Submitted:
29 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
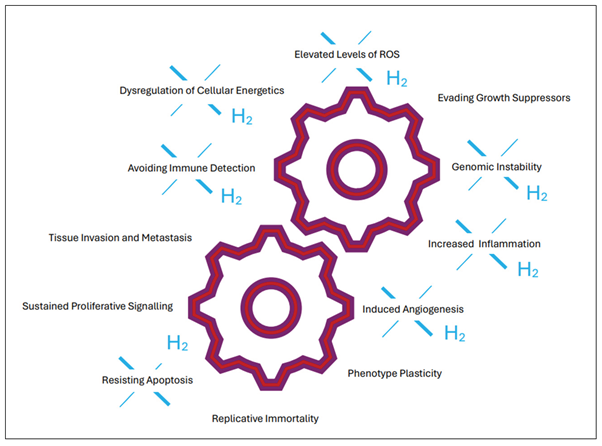
1. Introduction
2. Oxyhydrogen Therapies:
3. Mechanisms of Action: Oxygen
4. Mechanisms of Action: Hydrogen
5. Hydrogen Therapies and Lung Cancer
6. Hydrogen Therapies and Breast Cancer
7. Hydrogen Therapies and Colorectal Cancer
8. Future Perspectives
9. Summary and Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F. , Laversanne, M., Sung, H., Ferlay, J., Siegel, R.L., Soerjomataram, I. and Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 2024, 74, 229–263. [Google Scholar] [PubMed]
- Labrie, M. , Brugge, J.S., Mills, G.B. and Zervantonakis, I.K. Therapy resistance: opportunities created by adaptive responses to targeted therapies in cancer. Nat. revs. Cancer 2022, 22, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y. , Zhang, Y., Wang, Y., Chen, Y., Fan, W., Zhou, J., Qiao, J. and Wei, Y. Hydrogen, a novel therapeutic molecule, regulates oxidative stress, inflammation, and apoptosis. Frontiers in physiology 2021, 12, 789507. [Google Scholar] [CrossRef] [PubMed]
- Schulz, W.A. Cell Death and Replicative Senescence in Cancer. In Mol. Bio. of Human Cancers; Springer International Publishing: Cham, 2023; pp. 153–175. [Google Scholar]
- Ohta, S. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods in enzymology 2015, 555, 289–317. [Google Scholar] [PubMed]
- Kura, B. , Bagchi, A.K., Singal, P.K., Barancik, M., LeBaron, T.W., Valachova, K., Šoltés, L. and Slezák, J. Molecular hydrogen: Potential in mitigating oxidative-stress-induced radiation injury. Canadian Jrnl of Phys. and Pharm. 2019, 97, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N. and Cotter, T.G. ROS signalling in the biology of cancer. In Seminars in cell & developmental biology; Academic Press: Cambridge, MA, USA, 2018; Volume 80, pp. 50–64. [Google Scholar]
- Chen, P. , Ni, W., Xie, T. and Sui, X., 2019. Meta-Analysis of 5-Fluorouracil-Based Chemotherapy Combined With Traditional Chinese Medicines for Colorectal Cancer Treatment. Integrative cancer therapies 2019, 18, 1534735419828824. [Google Scholar] [CrossRef]
- Asgharzadeh, F. , Tarnava, A., Mostafapour, A., Khazaei, M. and LeBaron, T.W. (2022) Hydrogen-rich water exerts anti-tumor effects comparable to 5-fluorouracil in a colorectal cancer xenograft model. World Jrnl of Gastro Onc. 2022, 14, 242. [Google Scholar] [CrossRef]
- Nakashima-Kamimura, N. , Mori, T., Ohsawa, I., Asoh, S. and Ohta, S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemo. and Pharm. 2009, 64, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Russell, G. , Thomas, A.D., Nenov, A., Mannings, G. and Hancock, J.T. The therapeutic potential of oxyhydrogen gas in oncology: A study on Epstein–Barr Virus-immortalised B-Lymphoblastoid (TK6) cells. Hydrogen, 2023; 4, 746–759. [Google Scholar]
- Chen, J.B. , Kong, X.F., Qian, W., Mu, F., Lu, T.Y., Lu, Y.Y. and Xu, K.C. Two weeks of hydrogen inhalation can significantly reverse adaptive and innate immune system senescence patients with advanced non-small cell lung cancer: a self-controlled study. Med. Gas Res. 2020, 10, 149. [Google Scholar]
- Tamura, T. , Suzuki, M., Hayashida, K., Kobayashi, Y., Yoshizawa, J., Shibusawa, T., Sano, M., Hori, S. and Sasaki, J. Hydrogen gas inhalation alleviates oxidative stress in patients with post-cardiac arrest syndrome. Jrnl of Clin. Biochem and Nut. 2020, 67, 214–221. [Google Scholar] [CrossRef]
