Submitted:
23 July 2024
Posted:
25 July 2024
You are already at the latest version
Abstract
Keywords:
1. Attachment
2. Fusion
3. Capsid Transport and Uncoating
4. Reverse Transcription
5. Integration
6. Transcription
7. Nuclear Export of Viral mRNA
8. Translation
9. Assembly and Egress
10. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blut), G.A.C.B. (Arbeitskreis; Blood’, S. ‘Assessment of P.T. by Human Immunodeficiency Virus (HIV). Transfus. Med. Hemotherapy 2016, 43, 203. [CrossRef]
- Ganser-Pornillos, B.K.; Yeager, M.; Pornillos, O. Assembly and Architecture of HIV. In Viral Molecular Machines; Rossmann, M.G., Rao, V.B., Eds.; Springer US: Boston, MA, 2012; pp. 441–465 ISBN 978-1-4614-0980-9.
- Wilen, C.B.; Tilton, J.C.; Doms, R.W. HIV: Cell Binding and Entry. Cold Spring Harb. Perspect. Med. 2012, 2, a006866. [CrossRef]
- Arrildt, K.T.; Joseph, S.B.; Swanstrom, R. The HIV-1 Env Protein: A Coat of Many Colors. Curr. HIV/AIDS Rep. 2012, 9, 52–63. [CrossRef]
- Yoon, V.; Fridkis-Hareli, M.; Munisamy, S.; Lee, J.; Anastasiades, D.; Stevceva, L. The GP120 Molecule of HIV-1 and Its Interaction with T Cells. Curr. Med. Chem. 2010, 17, 741–749. [CrossRef]
- Negi, G.; Sharma, A.; Dey, M.; Dhanawat, G.; Parveen, N. Membrane Attachment and Fusion of HIV-1, Influenza A, and SARS-CoV-2: Resolving the Mechanisms with Biophysical Methods. Biophys. Rev. 2022, 14, 1109–1140. [CrossRef]
- Sattentau, Q.J.; Weiss, R.A. The CD4 Antigen: Physiological Ligand and HIV Receptor. Cell 1988, 52, 631–633. [CrossRef]
- Doms, R.W.; Moore, J.P. HIV-1 Membrane Fusion. J. Cell Biol. 2000, 151, f9–f14.
- Lee, B.; Sharron, M.; Montaner, L.J.; Weissman, D.; Doms, R.W. Quantification of CD4, CCR5, and CXCR4 Levels on Lymphocyte Subsets, Dendritic Cells, and Differentially Conditioned Monocyte-Derived Macrophages. Proc. Natl. Acad. Sci. 1999, 96, 5215–5220. [CrossRef]
- Clapham, Paul R.; Reeves, Jacqueline D.; Simmons, Graham; Dejucq, Natalie; Hibbitts, S.; Aine, McKnight HIV Coreceptors, Cell Tropism and Inhibition by Chemokine Receptor Ligands. Mol. Membr. Biol. 1999, 16, 49–55. [CrossRef]
- Schaeffer, E.; Geleziunas, R.; Greene, W.C. Human Immunodeficiency Virus Type 1 Nef Functions at the Level of Virus Entry by Enhancing Cytoplasmic Delivery of Virions. J. Virol. 2001, 75, 2993–3000. [CrossRef]
- Clapham, P.R.; McKnight, Á. HIV-1 Receptors and Cell Tropism. Br. Med. Bull. 2001, 58, 43–59. [CrossRef]
- Olinger, G.G.; Saifuddin, M.; Spear, G.T. CD4-Negative Cells Bind Human Immunodeficiency Virus Type 1 and Efficiently Transfer Virus to T Cells. J. Virol. 2000, 74, 8550–8557.
- Schnittman, S.M.; Lane, H.C.; Greenhouse, J.; Justement, J.S.; Baseler, M.; Fauci, A.S. Preferential Infection of CD4+ Memory T Cells by Human Immunodeficiency Virus Type 1: Evidence for a Role in the Selective T-Cell Functional Defects Observed in Infected Individuals. Proc. Natl. Acad. Sci. U. S. A. 1990, 87, 6058–6062. [CrossRef]
- Joseph, S.B.; Arrildt, K.T.; Sturdevant, C.B.; Swanstrom, R. HIV-1 Target Cells in the CNS. J. Neurovirol. 2015, 21, 276–289. [CrossRef]
- Blanco, J.; Barretina, J.; Gutiérrez, A.; Armand-Ugón, M.; Cabrera, C.; Clotet, B.; Esté, J.A. Preferential Attachment of HIV Particles to Activated and CD45RO+CD4+ T Cells. AIDS Res. Hum. Retroviruses 2002, 18, 27–38. [CrossRef]
- Spina, C.A.; Prince, H.E.; Richman, D.D. Preferential Replication of HIV-1 in the CD45RO Memory Cell Subset of Primary CD4 Lymphocytes in Vitro. Available online: https://www.jci.org/articles/view/119342/pdf (accessed on 13 May 2024).
- Brenchley, J.M.; Hill, B.J.; Ambrozak, D.R.; Price, D.A.; Guenaga, F.J.; Casazza, J.P.; Kuruppu, J.; Yazdani, J.; Migueles, S.A.; Connors, M.; et al. T-Cell Subsets That Harbor Human Immunodeficiency Virus (HIV) In Vivo: Implications for HIV Pathogenesis. J. Virol. 2004, 78, 1160–1168. [CrossRef]
- Stevenson, M.; Stanwick, T.L.; Dempsey, M.P.; Lamonica, C.A. HIV-1 Replication Is Controlled at the Level of T Cell Activation and Proviral Integration. EMBO J. 1990, 9, 1551–1560. [CrossRef]
- Ostrowski, M.A.; Chun, T.-W.; Justement, S.J.; Motola, I.; Spinelli, M.A.; Adelsberger, J.; Ehler, L.A.; Mizell, S.B.; Hallahan, C.W.; Fauci, A.S. Both Memory and CD45RA+/CD62L+ Naive CD4+ T Cells Are Infected in Human Immunodeficiency Virus Type 1-Infected Individuals. J. Virol. 1999, 73, 6430–6435. [CrossRef]
- Chun, T.W.; Engel, D.; Berrey, M.M.; Shea, T.; Corey, L.; Fauci, A.S. Early Establishment of a Pool of Latently Infected, Resting CD4(+) T Cells during Primary HIV-1 Infection. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 8869–8873. [CrossRef]
- Chavez, L.; Calvanese, V.; Verdin, E. HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells. PLOS Pathog. 2015, 11, e1004955. [CrossRef]
- Cenker, J.J.; Stultz, R.D.; McDonald, D. Brain Microglial Cells Are Highly Susceptible to HIV-1 Infection and Spread. AIDS Res. Hum. Retroviruses 2017, 33, 1155–1165. [CrossRef]
- Koppensteiner, H.; Brack-Werner, R.; Schindler, M. Macrophages and Their Relevance in Human Immunodeficiency Virus Type I Infection. Retrovirology 2012, 9, 82. [CrossRef]
- Loré, K.; Smed-Sörensen, A.; Vasudevan, J.; Mascola, J.R.; Koup, R.A. Myeloid and Plasmacytoid Dendritic Cells Transfer HIV-1 Preferentially to Antigen-Specific CD4+ T Cells. J. Exp. Med. 2005, 201, 2023–2033. [CrossRef]
- Manches, O.; Frleta, D.; Bhardwaj, N. Dendritic Cells in Progression and Pathology of HIV Infection. Trends Immunol. 2014, 35, 114–122. [CrossRef]
- Gill, V.; Shattock, R.J.; Freeman, A.R.; Robinson, G.; Griffin, G.E.; Gordon-Smith, E.C.; Gibson, F.M. Macrophages Are the Major Target Cell for HIV Infection in Long-Term Marrow Culture and Demonstrate Dual Susceptibility to Lymphocytotropic and Monocytotropic Strains of HIV-1. Br. J. Haematol. 1996, 93, 30–37. [CrossRef]
- Campbell, J.H.; Hearps, A.C.; Martin, G.E.; Williams, K.C.; Crowe, S.M. The Importance of Monocytes and Macrophages in HIV Pathogenesis, Treatment, and Cure. AIDS 2014, 28, 2175. [CrossRef]
- Calantone, N.; Wu, F.; Klase, Z.; Deleage, C.; Perkins, M.; Matsuda, K.; Thompson, E.A.; Ortiz, A.M.; Vinton, C.L.; Ourmanov, I.; et al. Tissue Myeloid Cells in SIV-Infected Primates Acquire Viral DNA through Phagocytosis of Infected T Cells. Immunity 2014, 41, 493–502. [CrossRef]
- Kazazi, F.; Mathijs, J.-M.; Foley, P.; Cunningham, A.L. Variations in CD4 Expression by Human Monocytes and Macrophages and Their Relationship to Infection with the Human Immunodeficiency Virus. J. Gen. Virol. 1989, 70, 2661–2672. [CrossRef]
- Quitadamo, B.; Peters, P.J.; Repik, A.; O’Connell, O.; Mou, Z.; Koch, M.; Somasundaran, M.; Brody, R.; Luzuriaga, K.; Wallace, A.; et al. HIV-1 R5 Macrophage-Tropic Envelope Glycoprotein Trimers Bind CD4 with High Affinity, While the CD4 Binding Site on Non-Macrophage-Tropic, T-Tropic R5 Envelopes Is Occluded. J. Virol. 2018, 92, e00841-17. [CrossRef]
- Joseph, S.B.; Arrildt, K.T.; Swanstrom, A.E.; Schnell, G.; Lee, B.; Hoxie, J.A.; Swanstrom, R. Quantification of Entry Phenotypes of Macrophage-Tropic HIV-1 across a Wide Range of CD4 Densities. J. Virol. 2014, 88, 1858–1869. [CrossRef]
- Arrildt, K.T.; LaBranche, C.C.; Joseph, S.B.; Dukhovlinova, E.N.; Graham, W.D.; Ping, L.-H.; Schnell, G.; Sturdevant, C.B.; Kincer, L.P.; Mallewa, M.; et al. Phenotypic Correlates of HIV-1 Macrophage Tropism. J. Virol. 2015, 89, 11294–11311. [CrossRef]
- Maddon, P.J.; McDougal, J.S.; Clapham, P.R.; Dalgleish, A.G.; Jamal, S.; Weiss, R.A.; Axel, R. HIV Infection Does Not Require Endocytosis of Its Receptor, CD4. Cell 1988, 54, 865–874. [CrossRef]
- Deng, H.; Liu, R.; Ellmeier, W.; Choe, S.; Unutmaz, D.; Burkhart, M.; Marzio, P.D.; Marmon, S.; Sutton, R.E.; Hill, C.M.; et al. Identification of a Major Co-Receptor for Primary Isolates of HIV-1. Nature 1996, 381, 661–666. [CrossRef]
- Dragic, T.; Litwin, V.; Allaway, G.P.; Martin, S.R.; Huang, Y.; Nagashima, K.A.; Cayanan, C.; Maddon, P.J.; Koup, R.A.; Moore, J.P.; et al. HIV-1 Entry into CD4+ Cells Is Mediated by the Chemokine Receptor CC-CKR-5. Nature 1996, 381, 667–673. [CrossRef]
- Alkhatib, G.; Combadiere, C.; Broder, C.C.; Feng, Y.; Kennedy, P.E.; Murphy, P.M.; Berger, E.A. CC CKR5: A RANTES, MIP-1alpha, MIP-1beta Receptor as a Fusion Cofactor for Macrophage-Tropic HIV-1. Science 1996, 272, 1955–1958. [CrossRef]
- Feng, Y.; Broder, C.C.; Kennedy, P.E.; Berger, E.A. HIV-1 Entry Cofactor: Functional cDNA Cloning of a Seven-Transmembrane, G Protein-Coupled Receptor. Science 1996, 272, 872–877. [CrossRef]
- Doranz, B.J.; Rucker, J.; Yi, Y.; Smyth, R.J.; Samson, M.; Peiper, S.C.; Parmentier, M.; Collman, R.G.; Doms, R.W. A Dual-Tropic Primary HIV-1 Isolate That Uses Fusin and the β-Chemokine Receptors CKR-5, CKR-3, and CKR-2b as Fusion Cofactors. Cell 1996, 85, 1149–1158. [CrossRef]
- He, J.; Chen, Y.; Farzan, M.; Choe, H.; Ohagen, A.; Gartner, S.; Busciglio, J.; Yang, X.; Hofmann, W.; Newman, W.; et al. CCR3 and CCR5 Are Co-Receptors for HIV-1 Infection of Microglia. Nature 1997, 385, 645–649. [CrossRef]
- Choe, H.; Farzan, M.; Sun, Y.; Sullivan, N.; Rollins, B.; Ponath, P.D.; Wu, L.; Mackay, C.R.; LaRosa, G.; Newman, W.; et al. The β-Chemokine Receptors CCR3 and CCR5 Facilitate Infection by Primary HIV-1 Isolates. Cell 1996, 85, 1135–1148. [CrossRef]
- Parrish, N.F.; Gao, F.; Li, H.; Giorgi, E.E.; Barbian, H.J.; Parrish, E.H.; Zajic, L.; Iyer, S.S.; Decker, J.M.; Kumar, A.; et al. Phenotypic Properties of Transmitted Founder HIV-1. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 6626–6633. [CrossRef]
- Keele, B.F.; Giorgi, E.E.; Salazar-Gonzalez, J.F.; Decker, J.M.; Pham, K.T.; Salazar, M.G.; Sun, C.; Grayson, T.; Wang, S.; Li, H.; et al. Identification and Characterization of Transmitted and Early Founder Virus Envelopes in Primary HIV-1 Infection. Proc. Natl. Acad. Sci. 2008, 105, 7552–7557. [CrossRef]
- Berger, E.A.; Murphy, P.M.; Farber, J.M. CHEMOKINE RECEPTORS AS HIV-1 CORECEPTORS: Roles in Viral Entry, Tropism, and Disease. Annu. Rev. Immunol. 1999, 17, 657–700. [CrossRef]
- Bleul, C.C.; Wu, L.; Hoxie, J.A.; Springer, T.A.; Mackay, C.R. The HIV Coreceptors CXCR4 and CCR5 Are Differentially Expressed and Regulated on Human T Lymphocytes. Proc. Natl. Acad. Sci. 1997, 94, 1925–1930. [CrossRef]
- Weinberger, A.D.; Perelson, A.S. Persistence and Emergence of X4 Virus in HIV Infection. Math. Biosci. Eng. MBE 2011, 8, 605–626.
- Wu, L.; Paxton, W.A.; Kassam, N.; Ruffing, N.; Rottman, J.B.; Sullivan, N.; Choe, H.; Sodroski, J.; Newman, W.; Koup, R.A.; et al. CCR5 Levels and Expression Pattern Correlate with Infectability by Macrophage-Tropic HIV-1, In Vitro. J. Exp. Med. 1997, 185, 1681–1692. [CrossRef]
- Cashin, K.; Roche, M.; Sterjovski, J.; Ellett, A.; Gray, L.R.; Cunningham, A.L.; Ramsland, P.A.; Churchill, M.J.; Gorry, P.R. Alternative Coreceptor Requirements for Efficient CCR5- and CXCR4-Mediated HIV-1 Entry into Macrophages. J. Virol. 2011, 85, 10699–10709. [CrossRef]
- Jayakumar, P.; Berger, I.; Autschbach, F.; Weinstein, M.; Funke, B.; Verdin, E.; Goldsmith, M.A.; Keppler, O.T. Tissue-Resident Macrophages Are Productively Infected Ex Vivo by Primary X4 Isolates of Human Immunodeficiency Virus Type 1. J. Virol. 2005, 79, 5220–5226. [CrossRef]
- Borrajo, A.; Ranazzi, A.; Pollicita, M.; Bellocchi, M.C.; Salpini, R.; Mauro, M.V.; Ceccherini-Silberstein, F.; Perno, C.F.; Svicher, V.; Aquaro, S. Different Patterns of HIV-1 Replication in MACROPHAGES Is Led by Co-Receptor Usage. Medicina (Mex.) 2019, 55, 297. [CrossRef]
- Bonner, X.; Sondgeroth, A.; McCue, A.; Nicely, N.; Tripathy, A.; Spielvogel, E.; Moeser, M.; Ke, R.; Leiderman, K.; Joseph, S.B.; et al. Stoichiometry for Entry and Binding Properties of the Env Protein of R5 T Cell-Tropic HIV-1 and Its Evolutionary Variant of Macrophage-Tropic HIV-1. mBio 2024, 15, e0032124. [CrossRef]
- Okoye, A.A.; Picker, L.J. CD4+ T Cell Depletion in HIV Infection: Mechanisms of Immunological Failure. Immunol. Rev. 2013, 254, 54–64. [CrossRef]
- Douek, D.C.; Picker, L.J.; Koup, R.A. T Cell Dynamics in HIV-1 Infection. Annu. Rev. Immunol. 2003, 21, 265–304. [CrossRef]
- Blaak, H.; van’t Wout, A.B.; Brouwer, M.; Hooibrink, B.; Hovenkamp, E.; Schuitemaker, H. In Vivo HIV-1 Infection of CD45RA+CD4+ T Cells Is Established Primarily by Syncytium-Inducing Variants and Correlates with the Rate of CD4+ T Cell Decline. Proc. Natl. Acad. Sci. 2000, 97, 1269–1274. [CrossRef]
- Dejucq, N. HIV-1 Replication in CD4+ T Cell Lines: The Effects of Adaptation on Co-Receptor Use, Tropism, and Accessory Gene Function. J. Leukoc. Biol. 2000, 68, 331–337.
