Submitted:
31 July 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
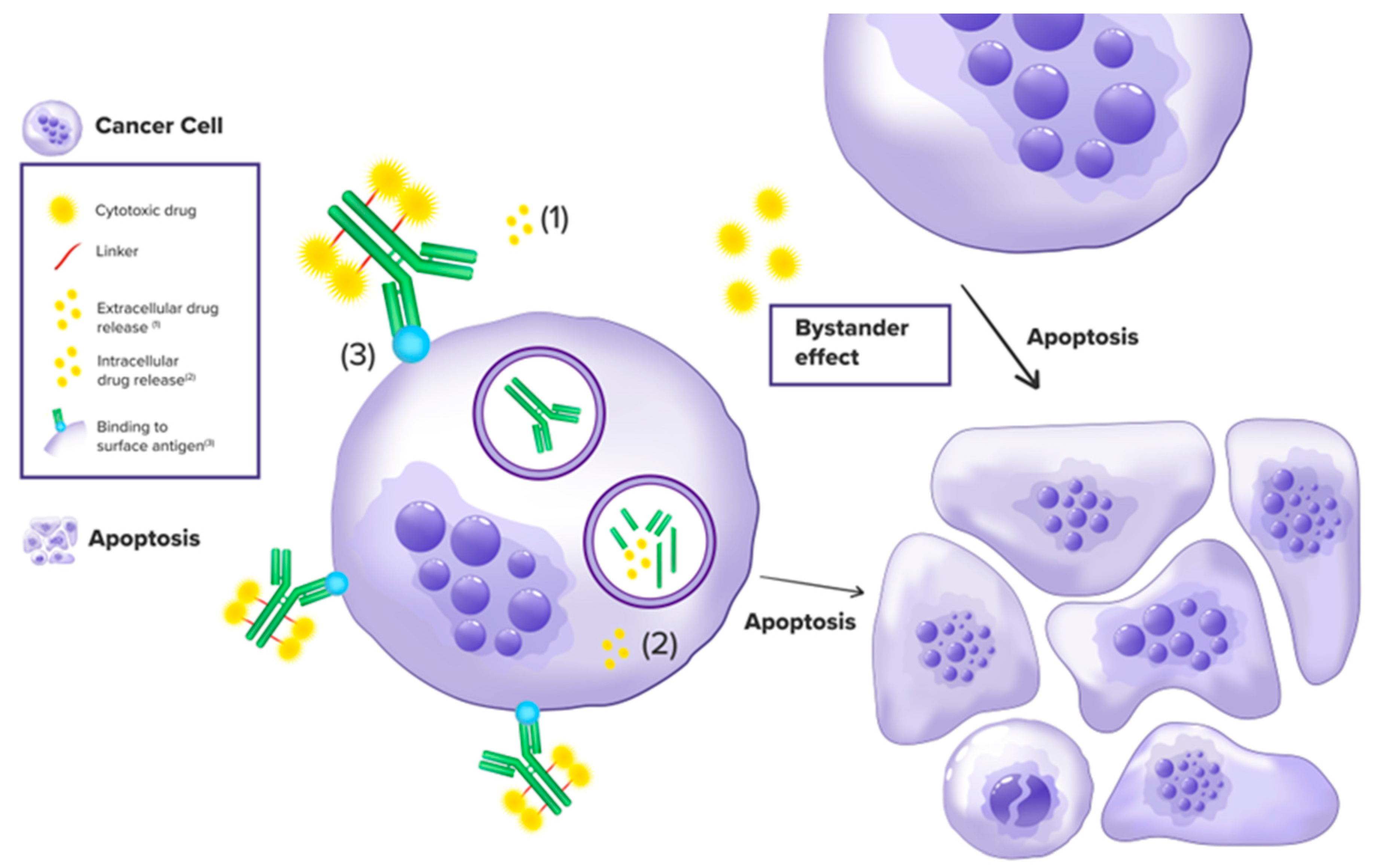
2. Mechanisms of Action, Toxicity and Resistance
2.1. Mechanisms of Action
2.2. Toxicity
2.3. Resistance
3. ADCs and Urothelial Cancer
3.1. Nectin 4
3.1.1. Enforumab Vedotin
3.2. TROP-2
3.2.1. Sagituzumab Govitecan
3.2.2. Datopotamab Deruxtecan
3.3. HER2
3.3.1. Trastuzumab Deruxtecan
3.3.2. Disitamab Vedotin
3.4. EPCAM
3.4.1. Oportuzumab Monatox (Vicineum)
3.5. Tissue Factor
3.5.1. Tisotumab Vedotin
4. Discussion and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R. L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M. D.; Chen, G. J.; Oh, W. K.; Bellmunt, J.; Roth, B. J.; Petrioli, R.; Dogliotti, L.; Dreicer, R.; Sonpavde, G. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol 2012, 23, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Sonpavde, G.; Pond, G. R.; Choueiri, T. K.; Mullane, S.; Niegisch, G.; Albers, P.; Necchi, A.; Di Lorenzo, G.; Buonerba, C.; Rozzi, A.; et al. Single-agent Taxane Versus Taxane-containing Combination Chemotherapy as Salvage Therapy for Advanced Urothelial Carcinoma. Eur Urol 2016, 69, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Pistamaltzian, N.; Tzannis, K.; Pissanidou, V.; Peroukidis, S.; Milaki, G.; Karavasilis, V.; Mitsogiannis, I.; Varkarakis, I.; Papatsoris, A.; Dellis, A.; et al. Treatment of relapsed urothelial bladder cancer with vinflunine: real-world evidence by the Hellenic Genitourinary Cancer Group. Anticancer Drugs 2016, 27, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Balar, A. V.; Castellano, D. E.; Grivas, P.; Vaughn, D. J.; Powles, T.; Vuky, J.; Fradet, Y.; Lee, J. L.; Fong, L.; Vogelzang, N. J.; et al. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Ann Oncol 2023, 34, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Durán, I.; van der Heijden, M. S.; Loriot, Y.; Vogelzang, N. J.; De Giorgi, U.; Oudard, S.; Retz, M. M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E. R.; Vaena, D.; Grimm, M. O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017, 18, 312–322. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, MS.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, JP.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. CheckMate 901 Trial Investigators. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef]
- Powles, T.; Park, SH.; Caserta, C.; Valderrama, BP.; Gurney, H.; Ullén, A.; Loriot, Y.; Sridhar, SS.; Sternberg, CN.; Bellmunt, J.; et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up. J Clin Oncol. 2023, 41, 3486–3492. [Google Scholar] [CrossRef]
- Bajorin, DF.; Witjes, JA.; Gschwend, JE.; Schenker, M.; Valderrama, BP.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, SF.; Park, SH.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2021, 384(22), 2102–2114. [Google Scholar] [CrossRef]
- Galsky M., :; et al. Extended Follow-up from CheckMate 274 Including the First Report of Overall Survival Outcomes. Presented during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th 2024. [Google Scholar]
- Loriot, Y.; Matsubara, N.; Park, S. H.; Huddart, R. A.; Burgess, E. F.; Houede, N.; Banek, S.; Guadalupi, V.; Ku, J. H.; Valderrama, B. P.; et al. Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2023, 389, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S. E.; VanderWeele, D. J. Antibody-drug conjugates and predictive biomarkers in advanced urothelial carcinoma. Front Oncol 2022, 12, 1069356. [Google Scholar] [CrossRef] [PubMed]