- Liu, B. , Jiang, X., Xie, Y., Jia, X., Zhang, J., Xue, Y. and Qin, S. The effect of a low dose hydrogen-oxygen mixture inhalation in midlife/older adults with hypertension: A randomized, placebo-controlled trial. Front. in Pharm. 2022, 13, 1025487. [Google Scholar]
- Zheng, Z.G. , Sun, W.Z., Hu, J.Y., Jie, Z.J., Xu, J.F., Cao, J., Song, Y.L., Wang, C.H., Wang, J., Zhao, H. and Guo, Z.L. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: results of a multicenter, randomized, double-blind, parallel-group controlled trial. Resp. Res. 2021, 22, 1–12. [Google Scholar]
- Shang, L. , Xie, F., Li, J., Zhang, Y., Liu, M., Zhao, P., Ma, X. and Lebaron, T.W. Therapeutic potential of molecular hydrogen in ovarian cancer. Trans. Cancer Res. 2018, 7. [Google Scholar] [CrossRef]
- Akagi, J. and Baba, H. Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis. Onc. Reps. 2019, 41, 301–311. [Google Scholar]
- Yang, Y. , Liu, P.Y., Bao, W., Chen, S.J., Wu, F.S. and Zhu, P.Y. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC cancer 2020, 20, 1–19. [Google Scholar]
- Zan, R. , Wang, H., Cai, W., Ni, J., Luthringer-Feyerabend, B.J., Wang, W., Peng, H., Ji, W., Yan, J., Xia, J. and Song, Y. Controlled release of hydrogen by implantation of magnesium induces P53-mediated tumor cells apoptosis. Bioact. Materials, 2022; 9, 385–396. [Google Scholar]
- Singh, R. and Manna, P.P. Reactive oxygen species in cancer progression and its role in therapeutics. Explo. of Med. 2022, 3, 43–57. [Google Scholar] [CrossRef]
- Iio, A. , Ito, M., Itoh, T., Terazawa, R., Fujita, Y., Nozawa, Y., Ohsawa, I., Ohno, K. and Ito, M. Molecular hydrogen attenuates fatty acid uptake and lipid accumulation through downregulating CD36 expression in HepG2 cells. Med. Gas Res 2013, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Frajese, G.V. , Benvenuto, M., Mattera, R., Giampaoli, S., Ambrosin, E., Bernardini, R., Giganti, M.G., Albonici, L., Dus, I., Manzari, V. and Modesti, A. Electrochemically reduced water delays mammary tumors growth in mice and inhibits breast cancer cells survival in vitro. Evi.-Based Compl. and Alt. Med. 2018; 4753507. [Google Scholar]
- Wang, D. , Wang, L., Zhang, Y., Zhao, Y. and Chen, G. Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed. & Pharma. 2018, 104, 788–797. [Google Scholar]
- Zhang, X. , Tao, G., Zhao, Y.N., Xing, S., Jiang, J., Liu, B. and Qin, S. Molecular hydrogen inhibits colorectal cancer growth via the AKT/SCD1 signaling pathway. BioMed Res. Int. 2024; 8024452. [Google Scholar]
- Lee, J.W. , Kim, J.I., Lee, Y.A., Lee, D.H., Song, C.S., Cho, Y.J. and Han, J.S., Inhaled hydrogen gas therapy for prevention of testicular ischemia/reperfusion injury in rats. Jrnl. of Ped. Surg. 2012, 47, 736–742. [Google Scholar] [CrossRef]
- Runtuwene, J. , Amitani, H., Amitani, M., Asakawa, A., Cheng, K.C. and Inui, A. Hydrogen–water enhances 5-fluorouracil-induced inhibition of colon cancer. PeerJ, 2015, 3, 859. [Google Scholar] [CrossRef]
- Yang, W.C. , Zhang, Y.R., Yu, J.X., Yang, Y., Liu, X.N., Zhang, X., Zhai, J.Y., Lai, P.C. and Wang, Q.S. Hydrogen-oxygen therapy improves postoperative pulmonary functions and accelerates recovery through attenuating inflammatory reactions and oxidative stress in patients undergoing lung surgery. [Preprint] Researchsquare 2022. [CrossRef]
