Submitted:
01 August 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
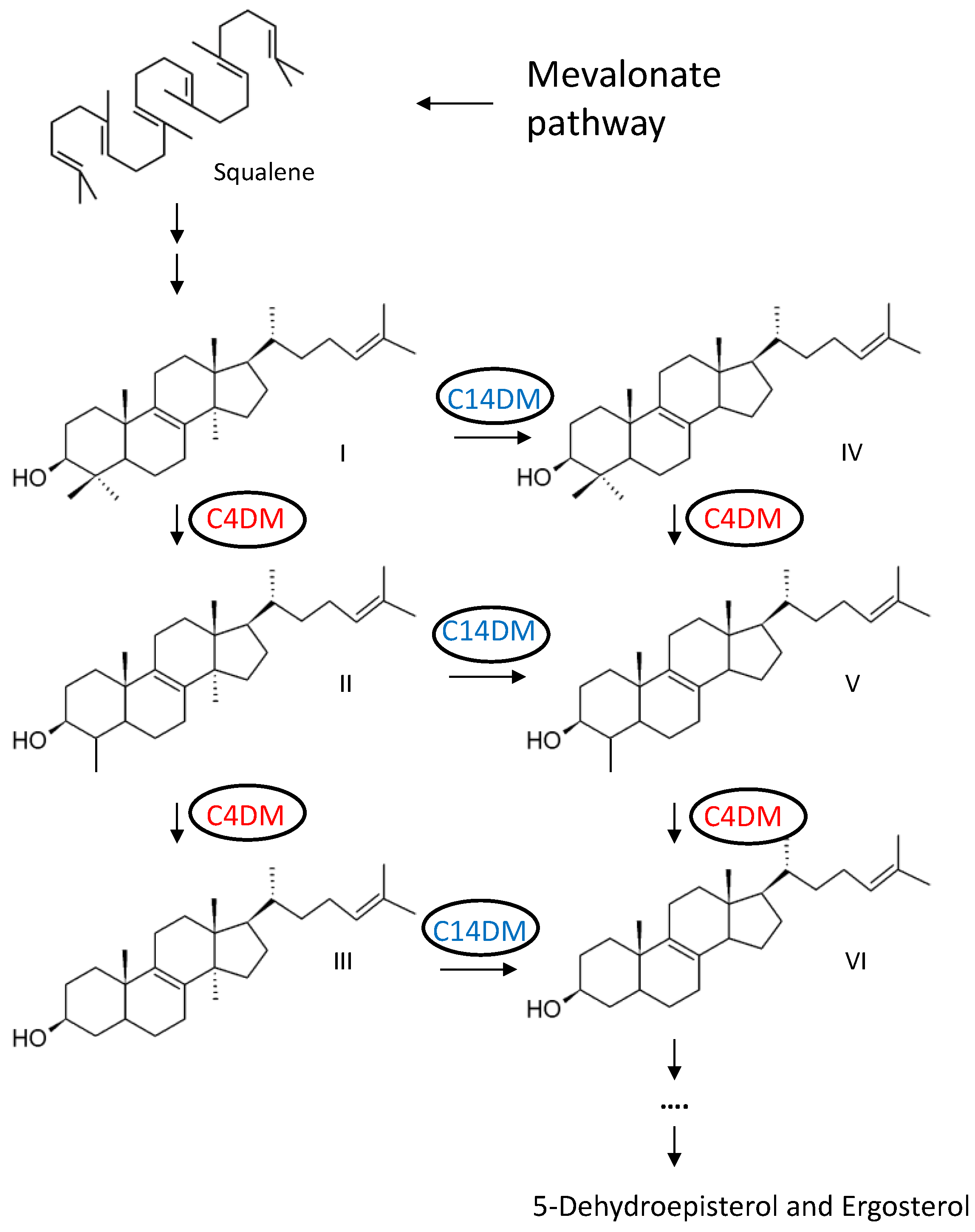
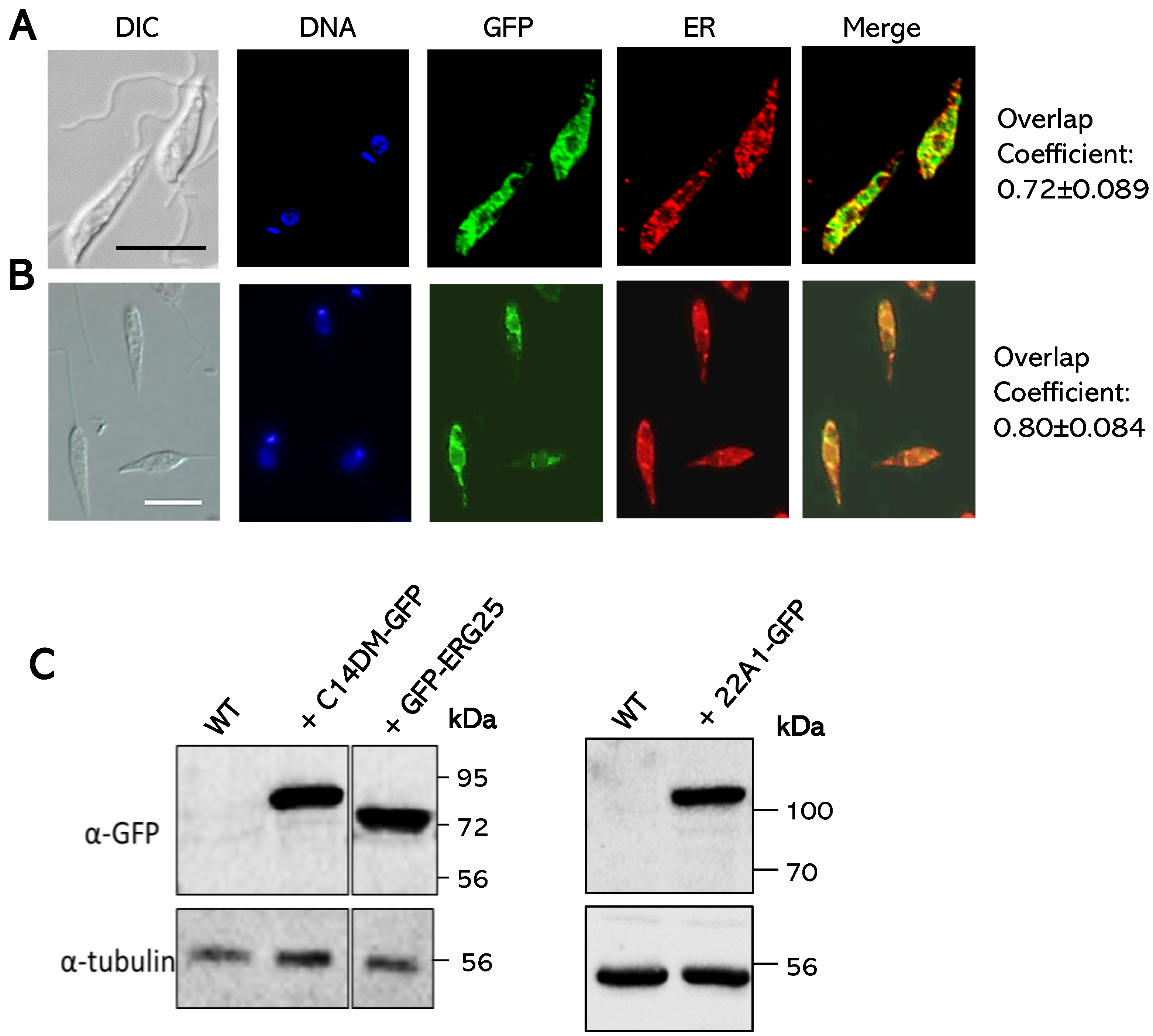
2.1. Identification and Cellular Localization of ERG25 and CYP5122A1 (22A1) in L. major
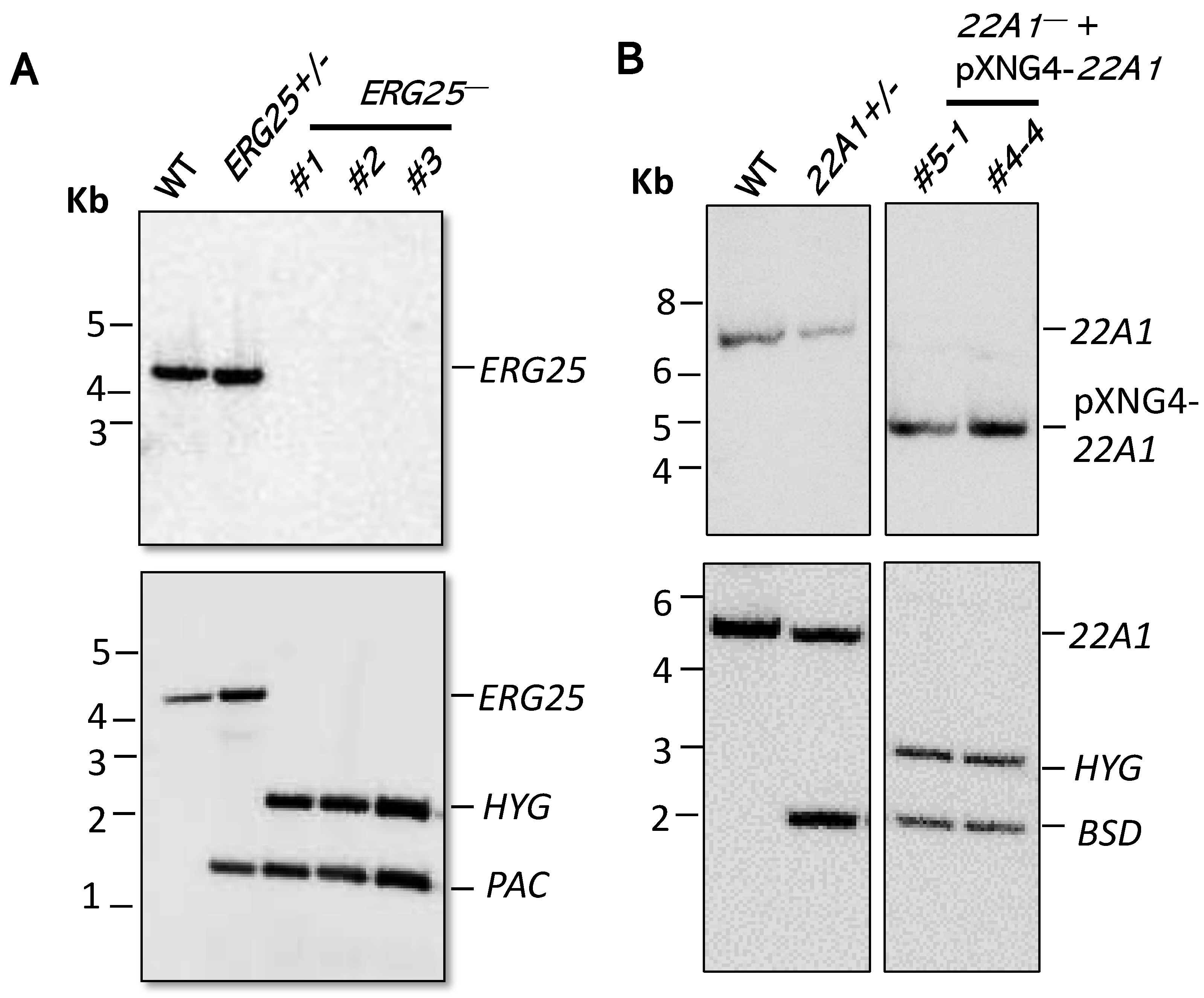
2.2. Successful Generation of ERG25-Null but not 22A1-Null Mutant in L. major
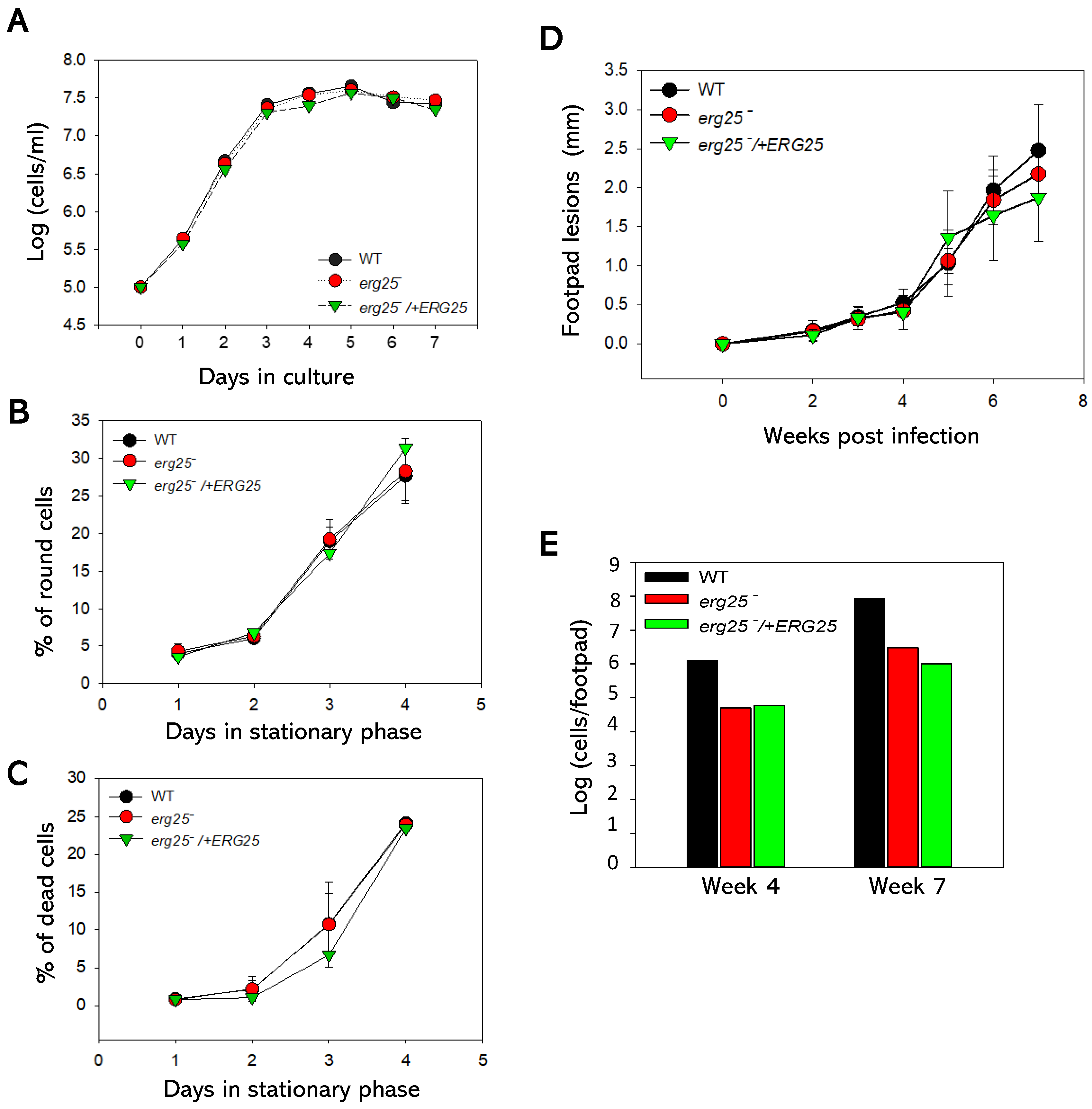
2.3. ERG25 is not Required for the Growth or Virulence of L. major Promastigotes.
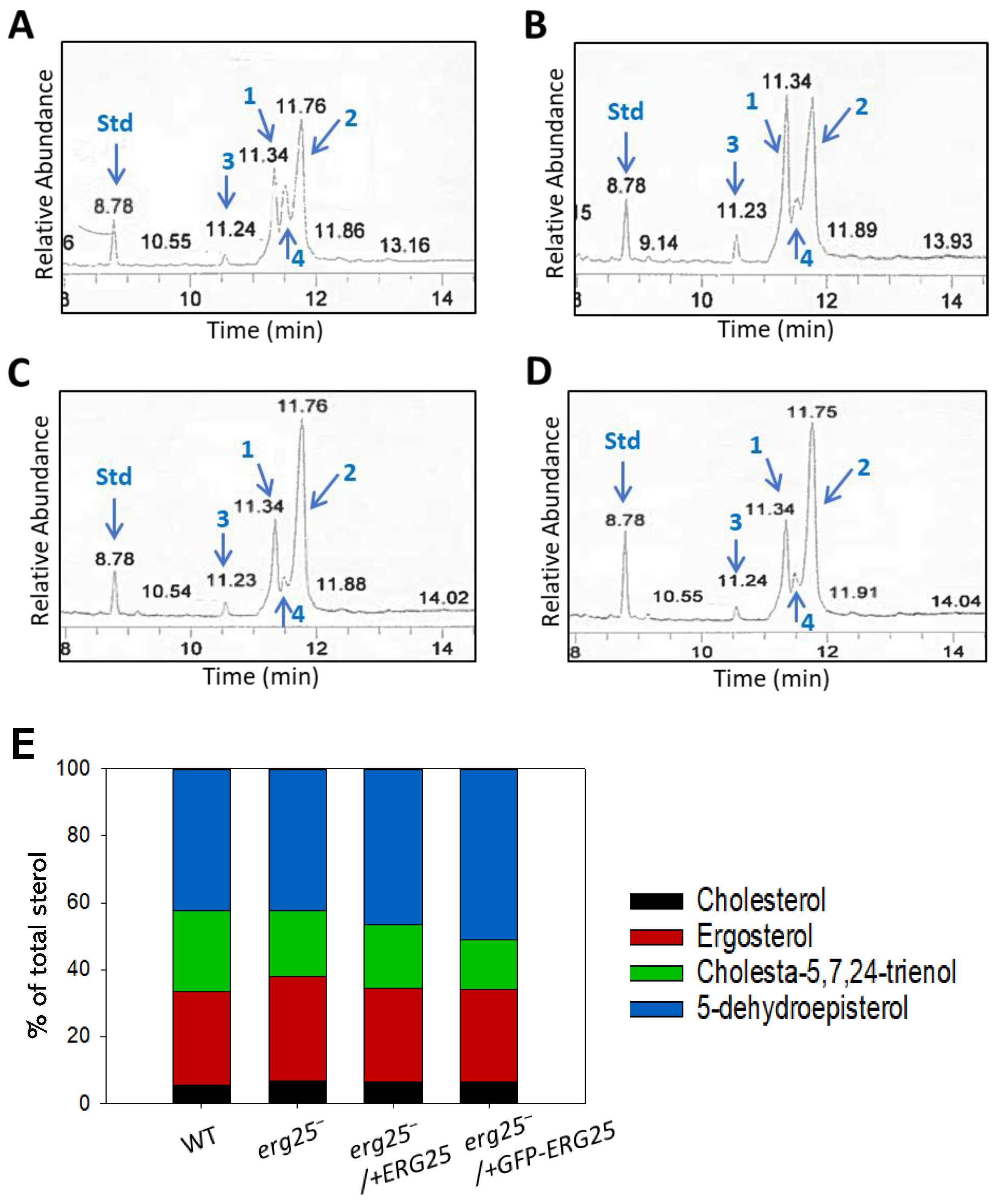
2.4. ERG25 Deletion or Overexpression Does Not Affect Sterol Composition in L. major
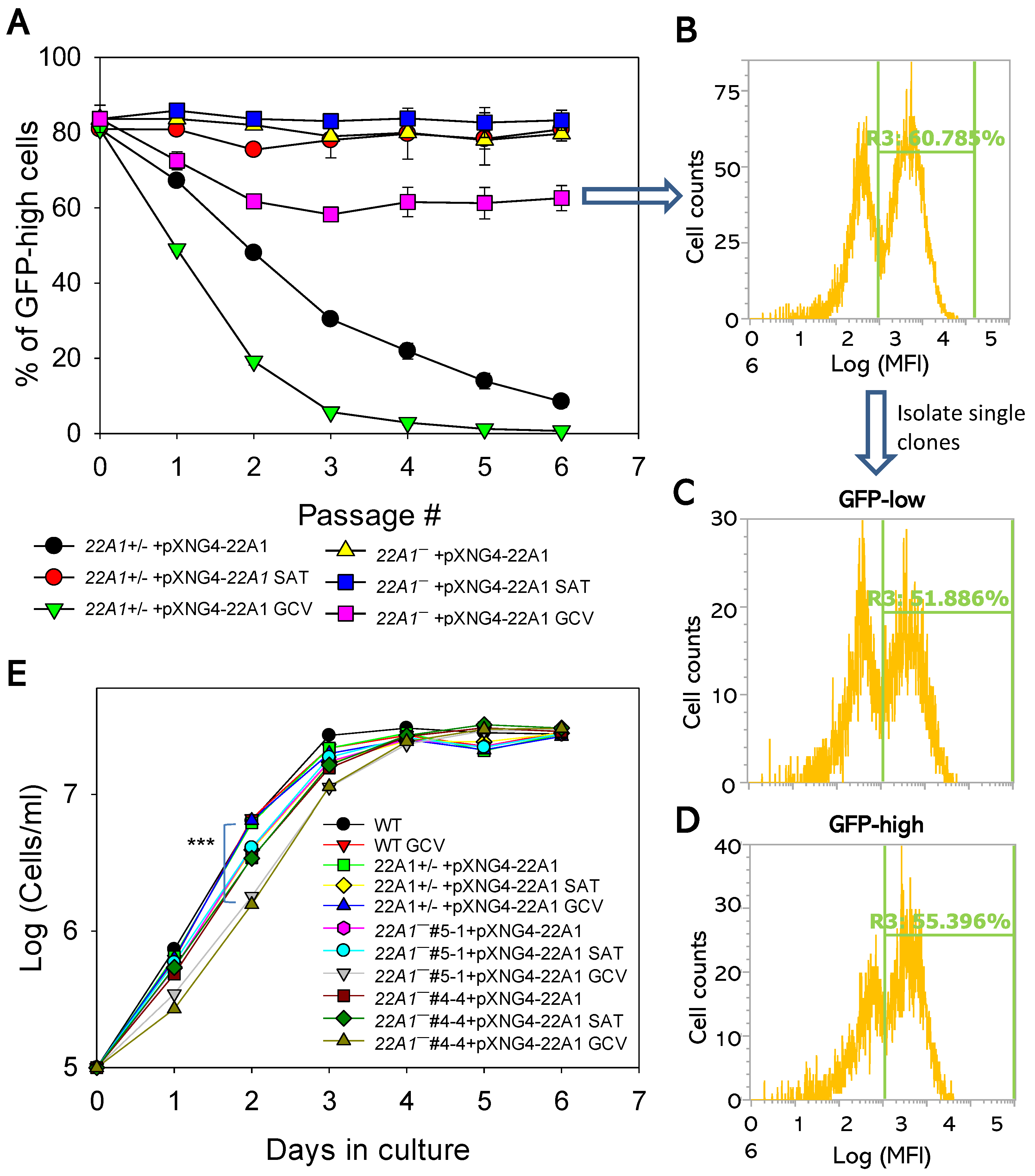
2.522. A1 is Indispensable during the Promastigote Stage of L. major
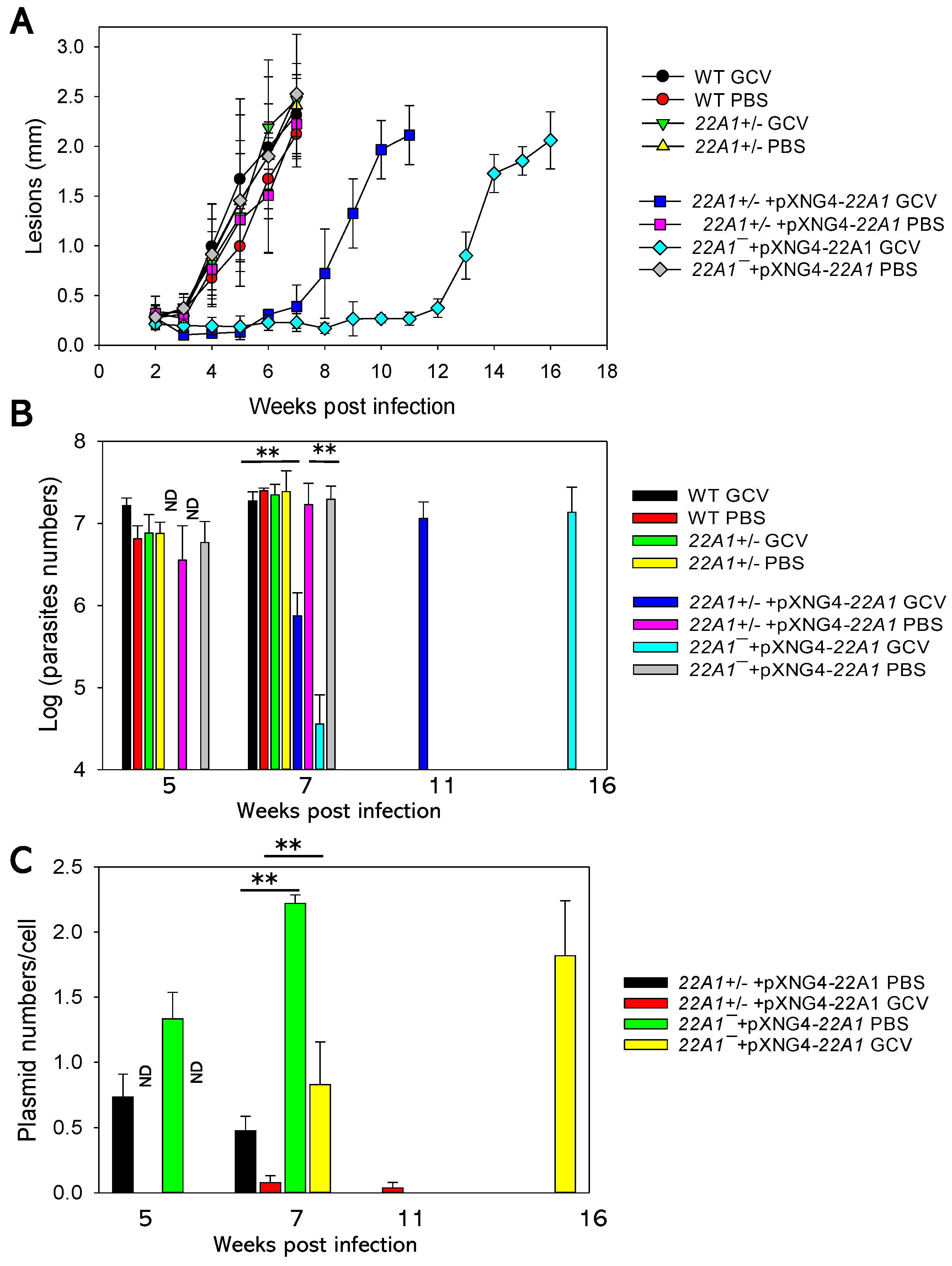
2.622. A1 is Essential for L. major during the Amastigote Stage