- Birrer, M. J.; Moore, K. N.; Betella, I.; Bates, R. C. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J Natl Cancer Inst 2019, 111, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Natsume, A.; Niwa, R.; Satoh, M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug Des Devel Ther 2009, 3, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xie, G.; Deng, X.; Zhang, Y.; Jia, Z.; Huang, Z. Antibody-drug conjugates in urinary tumors: clinical application, challenge, and perspectives. Front Oncol 2023, 13, 1259784. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shen, Z.; Zhao, W.; Lu, J.; Song, Y.; Shen, L.; Lu, Y.; Wu, M.; Shi, Q.; Zhuang, W.; et al. Rational Identification of Novel Antibody-Drug Conjugate with High Bystander Killing Effect against Heterogeneous Tumors. Adv Sci (Weinh) 2024, 11, e2306309. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, F.; Dal Bo, M.; Macor, P.; Toffoli, G. A comprehensive overview on antibody-drug conjugates: from the conceptualization to cancer therapy. Front Pharmacol 2023, 14, 1274088. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, E.; Fujita, K.; Nakano, K.; Kuwahara, K.; Minami, T.; Kato, T.; Hatano, K.; Kawashima, A.; Uemura, M.; Takao, T.; et al. Trop-2 in Upper Tract Urothelial Carcinoma. Curr Oncol 2022, 29, 3911–3921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Shen, Q.; Yin, W.; Huang, H.; Liu, Y.; Ni, Q. High expression of Nectin-4 is associated with unfavorable prognosis in gastric cancer. Oncol Lett 2018, 15, 8789–8795. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Wang, L.; Wu, Y.; Hao, J.; Wang, Z.; Lu, W.; Wang, X. A.; Zhang, F.; Cao, Y.; et al. A novel PI3K/AKT signaling axis mediates Nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth. Cancer Lett 2016, 375, 179–189. [Google Scholar] [CrossRef]
- Shafique, M. A.; Haseeb, A.; Siddiq, M. A.; Mussarat, A.; Rangwala, H. S.; Mustafa, M. S. Current and Emerging Treatments for Urothelial Carcinoma: A Focus on Enfortumab Vedotin. Cancer Manag Res 2023, 15, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Challita-Eid, P. M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D. R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, B. A.; Catalano, M.; Maiello, E.; Roviello, G. Enfortumab vedotin in metastatic urothelial carcinoma: the solution EVentually? Front Oncol 2023, 13, 1254906. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J. E.; Heath, E.; Perez, R.; Merchan, J.; Lang, J.; Ruether, D.; Petrylak, D.; Sangha, R.; Smith, D. C.; Sridhar, S.; et al. Interim analysis of a phase I dose escalation trial of ASG-22CE (ASG-22ME; enfortumab vedotin), an antibody drug conjugate (ADC), in patients (Pts) with metastatic urothelial cancer (mUC). Annals of oncology 2016, 27, vi273–vi273. [Google Scholar] [CrossRef]
- Rosenberg, J. E.; O'Donnell, P. H.; Balar, A. V.; McGregor, B. A.; Heath, E. I.; Yu, E. Y.; Galsky, M. D.; Hahn, N. M.; Gartner, E. M.; Pinelli, J. M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2019, 37, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Yu, E. Y.; Petrylak, D. P.; O'Donnell, P. H.; Lee, J. L.; van der Heijden, M. S.; Loriot, Y.; Stein, M. N.; Necchi, A.; Kojima, T.; Harrison, M. R.; et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2021, 22, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J. E.; Powles, T.; Sonpavde, G. P.; Loriot, Y.; Duran, I.; Lee, J. L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Mamtani, R.; et al. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann Oncol 2023, 34, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Astellas Pharma US, I. Padcev (enfortumab vedotin-ejfv) with Keytruda (pembrolizumab) Approved by FDA as the First and Only ADC Plus PD-1 to Treat Advanced Bladder Cancer. 2023. https://www.drugs.com/newdrugs/padcev-enfortumab-vedotin-ejfv-keytruda-pembrolizumab-approved-fda-first-only-adc-plus-pd-1-6161.html (accessed 2023).