- Connor, R.I.; Sheridan, K.E.; Ceradini, D.; Choe, S.; Landau, N.R. Change in Coreceptor Use Correlates with Disease Progression in HIV-1–Infected Individuals. J. Exp. Med. 1997, 185, 621–628. [CrossRef]
- Weinberger, A.D.; Perelson, A.S.; Ribeiro, R.M.; Weinberger, L.S. Accelerated Immunodeficiency by Anti-CCR5 Treatment in HIV Infection. PLoS Comput. Biol. 2009, 5, e1000467. [CrossRef]
- Ivetic, A.; Hoskins Green, H.L.; Hart, S.J. L-Selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front. Immunol. 2019, 10. [CrossRef]
- Kononchik, J.; Ireland, J.; Zou, Z.; Segura, J.; Holzapfel, G.; Chastain, A.; Wang, R.; Spencer, M.; He, B.; Stutzman, N.; et al. HIV-1 Targets L-Selectin for Adhesion and Induces Its Shedding for Viral Release. Nat. Commun. 2018, 9, 2825. [CrossRef]
- Sallusto, F.; Schaerli, P.; Loetscher, P.; Schaniel, C.; Lenig, D.; Mackay, C.R.; Qin, S.; Lanzavecchia, A. Rapid and Coordinated Switch in Chemokine Receptor Expression during Dendritic Cell Maturation. Eur. J. Immunol. 1998, 28, 2760–2769. [CrossRef]
- Turville, S.G.; Cameron, P.U.; Handley, A.; Lin, G.; Pöhlmann, S.; Doms, R.W.; Cunningham, A.L. Diversity of Receptors Binding HIV on Dendritic Cell Subsets. Nat. Immunol. 2002, 3, 975–983. [CrossRef]
- McIlroy, D.; Autran, B.; Cheynier, R.; Wain-Hobson, S.; Clauvel, J.P.; Oksenhendler, E.; Debré, P.; Hosmalin, A. Infection Frequency of Dendritic Cells and CD4+ T Lymphocytes in Spleens of Human Immunodeficiency Virus-Positive Patients. J. Virol. 1995, 69, 4737–4745. [CrossRef]
- Felts, R.L.; Narayan, K.; Estes, J.D.; Shi, D.; Trubey, C.M.; Fu, J.; Hartnell, L.M.; Ruthel, G.T.; Schneider, D.K.; Nagashima, K.; et al. 3D Visualization of HIV Transfer at the Virological Synapse between Dendritic Cells and T Cells. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 13336–13341. [CrossRef]
- Fortin, J.F.; Cantin, R.; Lamontagne, G.; Tremblay, M. Host-Derived ICAM-1 Glycoproteins Incorporated on Human Immunodeficiency Virus Type 1 Are Biologically Active and Enhance Viral Infectivity. J. Virol. 1997, 71, 3588–3596. [CrossRef]
- Rizzuto, C.D.; Sodroski, J.G. Contribution of Virion ICAM-1 to Human Immunodeficiency Virus Infectivity and Sensitivity to Neutralization. J. Virol. 1997, 71, 4847–4851. [CrossRef]
- Bounou, S.; Giguere, J.-F.; Cantin, R.; Gilbert, C.; Imbeault, M.; Martin, G.; Tremblay, M.J. The Importance of Virus-Associated Host ICAM-1 in Human Immunodeficiency Virus Type 1 Dissemination Depends on the Cellular Context. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 1294–1296. [CrossRef]
- Ohno, H.; Aguilar, R.C.; Fournier, M.C.; Hennecke, S.; Cosson, P.; Bonifacino, J.S. Interaction of Endocytic Signals from the HIV-1 Envelope Glycoprotein Complex with Members of the Adaptor Medium Chain Family. Virology 1997, 238, 305–315. [CrossRef]
- Boge, M.; Wyss, S.; Bonifacino, J.S.; Thali, M. A Membrane-Proximal Tyrosine-Based Signal Mediates Internalization of the HIV-1 Envelope Glycoprotein via Interaction with the AP-2 Clathrin Adaptor. J. Biol. Chem. 1998, 273, 15773–15778. [CrossRef]
- Zaitseva, E.; Zaitsev, E.; Melikov, K.; Arakelyan, A.; Marin, M.; Villasmil, R.; Margolis, L.B.; Melikyan, G.B.; Chernomordik, L.V. FUSION STAGE OF HIV-1 ENTRY DEPENDS ON VIRUS-INDUCED CELL SURFACE EXPOSURE OF PHOSPHATIDYLSERINE. Cell Host Microbe 2017, 22, 99-110.e7. [CrossRef]
- Callahan, M.K.; Popernack, P.M.; Tsutsui, S.; Truong, L.; Schlegel, R.A.; Henderson, A.J. Phosphatidylserine on HIV Envelope Is a Cofactor for Infection of Monocytic Cells1. J. Immunol. 2003, 170, 4840–4845. [CrossRef]
- Lu, J.; Pan, Q.; Rong, L.; Liu, S.-L.; Liang, C. The IFITM Proteins Inhibit HIV-1 Infection. J. Virol. 2011, 85, 2126–2137. [CrossRef]
- Foster, T.L.; Wilson, H.; Iyer, S.S.; Coss, K.; Doores, K.; Smith, S.; Kellam, P.; Finzi, A.; Borrow, P.; Hahn, B.H.; et al. Resistance of Transmitted Founder HIV-1 to IFITM-Mediated Restriction. Cell Host Microbe 2016, 20, 429–442. [CrossRef]
- Tartour, K.; Nguyen, X.-N.; Appourchaux, R.; Assil, S.; Barateau, V.; Bloyet, L.-M.; Gaillard, J.B.; Confort, M.-P.; Escudero-Perez, B.; Gruffat, H.; et al. Interference with the Production of Infectious Viral Particles and Bimodal Inhibition of Replication Are Broadly Conserved Antiviral Properties of IFITMs. PLOS Pathog. 2017, 13, e1006610. [CrossRef]
- Wang, Y.; Pan, Q.; Ding, S.; Wang, Z.; Yu, J.; Finzi, A.; Liu, S.-L.; Liang, C. The V3 Loop of HIV-1 Env Determines Viral Susceptibility to IFITM3 Impairment of Viral Infectivity. J. Virol. 2017, 91, 10.1128/jvi.02441-16. [CrossRef]
- Amini-Bavil-Olyaee, S.; Choi, Y.J.; Lee, J.H.; Shi, M.; Huang, I.-C.; Farzan, M.; Jung, J.U. The Antiviral Effector IFITM3 Disrupts Intracellular Cholesterol Homeostasis to Block Viral Entry. Cell Host Microbe 2013, 13, 452–464. [CrossRef]
- Silva-Januário, M.E. da; Costa, C.S. da; Tavares, L.A.; Oliveira, A.K.; Januário, Y.C.; Carvalho, A.N. de; Cassiano, M.H.A.; Rodrigues, R.L.; Miller, M.E.; Palameta, S.; et al. HIV-1 Nef Changes the Proteome of T Cells Extracellular Vesicles Depleting IFITMs and Other Antiviral Factors. Mol. Cell. Proteomics 2023, 22. [CrossRef]
- Compton, A.A.; Bruel, T.; Porrot, F.; Mallet, A.; Sachse, M.; Euvrard, M.; Liang, C.; Casartelli, N.; Schwartz, O. IFITM Proteins Incorporated into HIV-1 Virions Impair Viral Fusion and Spread. Cell Host Microbe 2014, 16, 736–747. [CrossRef]
- Inuzuka, M.; Hayakawa, M.; Ingi, T. Serinc, an Activity-Regulated Protein Family, Incorporates Serine into Membrane Lipid Synthesis. J. Biol. Chem. 2005, 280, 35776–35783. [CrossRef]
- Usami, Y.; Wu, Y.; Göttlinger, H.G. SERINC3 and SERINC5 Restrict HIV-1 Infectivity and Are Counteracted by Nef. Nature 2015, 526, 218–223. [CrossRef]
- Sood, C.; Marin, M.; Chande, A.; Pizzato, M.; Melikyan, G.B. SERINC5 Protein Inhibits HIV-1 Fusion Pore Formation by Promoting Functional Inactivation of Envelope Glycoproteins. J. Biol. Chem. 2017, 292, 6014–6026. [CrossRef]
- Beitari, S.; Ding, S.; Pan, Q.; Finzi, A.; Liang, C. Effect of HIV-1 Env on SERINC5 Antagonism. J. Virol. 2017, 91, 10.1128/jvi.02214-16. [CrossRef]
- Grover, J.R.; Veatch, S.L.; Ono, A. Basic Motifs Target PSGL-1, CD43, and CD44 to Plasma Membrane Sites Where HIV-1 Assembles. J. Virol. 2015, 89, 454–467. [CrossRef]
- Fu, Y.; He, S.; Waheed, A.A.; Dabbagh, D.; Zhou, Z.; Trinité, B.; Wang, Z.; Yu, J.; Wang, D.; Li, F.; et al. PSGL-1 Restricts HIV-1 Infectivity by Blocking Virus Particle Attachment to Target Cells. Proc. Natl. Acad. Sci. 2020, 117, 9537–9545. [CrossRef]
- Suzuki, Y.; Craigie, R. The Road to Chromatin — Nuclear Entry of Retroviruses. Nat. Rev. Microbiol. 2007, 5, 187–196. [CrossRef]
- Hulme, A.E.; Perez, O.; Hope, T.J. Complementary Assays Reveal a Relationship between HIV-1 Uncoating and Reverse Transcription. Proc. Natl. Acad. Sci. 2011, 108, 9975–9980. [CrossRef]
- Mamede, J.I.; Cianci, G.C.; Anderson, M.R.; Hope, T.J. Early Cytoplasmic Uncoating Is Associated with Infectivity of HIV-1. Proc. Natl. Acad. Sci. 2017, 114, E7169–E7178. [CrossRef]
- Arhel, N.J.; Souquere-Besse, S.; Munier, S.; Souque, P.; Guadagnini, S.; Rutherford, S.; Prévost, M.; Allen, T.D.; Charneau, P. HIV-1 DNA Flap Formation Promotes Uncoating of the Pre-integration Complex at the Nuclear Pore. EMBO J. 2007, 26, 3025–3037. [CrossRef]
- Müller, T.G.; Zila, V.; Peters, K.; Schifferdecker, S.; Stanic, M.; Lucic, B.; Laketa, V.; Lusic, M.; Müller, B.; Kräusslich, H.-G. HIV-1 Uncoating by Release of Viral cDNA from Capsid-like Structures in the Nucleus of Infected Cells. eLife 2021, 10, e64776. [CrossRef]
- Selyutina, A.; Persaud, M.; Lee, K.; KewalRamani, V.; Diaz-Griffero, F. Nuclear Import of the HIV-1 Core Precedes Reverse Transcription and Uncoating. Cell Rep. 2020, 32, 108201. [CrossRef]
- Gifford, L.B.; Melikyan, G.B. HIV-1 Capsid Uncoating Is a Multistep Process That Proceeds through Defect Formation Followed by Disassembly of the Capsid Lattice. ACS Nano 2024, 18, 2928–2947. [CrossRef]
- Müller, T.G.; Zila, V.; Müller, B.; Kräusslich, H.-G. Nuclear Capsid Uncoating and Reverse Transcription of HIV-1. Annu. Rev. Virol. 2022, 9, 261–284. [CrossRef]
- Rensen, E.; Mueller, F.; Scoca, V.; Parmar, J.J.; Souque, P.; Zimmer, C.; Di Nunzio, F. Clustering and Reverse Transcription of HIV-1 Genomes in Nuclear Niches of Macrophages. EMBO J. 2021, 40, e105247. [CrossRef]
- Sumner, R.P.; Harrison, L.; Touizer, E.; Peacock, T.P.; Spencer, M.; Zuliani-Alvarez, L.; Towers, G.J. Disrupting HIV-1 Capsid Formation Causes cGAS Sensing of Viral DNA. EMBO J. 2020, 39, e103958. [CrossRef]
- Campbell, E.M.; Hope, T.J. HIV-1 Capsid: The Multifaceted Key Player in HIV-1 Infection. Nat. Rev. Microbiol. 2015, 13, 471–483. [CrossRef]
- Rebensburg, S.V.; Wei, G.; Larue, R.C.; Lindenberger, J.; Francis, A.C.; Annamalai, A.S.; Morrison, J.; Shkriabai, N.; Huang, S.-W.; KewalRamani, V.; et al. Sec24C Is an HIV-1 Host Dependency Factor Crucial for Virus Replication. Nat. Microbiol. 2021, 6, 435–444. [CrossRef]
- Guth, C.A.; Sodroski, J. Contribution of PDZD8 to Stabilization of the Human Immunodeficiency Virus Type 1 Capsid. J. Virol. 2014, 88, 4612–4623. [CrossRef]
- Malikov, V.; da Silva, E.S.; Jovasevic, V.; Bennett, G.; de Souza Aranha Vieira, D.A.; Schulte, B.; Diaz-Griffero, F.; Walsh, D.; Naghavi, M.H. HIV-1 Capsids Bind and Exploit the Kinesin-1 Adaptor FEZ1 for Inward Movement to the Nucleus. Nat. Commun. 2015, 6, 6660. [CrossRef]
- Huang, P.-T.; Summers, B.J.; Xu, C.; Perilla, J.R.; Malikov, V.; Naghavi, M.H.; Xiong, Y. FEZ1 Is Recruited to a Conserved Cofactor Site on Capsid to Promote HIV-1 Trafficking. Cell Rep. 2019, 28, 2373-2385.e7. [CrossRef]
- Carnes, S.K.; Zhou, J.; Aiken, C. HIV-1 Engages a Dynein-Dynactin-BICD2 Complex for Infection and Transport to the Nucleus. J. Virol. 2018, 92, 10.1128/jvi.00358-18. [CrossRef]
- Dharan, A.; Opp, S.; Abdel-Rahim, O.; Keceli, S.K.; Imam, S.; Diaz-Griffero, F.; Campbell, E.M. Bicaudal D2 Facilitates the Cytoplasmic Trafficking and Nuclear Import of HIV-1 Genomes during Infection. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, E10707–E10716. [CrossRef]
- Dharan, A.; Talley, S.; Tripathi, A.; Mamede, J.I.; Majetschak, M.; Hope, T.J.; Campbell, E.M. KIF5B and Nup358 Cooperatively Mediate the Nuclear Import of HIV-1 during Infection. PLOS Pathog. 2016, 12, e1005700. [CrossRef]
- Opp, S.; Martinez-Lopez, A.; Fricke, T.; Buffone, C.; Severgnini, M.; Cifola, I.; Frabetti, S.; Skorupka, K.; Zadrozny, K.K.; Ganser-Pornillos, B.K.; et al. Nup153 Unlocks the Nuclear Pore Complex for HIV-1 Nuclear Import in Non-Dividing Cells 2018.