- Sarmiento-Salinas, F.L. , Perez-Gonzalez, A., Acosta-Casique, A., Ix-Ballote, A., Diaz, A., Treviño, S., Rosas-Murrieta, N.H., Millán-Perez-Peña, L. and Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, D. , Gibson, A. and Martin, S.A., 2021. The emerging relationship between metabolism and DNA repair. Cell Cycle 2021, 20, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A. , Pérez de la Lastra, J.M., Plou, F.J. and Pérez-Lebeña, E., 2021. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. Jrnl of Mol Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Hanley, R. , Pagliari, F., Garcia-Calderón, D., Fernandes Guerreiro, J., Genard, G., Jansen, J., Nisticò, C., Marafioti, M.G., Tirinato, L. and Seco, J. Radio-resistance of hypoxic tumors: exploring the effects of oxygen and X-ray radiation on non-small lung cancer cell lines. Radiation Onc. 2023, 18, 81. [Google Scholar]
- Kirtonia, A. , Sethi, G. and Garg, M. The multifaceted role of reactive oxygen species in tumorigenesis. Cellular and Mol. Life Sci. 2020, 77, 4459–4483. [Google Scholar] [CrossRef] [PubMed]
- Emami Nejad, A. , Najafgholian, S., Rostami, A., Sistani, A., Shojaeifar, S., Esparvarinha, M., Nedaeinia, R., Haghjooy Javanmard, S., Taherian, M., Ahmadlou, M. and Salehi, R., 2021. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 2021, 21, 1–26. [Google Scholar]
- Kopecka, J. , Salaroglio, I.C., Perez-Ruiz, E., Sarmento-Ribeiro, A.B., Saponara, S., De Las Rivas, J. and Riganti, C. Hypoxia as a driver of resistance to immunotherapy. Drug Resistance Updates, 2021, 59, 100787. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A. , Lecarpentier, Y. and Vallée, J.N. The key role of the WNT/β-catenin pathway in metabolic reprogramming in cancers under normoxic conditions. Cancers 2021, 13, 5557. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Campos, A.B. , Zamudio-Martinez, E., Delgado-Bellido, D., Fernández-Cortés, M., Montuenga, L.M., Oliver, F.J. and Garcia-Diaz, A. Implications of hyperoxia over the tumor microenvironment: an overview highlighting the importance of the immune system. Cancers 2022, 14, 2740. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, X. and Venkitaraman, A.R. A mitochondrial response to oxidative stress mediated by unscheduled RNA-DNA hybrids (R-loops). Mol. & Cell. Onc. 2021, 8, 2007028. [Google Scholar]
- Nakamura, H. and Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, G. , Oni, A.O. and Kumar, A. Blending blue hydrogen with natural gas for direct consumption: Examining the effect of hydrogen concentration on transportation and well-to-combustion greenhouse gas emissions. Int. Jrnl of Hydrogen Energy 2021, 46, 19202–19216. [Google Scholar] [CrossRef]
- Piché-Choquette, S. and Constant, P. Molecular hydrogen, a neglected key driver of soil biogeochemical processes. A and Env. Micro. 2019, 85, 2418. [Google Scholar]
- Martin, W. and Müller, M. The hydrogen hypothesis for the first eukaryote. Nature 1998, 392, 37–41. [Google Scholar] [CrossRef]
- Sousa, F.L. , Neukirchen, S., Allen, J.F., Lane, N. and Martin, W.F. Lokiarchaeon is hydrogen dependent. Nature Micro. 2016, 1, 1–3. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.F. , Holbrook, W.P. and Eastwood, M.A. Methane and hydrogen production by human intestinal anaerobic bacteria. Acta Patho. Micro. Scandi. Series B: Micro. 1982, 90, 257–260. [Google Scholar]
- Xue, D. , Zhou, X. and Qiu, J., 2020. Emerging role of NRF2 in ROS-mediated tumor chemoresistance. Biomed. & Pharmaco. 2020, 131, 110676. [Google Scholar]
- Slezak, J. , Kura, B., LeBaron, T.W., Singal, P.K., Buday, J. and Barancik, M. Oxidative stress and pathways of molecular hydrogen effects in medicine. Curr. Pharma. Des. 2021, 27, 610–625. [Google Scholar] [CrossRef]