- AGENCY, E. M.; Padcev : EPAR - Product information. 29/11/2023. https://www.ema.europa.eu/en/medicines/human/EPAR/padcev.
- O'Donnell, P. H.; Milowsky, M. I.; Petrylak, D. P.; Hoimes, C. J.; Flaig, T. W.; Mar, N.; Moon, H. H.; Friedlander, T. W.; McKay, R. R.; Bilen, M. A.; et al. Enfortumab Vedotin With or Without Pembrolizumab in Cisplatin-Ineligible Patients With Previously Untreated Locally Advanced or Metastatic Urothelial Cancer. J Clin Oncol 2023, 41, 4107–4117. [Google Scholar] [CrossRef] [PubMed]
- Hoimes, C. J.; Flaig, T. W.; Milowsky, M. I.; Friedlander, T. W.; Bilen, M. A.; Gupta, S.; Srinivas, S.; Merchan, J. R.; McKay, R. R.; Petrylak, D. P.; et al. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. J Clin Oncol 2023, 41, 22–31. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B. P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S. H.; Shin, S. J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med 2024, 390, 875–888. [Google Scholar] [CrossRef]
- Niegisch, G. Enfortumab Vedotin and Pembrolizumab - A New Perspective on Urothelial Cancer. N Engl J Med 2024, 390, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D. M.; Sharkey, R. M. Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan. MAbs 2019, 11, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer 2015, 6, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J. M.; Tenchov, R.; Bird, R.; Iyer, K. A.; Ralhan, K.; Rodriguez, Y.; Zhou, Q. A. The Evolving Landscape of Antibody–Drug Conjugates: In Depth Analysis of Recent Research Progress. Bioconjugate Chemistry 2023, 34, 1951–2000. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D. M.; Sharkey, R. M. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin Biol Ther 2020, 20, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Song, C. H.; Jeong, M.; In, H.; Kim, J. H.; Lin, C.-W.; Han, K. H. Trends in the Development of Antibody-Drug Conjugates for Cancer Therapy. Antibodies 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lim, K. S.; Blackburn, B. J.; Yun, J.; Putnam, C. W.; Bull, D. A.; Won, Y. W. The Potential of Topoisomerase Inhibitor-Based Antibody-Drug Conjugates. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Messersmith, W. A.; Kio, E. A.; Berlin, J. D.; Vahdat, L.; Masters, G. A.; Moroose, R.; Santin, A. D.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol 2021, 32, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Petrylak, D. P.; Rezazadeh Kalebasty, A.; Fléchon, A.; Jain, R. K.; Gupta, S.; Bupathi, M.; Beuzeboc, P.; Palmbos, P.; Balar, A. V.; et al. TROPHY-U-01, a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors: updated safety and efficacy outcomes. Ann Oncol 2024, 35, 392–401. [Google Scholar] [CrossRef]
- Grivas, P.; Pouessel, D.; Park, C. H.; Barthelemy, P.; Bupathi, M.; Petrylak, D. P.; Agarwal, N.; Gupta, S.; Fléchon, A.; Ramamurthy, C.; et al. Sacituzumab Govitecan in Combination With Pembrolizumab for Patients With Metastatic Urothelial Cancer That Progressed After Platinum-Based Chemotherapy: TROPHY-U-01 Cohort 3. J Clin Oncol 2024, 42, 1415–1425. [Google Scholar] [CrossRef]
- Paz-Ares, L. G.; Juan-Vidal, O.; Mountzios, G. S.; Felip, E.; Reinmuth, N.; Marinis, F. d.; Girard, N.; Patel, V. M.; Takahama, T.; Owen, S. P.; et al. Sacituzumab Govitecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non–Small Cell Lung Cancer: The Randomized, Open-Label Phase III EVOKE-01 Study. Journal of Clinical Oncology 0 (0), JCO.24.00733. [CrossRef]
- Okajima, D.; Yasuda, S.; Maejima, T.; Karibe, T.; Sakurai, K.; Aida, T.; Toki, T.; Yamaguchi, J.; Kitamura, M.; Kamei, R.; et al. Datopotamab Deruxtecan, a Novel TROP2-directed Antibody-drug Conjugate, Demonstrates Potent Antitumor Activity by Efficient Drug Delivery to Tumor Cells. Mol Cancer Ther 2021, 20, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Lisberg, A.; Drakaki, A.; Meric-Bernstam, F.; Alhalabi, O.; Kojima, T.; Kato, M.; Spira, A. I.; Salkeni, M. A.; Heist, R.; Gao, X.; et al. Datopotamab deruxtecan in locally advanced/metastatic urothelial cancer: Preliminary results from the phase 1 TROPION-PanTumor01 study. Journal of Clinical Oncology 2024, 42, 603–603. [Google Scholar] [CrossRef]