- Fernandez, J.; Machado, A.K.; Lyonnais, S.; Chamontin, C.; Gärtner, K.; Léger, T.; Henriquet, C.; Garcia, C.; Portilho, D.M.; Pugnière, M.; et al. Transportin-1 Binds to the HIV-1 Capsid via a Nuclear Localization Signal and Triggers Uncoating. Nat. Microbiol. 2019, 4, 1840–1850. [CrossRef]
- Shah, V.B.; Shi, J.; Hout, D.R.; Oztop, I.; Krishnan, L.; Ahn, J.; Shotwell, M.S.; Engelman, A.; Aiken, C. The Host Proteins Transportin SR2/TNPO3 and Cyclophilin A Exert Opposing Effects on HIV-1 Uncoating. J. Virol. 2013, 87, 422–432. [CrossRef]
- Misumi, S.; Inoue, M.; Dochi, T.; Kishimoto, N.; Hasegawa, N.; Takamune, N.; Shoji, S. Uncoating of Human Immunodeficiency Virus Type 1 Requires Prolyl Isomerase Pin1. J. Biol. Chem. 2010, 285, 25185–25195. [CrossRef]
- McDonald, D.; Vodicka, M.A.; Lucero, G.; Svitkina, T.M.; Borisy, G.G.; Emerman, M.; Hope, T.J. Visualization of the Intracellular Behavior of HIV in Living Cells. J. Cell Biol. 2002, 159, 441–452. [CrossRef]
- Nigro, P.; Pompilio, G.; Capogrossi, M.C. Cyclophilin A: A Key Player for Human Disease. Cell Death Dis. 2013, 4, e888–e888. [CrossRef]
- Luban, J.; Bossolt, K.L.; Franke, E.K.; Kalpana, G.V.; Goff, S.P. Human Immunodeficiency Virus Type 1 Gag Protein Binds to Cyclophilins A and B. Cell 1993, 73, 1067–1078. [CrossRef]
- Franke, E.K.; Yuan, H.E.; Luban, J. Specific Incorporation of Cyclophilin A into HIV-1 Virions. Nature 1994, 372, 359–362. [CrossRef]
- Thali, M.; Bukovsky, A.; Kondo, E.; Rosenwirth, B.; Walsh, C.T.; Sodroski, J.; Göttlinger, H.G. Functional Association of Cyclophilin A with HIV-1 Virions. Nature 1994, 372, 363–365. [CrossRef]
- Braaten, D.; Luban, J. Cyclophilin A Regulates HIV-1 Infectivity, as Demonstrated by Gene Targeting in Human T Cells. EMBO J. 2001, 20, 1300–1309. [CrossRef]
- Padron, A.; Prakash, P.; Pandhare, J.; Luban, J.; Aiken, C.; Balasubramaniam, M.; Dash, C. Emerging Role of Cyclophilin A in HIV-1 Infection: From Producer Cell to the Target Cell Nucleus. J. Virol. 2023, 97, e00732-23. [CrossRef]
- Gamble, T.R.; Vajdos, F.F.; Yoo, S.; Worthylake, D.K.; Houseweart, M.; Sundquist, W.I.; Hill, C.P. Crystal Structure of Human Cyclophilin A Bound to the Amino-Terminal Domain of HIV-1 Capsid. Cell 1996, 87, 1285–1294. [CrossRef]
- Perez-Caballero, D.; Hatziioannou, T.; Zhang, F.; Cowan, S.; Bieniasz, P.D. Restriction of Human Immunodeficiency Virus Type 1 by TRIM-CypA Occurs with Rapid Kinetics and Independently of Cytoplasmic Bodies, Ubiquitin, and Proteasome Activity. J. Virol. 2005, 79, 15567–15572. [CrossRef]
- Hulme, A.E.; Kelley, Z.; Okocha, E.A.; Hope, T.J. Identification of Capsid Mutations That Alter the Rate of HIV-1 Uncoating in Infected Cells. J. Virol. 2014, 89, 643–651. [CrossRef]
- Liu, C.; Perilla, J.R.; Ning, J.; Lu, M.; Hou, G.; Ramalho, R.; Himes, B.A.; Zhao, G.; Bedwell, G.J.; Byeon, I.-J.; et al. Cyclophilin A Stabilizes the HIV-1 Capsid through a Novel Non-Canonical Binding Site. Nat. Commun. 2016, 7, 10714. [CrossRef]
- Selyutina, A.; Persaud, M.; Simons, L.M.; Bulnes-Ramos, A.; Buffone, C.; Martinez-Lopez, A.; Scoca, V.; Di Nunzio, F.; Hiatt, J.; Marson, A.; et al. Cyclophilin A Prevents HIV-1 Restriction in Lymphocytes by Blocking Human TRIM5α Binding to the Viral Core. Cell Rep. 2020, 30, 3766-3777.e6. [CrossRef]
- Kim, K.; Dauphin, A.; Komurlu, S.; McCauley, S.M.; Yurkovetskiy, L.; Carbone, C.; Diehl, W.E.; Strambio-De-Castillia, C.; Campbell, E.M.; Luban, J. Cyclophilin A Protects HIV-1 from Restriction by Human TRIM5α. Nat. Microbiol. 2019, 4, 2044–2051. [CrossRef]
- Hatziioannou, T.; Perez-Caballero, D.; Cowan, S.; Bieniasz, P.D. Cyclophilin Interactions with Incoming Human Immunodeficiency Virus Type 1 Capsids with Opposing Effects on Infectivity in Human Cells. J. Virol. 2005, 79, 176–183. [CrossRef]
- Li, Y.; Kar, A.K.; Sodroski, J. Target Cell Type-Dependent Modulation of Human Immunodeficiency Virus Type 1 Capsid Disassembly by Cyclophilin A. J. Virol. 2009, 83, 10951–10962. [CrossRef]
- Mallery, D.L.; Márquez, C.L.; McEwan, W.A.; Dickson, C.F.; Jacques, D.A.; Anandapadamanaban, M.; Bichel, K.; Towers, G.J.; Saiardi, A.; Böcking, T.; et al. IP6 Is an HIV Pocket Factor That Prevents Capsid Collapse and Promotes DNA Synthesis. eLife 2018, 7, e35335. [CrossRef]
- Papa, G.; Albecka, A.; Mallery, D.; Vaysburd, M.; Renner, N.; James, L.C. IP6-Stabilised HIV Capsids Evade cGAS/STING-Mediated Host Immune Sensing. EMBO Rep. 2023, 24, e56275. [CrossRef]
- Bejarano, D.A.; Peng, K.; Laketa, V.; Börner, K.; Jost, K.L.; Lucic, B.; Glass, B.; Lusic, M.; Müller, B.; Kräusslich, H.-G. HIV-1 Nuclear Import in Macrophages Is Regulated by CPSF6-Capsid Interactions at the Nuclear Pore Complex. eLife 2019, 8, e41800. [CrossRef]
- Chin, C.R.; Perreira, J.M.; Savidis, G.; Portmann, J.M.; Aker, A.M.; Feeley, E.M.; Smith, M.C.; Brass, A.L. Direct Visualization of HIV-1 Replication Intermediates Shows That Capsid and CPSF6 Modulate HIV-1 Intra-Nuclear Invasion and Integration. Cell Rep. 2015, 13, 1717–1731. [CrossRef]
- Francis, A.C.; Marin, M.; Singh, P.K.; Achuthan, V.; Prellberg, M.J.; Palermino-Rowland, K.; Lan, S.; Tedbury, P.R.; Sarafianos, S.G.; Engelman, A.N.; et al. HIV-1 Replication Complexes Accumulate in Nuclear Speckles and Integrate into Speckle-Associated Genomic Domains. Nat. Commun. 2020, 11, 3505. [CrossRef]
- Achuthan, V.; Perreira, J.M.; Ahn, J.J.; Brass, A.L.; Engelman, A.N. Capsid-CPSF6 Interaction: Master Regulator of Nuclear HIV-1 Positioning and Integration. J. Life Sci. Westlake Village Calif 2019, 1, 39–45. [CrossRef]
- Bukrinsky, M.I.; Sharova, N.; Dempsey, M.P.; Stanwick, T.L.; Bukrinskaya, A.G.; Haggerty, S.; Stevenson, M. Active Nuclear Import of Human Immunodeficiency Virus Type 1 Preintegration Complexes. Proc. Natl. Acad. Sci. U. S. A. 1992, 89, 6580–6584. [CrossRef]
- Ananth, S.; Ambiel, I.; Schifferdecker, S.; Müller, T.G.; Wratil, P.R.; Mejias-Perez, E.; Kräusslich, H.-G.; Müller, B.; Keppler, O.T.; Fackler, O.T. Spatial Resolution of HIV-1 Post-Entry Steps in Resting CD4 T Cells. Cell Rep. 2024, 43. [CrossRef]
- Sowd, G.A.; Serrao, E.; Wang, H.; Wang, W.; Fadel, H.J.; Poeschla, E.M.; Engelman, A.N. A Critical Role for Alternative Polyadenylation Factor CPSF6 in Targeting HIV-1 Integration to Transcriptionally Active Chromatin. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E1054-1063. [CrossRef]
- Stremlau, M.; Owens, C.M.; Perron, M.J.; Kiessling, M.; Autissier, P.; Sodroski, J. The Cytoplasmic Body Component TRIM5alpha Restricts HIV-1 Infection in Old World Monkeys. Nature 2004, 427, 848–853. [CrossRef]
- Li, Y.; Li, X.; Stremlau, M.; Lee, M.; Sodroski, J. Removal of Arginine 332 Allows Human TRIM5alpha to Bind Human Immunodeficiency Virus Capsids and to Restrict Infection. J. Virol. 2006, 80, 6738–6744. [CrossRef]
- Black, L.R.; Aiken, C. TRIM5alpha Disrupts the Structure of Assembled HIV-1 Capsid Complexes in Vitro. J. Virol. 2010, 84, 6564–6569. [CrossRef]
- Jimenez-Guardeño, J.M.; Apolonia, L.; Betancor, G.; Malim, M.H. Immunoproteasome Activation Enables Human TRIM5α Restriction of HIV-1. Nat. Microbiol. 2019, 4, 933–940. [CrossRef]
- Perez-Caballero, D.; Hatziioannou, T.; Yang, A.; Cowan, S.; Bieniasz, P.D. Human Tripartite Motif 5alpha Domains Responsible for Retrovirus Restriction Activity and Specificity. J. Virol. 2005, 79, 8969–8978. [CrossRef]
- Ribeiro, C.M.S.; Sarrami-Forooshani, R.; Setiawan, L.C.; Zijlstra-Willems, E.M.; van Hamme, J.L.; Tigchelaar, W.; van der Wel, N.N.; Kootstra, N.A.; Gringhuis, S.I.; Geijtenbeek, T.B.H. Receptor Usage Dictates HIV-1 Restriction by Human TRIM5α in Dendritic Cell Subsets. Nature 2016, 540, 448–452. [CrossRef]
- Pertel, T.; Hausmann, S.; Morger, D.; Züger, S.; Guerra, J.; Lascano, J.; Reinhard, C.; Santoni, F.A.; Uchil, P.D.; Chatel, L.; et al. TRIM5 Is an Innate Immune Sensor for the Retrovirus Capsid Lattice. Nature 2011, 472, 361–365. [CrossRef]
- Lascano, J.; Uchil, P.D.; Mothes, W.; Luban, J. TRIM5 Retroviral Restriction Activity Correlates with the Ability To Induce Innate Immune Signaling. J. Virol. 2016, 90, 308–316. [CrossRef]
- Fletcher, A.J.; Vaysburd, M.; Maslen, S.; Zeng, J.; Skehel, J.M.; Towers, G.J.; James, L.C. Trivalent RING Assembly on Retroviral Capsids Activates TRIM5 Ubiquitination and Innate Immune Signaling. Cell Host Microbe 2018, 24, 761-775.e6. [CrossRef]
- Yoh, S.M.; Schneider, M.; Seifried, J.; Soonthornvacharin, S.; Akleh, R.E.; Olivieri, K.C.; De Jesus, P.D.; Ruan, C.; de Castro, E.; Ruiz, P.A.; et al. PQBP1 Is a Proximal Sensor of the cGAS-Dependent Innate Response to HIV-1. Cell 2015, 161, 1293–1305. [CrossRef]
- Yoh, S.M.; Mamede, J.I.; Lau, D.; Ahn, N.; Sánchez-Aparicio, M.T.; Temple, J.; Tuckwell, A.; Fuchs, N.V.; Cianci, G.C.; Riva, L.; et al. Recognition of HIV-1 Capsid by PQBP1 Licenses an Innate Immune Sensing of Nascent HIV-1 DNA. Mol. Cell 2022, 82, 2871-2884.e6. [CrossRef]
- Yuan, T.; Yao, W.; Tokunaga, K.; Yang, R.; Sun, B. An HIV-1 Capsid Binding Protein TRIM11 Accelerates Viral Uncoating. Retrovirology 2016, 13, 72. [CrossRef]
- Staeheli, P.; Haller, O. Human MX2/MxB: A Potent Interferon-Induced Postentry Inhibitor of Herpesviruses and HIV-1. J. Virol. 2018, 92, 10.1128/jvi.00709-18. [CrossRef]
- Kane, M.; Yadav, S.S.; Bitzegeio, J.; Kutluay, S.B.; Zang, T.; Wilson, S.J.; Schoggins, J.W.; Rice, C.M.; Yamashita, M.; Hatziioannou, T.; et al. MX2 Is an Interferon-Induced Inhibitor of HIV-1 Infection. Nature 2013, 502, 563–566. [CrossRef]
- Smaga, S.S.; Xu, C.; Summers, B.J.; Digianantonio, K.M.; Perilla, J.R.; Xiong, Y. MxB Restricts HIV-1 by Targeting the Tri-Hexamer Interface of the Viral Capsid. Struct. Lond. Engl. 1993 2019, 27, 1234-1245.e5. [CrossRef]
- Fricke, T.; White, T.E.; Schulte, B.; de Souza Aranha Vieira, D.A.; Dharan, A.; Campbell, E.M.; Brandariz-Nuñez, A.; Diaz-Griffero, F. MxB Binds to the HIV-1 Core and Prevents the Uncoating Process of HIV-1. Retrovirology 2014, 11, 68. [CrossRef]
- Melén, K.; Keskinen, P.; Ronni, T.; Sareneva, T.; Lounatmaa, K.; Julkunen, I. Human MxB Protein, an Interferon-Alpha-Inducible GTPase, Contains a Nuclear Targeting Signal and Is Localized in the Heterochromatin Region beneath the Nuclear Envelope. J. Biol. Chem. 1996, 271, 23478–23486. [CrossRef]
- Betancor, G. You Shall Not Pass: MX2 Proteins Are Versatile Viral Inhibitors. Vaccines 2023, 11, 930. [CrossRef]
- Maillet, S.; Fernandez, J.; Decourcelle, M.; El Koulali, K.; Blanchet, F.P.; Arhel, N.J.; Maarifi, G.; Nisole, S. Daxx Inhibits HIV-1 Reverse Transcription and Uncoating in a SUMO-Dependent Manner. Viruses 2020, 12, 636. [CrossRef]