2.722. A1 Overexpression Confers Resistance to Posaconazole and DB766 but Does Not Affect Sterol Composition or Stress Response in L. major
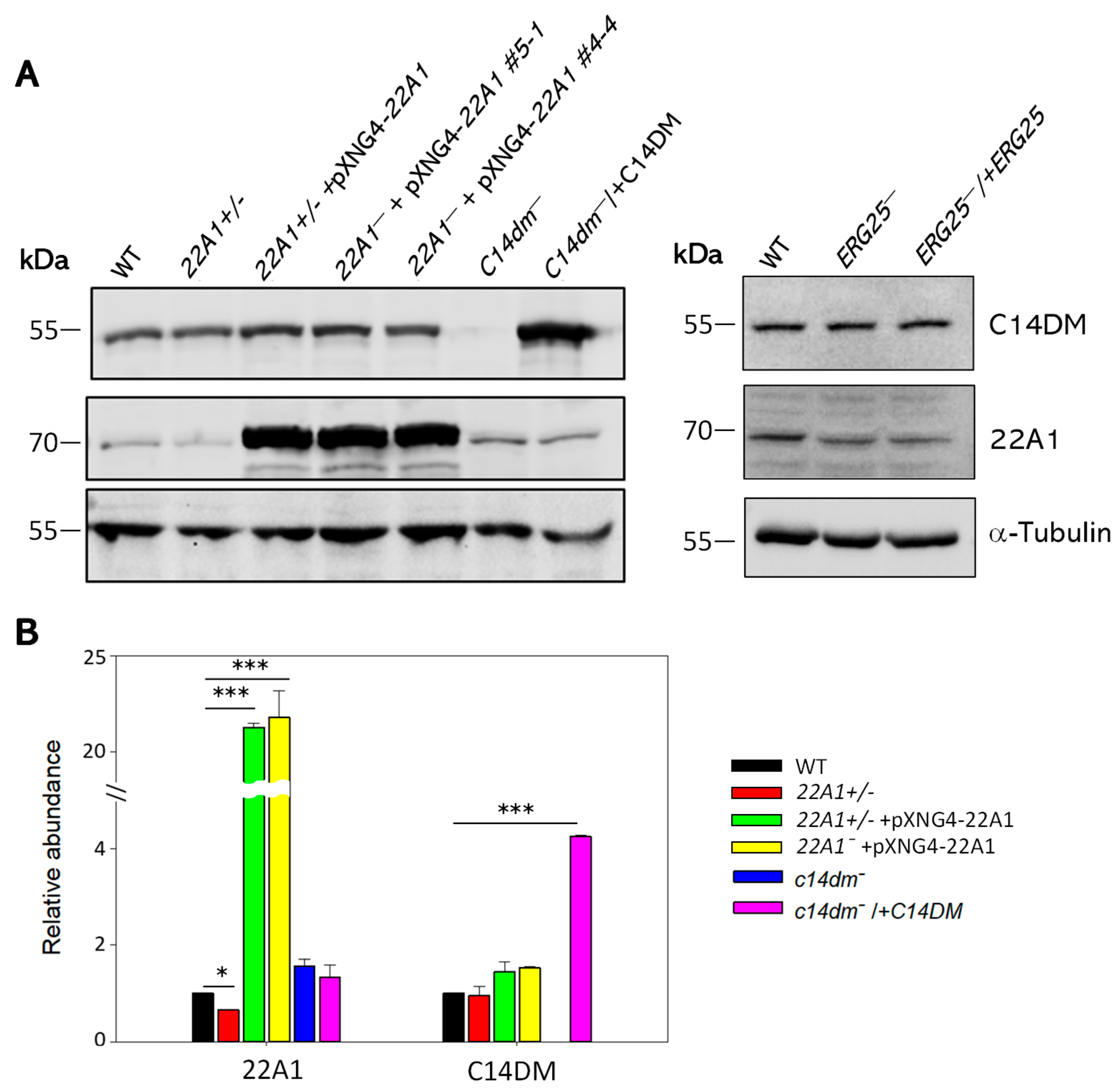
2.8. Genetic Manipulation of 22A1 or ERG25 Do Not Affect the Expression Level of C14DM
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Molecular Constructs
4.3. Leishmania Promastigote Culture and Growth Rate Measurement
4.4. Leishmania Genetic Manipulation and Southern Blot
4.5. Sterol Analysis by Gas Chromatography and Mass Spectrometry (GC-MS) and Liquid Chromatograph Tandem Mass Spectrometry (LC-MS/MS)
4.6. Immunofluorescence Microscopy and Western Blot
4.7. GCV Treatment of Promastigotes
4.8. Mouse Footpad Infections
4.9. QPCR Analyses
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Animal use