- Zhu, K.; Chang, Y.; Zhao, D.; Guo, A.; Cao, J.; Wu, C.; Guan, Y.; Ding, S. Expression of HER2 in high-grade urothelial carcinoma based on Chinese expert consensus and the clinical effects of disitamab vedotin-tislelizumab combination therapy in the treatment of advanced patients. Front Pharmacol 2024, 15, 1355081. [Google Scholar] [CrossRef] [PubMed]
- Aggen, D. H.; Shah, N. J.; Zheng, J.; Alam, S. M.; Balar, O.; Niederhausern, A.; Regazzi, A. M.; Ratna, N.; Funt, S. A.; Teo, M. Y.; et al. HER2 and PD-L1 immunohistochemistry (IHC) expression, and HER2 genomic alterations: Associations and clinical outcomes for advanced bladder cancer. Journal of Clinical Oncology 2024, 42, 538–538. [Google Scholar] [CrossRef]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016, 107, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Werner, L.; Bamias, A.; Fay, A. P.; Park, R. S.; Riester, M.; Selvarajah, S.; Barletta, J. A.; Berman, D. M.; de Muga, S.; et al. HER2 as a target in invasive urothelial carcinoma. Cancer Med 2015, 4, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Oh, D. Y.; Bang, Y. J. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol 2020, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Nally, E.; Young, M.; Wells, C.; Fairhead, R.; Baines, K.; Cheney-Lowe, H.; Jackson-Spence, F.; Powles, T. Is HER2 the New NECTIN4 in Advanced Urothelial Cancer? Eur Urol Focus 2024, 10, 219–221. [Google Scholar] [CrossRef]
- Li, B. T.; Meric-Bernstam, F.; Bardia, A.; Naito, Y.; Siena, S.; Aftimos, P.; Anderson, I.; Curigliano, G.; de Miguel, M.; Kalra, M.; et al. Trastuzumab deruxtecan in patients with solid tumours harbouring specific activating HER2 mutations (DESTINY-PanTumor01): an international, phase 2 study. Lancet Oncol 2024, 25, 707–719. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D. Y.; Banerjee, S.; González-Martín, A.; Jung, K. H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive solid tumors. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2 (accessed 2024).
- Kim, J. H.; Chang, I. H. A novel strategy for treatment of bladder cancer: Antibody-drug conjugates. Investig Clin Urol 2022, 63, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Liu, Y.; Zhou, X.; Shen, P.; Xue, R.; Zhang, M. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv 2022, 29, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Zhu, C.; Yu, P.; Wang, X.; Wang, Y.; Wang, J.; Yu, J.; Wang, K. Emerging strategy for the treatment of urothelial carcinoma: Advances in antibody-drug conjugates combination therapy. Biomed Pharmacother 2024, 171, 116152. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Sun, M.; Getz, T.; Ho, B.; Nauseef, J. T.; Tagawa, S. T. Antibody-drug conjugates for urothelial carcinoma. Urol Oncol 2023, 41, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, L.; He, Z.; Shi, Y.; Luo, H.; Han, W.; Yao, X.; Shi, B.; Liu, J.; Hu, C.; et al. Efficacy and Safety of Disitamab Vedotin in Patients With Human Epidermal Growth Factor Receptor 2-Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J Clin Oncol 2024, 42, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Zhou, L.; Yang, K.; Zhang, S.; Xu, H.; Yan, X.; Li, S.; Li, J.; Cui, C.; Chi, Z.; et al. Disitamab vedotin, a novel humanized anti-HER2 antibody-drug conjugate (ADC), combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma: An open-label phase 1b/2 study. Journal of Clinical Oncology 2023, 41, 4566–4566. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, R.; Yu, C.; Hong, Z.; Lin, L.; Li, T.; Chen, J. Disitamab vedotin in combination with immune checkpoint inhibitors for locally and locally advanced bladder urothelial carcinoma: a two-center's real-world study. Front Pharmacol 2023, 14, 1230395. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Yu, E. Y.; Iyer, G.; Campbell, M. T.; Loriot, Y.; Santis, M. D.; O'Donnell, P. H.; Burgess, E. F.; Necchi, A.; Krieger, L. E. M.; et al. Phase 2 clinical study evaluating the efficacy and safety of disitamab vedotin with or without pembrolizumab in patients with HER2-expressing urothelial carcinoma (RC48G001). Journal of Clinical Oncology 2023, 41, TPS594–TPS594. [Google Scholar] [CrossRef]