- Yang, Y.; Fricke, T.; Diaz-Griffero, F. Inhibition of Reverse Transcriptase Activity Increases Stability of the HIV-1 Core. J. Virol. 2013, 87, 683–687. [CrossRef]
- Cosnefroy, O.; Murray, P.J.; Bishop, K.N. HIV-1 Capsid Uncoating Initiates after the First Strand Transfer of Reverse Transcription. Retrovirology 2016, 13, 58. [CrossRef]
- Iordanskiy, S.; Bukrinsky, M. Reverse Transcription Complex: The Key Player of the Early Phase of HIV Replication. Future Virol. 2007, 2, 49–64. [CrossRef]
- Arhel, N. Revisiting HIV-1 Uncoating. Retrovirology 2010, 7, 96. [CrossRef]
- Jacques, D.A.; McEwan, W.A.; Hilditch, L.; Price, A.J.; Towers, G.J.; James, L.C. HIV-1 Uses Dynamic Capsid Pores to Import Nucleotides and Fuel Encapsidated DNA Synthesis. Nature 2016, 536, 349–353. [CrossRef]
- Basu, V.P.; Song, M.; Gao, L.; Rigby, S.T.; Hanson, M.N.; Bambara, R.A. Strand Transfer Events during HIV-1 Reverse Transcription. Virus Res. 2008, 134, 19–38. [CrossRef]
- Bukrinskaya, A.; Brichacek, B.; Mann, A.; Stevenson, M. Establishment of a Functional Human Immunodeficiency Virus Type 1 (HIV-1) Reverse Transcription Complex Involves the Cytoskeleton. J. Exp. Med. 1998, 188, 2113–2125. [CrossRef]
- Larguet, F.; Caté, C.; Barbeau, B.; Rassart, E.; Edouard, E. Histone Deacetylase 1 Interacts with HIV-1 Integrase and Modulates Viral Replication. Virol. J. 2019, 16, 138. [CrossRef]
- Sorin, M.; Cano, J.; Das, S.; Mathew, S.; Wu, X.; Davies, K.P.; Shi, X.; Cheng, S.-W.G.; Ott, D.; Kalpana, G.V. Recruitment of a SAP18-HDAC1 Complex into HIV-1 Virions and Its Requirement for Viral Replication. PLoS Pathog. 2009, 5, e1000463. [CrossRef]
- Sorin, M.; Yung, E.; Wu, X.; Kalpana, G.V. HIV-1 Replication in Cell Lines Harboring INI1/hSNF5 Mutations. Retrovirology 2006, 3, 56. [CrossRef]
- Takahashi, H.; Matsuda, M.; Kojima, A.; Sata, T.; Andoh, T.; Kurata, T.; Nagashima, K.; Hall, W.W. Human Immunodeficiency Virus Type 1 Reverse Transcriptase: Enhancement of Activity by Interaction with Cellular Topoisomerase I. Proc. Natl. Acad. Sci. 1995, 92, 5694–5698. [CrossRef]
- Staszewski, J.; Lazarewicz, N.; Konczak, J.; Migdal, I.; Maciaszczyk-Dziubinska, E. UPF1—From mRNA Degradation to Human Disorders. Cells 2023, 12, 419. [CrossRef]
- Serquiña, A.K.P.; Das, S.R.; Popova, E.; Ojelabi, O.A.; Roy, C.K.; Göttlinger, H.G. UPF1 Is Crucial for the Infectivity of Human Immunodeficiency Virus Type 1 Progeny Virions. J. Virol. 2013, 87, 8853–8861. [CrossRef]
- Lemay, J.; Maidou-Peindara, P.; Bader, T.; Ennifar, E.; Rain, J.-C.; Benarous, R.; Liu, L.X. HuR Interacts with Human Immunodeficiency Virus Type 1 Reverse Transcriptase, and Modulates Reverse Transcription in Infected Cells. Retrovirology 2008, 5, 47. [CrossRef]
- Lemay, J.; Maidou-Peindara, P.; Cancio, R.; Ennifar, E.; Coadou, G.; Maga, G.; Rain, J.-C.; Benarous, R.; Liu, L.X. AKAP149 Binds to HIV-1 Reverse Transcriptase and Is Involved in the Reverse Transcription. J. Mol. Biol. 2008, 383, 783–796. [CrossRef]
- Warren, K.; Warrilow, D.; Meredith, L.; Harrich, D. Reverse Transcriptase and Cellular Factors: Regulators of HIV-1 Reverse Transcription. Viruses 2009, 1, 873–894. [CrossRef]
- Meister, G.; Bühler, D.; Pillai, R.; Lottspeich, F.; Fischer, U. A Multiprotein Complex Mediates the ATP-Dependent Assembly of Spliceosomal U snRNPs. Nat. Cell Biol. 2001, 3, 945–949. [CrossRef]
- Hamamoto, S.; Nishitsuji, H.; Amagasa, T.; Kannagi, M.; Masuda, T. Identification of a Novel Human Immunodeficiency Virus Type 1 Integrase Interactor, Gemin2, That Facilitates Efficient Viral cDNA Synthesis In Vivo. J. Virol. 2006, 80, 5670–5677. [CrossRef]
- Mazur, D.J.; Perrino, F.W. Identification and Expression of the TREX1 and TREX2 cDNA Sequences Encoding Mammalian 3′→5′ Exonucleases*. J. Biol. Chem. 1999, 274, 19655–19660. [CrossRef]
- Hasan, M.; Yan, N. Safeguard against DNA Sensing: The Role of TREX1 in HIV-1 Infection and Autoimmune Diseases. Front. Microbiol. 2014, 5, 193. [CrossRef]
- Yan, N.; Regalado-Magdos, A.D.; Stiggelbout, B.; Lee-Kirsch, M.A.; Lieberman, J. The Cytosolic Exonuclease TREX1 Inhibits the Innate Immune Response to Human Immunodeficiency Virus Type 1. Nat. Immunol. 2010, 11, 1005–1013. [CrossRef]
- Radetskyy, R.; Daher, A.; Gatignol, A. ADAR1 and PKR, Interferon Stimulated Genes with Clashing Effects on HIV-1 Replication. Cytokine Growth Factor Rev. 2018, 40, 48–58. [CrossRef]
- Cuadrado, E.; Booiman, T.; van Hamme, J.L.; Jansen, M.H.; van Dort, K.A.; Vanderver, A.; Rice, G.I.; Crow, Y.J.; Kootstra, N.A.; Kuijpers, T.W. ADAR1 Facilitates HIV-1 Replication in Primary CD4+ T Cells. PloS One 2015, 10, e0143613. [CrossRef]
- Liddicoat, B.J.; Piskol, R.; Chalk, A.M.; Ramaswami, G.; Higuchi, M.; Hartner, J.C.; Li, J.B.; Seeburg, P.H.; Walkley, C.R. RNA Editing by ADAR1 Prevents MDA5 Sensing of Endogenous dsRNA as Nonself. Science 2015, 349, 1115–1120. [CrossRef]
- Friedrich, B.M.; Murray, J.L.; Li, G.; Sheng, J.; Hodge, T.W.; Rubin, D.H.; O’Brien, W.A.; Ferguson, M.R. A Functional Role for ADAM10 in Human Immunodeficiency Virus Type-1 Replication. Retrovirology 2011, 8, 32. [CrossRef]
- Endsley, M.A.; Somasunderam, A.D.; Li, G.; Oezguen, N.; Thiviyanathan, V.; Murray, J.L.; Rubin, D.H.; Hodge, T.W.; O’Brien, W.A.; Lewis, B.; et al. Nuclear Trafficking of the HIV-1 Pre-Integration Complex Depends on the ADAM10 Intracellular Domain. Virology 2014, 0, 60–66. [CrossRef]
- Zhang, H.; Dornadula, G.; Orenstein, J.; Pomerantz, R.J. Morphologic Changes in Human Immunodeficiency Virus Type 1 Virions Secondary to Intravirion Reverse Transcription: Evidence Indicating That Reverse Transcription May Not Take Place within the Intact Viral Core. J. Hum. Virol. 2000, 3, 165–172.
- Ao, Z.; Danappa Jayappa, K.; Wang, B.; Zheng, Y.; Kung, S.; Rassart, E.; Depping, R.; Kohler, M.; Cohen, E.A.; Yao, X. Importin A3 Interacts with HIV-1 Integrase and Contributes to HIV-1 Nuclear Import and Replication. J. Virol. 2010, 84, 8650–8663. [CrossRef]
- Gallay, P.; Hope, T.; Chin, D.; Trono, D. HIV-1 Infection of Nondividing Cells through the Recognition of Integrase by the Importin/Karyopherin Pathway. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 9825–9830.
- Solis, M.; Nakhaei, P.; Jalalirad, M.; Lacoste, J.; Douville, R.; Arguello, M.; Zhao, T.; Laughrea, M.; Wainberg, M.A.; Hiscott, J. RIG-I-Mediated Antiviral Signaling Is Inhibited in HIV-1 Infection by a Protease-Mediated Sequestration of RIG-I. J. Virol. 2011, 85, 1224–1236. [CrossRef]
- Thoresen, D.; Wang, W.; Galls, D.; Guo, R.; Xu, L.; Pyle, A.M. The Molecular Mechanism of RIG-I Activation and Signaling. Immunol. Rev. 2021, 304, 154–168. [CrossRef]
- Guney, M.H.; Nagalekshmi, K.; McCauley, S.M.; Carbone, C.; Aydemir, O.; Luban, J. IFIH1 (MDA5) Is Required for Innate Immune Detection of Intron-Containing RNA Expressed from the HIV-1 Provirus. BioRxiv Prepr. Serv. Biol. 2023, 2023.11.17.567619. [CrossRef]
- Pujantell, M.; Badia, R.; Ramirez, C.; Puig, T.; Clotet, B.; Ballana, E.; Esté, J.A.; Riveira-Muñoz, E. Long-Term HIV-1 Infection Induces an Antiviral State in Primary Macrophages. Antiviral Res. 2016, 133, 145–155. [CrossRef]
- Chattergoon, M.A.; Latanich, R.; Quinn, J.; Winter, M.E.; Buckheit, R.W.; Blankson, J.N.; Pardoll, D.; Cox, A.L. HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-like Receptors without Induction of Type 1 Interferon. PLoS Pathog. 2014, 10, e1004082. [CrossRef]
- Jakobsen, M.R.; Bak, R.O.; Andersen, A.; Berg, R.K.; Jensen, S.B.; Tengchuan, J.; Laustsen, A.; Hansen, K.; Ostergaard, L.; Fitzgerald, K.A.; et al. IFI16 Senses DNA Forms of the Lentiviral Replication Cycle and Controls HIV-1 Replication. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, E4571-4580. [CrossRef]
- Itell, H.L.; Humes, D.; Overbaugh, J. Several Cell-Intrinsic Effectors Drive Type I Interferon-Mediated Restriction of HIV-1 in Primary CD4+ T Cells. Cell Rep. 2023, 42, 112556. [CrossRef]
- Bosso, M.; Bozzo, C.P.; Volcic, M.; Kirchhoff, F. IFI16 Knockdown in Primary HIV-1 Target Cells. STAR Protoc. 2021, 2, 100236. [CrossRef]
- Gao, D.; Wu, J.; Wu, Y.-T.; Du, F.; Aroh, C.; Yan, N.; Sun, L.; Chen, Z.J. Cyclic GMP-AMP Synthase Is an Innate Immune Sensor of HIV and Other Retroviruses. Science 2013, 341, 903–906. [CrossRef]
- Elsner, C.; Ponnurangam, A.; Kazmierski, J.; Zillinger, T.; Jansen, J.; Todt, D.; Döhner, K.; Xu, S.; Ducroux, A.; Kriedemann, N.; et al. Absence of cGAS-Mediated Type I IFN Responses in HIV-1–Infected T Cells. Proc. Natl. Acad. Sci. 2020, 117, 19475–19486. [CrossRef]
- Lahaye, X.; Satoh, T.; Gentili, M.; Cerboni, S.; Conrad, C.; Hurbain, I.; El Marjou, A.; Lacabaratz, C.; Lelièvre, J.-D.; Manel, N. The Capsids of HIV-1 and HIV-2 Determine Immune Detection of the Viral cDNA by the Innate Sensor cGAS in Dendritic Cells. Immunity 2013, 39, 1132–1142. [CrossRef]
- Manel, N.; Hogstad, B.; Wang, Y.; Levy, D.E.; Unutmaz, D.; Littman, D.R. A Cryptic Sensor for HIV-1 Activates Antiviral Innate Immunity in Dendritic Cells. Nature 2010, 467, 214–217. [CrossRef]
- Beignon, A.-S.; McKenna, K.; Skoberne, M.; Manches, O.; DaSilva, I.; Kavanagh, D.G.; Larsson, M.; Gorelick, R.J.; Lifson, J.D.; Bhardwaj, N. Endocytosis of HIV-1 Activates Plasmacytoid Dendritic Cells via Toll-like Receptor– Viral RNA Interactions. J. Clin. Invest. 2005, 115, 3265–3275. [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [CrossRef]
- Goujon, C.; Malim, M.H. Characterization of the Alpha Interferon-Induced Postentry Block to HIV-1 Infection in Primary Human Macrophages and T Cells. J. Virol. 2010, 84, 9254–9266. [CrossRef]
- Sheehy, A.M.; Gaddis, N.C.; Choi, J.D.; Malim, M.H. Isolation of a Human Gene That Inhibits HIV-1 Infection and Is Suppressed by the Viral Vif Protein. Nature 2002, 418, 646–650. [CrossRef]
- Stopak, K.; de Noronha, C.; Yonemoto, W.; Greene, W.C. HIV-1 Vif Blocks the Antiviral Activity of APOBEC3G by Impairing Both Its Translation and Intracellular Stability. Mol. Cell 2003, 12, 591–601. [CrossRef]
- Zhang, H.; Yang, B.; Pomerantz, R.J.; Zhang, C.; Arunachalam, S.C.; Gao, L. The Cytidine Deaminase CEM15 Induces Hypermutation in Newly Synthesized HIV-1 DNA. Nature 2003, 424, 94–98. [CrossRef]
- Hultquist, J.F.; Lengyel, J.A.; Refsland, E.W.; LaRue, R.S.; Lackey, L.; Brown, W.L.; Harris, R.S. Human and Rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H Demonstrate a Conserved Capacity to Restrict Vif-Deficient HIV-1. J. Virol. 2011, 85, 11220–11234. [CrossRef]