- Roy, Z. , Bansal, R., Siddiqui, L. and Chaudhary, N. Understanding the role of free radicals and antioxidant enzymes in human diseases. Curr. Pharma. Biotech. 2023, 24, 1265–1276. [Google Scholar]
- Ahmad, A. , Baig, A.A., Hussain, M., Saeed, M.U., Bilal, M., Ahmed, N., Chopra, H., Hassan, M., Rachamalla, M., Putnala, S.K. and Khaliq, M. Narrative on Hydrogen Therapy and its Clinical Applications: Safety and Efficacy. Curr. Pharma. Des. 2022, 28, 2519–2537. [Google Scholar] [CrossRef]
- Barancik, M. , Kura, B., LeBaron, T.W., Bolli, R., Buday, J. and Slezak, J. Molecular and cellular mechanisms associated with effects of molecular hydrogen in cardiovascular and central nervous systems. Antioxidants 2020, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, M. , Sobue, S., Ito, M., Ito, M., Hirayama, M. and Ohno, K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen-comprehensive review of 321 original articles. Med. Gas Res. 2015, 5, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gharib, B. , Hanna, S., Abdallahi, O.M., Lepidi, H., Gardette, B. and De Reggi, M. Anti-inflammatory properties of molecular hydrogen: investigation on parasite-induced liver inflammation. Comptes Rendus de l’Académie des Sciences-Series III-Sciences de la Vie. 2001, 324, 719–724. [Google Scholar]
- Huang, L. Molecular hydrogen: a therapeutic antioxidant and beyond. Med. Gas Res. 2016, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Satta, H. , Iwamoto, T., Kawai, Y., Koguchi, N., Shibata, K., Kobayashi, N., Yoshida, M. and Nakayama, M. Amelioration of hemodialysis-induced oxidative stress and fatigue with a hemodialysis system employing electrolyzed water containing molecular hydrogen. Renal Replacement Ther. 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R. , Schock, B., Chalbatani, G.M., Zarandi, P.K., Jalali, S.A. and Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes & diseases 2021, 8, 287–297. [Google Scholar]
- DiDonato, J.A. , Mercurio, F. and Karin, M., 2012. NF-κB and the link between inflammation and cancer. Immuno. Revs. 2012, 246, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Verzella, D. , Pescatore, A., Capece, D., Vecchiotti, D., Ursini, M.V., Franzoso, G., Alesse, E. and Zazzeroni, F. Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death & Dis. 2020, 11, 210. [Google Scholar]
- Liu, M.Y. , Xie, F., Zhang, Y., Wang, T.T., Ma, S.N., Zhao, P.X., Zhang, X., Lebaron, T.W., Yan, X.L. and Ma, X.M. Molecular hydrogen suppresses glioblastoma growth via inducing the glioma stem-like cell differentiation. Stem Cell Res. & Ther. 2019; 10, 145. [Google Scholar]
- Yang, Q. , Ji, G., Pan, R., Zhao, Y. and Yan, P., 2017. Protective effect of hydrogen-rich water on liver function of colorectal cancer patients treated with mFOLFOX6 chemotherapy. Mol. and Clin. Onco. 2017, 7, 891–896. [Google Scholar] [CrossRef]
- Hirano, S.I. , Ichikawa, Y., Sato, B., Yamamoto, H., Takefuji, Y. and Satoh, F., 2021. Molecular hydrogen as a potential clinically applicable radioprotective agent. Int. Jrnl. Of Mol. Sci. 2021; 22, 4566. [Google Scholar]
- Zhou, W. , Zhang, J., Chen, W. and Miao, C. Prospects of molecular hydrogen in cancer prevention and treatment. Jrnl of Cancer Res. and Clin. Onco. 2024, 150, 170. [Google Scholar] [CrossRef]
- Begum, R. , Kim, C.S., Fadriquela, A., Bajgai, J., Jing, X., Kim, D.H., Kim, S.K. and Lee, K.J. Molecular hydrogen protects against oxidative stress-induced RAW 264.7 macrophage cells through the activation of Nrf2 and inhibition of MAPK signaling pathway. Mol. & Cell. Tox. 2020; 16, 103–118. [Google Scholar]
