. Vagizova, P. A. Stepanov, Yu. N. Khakimullin // Proceedings of the III All-Russian scientific conference “Physicochemistry of polymer processing processes”, - Ivanovo, IHTU - 2006 - pp. 92.
- Vagizova, R. R., Features of obtaining radiation reclaimed butyl rubber under γ-irradiation and under the action of accelerated electrons / R. R. Vagizova, Yu. N. Khakimullin // Abstracts of the IV All-Russian Karginsky Conference “Polymer Science in the 21st Century” Moscow - 2007.pp234-339.
- Harmful Substances in Industry. T. Z. Ed. by N. V. Lazarev and I. D. Gadaskina. - L.: Chemistry, 1977, pp. 303-307.
- Polymer Composite Materials: Structure, Properties, Technology: Textbook // M. L. Kerber et al.; Ed. by A. A. Berlin. St. Petersburg: Profession,2008. 560 .P.
- Jafarov V.D., Babaeva G.R., Veliyev I.V. Creation of highly filled compositions based on LDPE, kaolin and polymer dressing // Bulletin of the Azerbaijan Engineering Academy, 2013. Vol. 5. No. 2. pp. 83-87.
- Jafarov V.D. Study of the influence of fillers on the supramolecular structure and properties of polyethylene composites. // Processes of petrochemistry and oil refining, 2005. No. 2. pp. 63-81.
- Babaeva G.R., Bektashi S.A., Alkhazov T.I. and others. Influence of particle dispersion on the strength characteristics of highly filled polymer composites based on low-density polyethylene. / Collection of materials of the International scientific and practical conference dedicated to the 1150th anniversary of the Persian-Tajik scientist-encyclopedist, physician, alchemist and philosopher Abu Bakr Muhammad ibn Zakariya Razi, Dushanbe, May 27-28, 2015. pp.148-149.
- Andruz J., Brimblecombe P., Gickels T., Liss P. Introduction to Environmental Chemistry (translated from English) Moscow, Mir, 1999, 271 P.
- Man and His Environment. Reader. Edited by G. V. Lisichkin and N. N. Chernov. Moscow: Mir, 2003. 460 P.
- Shixaliyev K., Maharramov T., Alizade T., Mustafayev A. PHYSICO-MECHANICAL PROPERTIES OF PETROLEUM ROAD BITUMEN MODIFIED ON THE BASIS OF STYRENE-BUTADIENE BUTYL RUBBE, Journal “WORLD OF CONFERENCES” (Philadelphia, USA 2024), Page: 34-39.
| RECIPE | ||
| Main components |
Standard resin mixture prepared based on polybutylene terephthalate (100 parts by mass, part by mass according to rubber) |
The rubber mixture prepared based on polybutylene terephthalate modified with nanomaterials (100 parts by mass, part by mass by rubber) |
| Polietilen tereftalat | 100 | 90 |
| tree resin | - | 2.5 |
| Astragalus | - | 10 |
| Petroleum bitumen | 5.0 | 5.0 |
| Zinc oxide | 3.0 | 3.0 |
| Stearic acid | 2.0 | 2.0 |
| Sulphur | 2.0 | 2.0 |
| Tiuram | 1.5 | 1.5 |
| Sulfenamide | 1.0 | 1.0 |
| Neazon-D Technical carbon H330 |
3.0 30 |
3.0 30 |
| The main indicators of vulcanization | Death limit | Requirements of the standard | The result obtained in practice |
| Relative elongation | % | 300 | 500 |
| Relative residual deformation | % | 3,9 | 2,9 |
| Elasticity (Bending Modulus) | GPa | 3 | 4 |
| Hardness Rockwell M | GPa | 70 | 90 |
| Hardness Area D | GPa | 90 | 95 |
| Stiffness (bending modulus) | GPa | 3 | 4 |
| Ultimate breaking strength | MPa | 40 | 50 |
| Durability (At Room Temp Notched Izod Effect) |
J/m | 29 | 45 |
| Low Temperature Hardness | J/m | 45 | 87 |
| Yung’s Modulus | GPa | 2 | 3 |
| Electrical property | Electrical property of vulcanizate | Electrical property of vulcanizate | Electrical property of vulcanizate |
| Arc Resistance | sec | 123 | 180 |
| Dielectric constant | 2.9 | 4 | |
| Dielectric Strength | kV/mm | 23 | 31 |
| Dissipation factor | 10-200 x 10 -4 | 10-200 x 10 -4 | 10-200 x 10 -4 |
| Volume resistance | Ohm.sm | 15 x 10 15 | 18 x 10 15 |
| Shrinkage | % | 0,6 | 2,1 |
| Water absorption 24 hours | % | 0,11 | 0,24 |
| Density | q/sm 3 | 1,3 | 1,39 |
| Glass transition temperature | °C | 55 | 64 |
| Coefficient of linear thermal expansion | °C | 6 x 10 -5 | 10 x 10 -5 |
| Thermal conductivity | Vt/mK | 0,21 | 0,27 |
| Resistance to fire (LOI) | % | 20 | 23 |
| Learning class | HB | ||
| Brittle transition temperature | °C | -32 | -39 |
| HDT @0.46 MPa (67 psi) | °C | 123 | 148 |
| HDT @1.8 MPa (264 psi) | °C | 50 | 85 |
| Maximum Continuous Service | °C | 90 | 141 |
| Minimum Operating Temperature | °C | -34 | -40 |
| No | Name of key indicators | Key indicators | |||||
| 1 | Requirements of the standard | Results from the experiment | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Dartilmada şərti möhkəmlik, MPa | 17 | 16,7 | 16,6 | 18.1 | 18,9 | 17,9 | |
| 2 | Relative elongation, % | 320 | 330 | 300 | 360 | 380 | 395 |
| 3 | Relative residual deformation,% | 12 | 14 | 12 | 12 | 12,2 | 12,5 |
| 4 | Tensile strength, kN/m | 65 | 64 | 68 | 68,3 | 69,6 | 69,2 |
| 5 | Friction, cm3/Wh | 59 | 60 | 56 | 60 | 59 | 58,5 |
| 6 | Metal bond strength, MPa:steel-3brass | 5,8- | 5,4- | 5,53,6 | 85,1 | 9,26,2 | 9,86,9 |
| 7 | Brittleness temperature, oC | -11 | - | 28 | 26 | 25 | 24 |
| 8 | TM-2 üzrə möhkəmlik, şərti vahid | 82 | 80 | 78 | 82 | 81,6 | 82,1 |
| 9 | 2525oC for 24 hoursdegree of swelling at temperature, %:in an isooctane-toluene mixture(70:30)in gasoline-benzene mixture (3:1) | 12- | 14- | 14,523,5 | 10,114,7 | 10,213,9 | 10,914,1 |
| 10 | 25oC for 48 hoursheat aging at tempcoefficients | 0,850,64 | 0,870,62 | 1,030,88 | 0,920,83 | 0,940,86 | 0.950.90 |
| 11 | Elasticity on jump, % | 10 | 11 | 14 | 13,7 | 14 | 13.8 |
| 12 | 25oC for 27 hoursozone resistance at temp(deformation 20%, ozonehardness 0.015 %) | is falling apart | is falling apart | is falling apart | is falling apart | is falling apart | is falling apart |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