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pace, D. Leishmaniasis. The Journal of infection 2014, 69 Suppl 1, S10-18. [CrossRef]
- Croft, S.L.; Olliaro, P. Leishmaniasis chemotherapy--challenges and opportunities. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2011, 17, 1478-1483. [CrossRef]
- Beach, D.H.; Goad, L.J.; Holz, G.G., Jr. Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Molecular and biochemical parasitology 1988, 31, 149-162. [CrossRef]
- Lepesheva, G.I.; Waterman, M.R. Sterol 14alpha-demethylase (CYP51) as a therapeutic target for human trypanosomiasis and leishmaniasis. Curr Top Med Chem 2011, 11, 2060-2071. [CrossRef]
- Lepesheva, G.I.; Waterman, M.R. Structural basis for conservation in the CYP51 family. Biochimica et biophysica acta 2011, 1814, 88-93. [CrossRef]
- Xu, W.; Hsu, F.F.; Baykal, E.; Huang, J.; Zhang, K. Sterol Biosynthesis Is Required for Heat Resistance but Not Extracellular Survival in Leishmania. PLoS pathogens 2014, 10, e1004427. [CrossRef]
- Mukherjee, S.; Moitra, S.; Xu, W.; Hernandez, V.; Zhang, K. Sterol 14-alpha-demethylase is vital for mitochondrial functions and stress tolerance in Leishmania major. PLoS pathogens 2020, 16, e1008810. [CrossRef]
- Bard, M.; Bruner, D.A.; Pierson, C.A.; Lees, N.D.; Biermann, B.; Frye, L.; Koegel, C.; Barbuch, R. Cloning and characterization of ERG25, the Saccharomyces cerevisiae gene encoding C-4 sterol methyl oxidase. Proceedings of the National Academy of Sciences of the United States of America 1996, 93, 186-190. [CrossRef]