- Liu, K. , Ou, J., Wang, S., Gao, J., Liu, L., Ye, Y., Wilson, D.A., Hu, Y., Peng, F. and Tu, Y. Magnesium-based micromotors for enhanced active and synergistic hydrogen chemotherapy. Appl. Mat. Tdy. 2020, 20, 100694. [Google Scholar]
- Artamonov, M.Y. , Martusevich, A.K., Pyatakovich, F.A., Minenko, I.A., Dlin, S.V. and LeBaron, T.W. Molecular hydrogen: from molecular effects to stem cells management and tissue regeneration. Antioxidants 2023, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C. , Buse, M., Busuioc, C., Drula, R., Gulei, D., Raduly, L., Rusu, A., Irimie, A., Atanasov, A.G., Slaby, O. and Ionescu, C. A comprehensive review on MAPK: a promising therapeutic target in cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- You, I.S.; Sharma, S.; Fadriquela, A.; Bajgai, J.; Thi, T.T.; Rahman, M.H.; Sung, J.; Kwon, H.U.; Lee, S.Y.; Kim, C.S.; et al. Antioxidant properties of hydrogen gas attenuates oxidative stress in airway epithelial cells. Molecules 2021, 26, 6375. [Google Scholar] [CrossRef] [PubMed]
- Chu, J. , Gao, J., Wang, J., Li, L., Chen, G., Dang, J., Wang, Z., Jin, Z. and Liu, X. Mechanism of hydrogen on cervical cancer suppression revealed by high-throughput RNA sequencing. Onco. Rpts. 2021, 46, 1–11. [Google Scholar]
- Zhu, B. , Cui, H. and Xu, W. Hydrogen inhibits the proliferation and migration of gastric cancer cells by modulating lncRNA MALAT1/miR-124-3p/EZH2 axis. Cancer Cell Int. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meng, J. , Liu, L., Wang, D., Yan, Z. and Chen, G. Hydrogen gas represses the progression of lung cancer via down-regulating CD47. Biosci. Rpts. 2020, 40, 20192761. [Google Scholar]
- Wang, F. , Shu, X., Meszoely, I., Pal, T., Mayer, I.A., Yu, Z., Zheng, W., Bailey, C.E. and Shu, X.O. Overall mortality after diagnosis of breast cancer in men vs women. JAMA onco. 2019, 5, 1589–1596. [Google Scholar] [CrossRef]
- Arnold, M. , Morgan, E., Rumgay, H., Mafra, A., Singh, D., Laversanne, M., Vignat, J., Gralow, J.R., Cardoso, F., Siesling, S. and Soerjomataram, I. Current and future burden of breast cancer: Global statistics for 2020 and 2040. The Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Tchen, N. , Juffs, H.G., Downie, F.P., Yi, Q.L., Hu, H., Chemerynsky, I., Clemons, M., Crump, M., Goss, P.E., Warr, D. and Tweedale, M.E. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. Jrnl. of Clin. Onco. 2003, 21, 4175–4183. [Google Scholar] [CrossRef]
- Smolarz, B. , Nowak, A.Z. and Romanowicz, H. Breast cancer—epidemiology, classification, pathogenesis and treatment (review of literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- McCart Reed, A.E. , Kalinowski, L., Simpson, P.T. and Lakhani, S.R. Invasive lobular carcinoma of the breast: the increasing importance of this special subtype. Breast Cancer Res. 2021, 23, 1–16. [Google Scholar] [CrossRef]
- Magno, E. and Bussard, K.M. A Representative Clinical Course of Progression, with Molecular Insights, of Hormone Receptor-Positive, HER2-Negative Bone Metastatic Breast Cancer. Int. Jrnl. of Mol. Sci. 2014, 25, 3407. [Google Scholar] [CrossRef]
- Derakhshan, F. and Reis-Filho, J.S. Pathogenesis of triple-negative breast cancer. Ann. Rev. of Path.: Mechs. of Dis. 2022, 17, 181–204. [Google Scholar] [CrossRef]