- Harris, R.S.; Bishop, K.N.; Sheehy, A.M.; Craig, H.M.; Petersen-Mahrt, S.K.; Watt, I.N.; Neuberger, M.S.; Malim, M.H. DNA Deamination Mediates Innate Immunity to Retroviral Infection. Cell 2003, 113, 803–809. [CrossRef]
- Harris, R.S.; Petersen-Mahrt, S.K.; Neuberger, M.S. RNA Editing Enzyme APOBEC1 and Some of Its Homologs Can Act as DNA Mutators. Mol. Cell 2002, 10, 1247–1253. [CrossRef]
- Khan, M.A.; Kao, S.; Miyagi, E.; Takeuchi, H.; Goila-Gaur, R.; Opi, S.; Gipson, C.L.; Parslow, T.G.; Ly, H.; Strebel, K. Viral RNA Is Required for the Association of APOBEC3G with Human Immunodeficiency Virus Type 1 Nucleoprotein Complexes. J. Virol. 2005, 79, 5870–5874. [CrossRef]
- Yu, Q.; König, R.; Pillai, S.; Chiles, K.; Kearney, M.; Palmer, S.; Richman, D.; Coffin, J.M.; Landau, N.R. Single-Strand Specificity of APOBEC3G Accounts for Minus-Strand Deamination of the HIV Genome. Nat. Struct. Mol. Biol. 2004, 11, 435–442. [CrossRef]
- Miyagi, E.; Schwartzkopff, F.; Plishka, R.; Buckler-White, A.; Clouse, K.A.; Strebel, K. APOBEC3G-Independent Reduction in Virion Infectivity during Long-Term HIV-1 Replication in Terminally Differentiated Macrophages. Virology 2008, 379, 266–274. [CrossRef]
- Malim, M.H. APOBEC Proteins and Intrinsic Resistance to HIV-1 Infection. Philos. Trans. R. Soc. B Biol. Sci. 2008, 364, 675–687. [CrossRef]
- Mangeat, B.; Turelli, P.; Caron, G.; Friedli, M.; Perrin, L.; Trono, D. Broad Antiretroviral Defence by Human APOBEC3G through Lethal Editing of Nascent Reverse Transcripts. Nature 2003, 424, 99–103. [CrossRef]
- Adolph, M.B.; Ara, A.; Feng, Y.; Wittkopp, C.J.; Emerman, M.; Fraser, J.S.; Chelico, L. Cytidine Deaminase Efficiency of the Lentiviral Viral Restriction Factor APOBEC3C Correlates with Dimerization. Nucleic Acids Res. 2017, 45, 3378–3394. [CrossRef]
- Holmes, R.K.; Malim, M.H.; Bishop, K.N. APOBEC-Mediated Viral Restriction: Not Simply Editing? Trends Biochem. Sci. 2007, 32, 118–128. [CrossRef]
- Wang, X.; Ao, Z.; Chen, L.; Kobinger, G.; Peng, J.; Yao, X. The Cellular Antiviral Protein APOBEC3G Interacts with HIV-1 Reverse Transcriptase and Inhibits Its Function during Viral Replication. J. Virol. 2012, 86, 3777–3786. [CrossRef]
- Bishop, K.N.; Holmes, R.K.; Malim, M.H. Antiviral Potency of APOBEC Proteins Does Not Correlate with Cytidine Deamination. J. Virol. 2006, 80, 8450–8458. [CrossRef]
- Gillick, K.; Pollpeter, D.; Phalora, P.; Kim, E.-Y.; Wolinsky, S.M.; Malim, M.H. Suppression of HIV-1 Infection by APOBEC3 Proteins in Primary Human CD4+ T Cells Is Associated with Inhibition of Processive Reverse Transcription as Well as Excessive Cytidine Deamination. J. Virol. 2013, 87, 1508–1517. [CrossRef]
- Newman, E.N.C.; Holmes, R.K.; Craig, H.M.; Klein, K.C.; Lingappa, J.R.; Malim, M.H.; Sheehy, A.M. Antiviral Function of APOBEC3G Can Be Dissociated from Cytidine Deaminase Activity. Curr. Biol. 2005, 15, 166–170. [CrossRef]
- Guo, F.; Cen, S.; Niu, M.; Saadatmand, J.; Kleiman, L. Inhibition of tRNA₃(Lys)-Primed Reverse Transcription by Human APOBEC3G during Human Immunodeficiency Virus Type 1 Replication. J. Virol. 2006, 80, 11710–11722. [CrossRef]
- Liu, Y.; Fu, Y.; Wang, Q.; Li, M.; Zhou, Z.; Dabbagh, D.; Fu, C.; Zhang, H.; Li, S.; Zhang, T.; et al. Proteomic Profiling of HIV-1 Infection of Human CD4+ T Cells Identifies PSGL-1 as an HIV Restriction Factor. Nat. Microbiol. 2019, 4, 813–825. [CrossRef]
- Liu, Y.; Song, Y.; Zhang, S.; Diao, M.; Huang, S.; Li, S.; Tan, X. PSGL-1 Inhibits HIV-1 Infection by Restricting Actin Dynamics and Sequestering HIV Envelope Proteins. Cell Discov. 2020, 6, 1–15. [CrossRef]
- Liu, L.; Oliveira, N.M.M.; Cheney, K.M.; Pade, C.; Dreja, H.; Bergin, A.-M.H.; Borgdorff, V.; Beach, D.H.; Bishop, C.L.; Dittmar, M.T.; et al. A Whole Genome Screen for HIV Restriction Factors. Retrovirology 2011, 8, 94. [CrossRef]
- Yan, J.; Shun, M.-C.; Hao, C.; Zhang, Y.; Qian, J.; Hrecka, K.; DeLucia, M.; Monnie, C.; Ahn, J.; Skowronski, J. HIV-1 Vpr Reprograms CLR4DCAF1 E3 Ubiquitin Ligase to Antagonize Exonuclease 1-Mediated Restriction of HIV-1 Infection. mBio 2018, 9, e01732-18. [CrossRef]
- Yan, J.; Shun, M.-C.; Zhang, Y.; Hao, C.; Skowronski, J. HIV-1 Vpr Counteracts HLTF-Mediated Restriction of HIV-1 Infection in T Cells. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 9568–9577. [CrossRef]
- Burdick, R.; Smith, J.L.; Chaipan, C.; Friew, Y.; Chen, J.; Venkatachari, N.J.; Delviks-Frankenberry, K.A.; Hu, W.-S.; Pathak, V.K. P Body-Associated Protein Mov10 Inhibits HIV-1 Replication at Multiple Stages. J. Virol. 2010, 84, 10241–10253. [CrossRef]
- Xie, L.; Chen, L.; Zhong, C.; Yu, T.; Ju, Z.; Wang, M.; Xiong, H.; Zeng, Y.; Wang, J.; Hu, H.; et al. MxB Impedes the NUP358-Mediated HIV-1 Pre-Integration Complex Nuclear Import and Viral Replication Cooperatively with CPSF6. Retrovirology 2020, 17, 16. [CrossRef]
- Marno, K.M.; Ogunkolade, B.W.; Pade, C.; Oliveira, N.M.M.; O’Sullivan, E.; McKnight, Á. Novel Restriction Factor RNA-Associated Early-Stage Anti-Viral Factor (REAF) Inhibits Human and Simian Immunodeficiency Viruses. Retrovirology 2014, 11, 3. [CrossRef]
- Jackson-Jones, K.A.; McKnight, Á.; Sloan, R.D. The Innate Immune Factor RPRD2/REAF and Its Role in the Lv2 Restriction of HIV. mBio 2023, 14, e02572-21. [CrossRef]
- Sonza, S.; Maerz, A.; Deacon, N.; Meanger, J.; Mills, J.; Crowe, S. Human Immunodeficiency Virus Type 1 Replication Is Blocked Prior to Reverse Transcription and Integration in Freshly Isolated Peripheral Blood Monocytes. J. Virol. 1996, 70, 3863–3869. [CrossRef]
- O’Brien, W.A.; Namazi, A.; Kalhor, H.; Mao, S.H.; Zack, J.A.; Chen, I.S. Kinetics of Human Immunodeficiency Virus Type 1 Reverse Transcription in Blood Mononuclear Phagocytes Are Slowed by Limitations of Nucleotide Precursors. J. Virol. 1994, 68, 1258–1263. [CrossRef]
- Collin, M.; Gordon, S. The Kinetics of Human Immunodeficiency Virus Reverse Transcription Are Slower in Primary Human Macrophages than in a Lymphoid Cell Line. Virology 1994, 200, 114–120. [CrossRef]
- Laguette, N.; Sobhian, B.; Casartelli, N.; Ringeard, M.; Chable-Bessia, C.; Ségéral, E.; Yatim, A.; Emiliani, S.; Schwartz, O.; Benkirane, M. SAMHD1 Is the Dendritic- and Myeloid-Cell-Specific HIV-1 Restriction Factor Counteracted by Vpx. Nature 2011, 474, 654–657. [CrossRef]
- Ballana, E.; Esté, J.A. SAMHD1: At the Crossroads of Cell Proliferation, Immune Responses, and Virus Restriction. Trends Microbiol. 2015, 23, 680–692. [CrossRef]
- Berger, A.; Sommer, A.F.R.; Zwarg, J.; Hamdorf, M.; Welzel, K.; Esly, N.; Panitz, S.; Reuter, A.; Ramos, I.; Jatiani, A.; et al. SAMHD1-Deficient CD14+ Cells from Individuals with Aicardi-Goutières Syndrome Are Highly Susceptible to HIV-1 Infection. PLOS Pathog. 2011, 7, e1002425. [CrossRef]
- Amie, S.M.; Noble, E.; Kim, B. Intracellular Nucleotide Levels and the Control of Retroviral Infections. Virology 2013, 436, 247–254. [CrossRef]
- Franzolin, E.; Pontarin, G.; Rampazzo, C.; Miazzi, C.; Ferraro, P.; Palumbo, E.; Reichard, P.; Bianchi, V. The Deoxynucleotide Triphosphohydrolase SAMHD1 Is a Major Regulator of DNA Precursor Pools in Mammalian Cells. Proc. Natl. Acad. Sci. 2013, 110, 14272–14277. [CrossRef]
- Lahouassa, H.; Daddacha, W.; Hofmann, H.; Ayinde, D.; Logue, E.C.; Dragin, L.; Bloch, N.; Maudet, C.; Bertrand, M.; Gramberg, T.; et al. SAMHD1 Restricts the Replication of Human Immunodeficiency Virus Type 1 by Depleting the Intracellular Pool of Deoxynucleoside Triphosphates. Nat. Immunol. 2012, 13, 223–228. [CrossRef]
- Descours, B.; Cribier, A.; Chable-Bessia, C.; Ayinde, D.; Rice, G.; Crow, Y.; Yatim, A.; Schwartz, O.; Laguette, N.; Benkirane, M. SAMHD1 Restricts HIV-1 Reverse Transcription in Quiescent CD4(+) T-Cells. Retrovirology 2012, 9, 87. [CrossRef]
- Baldauf, H.-M.; Pan, X.; Erikson, E.; Schmidt, S.; Daddacha, W.; Burggraf, M.; Schenkova, K.; Ambiel, I.; Wabnitz, G.; Gramberg, T.; et al. SAMHD1 Restricts HIV-1 Infection in Resting CD4+ T Cells. Nat. Med. 2012, 18, 1682–1688. [CrossRef]
- Diamond, T.L.; Roshal, M.; Jamburuthugoda, V.K.; Reynolds, H.M.; Merriam, A.R.; Lee, K.Y.; Balakrishnan, M.; Bambara, R.A.; Planelles, V.; Dewhurst, S.; et al. Macrophage Tropism of HIV-1 Depends on Efficient Cellular dNTP Utilization by Reverse Transcriptase. J. Biol. Chem. 2004, 279, 51545–51553. [CrossRef]
- Hrecka, K.; Hao, C.; Gierszewska, M.; Swanson, S.K.; Kesik-Brodacka, M.; Srivastava, S.; Florens, L.; Washburn, M.P.; Skowronski, J. Vpx Relieves Inhibition of HIV-1 Infection of Macrophages Mediated by the SAMHD1 Protein. Nature 2011, 474, 658–661. [CrossRef]
- Hasanshahi, Z.; Dehghani, B.; Hashempour, A. Interaction Between Vpx and SAMHD1, Vital for SAMHD1 Inhibition. AIDS Res. Hum. Retroviruses 2023. [CrossRef]
- Plitnik, T.; Sharkey, M.E.; Mahboubi, B.; Kim, B.; Stevenson, M. Incomplete Suppression of HIV-1 by SAMHD1 Permits Efficient Macrophage Infection. Pathog. Immun. 2018, 3, 197–223. [CrossRef]
- White, T.E.; Brandariz-Nuñez, A.; Valle-Casuso, J.C.; Amie, S.; Nguyen, L.A.; Kim, B.; Tuzova, M.; Diaz-Griffero, F. The Retroviral Restriction Ability of SAMHD1, but Not Its Deoxynucleotide Triphosphohydrolase Activity, Is Regulated by Phosphorylation. Cell Host Microbe 2013, 13, 441–451. [CrossRef]
- Beloglazova, N.; Flick, R.; Tchigvintsev, A.; Brown, G.; Popovic, A.; Nocek, B.; Yakunin, A.F. Nuclease Activity of the Human SAMHD1 Protein Implicated in the Aicardi-Goutieres Syndrome and HIV-1 Restriction. J. Biol. Chem. 2013, 288, 8101–8110. [CrossRef]
- Choi, J.; Ryoo, J.; Oh, C.; Hwang, S.; Ahn, K. SAMHD1 Specifically Restricts Retroviruses through Its RNase Activity. Retrovirology 2015, 12, 46. [CrossRef]
- Pauls, E.; Ruiz, A.; Riveira-Muñoz, E.; Permanyer, M.; Badia, R.; Clotet, B.; Keppler, O.T.; Ballana, E.; Este, J.A. P21 Regulates the HIV-1 Restriction Factor SAMHD1. Proc. Natl. Acad. Sci. 2014, 111, E1322–E1324. [CrossRef]
- Valle-Casuso, J.C.; Allouch, A.; David, A.; Lenzi, G.M.; Studdard, L.; Barré-Sinoussi, F.; Müller-Trutwin, M.; Kim, B.; Pancino, G.; Sáez-Cirión, A. P21 Restricts HIV-1 in Monocyte-Derived Dendritic Cells through the Reduction of Deoxynucleoside Triphosphate Biosynthesis and Regulation of SAMHD1 Antiviral Activity. J. Virol. 2017, 91, 10.1128/jvi.01324-17. [CrossRef]
- Chen, H.; Li, C.; Huang, J.; Cung, T.; Seiss, K.; Beamon, J.; Carrington, M.F.; Porter, L.C.; Burke, P.S.; Yang, Y.; et al. CD4+ T Cells from Elite Controllers Resist HIV-1 Infection by Selective Upregulation of P21 Available online: https://www.jci.org/articles/view/44539/pdf (accessed on 24 May 2024).