- Galsky, M.; Grande, E.; Necchi, A.; Koontz, M.; Iyer, G.; Campbell, M.; Drakaki, A.; Loriot, Y.; Sokolowski, K.; Zhang, W.; et al. Phase 3 open-label, randomized, controlled study of disitamab vedotin with pembrolizumab versus chemotherapy in patients with previously untreated locally advanced or metastatic urothelial carcinoma that expresses HER2 (DV-001). Journal of Clinical Oncology 2024, 42, TPS717–TPS717. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Y.; Chen, Z.; Zeng, X.; Hu, Z.; Yang, C. Neoadjuvant therapy with Disitamab vedotin in treating muscle-invasive bladder cancer: A case report. Heliyon 2023, 9, e15157. [Google Scholar] [CrossRef] [PubMed]
- Alameddine, R.; Mallea, P.; Shahab, F.; Zakharia, Y. Antibody Drug Conjugates in Bladder Cancer: Current Milestones and Future Perspectives. Curr Treat Options Oncol 2023, 24, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Sarfaty, M.; Rosenberg, J. E. Antibody-Drug Conjugates in Urothelial Carcinomas. Curr Oncol Rep 2020, 22. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, A.; Tucci, M.; Audisio, A.; Di Prima, L.; Pisano, C.; Turco, F.; Delcuratolo, M. D.; Di Maio, M.; Scagliotti, G. V.; Buttigliero, C. Antibody-Drug Conjugates in Urothelial Carcinoma: A New Therapeutic Opportunity Moves from Bench to Bedside. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.; Entwistle, J.; Cizeau, J.; Niforos, D.; Loewen, S.; Chapman, W.; MacDonald, G. C. A Phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCGrefractory and BCG-intolerant patients. Drug Des Devel Ther 2010, 4, 313–320. [Google Scholar] [CrossRef] [PubMed]
- R Dickstein, N. W., B Cowan, C Dunshee, M Franks, F Wolk, L Belkoff, S Castellucci, J Holzbeierlein, G Kulkarni, A Weizer, D Lamm, S Ali, J; Epstein, G. A., H Youssoufian, W Kassouf. VISTA, PHASE 3 TRIAL OF VICINIUM, AN EPCAM-TARGETED PSEUDOMONAS EXOTOXIN, IN BCG-UNRESPONSIVE NON-MUSCLE INVASIVE BLADDER CANCER. SESEN Bio. 2018. https://sesenbio.com/wp-content/uploads/2019/01/BLADDR_Congress_2018_Posterl.pdf (accessed 2018).
- Rivera-Marquez, G.; Walter, B.; Dolan, R.; Belfield, S.; Merino, M.; Gurram, S.; … Valera, V.A. MP16-20 FINAL RESULTS OF A PHASE I, SINGLE-ARM CLINICAL TRIAL OF THE COMBINATION OF DURVALUMAB AND VICINEUM IN BCG UNRESPONSIVE, HIGH-RISK NON-MUSCLE-INVASIVE BLADDER CANCER PATIENTS (NCT03258593). Journal of Urology 2024, 211. [Google Scholar] [CrossRef]
- de Bono, J. S.; Concin, N.; Hong, D. S.; Thistlethwaite, F. C.; Machiels, J. P.; Arkenau, H. T.; Plummer, R.; Jones, R. H.; Nielsen, D.; Windfeld, K.; et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1-2 trial. Lancet Oncol 2019, 20, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Hoimes, C.J.; Flaig, T.W.; Milowsky, M.I.; Friedlander, T.W.; Bilen, M.A.; Gupta, S.; Srinivas, S.; Merchan, J.R.; McKay, R.R.; Petrylak, D.P.; Sasse, C.; Moreno, B.H.; Yu, Y.; Carret, A.S.; Rosenberg, J.E. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2023, 41, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S. H.; Caserta, C.; Valderrama, B. P.; Gurney, H.; Ullén, A.; Loriot, Y.; Sridhar, S. S.; Sternberg, C. N.; Bellmunt, J.; et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up. J Clin Oncol 2023, 41, 3486–3492. [Google Scholar] [CrossRef]
- Gray, E.; Ulrich, M.; Epp, A.; Younan, P.; Sahetya, D.; Hensley, K.; Allred, S.; Huang, L. Y.; Hahn, J.; Gahnberg, K.; et al. SGN-B7H4V, an investigational vedotin ADC directed to the immune checkpoint ligand B7-H4, shows promising activity in preclinical models. J Immunother Cancer 2023, 11. [Google Scholar] [CrossRef]
- Oncology, A. o. annals of Oncology; 2022; pp. S808–S869. [Google Scholar]
- Li, C.; Zhu, J.; Yu, W. Efficacy and safety of disitamab vedotin combined with toripalimab as neoadjuvant treatment in patients with HER2 positive locally advanced muscle-invasive urothelial bladder cancer. Journal of Clinical Oncology 2024, 42, 631–631. [Google Scholar] [CrossRef]
- Bajorin, D. F.; Witjes, J. A.; Gschwend, J. E.; Schenker, M.; Valderrama, B. P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S. F.; Park, S. H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Hoimes, C.; Bedke, J.; Loriot, Y.; Nishiyama, H.; Fang, X.; Kataria, R.; Homet, B.; Galsky, M. KEYNOTE-B15/EV-304: Randomized phase 3 study of perioperative enfortumab vedotin plus pembrolizumab versus chemotherapy in cisplatin-eligible patients with muscle-invasive bladder cancer (MIBC). Journal of Clinical Oncology 2021, 39, TPS4587–TPS4587. [Google Scholar] [CrossRef]