- Gachotte, D.; Barbuch, R.; Gaylor, J.; Nickel, E.; Bard, M. Characterization of the Saccharomyces cerevisiae ERG26 gene encoding the C-3 sterol dehydrogenase (C-4 decarboxylase) involved in sterol biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 1998, 95, 13794-13799. [CrossRef]
- Gachotte, D.; Sen, S.E.; Eckstein, J.; Barbuch, R.; Krieger, M.; Ray, B.D.; Bard, M. Characterization of the Saccharomyces cerevisiae ERG27 gene encoding the 3-keto reductase involved in C-4 sterol demethylation. Proceedings of the National Academy of Sciences of the United States of America 1999, 96, 12655-12660. [CrossRef]
- Rahier, A. Dissecting the sterol C-4 demethylation process in higher plants. From structures and genes to catalytic mechanism. Steroids 2011, 76, 340-352. [CrossRef]
- Pascal, S.; Taton, M.; Rahier, A. Plant sterol biosynthesis. Identification and characterization of two distinct microsomal oxidative enzymatic systems involved in sterol C4-demethylation. The Journal of biological chemistry 1993, 268, 11639-11654. [CrossRef]
- Jin, Y.; Basu, S.; Feng, M.; Ning, Y.; Munasinghe, I.; Joachim, A.M.; Li, J.; Madden, R.; Burks, H.; Gao, P.; et al. CYP5122A1 encodes an essential sterol C4-methyl oxidase in Leishmania donovani and determines the antileishmanial activity of antifungal azoles. Res Sq 2023. [CrossRef]
- Verma, S.; Mehta, A.; Shaha, C. CYP5122A1, a novel cytochrome P450 is essential for survival of Leishmania donovani. PloS one 2011, 6, e25273. [CrossRef]
- La Rosa, C., et al. N-substituted-4-(pyridin-4-ylalkyl)piperazine-1-carboxamides and Related Compounds as Leishmania CYP51 and CYP5122A1 Inhibitors Bioorganic & medicinal chemistry 2024.