- Chen, J.B. , Kong, X.F., Lv, Y.Y., Qin, S.C., Sun, X.J., Mu, F., Lu, T.Y. and Xu, K.C. “Real world survey” of hydrogen-controlled cancer: a follow-up report of 82 advanced cancer patients. Med. Gas Res. 2019, 9, 115–121. [Google Scholar]
- Dohrn, N. and Klein, M.F. Colorectal cancer: current management and future perspectives. Brit. Jrnl of Surg. 2023, 110, 1256–1259. [Google Scholar] [CrossRef]
- Luo, H. , Chen, C.Y., Li, X., Zhang, X., Su, C.W., Liu, Y., Cao, T., Hao, L., Wang, M. and Kang, J.X. Increased lipogenesis is critical for self-renewal and growth of breast cancer stem cells: Impact of omega-3 fatty acids. Stem Cells, 2021, 39, 1660–1670. [Google Scholar] [CrossRef]
- Addeo, M. , Di Paola, G., Verma, H.K., Laurino, S., Russi, S., Zoppoli, P., Falco, G. and Mazzone, P. Gastric cancer stem cells: a glimpse on metabolic reprogramming. Front. in Onco. 2021, 11, 698394. [Google Scholar]
- Katoh, Y. , Yaguchi, T., Kubo, A., Iwata, T., Morii, K., Kato, D., Ohta, S., Satomi, R., Yamamoto, Y., Oyamada, Y. and Ouchi, K. Inhibition of stearoyl-CoA desaturase 1 (SCD1) enhances the antitumor T cell response through regulating β-catenin signaling in cancer cells and ER stress in T cells and synergizes with anti-PD-1 antibody. Jrnl for Immuno. of Cancer 2022, 10. [Google Scholar]
- Chen, J. , Wang, Y., Meng, W., Zhao, R., Lin, W., Xiao, H. and Liao, Y. Stearoyl-CoA Desaturases1 accelerates non-small cell lung cancer metastasis by promoting aromatase expression to improve estrogen synthesis. Int. Jrnl of Mol. Sci. 2023, 24, 6826. [Google Scholar] [CrossRef]
- Kim, S.A. , Jong, Y.C., Kang, M.S. and Yu, C.J., 2022. Antioxidation activity of molecular hydrogen via protoheme catalysis in vivo: an insight from ab initio calculations. Jrnl. of Mol. Modeling 2022, 28, 287. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular hydrogen may activate the transcription factor Nrf2 to alleviate oxidative stress through the hydrogen-targeted porphyrin. Aging Pathobio. and Therapeutics 2023, 25–32. [Google Scholar] [CrossRef]
- Russell, G. , May, J. and Hancock, J.T. An interplay of gases: Oxygen and hydrogen in biological systems. Oxygen 2024, 4, 37–52. [Google Scholar] [CrossRef]
- Hancock, J.T. , Russell, G., Craig, T.J., May, J., Morse, H.R. and Stamler, J.S. Understanding hydrogen: Lessons to be learned from physical interactions between the inert gases and the globin superfamily. Oxygen 2022, 2, 578–590. [Google Scholar] [CrossRef]
- Fan, X. , Jin, Y., Chen, G., Ma, X. and Zhang, L. Gut microbiota dysbiosis drives the development of colorectal cancer. Digestion 1964, 102, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Artemev, A. , Naik, S., Pougno, A., Honnavar, P. and Shanbhag, N.M. The association of microbiome dysbiosis with colorectal cancer. Cureus 2022, 14. [Google Scholar]
- Gagnière, J. , Raisch, J., Veziant, J., Barnich, N., Bonnet, R., Buc, E., Bringer, M.A., Pezet, D. and Bonnet, M., 2016. Gut microbiota imbalance and colorectal cancer. World Jrnl. of Gastroentero. 2016, 22, 501. [Google Scholar] [CrossRef]
- Russell, G. , Nenov, A., Kisher, H. and Hancock, J.T. Molecular hydrogen as medicine: An assessment of administration methods. Hydrogen, 2021, 2, 444–460. [Google Scholar] [CrossRef]
- Yang, Y. , Zhu, Y. and Xi, X. Antiinflammatory- and antitumor action of hydrogen via reactive oxygen species. Onco. Letters 2018, 16, 2771–2776. [Google Scholar]
- Wu, Y. , Yuan, M., Song, J., Chen, X. and Yang, H. Hydrogen gas from inflammation treatment to cancer therapy. ACS nano 2019, 13, 8505–8511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