- Powles, T.; Meeks, J.; Galsky, M.; Van der Heijden, M.; Nishiyama, H.; Al-Ahmadie, H.; Gupta, A.; Ye, J.; Donegan, S.; Ghiorghiu, D.; et al. A phase III, randomized, open label, multicenter, global study of efficacy and safety of durvalumab in combination with gemcitabine+cisplatin (G+C) for neoadjuvant treatment followed by durvalumab alone for adjuvant treatment in muscle-invasive bladder cancer (MIBC) (NIAGARA). Journal of Clinical Oncology 2019, 37, TPS4592–TPS4592. [Google Scholar] [CrossRef]
- Powles, T.; Drakaki, A.; Teoh, J.; Grande, E.; Fontes-Sousa, M.; Porta, C.; Wu, E.; Goluboff, E.; Ho, S.; Hois, S.; et al. A phase 3, randomized, open-label, multicenter, global study of the efficacy and safety of durvalumab (D) + tremelimumab (T) + enfortumab vedotin (EV) or D + EV for neoadjuvant treatment in cisplatin-ineligible muscle-invasive bladder cancer (MIBC) (VOLGA). Journal of Clinical Oncology 2022, 40, TPS579–TPS579. [Google Scholar] [CrossRef]
- Galsky, M. D.; Hoimes, C. J.; Necchi, A.; Shore, N.; Witjes, J. A.; Steinberg, G.; Bedke, J.; Nishiyama, H.; Fang, X.; Kataria, R.; et al. Perioperative pembrolizumab therapy in muscle-invasive bladder cancer: Phase III KEYNOTE-866 and KEYNOTE-905/EV-303. Future Oncol 2021, 17, 3137–3150. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J. E.; Sonpavde, G. P.; Loriot, Y.; Durán, I.; Lee, J. L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S. T.; Balar, A. V.; Petrylak, D. P.; Kalebasty, A. R.; Loriot, Y.; Fléchon, A.; Jain, R. K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J Clin Oncol 2021, 39, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Shin, S.J.; Kim, H.C.; Kim, Y.S.; Lee, H.J.; Keam, B.; Choi, Y.J.; Kim, Y.J.; Park, I.; Park, S.H.; Lee, J.L. Enfortumab vedotin-related pneumonitis is more common than expected and could lead to acute respiratory failure. Eur J Cancer. 2022, 174, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mathew Thomas, V.; Jo, Y.; Tripathi, N.; Roy, S.; Chigarira, B.; Narang, A.; Gebrael, G.; Hage Chehade, C.; Sayegh, N.; Galarza Fortuna, G.; et al. Treatment Patterns and Attrition With Lines of Therapy for Advanced Urothelial Carcinoma in the US. JAMA Netw Open 2024, 7, e249417. [Google Scholar] [CrossRef]
- Brown, J. R.; Koshkin, V. S. Therapies After Progression on Enfortumab Vedotin and Pembrolizumab: Navigating Second-line Options for Metastatic Urothelial Carcinoma in the New Treatment Landscape. Eur Urol Focus 2024, 10, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Nizam, A.; Jindal, T.; Jiang, C.; Alhalabi, O.; Bakaloudi, D.; Talukder, R.; Davidsohn, M.; Nguyen, C.; Oh, E.; Taylor, A.; et al. Outcomes in patients (pts) with advanced urothelial carcinoma (aUC) treated with enfortumab vedotin (EV) after switch maintenance avelumab (MAv) in the UNITE study. Journal of Clinical Oncology 2024, 42, 537–537. [Google Scholar] [CrossRef]
- Jiang, C.; Hwang, H.; Jindal, T.; Qiao, W.; Epstein, I.; Nguyen, C.; Gupta, S.; Shah, S.; Bilen, M.; Milowsky, M.; et al. Sequencing of erdafitinib (erda) and enfortumab vedotin (EV) in patients (pts) with fibroblast growth factor receptor (FGFR2/3) altered (alt) advanced urothelial cancer (aUC): Analysis of UNITE database. Journal of Clinical Oncology 2024, 42, 616–616. [Google Scholar] [CrossRef]
- Jindal, T.; Jiang, C. Y.; Alhalabi, O.; Bakaloudi, D. R.; Talukder, R.; Davidsohn, M. P.; Nizam, A.; Taylor, A. K.; Nguyen, C. B.; Kilari, D.; et al. Biomarkers of response to sacituzumab govitecan (SG) and efficacy after treatment with enfortumab vedotin (EV) in advanced urothelial carcinoma (aUC): Analysis of the UNITE study. Journal of Clinical Oncology 2023. [Google Scholar] [CrossRef]
- Vlachou, E.; Hahn, N.; Dabb, A.; Johnson, B.; Lefande, M.; Hoffman-Censits, J. Evaluation of clinical benefit of sacituzumab govitecan (SG) following enfortumab vedotin (EV) in advanced urothelial cancer (UC): Real-world experience. Journal of Clinical Oncology 2023, 41, 523–523. [Google Scholar] [CrossRef]
- Bellmunt, J.; Théodore, C.; Demkov, T.; Komyakov, B.; Sengelov, L.; Daugaard, G.; Caty, A.; Carles, J.; Jagiello-Gruszfeld, A.; Karyakin, O.; et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009, 27, 4454–4461. [Google Scholar] [CrossRef] [PubMed]