- Shi, B.; Sharifi, H.J.; DiGrigoli, S.; Kinnetz, M.; Mellon, K.; Hu, W.; de Noronha, C.M.C. Inhibition of HIV Early Replication by the P53 and Its Downstream Gene P21. Virol. J. 2018, 15, 53. [CrossRef]
- Bergamaschi, A.; David, A.; Le Rouzic, E.; Nisole, S.; Barré-Sinoussi, F.; Pancino, G. The CDK Inhibitor p21Cip1/WAF1 Is Induced by FcgammaR Activation and Restricts the Replication of Human Immunodeficiency Virus Type 1 and Related Primate Lentiviruses in Human Macrophages. J. Virol. 2009, 83, 12253–12265. [CrossRef]
- Allouch, A.; David, A.; Amie, S.M.; Lahouassa, H.; Chartier, L.; Margottin-Goguet, F.; Barré-Sinoussi, F.; Kim, B.; Sáez-Cirión, A.; Pancino, G. P21-Mediated RNR2 Repression Restricts HIV-1 Replication in Macrophages by Inhibiting dNTP Biosynthesis Pathway. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, E3997-4006. [CrossRef]
- Kennedy, E.M.; Amie, S.M.; Bambara, R.A.; Kim, B. Frequent Incorporation of Ribonucleotides during HIV-1 Reverse Transcription and Their Attenuated Repair in Macrophages. J. Biol. Chem. 2012, 287, 14280–14288. [CrossRef]
- Craigie, R.; Bushman, F.D. HIV DNA Integration. Cold Spring Harb. Perspect. Med. 2012, 2, a006890. [CrossRef]
- Sloan, R.D.; Wainberg, M.A. The Role of Unintegrated DNA in HIV Infection. Retrovirology 2011, 8, 52. [CrossRef]
- Cereseto, A.; Manganaro, L.; Gutierrez, M.I.; Terreni, M.; Fittipaldi, A.; Lusic, M.; Marcello, A.; Giacca, M. Acetylation of HIV-1 Integrase by P300 Regulates Viral Integration. EMBO J. 2005, 24, 3070–3081. [CrossRef]
- Achuthan, V.; Perreira, J.M.; Sowd, G.A.; Puray-Chavez, M.; McDougall, W.M.; Paulucci-Holthauzen, A.; Wu, X.; Fadel, H.J.; Poeschla, E.M.; Multani, A.S.; et al. Capsid-CPSF6 Interaction Licenses Nuclear HIV-1 Trafficking to Sites of Viral DNA Integration. Cell Host Microbe 2018, 24, 392-404.e8. [CrossRef]
- Vranckx, L.S.; Demeulemeester, J.; Saleh, S.; Boll, A.; Vansant, G.; Schrijvers, R.; Weydert, C.; Battivelli, E.; Verdin, E.; Cereseto, A.; et al. LEDGIN-Mediated Inhibition of Integrase-LEDGF/P75 Interaction Reduces Reactivation of Residual Latent HIV. EBioMedicine 2016, 8, 248–264. [CrossRef]
- Cherepanov, P.; Maertens, G.; Proost, P.; Devreese, B.; Van Beeumen, J.; Engelborghs, Y.; De Clercq, E.; Debyser, Z. HIV-1 Integrase Forms Stable Tetramers and Associates with LEDGF/P75 Protein in Human Cells. J. Biol. Chem. 2003, 278, 372–381. [CrossRef]
- Emiliani, S.; Mousnier, A.; Busschots, K.; Maroun, M.; Van Maele, B.; Tempé, D.; Vandekerckhove, L.; Moisant, F.; Ben-Slama, L.; Witvrouw, M.; et al. Integrase Mutants Defective for Interaction with LEDGF/P75 Are Impaired in Chromosome Tethering and HIV-1 Replication. J. Biol. Chem. 2005, 280, 25517–25523. [CrossRef]
- Shun, M.-C.; Raghavendra, N.K.; Vandegraaff, N.; Daigle, J.E.; Hughes, S.; Kellam, P.; Cherepanov, P.; Engelman, A. LEDGF/P75 Functions Downstream from Preintegration Complex Formation to Effect Gene-Specific HIV-1 Integration. Genes Dev. 2007, 21, 1767–1778. [CrossRef]
- Llano, M.; Vanegas, M.; Hutchins, N.; Thompson, D.; Delgado, S.; Poeschla, E.M. Identification and Characterization of the Chromatin-Binding Domains of the HIV-1 Integrase Interactor LEDGF/P75. J. Mol. Biol. 2006, 360, 760–773. [CrossRef]
- De Rijck, J.; Vandekerckhove, L.; Gijsbers, R.; Hombrouck, A.; Hendrix, J.; Vercammen, J.; Engelborghs, Y.; Christ, F.; Debyser, Z. Overexpression of the Lens Epithelium-Derived Growth Factor/P75 Integrase Binding Domain Inhibits Human Immunodeficiency Virus Replication. J. Virol. 2006, 80, 11498–11509. [CrossRef]
- Llano, M.; Saenz, D.T.; Meehan, A.; Wongthida, P.; Peretz, M.; Walker, W.H.; Teo, W.; Poeschla, E.M. An Essential Role for LEDGF/P75 in HIV Integration. Science 2006, 314, 461–464. [CrossRef]
- Marshall, H.M.; Ronen, K.; Berry, C.; Llano, M.; Sutherland, H.; Saenz, D.; Bickmore, W.; Poeschla, E.; Bushman, F.D. Role of PSIP1/LEDGF/P75 in Lentiviral Infectivity and Integration Targeting. PLoS ONE 2007, 2, e1340. [CrossRef]
- Farnet, C.M.; Bushman, F.D. HIV-1 cDNA Integration: Requirement of HMG I(Y) Protein for Function of Preintegration Complexes In Vitro. Cell 1997, 88, 483–492. [CrossRef]
- Li, L.; Yoder, K.; Hansen, M.S.T.; Olvera, J.; Miller, M.D.; Bushman, F.D. Retroviral cDNA Integration: Stimulation by HMG I Family Proteins. J. Virol. 2000, 74, 10965–10974. [CrossRef]
- Lesbats, P.; Botbol, Y.; Chevereau, G.; Vaillant, C.; Calmels, C.; Arneodo, A.; Andreola, M.-L.; Lavigne, M.; Parissi, V. Functional Coupling between HIV-1 Integrase and the SWI/SNF Chromatin Remodeling Complex for Efficient in Vitro Integration into Stable Nucleosomes. PLoS Pathog. 2011, 7, e1001280. [CrossRef]
- Davis, A.J.; Chen, B.P.C.; Chen, D.J. DNA-PK: A Dynamic Enzyme in a Versatile DSB Repair Pathway. DNA Repair 2014, 17, 21–29. [CrossRef]
- Daniel, R.; Katz, R.A.; Skalka, A.M. A Role for DNA-PK in Retroviral DNA Integration. Science 1999, 284, 644–647. [CrossRef]
- Li, L.; Olvera, J.M.; Yoder, K.E.; Mitchell, R.S.; Butler, S.L.; Lieber, M.; Martin, S.L.; Bushman, F.D. Role of the Non-homologous DNA End Joining Pathway in the Early Steps of Retroviral Infection. EMBO J. 2001, 20, 3272–3281. [CrossRef]
- Jeanson, L.; Subra, F.; Vaganay, S.; Hervy, M.; Marangoni, E.; Bourhis, J.; Mouscadet, J.-F. Effect of Ku80 Depletion on the Preintegrative Steps of HIV-1 Replication in Human Cells. Virology 2002, 300, 100–108. [CrossRef]
- Lau, A.; Swinbank, K.M.; Ahmed, P.S.; Taylor, D.L.; Jackson, S.P.; Smith, G.C.M.; O’Connor, M.J. Suppression of HIV-1 Infection by a Small Molecule Inhibitor of the ATM Kinase. Nat. Cell Biol. 2005, 7, 493–500. [CrossRef]
- Anderson, E.M.; Maldarelli, F. The Role of Integration and Clonal Expansion in HIV Infection: Live Long and Prosper. Retrovirology 2018, 15, 71. [CrossRef]
- Maroun, M.; Delelis, O.; Coadou, G.; Bader, T.; Ségéral, E.; Mbemba, G.; Petit, C.; Sonigo, P.; Rain, J.-C.; Mouscadet, J.-F.; et al. Inhibition of Early Steps of HIV-1 Replication by SNF5/Ini1 *. J. Biol. Chem. 2006, 281, 22736–22743. [CrossRef]
- Lau, A.; Kanaar, R.; Jackson, S.P.; O’Connor, M.J. Suppression of Retroviral Infection by the RAD52 DNA Repair Protein. EMBO J. 2004, 23, 3421–3429. [CrossRef]
- Yoder, K.; Sarasin, A.; Kraemer, K.; McIlhatton, M.; Bushman, F.; Fishel, R. The DNA Repair Genes XPB and XPD Defend Cells from Retroviral Infection. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 4622–4627. [CrossRef]
- Allouch, A.; Di Primio, C.; Alpi, E.; Lusic, M.; Arosio, D.; Giacca, M.; Cereseto, A. The TRIM Family Protein KAP1 Inhibits HIV-1 Integration. Cell Host Microbe 2011, 9, 484–495. [CrossRef]
- Marcello, A.; Zoppé, M.; Giacca, M. Multiple Modes of Transcriptional Regulation by the HIV-1 Tat Transactivator. IUBMB Life 2001, 51, 175–181. [CrossRef]
- Roebuck, K.A.; Saifuddin, M. Regulation of HIV-1 Transcription. Gene Expr. 2018, 8, 67–84.
- Van Lint, C.; Bouchat, S.; Marcello, A. HIV-1 Transcription and Latency: An Update. Retrovirology 2013, 10, 67. [CrossRef]
- Pendergrast, P.S.; Morrison, D.; Tansey, W.P.; Hernandez, N. Mutations in the Carboxy-Terminal Domain of TBP Affect the Synthesis of Human Immunodeficiency Virus Type 1 Full-Length and Short Transcripts Similarly. J. Virol. 1996, 70, 5025–5034. [CrossRef]
- Majello, B.; Napolitano, G.; De Luca, P.; Lania, L. Recruitment of Human TBP Selectively Activates RNA Polymerase II TATA-Dependent Promoters*. J. Biol. Chem. 1998, 273, 16509–16516. [CrossRef]
- Rice, A.P. Roles of CDKs in RNA Polymerase II Transcription of the HIV-1 Genome. Transcription 2019, 10, 111–117. [CrossRef]
- Chun, R.F.; Jeang, K.-T. Requirements for RNA Polymerase II Carboxyl-Terminal Domain for Activated Transcription of Human Retroviruses Human T-Cell Lymphotropic Virus I and HIV-1 *. J. Biol. Chem. 1996, 271, 27888–27894. [CrossRef]
- Mancebo, H.S.Y.; Lee, G.; Flygare, J.; Tomassini, J.; Luu, P.; Zhu, Y.; Peng, J.; Blau, C.; Hazuda, D.; Price, D.; et al. P-TEFb Kinase Is Required for HIV Tat Transcriptional Activation in Vivo and in Vitro. Genes Dev. 1997, 11, 2633–2644. [CrossRef]
- Zhu, Y.; Pe’ery, T.; Peng, J.; Ramanathan, Y.; Marshall, N.; Marshall, T.; Amendt, B.; Mathews, M.B.; Price, D.H. Transcription Elongation Factor P-TEFb Is Required for HIV-1 Tat Transactivation in Vitro. Genes Dev. 1997, 11, 2622–2632. [CrossRef]
- Gold, M.O.; Yang, X.; Herrmann, C.H.; Rice, A.P. PITALRE, the Catalytic Subunit of TAK, Is Required for Human Immunodeficiency Virus Tat Transactivation In Vivo. J. Virol. 1998, 72, 4448–4453. [CrossRef]
- Bruce, J.W.; Reddington, R.; Mathieu, E.; Bracken, M.; Young, J.A.T.; Ahlquist, P. ZASC1 Stimulates HIV-1 Transcription Elongation by Recruiting P-TEFb and TAT to the LTR Promoter. PLOS Pathog. 2013, 9, e1003712. [CrossRef]
- Herrmann, C.H.; Carroll, R.G.; Wei, P.; Jones, K.A.; Rice, A.P. Tat-Associated Kinase, TAK, Activity Is Regulated by Distinct Mechanisms in Peripheral Blood Lymphocytes and Promonocytic Cell Lines. J. Virol. 1998, 72, 9881–9888. [CrossRef]
- García-Martínez, L.F.; Mavankal, G.; Neveu, J.M.; Lane, W.S.; Ivanov, D.; Gaynor, R.B. Purification of a Tat-associated Kinase Reveals a TFIIH Complex That Modulates HIV-1 Transcription. EMBO J. 1997, 16, 2836–2850. [CrossRef]
- Schilbach, S.; Hantsche, M.; Tegunov, D.; Dienemann, C.; Wigge, C.; Urlaub, H.; Cramer, P. Structures of Transcription Pre-Initiation Complex with TFIIH and Mediator. Nature 2017, 551, 204–209. [CrossRef]
- Chen, D.; Zhou, Q. Tat Activates Human Immunodeficiency Virus Type 1 Transcriptional Elongation Independent of TFIIH Kinase. Mol. Cell. Biol. 1999, 19, 2863–2871. [CrossRef]
- Zhou, M.; Halanski, M.A.; Radonovich, M.F.; Kashanchi, F.; Peng, J.; Price, D.H.; Brady, J.N. Tat Modifies the Activity of CDK9 to Phosphorylate Serine 5 of the RNA Polymerase II Carboxyl-Terminal Domain during Human Immunodeficiency Virus Type 1 Transcription. Mol. Cell. Biol. 2000, 20, 5077–5086. [CrossRef]
- Zhou, M.; Nekhai, S.; Bharucha, D.C.; Kumar, A.; Ge, H.; Price, D.H.; Egly, J.-M.; Brady, J.N. TFIIH Inhibits CDK9 Phosphorylation during Human Immunodeficiency Virus Type 1 Transcription*. J. Biol. Chem. 2001, 276, 44633–44640. [CrossRef]
- Holloway, A.F.; Occhiodoro, F.; Mittler, G.; Meisterernst, M.; Shannon, M.F. Functional Interaction between the HIV Transactivator Tat and the Transcriptional Coactivator PC4 in T Cells. J. Biol. Chem. 2000, 275, 21668–21677. [CrossRef]
- Kim, H.-Y.; Choi, B.-S.; Kim, S.S.; Roh, T.-Y.; Park, J.; Yoon, C.-H. NUCKS1, a Novel Tat Coactivator, Plays a Crucial Role in HIV-1 Replication by Increasing Tat-Mediated Viral Transcription on the HIV-1 LTR Promoter. Retrovirology 2014, 11, 67. [CrossRef]
- Dorin, D.; Bonnet, M.C.; Bannwarth, S.; Gatignol, A.; Meurs, E.F.; Vaquero, C. The TAR RNA-Binding Protein, TRBP, Stimulates the Expression of TAR-Containing RNAs in Vitro and in Vivo Independently of Its Ability to Inhibit the dsRNA-Dependent Kinase PKR. J. Biol. Chem. 2003, 278, 4440–4448. [CrossRef]
- Christensen, H.S.; Daher, A.; Soye, K.J.; Frankel, L.B.; Alexander, M.R.; Lainé, S.; Bannwarth, S.; Ong, C.L.; Chung, S.W.L.; Campbell, S.M.; et al. Small Interfering RNAs against the TAR RNA Binding Protein, TRBP, a Dicer Cofactor, Inhibit Human Immunodeficiency Virus Type 1 Long Terminal Repeat Expression and Viral Production. J. Virol. 2007, 81, 5121–5131. [CrossRef]
- Cho, E.-J.; Takagi, T.; Moore, C.R.; Buratowski, S. mRNA Capping Enzyme Is Recruited to the Transcription Complex by Phosphorylation of the RNA Polymerase II Carboxy-Terminal Domain. Genes Dev. 1997, 11, 3319–3326.
- Chiu, Y.-L.; Ho, C.K.; Saha, N.; Schwer, B.; Shuman, S.; Rana, T.M. Tat Stimulates Cotranscriptional Capping of HIV mRNA. Mol. Cell 2002, 10, 585–597. [CrossRef]
- Zhou, M.; Deng, L.; Kashanchi, F.; Brady, J.N.; Shatkin, A.J.; Kumar, A. The Tat/TAR-Dependent Phosphorylation of RNA Polymerase II C-Terminal Domain Stimulates Cotranscriptional Capping of HIV-1 mRNA. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 12666–12671. [CrossRef]
- Chiu, Y.L.; Coronel, E.; Ho, C.K.; Shuman, S.; Rana, T.M. HIV-1 Tat Protein Interacts with Mammalian Capping Enzyme and Stimulates Capping of TAR RNA. J. Biol. Chem. 2001, 276, 12959–12966. [CrossRef]
- Wilusz, J. Putting an ‘End’ to HIV mRNAs: Capping and Polyadenylation as Potential Therapeutic Targets. AIDS Res. Ther. 2013, 10, 31. [CrossRef]
- Sertznig, H.; Hillebrand, F.; Erkelenz, S.; Schaal, H.; Widera, M. Behind the Scenes of HIV-1 Replication: Alternative Splicing as the Dependency Factor on the Quiet. Virology 2018, 516, 176–188. [CrossRef]
- Caputi, M.; Mayeda, A.; Krainer, A.R.; Zahler, A.M. hnRNP A/B Proteins Are Required for Inhibition of HIV-1 Pre-mRNA Splicing. EMBO J. 1999, 18, 4060–4067. [CrossRef]
- Bilodeau, P.S.; Domsic, J.K.; Mayeda, A.; Krainer, A.R.; Stoltzfus, C.M. RNA Splicing at Human Immunodeficiency Virus Type 1 3’ Splice Site A2 Is Regulated by Binding of hnRNP A/B Proteins to an Exonic Splicing Silencer Element. J. Virol. 2001, 75, 8487–8497. [CrossRef]
- Namer, L.S.; Harwig, A.; Heynen, S.P.; Das, A.T.; Berkhout, B.; Kaempfer, R. HIV Co-Opts a Cellular Antiviral Mechanism, Activation of Stress Kinase PKR by Its RNA, to Enable Splicing of Rev/Tat mRNA. Cell Biosci. 2023, 13, 28. [CrossRef]
- Jones, K.A.; Kadonaga, J.T.; Luciw, P.A.; Tjian, R. Activation of the AIDS Retrovirus Promoter by the Cellular Transcription Factor, Sp1. Science 1986, 232, 755–759. [CrossRef]
- Suñé, C.; García-Blanco, M.A. Sp1 Transcription Factor Is Required for in Vitro Basal and Tat-Activated Transcription from the Human Immunodeficiency Virus Type 1 Long Terminal Repeat. J. Virol. 1995, 69, 6572–6576. [CrossRef]
- Majello, B.; De Luca, P.; Hagen, G.; Suske, G.; Lania, L. Different Members of the Sp1 Multigene Family Exert Opposite Transcritional Regulation of the Long Terminal Repeat of HIV-1. Nucleic Acids Res. 1994, 22, 4914–4921. [CrossRef]
- IMATAKA, H.; MIZUNO, A.; FUJII-KURIYAMA, Y.; HAYAMI, M. Activation of the Human Immunodeficiency Virus Type 1 Long Terminal Repeat by BTEB, a GC Box-Binding Transcription Factor Available online: https://www.liebertpub.com/doi/10.1089/aid.1993.9.825 (accessed on 29 May 2024).