- Bangs, J.D.; Uyetake, L.; Brickman, M.J.; Balber, A.E.; Boothroyd, J.C. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. Journal of cell science 1993, 105 ( Pt 4), 1101-1113.
- Cruz, A.; Beverley, S.M. Gene replacement in parasitic protozoa. Nature 1990, 348, 171-173. [CrossRef]
- Mukherjee, S.; Xu, W.; Hsu, F.F.; Patel, J.; Huang, J.; Zhang, K. Sterol methyltransferase is required for optimal mitochondrial function and virulence in Leishmania major. Molecular microbiology 2019, 111, 65-81. [CrossRef]
- Ha, D.S.; Schwarz, J.K.; Turco, S.J.; Beverley, S.M. Use of the green fluorescent protein as a marker in transfected Leishmania. Molecular and biochemical parasitology 1996, 77, 57-64. [CrossRef]
- Murta, S.M.; Vickers, T.J.; Scott, D.A.; Beverley, S.M. Methylene tetrahydrofolate dehydrogenase/cyclohydrolase and the synthesis of 10-CHO-THF are essential in Leishmania major. Molecular microbiology 2009, 71, 1386-1401. [CrossRef]
- Wang, M.Z.; Zhu, X.; Srivastava, A.; Liu, Q.; Sweat, J.M.; Pandharkar, T.; Stephens, C.E.; Riccio, E.; Parman, T.; Munde, M.; et al. Novel arylimidamides for treatment of visceral leishmaniasis. Antimicrobial agents and chemotherapy 2010, 54, 2507-2516. [CrossRef]
- Ohvo-Rekila, H.; Ramstedt, B.; Leppimaki, P.; Slotte, J.P. Cholesterol interactions with phospholipids in membranes. Prog Lipid Res 2002, 41, 66-97. [CrossRef]
- Zhang, K.; Hsu, F.F.; Scott, D.A.; Docampo, R.; Turk, J.; Beverley, S.M. Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Molecular microbiology 2005, 55, 1566-1578. [CrossRef]
- Boitz, J.M.; Gilroy, C.A.; Olenyik, T.D.; Paradis, D.; Perdeh, J.; Dearman, K.; Davis, M.J.; Yates, P.A.; Li, Y.; Riscoe, M.K.; et al. Arginase Is Essential for Survival of Leishmania donovani Promastigotes but Not Intracellular Amastigotes. Infection and immunity 2017, 85. [CrossRef]
- Ilg, T. Proteophosphoglycans of Leishmania. Parasitology today (Personal ed 2000, 16, 489-497.
- Zhang, K. Balancing de novo synthesis and salvage of lipids by Leishmania amastigotes. Current opinion in microbiology 2021, 63, 98-103. [CrossRef]
- He, M.; Kratz, L.E.; Michel, J.J.; Vallejo, A.N.; Ferris, L.; Kelley, R.I.; Hoover, J.J.; Jukic, D.; Gibson, K.M.; Wolfe, L.A.; et al. Mutations in the human SC4MOL gene encoding a methyl sterol oxidase cause psoriasiform dermatitis, microcephaly, and developmental delay. The Journal of clinical investigation 2011, 121, 976-984. [CrossRef]
- Blosser, S.J.; Merriman, B.; Grahl, N.; Chung, D.; Cramer, R.A. Two C4-sterol methyl oxidases (Erg25) catalyse ergosterol intermediate demethylation and impact environmental stress adaptation in Aspergillus fumigatus. Microbiology (Reading) 2014, 160, 2492-2506. [CrossRef]
- Kennedy, M.A.; Johnson, T.A.; Lees, N.D.; Barbuch, R.; Eckstein, J.A.; Bard, M. Cloning and sequencing of the Candida albicans C-4 sterol methyl oxidase gene (ERG25) and expression of an ERG25 conditional lethal mutation in Saccharomyces cerevisiae. Lipids 2000, 35, 257-262. [CrossRef]
- Benveniste, P. Biosynthesis and accumulation of sterols. Annu Rev Plant Biol 2004, 55, 429-457. [CrossRef]
- Darnet, S.; Bard, M.; Rahier, A. Functional identification of sterol-4alpha-methyl oxidase cDNAs from Arabidopsis thaliana by complementation of a yeast erg25 mutant lacking sterol-4alpha-methyl oxidation. FEBS Lett 2001, 508, 39-43. [CrossRef]
- Lee, A.K.; Banta, A.B.; Wei, J.H.; Kiemle, D.J.; Feng, J.; Giner, J.-L.; Welander, P.V. C-4 sterol demethylation enzymes distinguish bacterial and eukaryotic sterol synthesis. Proceedings of the National Academy of Sciences 2018, 115, 5884-5889. [CrossRef]
- Ning, Y.; Frankfater, C.; Hsu, F.F.; Soares, R.P.; Cardoso, C.A.; Nogueira, P.M.; Lander, N.M.; Docampo, R.; Zhang, K. Lathosterol Oxidase (Sterol C-5 Desaturase) Deletion Confers Resistance to Amphotericin B and Sensitivity to Acidic Stress in Leishmania major. mSphere 2020, 5. [CrossRef]
- Cosentino, R.O.; Agüero, F. Genetic profiling of the isoprenoid and sterol biosynthesis pathway genes of Trypanosoma cruzi. PloS one 2014, 9, e96762. [CrossRef]
- Kapler, G.M.; Coburn, C.M.; Beverley, S.M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol 1990, 10, 1084-1094.
- Zhang, O.; Wilson, M.C.; Xu, W.; Hsu, F.F.; Turk, J.; Kuhlmann, F.M.; Wang, Y.; Soong, L.; Key, P.; Beverley, S.M.; et al. Degradation of host sphingomyelin is essential for Leishmania virulence. PLoS pathogens 2009, 5, e1000692. [CrossRef]
- Zhang, K., Barron, T., Turco, S. J., and Beverley, S. M. The LPG1 gene family of Leishmania major. Mol. Biochem. Parasitol. 2004, 136, 11-23. PMC Journal – In Process. [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of biological chemistry 1957, 226, 497-509.