- Sternschuss, M.; Toumbacaris, N.; Das, J. P.; Powles, T.; Bajorin, D. F.; Kotecha, R. R.; Laccetti, A. L.; Xiao, H.; Feld, E.; McHugh, D. J.; et al. Clinical outcomes of sacituzumab govitecan (SG) after prior exposure to enfortumab vedotin (EV) in patients with metastatic urothelial carcinoma (mUC). Journal of Clinical Oncology 2024, 42, 4581–4581. [Google Scholar] [CrossRef]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B. P.; Ravaud, A.; Shariat, S. F.; et al. ESMO Clinical Practice Guideline interim update on first-line therapy in advanced urothelial carcinoma. Ann Oncol 2024, 35, 485–490. [Google Scholar] [CrossRef]
- Vassiliou, V.; Katsila, T.; Sodergren, S. C.; Kardamakis, D. Radiotherapy in Metastatic Urothelial Carcinoma: Rationale and Clinical Applications. Anticancer Res 2022, 42, 3767–3778. [Google Scholar] [CrossRef]
| ADC UC | Year Approved | Target Receptor | Cleavable Linker | Payload | Payload Action |
|---|---|---|---|---|---|
| Enfortumab vedotin | 2019 FDA, 2022 EMA |
Nectin-4 | Enzyme | MMAE | Microtubule inhibitor |
| Sacituzumab govitecan | 2021 FDA, 2021 EMA |
Trop2 | Acid | SN-38 | Topoisomerase-DNA complex Inhibitor |
| Disitamab vedotin | 2020 FDA | HER2 | Protease | MMAE | Microtubule inhibitor |
| Trastuzumab deruxtecan | 2024 FDA | HER2 | Enzyme | Deruxtecan | Topoisomerase-DNA complex Inhibitor |
| Tisotumab vedotin | 2024 FDA | Tissue Factor (TF) | Protease | MMAE | Microtubule inhibitor |
| Trial, NCT number | Phase | Regimen | Study Population | Primary and/or Co-Primary End Points |
|---|---|---|---|---|
| EV-301, NCT03474107 |
III | EV | Evaluate Enfortumab Vedotin vs Chemotherapy in Subjects With Previously Treated Locally Advanced or Metastatic Urothelial Cancer | OS 12.88 months (m) (10.58 to 15.21) PSF1 5.55 m (5.32 to 5.82) ORR 40.6% (34.90 to 46.54) DCR 71.9% (66.30 to 76.99) DoR 7.39 m (5.59 to 9.46) |
| EV-201, Cohort 2, NCT03219333 | II | EV | Locally Advanced or Metastatic Urothelial Cancer Who Previously Received Immune Checkpoint Inhibitor (CPI) Therapy | ORR 51.7% (39.8 to 61.3) DoR 10.9 m (5.78 to NA) PFS 5.8 m (5.03 to 8.28) |
| EV-103, cohort H, NCT03288545 | I/II | EV | Neoadjuvant in cisplatin-ineligible MIBC naive to systemic therapy | pCR 36.4% (95% CI, 17.2, 59.3) |
| EV-103, cohort L, NCT03288545 | I/II | EV | Perioperative, previously untreated, cis-ineligible MIBC | pCR 34% (95%CI, 21.2, 48.8) |
| EV-103, Cohort A, NCT03288545 | I/II | EV + P | First line in platinum ineligible LA/mUC refractory to prior therapies. | ORR 73.3% (95% CI, 58.1-85.4) DCR 84.4% (95% CI: 70.5, 93.5) PFS 12.7 m (95% CI: 6.11, -) OS 26.1 m (95% CI: 15.51, -) |
| EV-103, Cohort B, NCT03288545 |
I/II | EV + P | Second line in LA/mUC refractory to platinum therapies. | ORR |
| EV-103, Cohort D, NCT03288545 |
I/II | EV + Cis | First line in platinum-eligible LA/mUC. | ORR |
| EV-103, Cohort E, NCT03288545 |
I/II | EV + Carbo | First line in cisplatin-ineligible, carboplatin-eligible LA/mUC. | ORR |
| EV-103, Cohort F, NCT03288545 |
I/II | EV + Gem | First and second line in platinum ineligible LA/mUC refractory to prior therapies | ORR |
| EV-103,CohortG, NCT03288545 | I/II | EV + Plt + P | First line in platinum-eligible LA/mUC | ORR |
| EV-103, Cohort J, NCT03288545 |
I/II | EV + P | Neoadjuvant in cisplatin-ineligible MIBC naive to systemic therapy | ORR |
| EV-302, NCT04223856 |
III | EV + P vs. Gem + Plat |
La/mUC and no prior systemic therapy for advanced disease who are ineligible for cisplatin-containing chemotherapy |
mOS 31.5m (95% CI, 25.4-not reached [NR]) |
| 516–003, Cohort 9, NCT03606174 | I/II | EV + P + Si | LA/mUC refractory to platinum and ICI therapies | ORR |
| KEYNOTE-905/EV-303, NCT03924895 |
III | EV + P vs. P vs. Cystectomy |
Neoadjuvant therapy or only surgery in cisplatin-ineligible MIBC; Arm A, neoadjuvant P followed by RC + PLND and adjuvant P, Arm B, RC + PLND followed by observation, Arm C, neoadjuvant EV + P followed by RC + PLND and adjuvant EV + P and adjuvant P. |
pCR |
| Keynote-B15/ EV-304, NCT04700124 | III | EV + P vs. CTX |
Neoadjuvant in cisplatin-eligible MIBC; Arm A, neoadjuvant EV + P followed by adjuvant EV + adjuvant P after RC + PLND Arm B, neoadjuvant Gem + Cis followed by observation after RC + PLND |