- Nabel, G.; Baltimore, D. An Inducible Transcription Factor Activates Expression of Human Immunodeficiency Virus in T Cells. Nature 1987, 326, 711–713. [CrossRef]
- Griffin, G.E.; Leung, K.; Folks, T.M.; Kunkel, S.; Nabel, G.J. Activation of HIV Gene Expression during Monocyte Differentiation by Induction of NF-kB. Nature 1989, 339, 70–73. [CrossRef]
- Conant, K.; Ma, M.; Nath, A.; Major, E.O. Extracellular Human Immunodeficiency Virus Type 1 Tat Protein Is Associated with an Increase in Both NF-Kappa B Binding and Protein Kinase C Activity in Primary Human Astrocytes. J. Virol. 1996, 70, 1384–1389. [CrossRef]
- Demarchi, F.; d’Adda di Fagagna, F.; Falaschi, A.; Giacca, M. Activation of Transcription Factor NF-kappaB by the Tat Protein of Human Immunodeficiency Virus Type 1. J. Virol. 1996, 70, 4427–4437. [CrossRef]
- Biswas, D.K.; Salas, T.R.; Wang, F.; Ahlers, C.M.; Dezube, B.J.; Pardee, A.B. A Tat-Induced Auto-up-Regulatory Loop for Superactivation of the Human Immunodeficiency Virus Type 1 Promoter. J. Virol. 1995, 69, 7437–7444. [CrossRef]
- Kretzschmar, M.; Meisterernst, M.; Scheidereit, C.; Li, G.; Roeder, R.G. Transcriptional Regulation of the HIV-1 Promoter by NF-Kappa B in Vitro. Genes Dev. 1992, 6, 761–774. [CrossRef]
- Chen, B.K.; Feinberg, M.B.; Baltimore, D. The kappaB Sites in the Human Immunodeficiency Virus Type 1 Long Terminal Repeat Enhance Virus Replication yet Are Not Absolutely Required for Viral Growth. J. Virol. 1997, 71, 5495–5504. [CrossRef]
- Liu, J.; Perkins, N.D.; Schmid, R.M.; Nabel, G.J. Specific NF-Kappa B Subunits Act in Concert with Tat to Stimulate Human Immunodeficiency Virus Type 1 Transcription. J. Virol. 1992, 66, 3883–3887. [CrossRef]
- Schmitz, M.L.; Stelzer, G.; Altmann, H.; Meisterernst, M.; Baeuerle, P.A. Interaction of the COOH-Terminal Transactivation Domain of P65 NF-Kappa B with TATA-Binding Protein, Transcription Factor IIB, and Coactivators. J. Biol. Chem. 1995, 270, 7219–7226. [CrossRef]
- Perkins, N.D.; Edwards, N.L.; Duckett, C.S.; Agranoff, A.B.; Schmid, R.M.; Nabel, G.J. A Cooperative Interaction between NF-Kappa B and Sp1 Is Required for HIV-1 Enhancer Activation. EMBO J. 1993, 12, 3551–3558.
- Duh, E.J.; Maury, W.J.; Folks, T.M.; Fauci, A.S.; Rabson, A.B. Tumor Necrosis Factor Alpha Activates Human Immunodeficiency Virus Type 1 through Induction of Nuclear Factor Binding to the NF-Kappa B Sites in the Long Terminal Repeat. Proc. Natl. Acad. Sci. 1989, 86, 5974–5978. [CrossRef]
- Granowitz, E.V.; Saget, B.M.; Wang, M.Z.; Dinarello, C.A.; Skolnik, P.R. Interleukin 1 Induces HIV-1 Expression in Chronically Infected U1 Cells: Blockade by Interleukin 1 Receptor Antagonist and Tumor Necrosis Factor Binding Protein Type 1. Mol. Med. 1995, 1, 667–677.
- Folks, T.M.; Justement, J.; Kinter, A.; Dinarello, C.A.; Fauci, A.S. Cytokine-Induced Expression of HIV-1 in a Chronically Infected Promonocyte Cell Line. Science 1987, 238, 800–802. [CrossRef]
- Finnegan, A.; Roebuck, K.A.; Nakai, B.E.; Gu, D.S.; Rabbi, M.F.; Song, S.; Landay, A.L. IL-10 Cooperates with TNF-Alpha to Activate HIV-1 from Latently and Acutely Infected Cells of Monocyte/Macrophage Lineage. J. Immunol. Baltim. Md 1950 1996, 156, 841–851.
- Li, J.M.; Shen, X.; Hu, P.P.; Wang, X.F. Transforming Growth Factor Beta Stimulates the Human Immunodeficiency Virus 1 Enhancer and Requires NF-kappaB Activity. Mol. Cell. Biol. 1998, 18, 110–121. [CrossRef]
- Dinter, H.; Chiu, R.; Imagawa, M.; Karin, M.; Jones, K.A. In Vitro Activation of the HIV-1 Enhancer in Extracts from Cells Treated with a Phorbol Ester Tumor Promoter. EMBO J. 1987, 6, 4067–4071. [CrossRef]
- Bassuk, A.G.; Anandappa, R.T.; Leiden, J.M. Physical Interactions between Ets and NF-kappaB/NFAT Proteins Play an Important Role in Their Cooperative Activation of the Human Immunodeficiency Virus Enhancer in T Cells. J. Virol. 1997, 71, 3563–3573. [CrossRef]
- Kundu, M.; Srinivasan, A.; Pomerantz, R.J.; Khalili, K. Evidence That a Cell Cycle Regulator, E2F1, down-Regulates Transcriptional Activity of the Human Immunodeficiency Virus Type 1 Promoter. J. Virol. 1995, 69, 6940–6946. [CrossRef]
- Verdin, E.; Becker, N.; Bex, F.; Droogmans, L.; Burny, A. Identification and Characterization of an Enhancer in the Coding Region of the Genome of Human Immunodeficiency Virus Type 1. Proc. Natl. Acad. Sci. 1990, 87, 4874–4878. [CrossRef]
- Van Lint, C.; Burny, A.; Verdin, E. The Intragenic Enhancer of Human Immunodeficiency Virus Type 1 Contains Functional AP-1 Binding Sites. J. Virol. 1991, 65, 7066–7072. [CrossRef]
- Colin, L.; Vandenhoudt, N.; Walque, S. de; Driessche, B.V.; Bergamaschi, A.; Martinelli, V.; Cherrier, T.; Vanhulle, C.; Guiguen, A.; David, A.; et al. The AP-1 Binding Sites Located in the Pol Gene Intragenic Regulatory Region of HIV-1 Are Important for Viral Replication. PLOS ONE 2011, 6, e19084. [CrossRef]
- Lu, Y.C.; Touzjian, N.; Stenzel, M.; Dorfman, T.; Sodroski, J.G.; Haseltine, W.A. Identification of Cis-Acting Repressive Sequences within the Negative Regulatory Element of Human Immunodeficiency Virus Type 1. J. Virol. 1990, 64, 5226–5229. [CrossRef]
- Van Lint, C.; Amella, C.A.; Emiliani, S.; John, M.; Jie, T.; Verdin, E. Transcription Factor Binding Sites Downstream of the Human Immunodeficiency Virus Type 1 Transcription Start Site Are Important for Virus Infectivity. J. Virol. 1997, 71, 6113–6127. [CrossRef]
- Yang, Z.; Engel, J.D. Human T Cell Transcription Factor GATA-3 Stimulates HIV-1 Expression. Nucleic Acids Res. 1993, 21, 2831–2836. [CrossRef]
- Sheridan, P.L.; Sheline, C.T.; Cannon, K.; Voz, M.L.; Pazin, M.J.; Kadonaga, J.T.; Jones, K.A. Activation of the HIV-1 Enhancer by the LEF-1 HMG Protein on Nucleosome-Assembled DNA in Vitro. Genes Dev. 1995, 9, 2090–2104. [CrossRef]
- Henderson, A.J.; Zou, X.; Calame, K.L. C/EBP Proteins Activate Transcription from the Human Immunodeficiency Virus Type 1 Long Terminal Repeat in Macrophages/Monocytes. J. Virol. 1995, 69, 5337–5344. [CrossRef]
- Henderson, A.J.; Connor, R.I.; Calame, K.L. C/EBP Activators Are Required for HIV-1 Replication and Proviral Induction in Monocytic Cell Lines. Immunity 1996, 5, 91–101. [CrossRef]
- Henderson, A.J.; Calame, K.L. CCAAT/Enhancer Binding Protein (C/EBP) Sites Are Required for HIV-1 Replication in Primary Macrophages but Not CD4+ T Cells. Proc. Natl. Acad. Sci. 1997, 94, 8714–8719. [CrossRef]
- Cherrier, T.; Le Douce, V.; Eilebrecht, S.; Riclet, R.; Marban, C.; Dequiedt, F.; Goumon, Y.; Paillart, J.-C.; Mericskay, M.; Parlakian, A.; et al. CTIP2 Is a Negative Regulator of P-TEFb. Proc. Natl. Acad. Sci. 2013, 110, 12655–12660. [CrossRef]
- Eilebrecht, S.; Le Douce, V.; Riclet, R.; Targat, B.; Hallay, H.; Van Driessche, B.; Schwartz, C.; Robette, G.; Van Lint, C.; Rohr, O.; et al. HMGA1 Recruits CTIP2-Repressed P-TEFb to the HIV-1 and Cellular Target Promoters. Nucleic Acids Res. 2014, 42, 4962–4971. [CrossRef]
- Duan, L.; Ozaki, I.; Oakes, J.W.; Taylor, J.P.; Khalili, K.; Pomerantz, R.J. The Tumor Suppressor Protein P53 Strongly Alters Human Immunodeficiency Virus Type 1 Replication. J. Virol. 1994, 68, 4302–4313.
- Gérard, A.; Ségéral, E.; Naughtin, M.; Abdouni, A.; Charmeteau, B.; Cheynier, R.; Rain, J.-C.; Emiliani, S. The Integrase Cofactor LEDGF/P75 Associates with Iws1 and Spt6 for Postintegration Silencing of HIV-1 Gene Expression in Latently Infected Cells. Cell Host Microbe 2015, 17, 107–117. [CrossRef]
- LIU, Y.-Z.; LATCHMAN, D.S. The Octamer-Binding Proteins Oct-1 and Oct-2 Repress the HIV Long Terminal Repeat Promoter and Its Transactivation by Tat. Biochem. J. 1997, 322, 155–158. [CrossRef]
- Fenard, D.; Houzet, L.; Bernard, E.; Tupin, A.; Brun, S.; Mougel, M.; Devaux, C.; Chazal, N.; Briant, L. Uracil DNA Glycosylase 2 Negatively Regulates HIV-1 LTR Transcription. Nucleic Acids Res. 2009, 37, 6008–6018. [CrossRef]
- Margolis, D.M.; Somasundaran, M.; Green, M.R. Human Transcription Factor YY1 Represses Human Immunodeficiency Virus Type 1 Transcription and Virion Production. J. Virol. 1994, 68, 905–910. [CrossRef]
- Jones, K.A.; Luciw, P.A.; Duchange, N. Structural Arrangements of Transcription Control Domains within the 5’-Untranslated Leader Regions of the HIV-1 and HIV-2 Promoters. Genes Dev. 1988, 2, 1101–1114. [CrossRef]
- Kato, H.; Horikoshi, M.; Roeder, R.G. Repression of HIV-1 Transcription by a Cellular Protein. Science 1991, 251, 1476–1479. [CrossRef]
- Parada, C.A.; Yoon, J.-B.; Roeder, R.G. A Novel LBP-1-Mediated Restriction of HIV-1 Transcription at the Level of Elongation in Vitro(∗). J. Biol. Chem. 1995, 270, 2274–2283. [CrossRef]
- Romerio, F.; Gabriel, M.N.; Margolis, D.M. Repression of Human Immunodeficiency Virus Type 1 through the Novel Cooperation of Human Factors YY1 and LSF. J. Virol. 1997, 71, 9375–9382. [CrossRef]
- Hotter, D.; Bosso, M.; Jønsson, K.L.; Krapp, C.; Stürzel, C.M.; Das, A.; Littwitz-Salomon, E.; Berkhout, B.; Russ, A.; Wittmann, S.; et al. IFI16 Targets the Transcription Factor Sp1 to Suppress HIV-1 Transcription and Latency Reactivation. Cell Host Microbe 2019, 25, 858-872.e13. [CrossRef]
- Kajaste-Rudnitski, A.; Marelli, S.S.; Pultrone, C.; Pertel, T.; Uchil, P.D.; Mechti, N.; Mothes, W.; Poli, G.; Luban, J.; Vicenzi, E. TRIM22 Inhibits HIV-1 Transcription Independently of Its E3 Ubiquitin Ligase Activity, Tat, and NF-kappaB-Responsive Long Terminal Repeat Elements. J. Virol. 2011, 85, 5183–5196. [CrossRef]
- Turrini, F.; Marelli, S.; Kajaste-Rudnitski, A.; Lusic, M.; Van Lint, C.; Das, A.T.; Harwig, A.; Berkhout, B.; Vicenzi, E. HIV-1 Transcriptional Silencing Caused by TRIM22 Inhibition of Sp1 Binding to the Viral Promoter. Retrovirology 2015, 12, 104. [CrossRef]
- Turrini, F.; Saliu, F.; Forlani, G.; Das, A.T.; Van Lint, C.; Accolla, R.S.; Berkhout, B.; Poli, G.; Vicenzi, E. Interferon-Inducible TRIM22 Contributes to Maintenance of HIV-1 Proviral Latency in T Cell Lines. Virus Res. 2019, 269, 197631. [CrossRef]
- Shi, Y.; Simpson, S.; Ahmed, S.K.; Chen, Y.; Tavakoli-Tameh, A.; Janaka, S.K.; Evans, D.T.; Serra-Moreno, R. The Antiviral Factor SERINC5 Impairs the Expression of Non-Self-DNA. Viruses 2023, 15, 1961. [CrossRef]
- Dupont, L.; Bloor, S.; Williamson, J.C.; Cuesta, S.M.; Shah, R.; Teixeira-Silva, A.; Naamati, A.; Greenwood, E.J.D.; Sarafianos, S.G.; Matheson, N.J.; et al. The SMC5/6 Complex Compacts and Silences Unintegrated HIV-1 DNA and Is Antagonized by Vpr. Cell Host Microbe 2021, 29, 792-805.e6. [CrossRef]
- Gallastegui, E.; Millán-Zambrano, G.; Terme, J.-M.; Chávez, S.; Jordan, A. Chromatin Reassembly Factors Are Involved in Transcriptional Interference Promoting HIV Latency. J. Virol. 2011, 85, 3187–3202. [CrossRef]
- Shirakawa, K.; Chavez, L.; Hakre, S.; Calvanese, V.; Verdin, E. Reactivation of Latent HIV by Histone Deacetylase Inhibitors. Trends Microbiol. 2013, 21, 277–285. [CrossRef]
- Le Douce, V.; Cherrier, T.; Riclet, R.; Rohr, O.; Schwartz, C. The Many Lives of CTIP2: From AIDS to Cancer and Cardiac Hypertrophy. J. Cell. Physiol. 2014, 229, 533–537. [CrossRef]
- Bruce, J.W.; Bracken, M.; Evans, E.; Sherer, N.; Ahlquist, P. ZBTB2 Represses HIV-1 Transcription and Is Regulated by HIV-1 Vpr and Cellular DNA Damage Responses. PLOS Pathog. 2021, 17, e1009364. [CrossRef]
- Schröder, H.C.; Wenger, R.; Kuchino, Y.; Müller, W.E. Modulation of Nuclear Matrix-Associated 2’,5’-Oligoadenylate Metabolism and Ribonuclease L Activity in H9 Cells by Human Immunodeficiency Virus. J. Biol. Chem. 1989, 264, 5669–5673.