- Feng, M.; Jin, Y.; Yang, S.; Joachim, A.M.; Ning, Y.; Mori-Quiroz, L.M.; Fromm, J.; Perera, C.; Zhang, K.; Werbovetz, K.A.; et al. Sterol profiling of Leishmania parasites using a new HPLC-tandem mass spectrometry-based method and antifungal azoles as chemical probes reveals a key intermediate sterol that supports a branched ergosterol biosynthetic pathway. Int J Parasitol Drugs Drug Resist 2022, 20, 27-42. [CrossRef]
- de Ibarra, A.A.; Howard, J.G.; Snary, D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology 1982, 85 (Pt 3), 523-531. [CrossRef]
- Titus, R.G.; Marchand, M.; Boon, T.; Louis, J.A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol 1985, 7, 545-555. [CrossRef]
- Basu, S.; Pawlowic, M.C.; Hsu, F.F.; Thomas, G.; Zhang, K. Ethanolaminephosphate cytidylyltransferase is essential for survival, lipid homeostasis and stress tolerance in Leishmania major. PLoS pathogens 2023, 19, e1011112. [CrossRef]
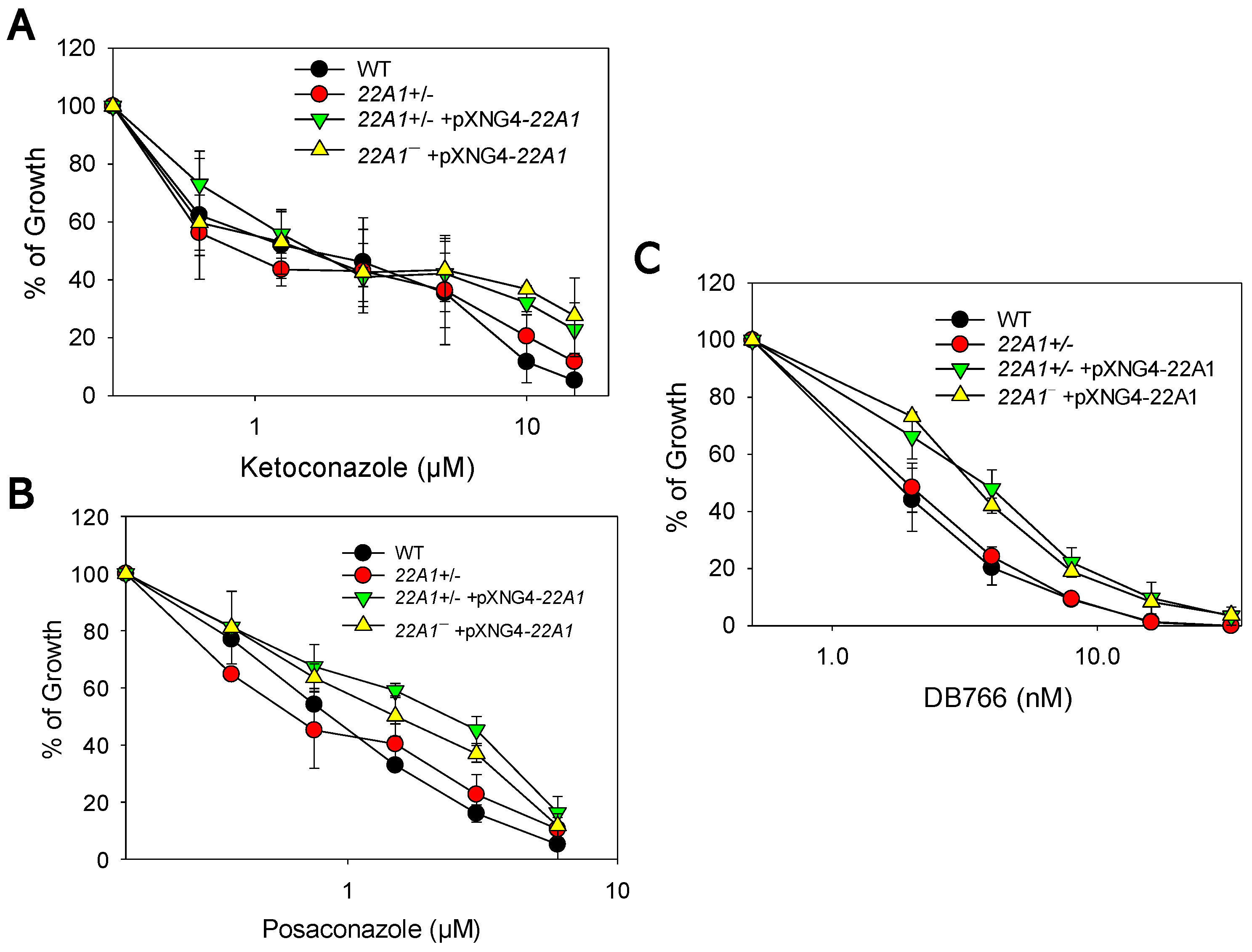
| Inhibitor | Effective concentration (EC) | WT | 22A1+/‒ | 22A1+/‒ +pXNG4-22A1 | 22A1‒ +pXNG4-22A1 |
| Posaconazole | EC25 (µM ± SD) | 0.29 ± 0.089 | 0.21 ± 0.041 | 0.48 ± 0.15 | 0.47 ± 0.14 |
| EC50 (µM ± SD) | 1.0 ± 0.096 | 0.74 ± 0.18 | 3.0 ± 1.0* | 1.7 ± 0.38* | |
| DB766 | EC25 (nM ± SD) | 0.90 ± 0.12 | 0.96 ± 0.16 | 1.6 ± 0.33 | 1.9 ± 0.094 |
| EC50 (nM ± SD) | 1.9 ± 0.31 | 2.04 ± 0.41 | 3.8 ± 0.68** | 3.5 ± 0.19** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