pCR, PFS, OS |
| VOLGA, NCT04960709 | III | EV + Du + Tr vs. EV + Du |
Neoadjuvant in cisplatin-ineligible systemic therapy-naive MIBC; Arm A, Du + T + EV, Arm B, Du + EV, Arm C, no neoadjuvant treatment (SoC) |
pCR, EFS |
| NCT04878029 | Ib | EV + Caboz | Cabozantinib in Combination With Enfortumab Vedotin (EV) in the Treatment of Locally Advanced or Metastatic Urothelial Cancer | recommended phase II dose (RP2D), ORR,PFS, OS |
| NCT05775471 | II | EV + P | Pembrolizumab and Enfortumab Vedotin with Pembrolizumab Prior to and After Radical Nephroureterectomy for High-Risk Upper Tract Urothelial Cancer. |
ORR, recurrence-free survival |
| NCT04963153 | I | EV + Erdb | Phase Ib Trial of Erdafitinib Combined With Enfortumab Vedotin Following Platinum and PD1/L1 Inhibitors for Metastatic Urothelial Carcinoma With FGFR2/3 Genetic Alterations | ORR, DoR, PFS, OS |
| SKB264, NCT04152499 | II | SKB264 ADC targeting TROP2 | La/Unresectable/Metastatic Solid Tumors who are refractory to Available Standard Therapies including Urothelial carcinoma. | ORR |
| SURE-01, NCT05226117 | II | SG | Neoadjuvant in cisplatin-ineligible MIBC naive to systemic therapy | pCR 36.4% (95% CI, 14.9%-64.8%) |
| NCT05581589 | II | Adj SG | Sacituzumab Govitecan as Neoadjuvant Therapy in Pts With Non-Urothelial Muscle Invasive Bladder Cancer |
pCR, RFS, OS |
| TROPiCS-4 / Immu132–13, NCT04527991 | III | SG vs CTX | LA/mUC refractory to platinum and anti-PD-1/PD-L1 Therapies | OS not met |
| Trophy-U-01, Cohort 4, NCT03547973 | II | SG + Cis + AVLM | Platinum naive and cisplatin-eligible LA/mUC with responders receiving Avelumab maintenance | ORR, PFS |
| Trophy-U-01, Cohort 4, NCT03547973 | II | SG + Cis + Zi | Platinum naive and cisplatin-eligible LA/mUC with responders receiving Avelumab maintenance | ORR, PFS |
| Trophy-U-01, Cohort 5, NCT03547973 | II | SG Zi or AVLM or Zi | LA/mUC maintenance therapy following Gem-Cis | ORR, PFS |
| Trophy-U-01, Cohort 6, NCT03547973 | II | SG or SG + Zi or SG + Zi + Do or GC | Cisplatin-ineligible, treatment-naive LA/mUC | ORR, PFS |
| Trophy-U-01, Cohort 3, NCT03547973 | II | SG + P | Sacituzumab Govitecan in Unresectable Locally Advanced/Metastatic Urothelial Cancer | ORR 41% (95% CI, 26.3 to 57.9; 20% CR rate), CBR 46% (95% CI, 30.7 to 62.6), mDOR 11.1 m (95% CI, 4.8 to not NE), mPFS 5.3 m (95% CI, 3.4 to 10.2) mOS 12.7 m (10.7-NE) |
| MORPHEUS-UC, NCT03869190 |
IB/II | SG + At | PD1-expressing LA/mUC refractory to platinum therapy | ORR, pCRR criteria not met |
| JAVELIN Bladder Medley, NCT05327530 |
II | AVLM + SoC vs SG + AVLM |
Maintenance Treatment in Participants with Locally Advanced or Metastatic Urothelial Carcinoma Whose Disease Did Not Progress with First Line Platinum-Containing Chemotherapy (JAVELIN Bladder Medley) Group B: Avelumab + Sacituzumab Govitecan |
PFS, OS, OR, DoR |
| DestinyPanTumor02, Cohort 2 NCT04482309 | II | T-DXd | HER2-positive solid tumor including bladder cancer | ORR 39% (24.2 to 55.5) mDoR 8.7m (4.3 to 11.8) PFS 7.0m (4.2 to 9.7) mOS 12.8m (11.2 to 15.1) |
| DestinyPanTumor01, NCT04639219 | II | T-DXd | Trastuzumab deruxtecan in patients with solid tumours harbouring specific activating HER2 mutations | ORR 29.4% (95% CI 20·8–39·3; 30 of 102 patients) |
| Keynote D78/RC48G001, NCT04879329, Cohort A, Cohort B. |
II b | DV | HER2-positive, platinum-refractory LA/mUC. HER2 low expressing, platinum refractory LA/mUC |
ORR |
| KeynoteD78/RC48G001 NCT04879329, Cohort C. |
IIb | DV vs. DV + P, |
HER2-positive, platinum eligible, treatment-naive LA/mUC | ORR |
| RC48-C016, NCT05302284 |
III | DV vs. Gem + Plat |
HER2-positive platinum-eligible treatment-naive LA/mUC; Arm A, DV, until PD, unacceptable toxicity, or voluntary withdrawal, Arm B, Gem + Cis or Carbo until loss of clinical benefit, unacceptable toxicity, investigator or participant decision to withdraw from therapy, or death. |
PFS, OS |
| NCT06210490 | II | DV + Rt vs SoC |
Efficacy and Safety of Disitamab Vedotin in Combination With Radiotherapy for the Adjuvant Treatment of HER2 Overexpressing UTUC Patients With High Risk Factors for Recurrence After Radical Surgery | DFS, OS, MFS, Local-Recurrence Free (LRF) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