- Brennan-Laun, S.E.; Ezelle, H.J.; Li, X.-L.; Hassel, B.A. RNase-L Control of Cellular mRNAs: Roles in Biologic Functions and Mechanisms of Substrate Targeting. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2014, 34, 275–288. [CrossRef]
- Martinand, C.; Montavon, C.; Salehzada, T.; Silhol, M.; Lebleu, B.; Bisbal, C. RNase L Inhibitor Is Induced during Human Immunodeficiency Virus Type 1 Infection and Down Regulates the 2-5A/RNase L Pathway in Human T Cells. J. Virol. 1999, 73, 290–296. [CrossRef]
- Nchioua, R.; Bosso, M.; Kmiec, D.; Kirchhoff, F. Cellular Factors Targeting HIV-1 Transcription and Viral RNA Transcripts. Viruses 2020, 12, 495. [CrossRef]
- Zhu, Y.; Chen, G.; Lv, F.; Wang, X.; Ji, X.; Xu, Y.; Sun, J.; Wu, L.; Zheng, Y.-T.; Gao, G. Zinc-Finger Antiviral Protein Inhibits HIV-1 Infection by Selectively Targeting Multiply Spliced Viral mRNAs for Degradation. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 15834–15839. [CrossRef]
- Chen, G.; Guo, X.; Lv, F.; Xu, Y.; Gao, G. P72 DEAD Box RNA Helicase Is Required for Optimal Function of the Zinc-Finger Antiviral Protein. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 4352–4357. [CrossRef]
- Schwerk, J.; Soveg, F.W.; Ryan, A.P.; Thomas, K.R.; Hatfield, L.D.; Ozarkar, S.; Forero, A.; Kell, A.M.; Roby, J.A.; So, L.; et al. RNA-Binding Protein Isoforms ZAP-S and ZAP-L Have Distinct Antiviral and Immune Resolution Functions. Nat. Immunol. 2019, 20, 1610–1620. [CrossRef]
- Zheng, X.; Wang, X.; Tu, F.; Wang, Q.; Fan, Z.; Gao, G. TRIM25 Is Required for the Antiviral Activity of Zinc Finger Antiviral Protein. J. Virol. 2017, 91, e00088-17. [CrossRef]
- Ficarelli, M.; Wilson, H.; Pedro Galão, R.; Mazzon, M.; Antzin-Anduetza, I.; Marsh, M.; Neil, S.J.; Swanson, C.M. KHNYN Is Essential for the Zinc Finger Antiviral Protein (ZAP) to Restrict HIV-1 Containing Clustered CpG Dinucleotides. eLife 2019, 8, e46767. [CrossRef]
- Ficarelli, M.; Antzin-Anduetza, I.; Hugh-White, R.; Firth, A.E.; Sertkaya, H.; Wilson, H.; Neil, S.J.D.; Schulz, R.; Swanson, C.M. CpG Dinucleotides Inhibit HIV-1 Replication through Zinc Finger Antiviral Protein (ZAP)-Dependent and -Independent Mechanisms. J. Virol. 2020, 94, e01337-19. [CrossRef]
- Kmiec, D.; Nchioua, R.; Sherrill-Mix, S.; Stürzel, C.M.; Heusinger, E.; Braun, E.; Gondim, M.V.P.; Hotter, D.; Sparrer, K.M.J.; Hahn, B.H.; et al. CpG Frequency in the 5’ Third of the Env Gene Determines Sensitivity of Primary HIV-1 Strains to the Zinc-Finger Antiviral Protein. mBio 2020, 11, e02903-19. [CrossRef]
- Takata, M.A.; Gonçalves-Carneiro, D.; Zang, T.M.; Soll, S.J.; York, A.; Blanco-Melo, D.; Bieniasz, P.D. CG Dinucleotide Suppression Enables Antiviral Defence Targeting Non-Self RNA. Nature 2017, 550, 124–127. [CrossRef]
- Yamasoba, D.; Sato, K.; Ichinose, T.; Imamura, T.; Koepke, L.; Joas, S.; Reith, E.; Hotter, D.; Misawa, N.; Akaki, K.; et al. N4BP1 Restricts HIV-1 and Its Inactivation by MALT1 Promotes Viral Reactivation. Nat. Microbiol. 2019, 4, 1532–1544. [CrossRef]
- Liu, S.; Qiu, C.; Miao, R.; Zhou, J.; Lee, A.; Liu, B.; Lester, S.N.; Fu, W.; Zhu, L.; Zhang, L.; et al. MCPIP1 Restricts HIV Infection and Is Rapidly Degraded in Activated CD4+ T Cells. Proc. Natl. Acad. Sci. 2013, 110, 19083–19088. [CrossRef]
- Triboulet, R.; Mari, B.; Lin, Y.-L.; Chable-Bessia, C.; Bennasser, Y.; Lebrigand, K.; Cardinaud, B.; Maurin, T.; Barbry, P.; Baillat, V.; et al. Suppression of MicroRNA-Silencing Pathway by HIV-1 During Virus Replication. Science 2007, 315, 1579–1582. [CrossRef]
- Nathans, R.; Chu, C.; Serquina, A.K.; Lu, C.-C.; Cao, H.; Rana, T.M. Cellular MicroRNA and P Bodies Modulate Host-HIV-1 Interactions. Mol. Cell 2009, 34, 696–709. [CrossRef]
- Wang, X.; Ye, L.; Hou, W.; Zhou, Y.; Wang, Y.-J.; Metzger, D.S.; Ho, W.-Z. Cellular microRNA Expression Correlates with Susceptibility of Monocytes/Macrophages to HIV-1 Infection. Blood 2009, 113, 671–674. [CrossRef]
- Toro-Ascuy, D.; Rojas-Araya, B.; Valiente-Echeverría, F.; Soto-Rifo, R. Interactions between the HIV-1 Unspliced mRNA and Host mRNA Decay Machineries. Viruses 2016, 8, 320. [CrossRef]
- Coyle, J.H.; Bor, Y.-C.; Rekosh, D.; Hammarskjold, M.-L. The Tpr Protein Regulates Export of mRNAs with Retained Introns That Traffic through the Nxf1 Pathway. RNA 2011, 17, 1344–1356. [CrossRef]
- Arnold, M.; Nath, A.; Hauber, J.; Kehlenbach, R.H. Multiple Importins Function as Nuclear Transport Receptors for the Rev Protein of Human Immunodeficiency Virus Type 1. J. Biol. Chem. 2006, 281, 20883–20890. [CrossRef]
- Izaurralde, E.; Stepinski, J.; Darzynkiewicz, E.; Mattaj, I.W. A Cap Binding Protein That May Mediate Nuclear Export of RNA Polymerase II-Transcribed RNAs. J. Cell Biol. 1992, 118, 1287–1295. [CrossRef]
- Izaurralde, E.; Lewis, J.; Gamberi, C.; Jarmolowski, A.; McGuigan, C.; Mattaj, I.W. A Cap-Binding Protein Complex Mediating U snRNA Export. Nature 1995, 376, 709–712. [CrossRef]
- Nawroth, I.; Mueller, F.; Basyuk, E.; Beerens, N.; Rahbek, U.L.; Darzacq, X.; Bertrand, E.; Kjems, J.; Schmidt, U. Stable Assembly of HIV-1 Export Complexes Occurs Cotranscriptionally. RNA 2014, 20, 1–8. [CrossRef]
- Sharma, A.; Yilmaz, A.; Marsh, K.; Cochrane, A.; Boris-Lawrie, K. Thriving under Stress: Selective Translation of HIV-1 Structural Protein mRNA during Vpr-Mediated Impairment of eIF4E Translation Activity. PLOS Pathog. 2012, 8, e1002612. [CrossRef]
- Fornerod, M.; Ohno, M.; Yoshida, M.; Mattaj, I.W. CRM1 Is an Export Receptor for Leucine-Rich Nuclear Export Signals. Cell 1997, 90, 1051–1060. [CrossRef]
- Malim, M.H.; Hauber, J.; Le, S.-Y.; Maizel, J.V.; Cullen, B.R. The HIV-1 Rev Trans-Activator Acts through a Structured Target Sequence to Activate Nuclear Export of Unspliced Viral mRNA. Nature 1989, 338, 254–257. [CrossRef]
- Fischer, U.; Meyer, S.; Teufel, M.; Heckel, C.; Lührmann, R.; Rautmann, G. Evidence That HIV-1 Rev Directly Promotes the Nuclear Export of Unspliced RNA. EMBO J. 1994, 13, 4105–4112. [CrossRef]
- Fischer, U.; Huber, J.; Boelens, W.C.; Mattaj, I.W.; Lührmann, R. The HIV-1 Rev Activation Domain Is a Nuclear Export Signal That Accesses an Export Pathway Used by Specific Cellular RNAs. Cell 1995, 82, 475–483. [CrossRef]
- Lai, M.-C.; Lee, Y.-H.W.; Tarn, W.-Y. The DEAD-Box RNA Helicase DDX3 Associates with Export Messenger Ribonucleoproteins as Well asTip-Associated Protein and Participates in Translational Control. Mol. Biol. Cell 2008, 19, 3847–3858. [CrossRef]
- Guo, J.; Zhu, Y.; Ma, X.; Shang, G.; Liu, B.; Zhang, K. Virus Infection and mRNA Nuclear Export. Int. J. Mol. Sci. 2023, 24, 12593. [CrossRef]
- Kuss, S.K.; Mata, M.A.; Zhang, L.; Fontoura, B.M.A. Nuclear Imprisonment: Viral Strategies to Arrest Host mRNA Nuclear Export. Viruses 2013, 5, 1824–1849. [CrossRef]
- Cavazza, T.; Vernos, I. The RanGTP Pathway: From Nucleo-Cytoplasmic Transport to Spindle Assembly and Beyond. Front. Cell Dev. Biol. 2016, 3. [CrossRef]
- Askjaer, P.; Jensen, T.H.; Nilsson, J.; Englmeier, L.; Kjems, J. The Specificity of the CRM1-Rev Nuclear Export Signal Interaction Is Mediated by RanGTP. J. Biol. Chem. 1998, 273, 33414–33422. [CrossRef]
- Liu, H.; Hu, P.-W.; Budhiraja, S.; Misra, A.; Couturier, J.; Lloyd, R.E.; Lewis, D.E.; Kimata, J.T.; Rice, A.P. PACS1 Is an HIV-1 Cofactor That Functions in Rev-Mediated Nuclear Export of Viral RNA. Virology 2020, 540, 88–96. [CrossRef]
- Kula, A.; Gharu, L.; Marcello, A. HIV-1 Pre-mRNA Commitment to Rev Mediated Export through PSF and Matrin 3. Virology 2013, 435, 329–340. [CrossRef]
- Jones, T.; Sheer, D.; Bevec, D.; Kappel, B.; Hauber, J.; Steinkasserer, A. The Human HIV-1 Rev Binding-Protein hRIP/Rab (HRB) Maps to Chromosome 2q36. Genomics 1997, 40, 198–199. [CrossRef]
- Li, J.; Tang, H.; Mullen, T.M.; Westberg, C.; Reddy, T.R.; Rose, D.W.; Wong-Staal, F. A Role for RNA Helicase A in Post-Transcriptional Regulation of HIV Type 1. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 709–714. [CrossRef]
- Budhiraja, S.; Liu, H.; Couturier, J.; Malovannaya, A.; Qin, J.; Lewis, D.E.; Rice, A.P. Mining the Human Complexome Database Identifies RBM14 as an XPO1-Associated Protein Involved in HIV-1 Rev Function. J. Virol. 2015, 89, 3557–3567. [CrossRef]
- Yedavalli, V.S.R.K.; Neuveut, C.; Chi, Y.; Kleiman, L.; Jeang, K.-T. Requirement of DDX3 DEAD Box RNA Helicase for HIV-1 Rev-RRE Export Function. Cell 2004, 119, 381–392. [CrossRef]
- Dayton, A.I. Within You, without You: HIV-1 Rev and RNA Export. Retrovirology 2004, 1, 35. [CrossRef]
- Fang, J.; Kubota, S.; Yang, B.; Zhou, N.; Zhang, H.; Godbout, R.; Pomerantz, R.J. A DEAD Box Protein Facilitates HIV-1 Replication as a Cellular Co-Factor of Rev. Virology 2004, 330, 471–480. [CrossRef]
- Li, J.; Liu, Y.; Kim, B.O.; He, J.J. Direct Participation of Sam68, the 68-Kilodalton Src-Associated Protein in Mitosis, in the CRM1-Mediated Rev Nuclear Export Pathway. J. Virol. 2002, 76, 8374–8382. [CrossRef]
- Schatz, O.; Oft, M.; Dascher, C.; Schebesta, M.; Rosorius, O.; Jaksche, H.; Dobrovnik, M.; Bevec, D.; Hauber, J. Interaction of the HIV-1 Rev Cofactor Eukaryotic Initiation Factor 5A with Ribosomal Protein L5. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 1607–1612. [CrossRef]
- Najera, I.; Krieg, M.; Karn, J. Synergistic Stimulation of HIV-1 Rev-Dependent Export of Unspliced mRNA to the Cytoplasm by hnRNP A1. J. Mol. Biol. 1999, 285, 1951–1964. [CrossRef]
- Fritz, C.C.; Zapp, M.L.; Green, M.R. A Human Nucleoporin-like Protein That Specifically Interacts with HIV Rev. Nature 1995, 376, 530–533. [CrossRef]
- Ajamian, L.; Abrahamyan, L.; Milev, M.; Ivanov, P.V.; Kulozik, A.E.; Gehring, N.H.; Mouland, A.J. Unexpected Roles for UPF1 in HIV-1 RNA Metabolism and Translation. RNA N. Y. N 2008, 14, 914–927. [CrossRef]
- Ajamian, L.; Abel, K.; Rao, S.; Vyboh, K.; García-de-Gracia, F.; Soto-Rifo, R.; Kulozik, A.E.; Gehring, N.H.; Mouland, A.J. HIV-1 Recruits UPF1 but Excludes UPF2 to Promote Nucleocytoplasmic Export of the Genomic RNA. Biomolecules 2015, 5, 2808–2839. [CrossRef]
- Park, E.; Maquat, L.E. Staufen-Mediated mRNA Decay. Wiley Interdiscip. Rev. RNA 2013, 4, 423–435. [CrossRef]
- Dugré-Brisson, S.; Elvira, G.; Boulay, K.; Chatel-Chaix, L.; Mouland, A.J.; DesGroseillers, L. Interaction of Staufen1 with the 5′ End of mRNA Facilitates Translation of These RNAs. Nucleic Acids Res. 2005, 33, 4797–4812. [CrossRef]
- Chatel-Chaix, L.; Clément, J.-F.; Martel, C.; Bériault, V.; Gatignol, A.; DesGroseillers, L.; Mouland, A.J. Identification of Staufen in the Human Immunodeficiency Virus Type 1 Gag Ribonucleoprotein Complex and a Role in Generating Infectious Viral Particles. Mol. Cell. Biol. 2004, 24, 2637–2648. [CrossRef]
- Banerjee, A.; Benjamin, R.; Balakrishnan, K.; Ghosh, P.; Banerjee, S. Human Protein Staufen-2 Promotes HIV-1 Proliferation by Positively Regulating RNA Export Activity of Viral Protein Rev. Retrovirology 2014, 11, 18. [CrossRef]
- Soto-Rifo, R.; Limousin, T.; Rubilar, P.S.; Ricci, E.P.; Décimo, D.; Moncorgé, O.; Trabaud, M.-A.; André, P.; Cimarelli, A.; Ohlmann, T. Different Effects of the TAR Structure on HIV-1 and HIV-2 Genomic RNA Translation. Nucleic Acids Res. 2012, 40, 2653–2667. [CrossRef]
- Parsyan, A.; Svitkin, Y.; Shahbazian, D.; Gkogkas, C.; Lasko, P.; Merrick, W.C.; Sonenberg, N. mRNA Helicases: The Tacticians of Translational Control. Nat. Rev. Mol. Cell Biol. 2011, 12, 235–245. [CrossRef]
- Ohlmann, T.; Mengardi, C.; López-Lastra, M. Translation Initiation of the HIV-1 mRNA. Translation 2014, 2, e960242. [CrossRef]
- Fujii, R.; Okamoto, M.; Aratani, S.; Oishi, T.; Ohshima, T.; Taira, K.; Baba, M.; Fukamizu, A.; Nakajima, T. A Role of RNA Helicase A in Cis-Acting Transactivation Response Element-Mediated Transcriptional Regulation of Human Immunodeficiency Virus Type 1. J. Biol. Chem. 2001, 276, 5445–5451. [CrossRef]
- Bolinger, C.; Sharma, A.; Singh, D.; Yu, L.; Boris-Lawrie, K. RNA Helicase A Modulates Translation of HIV-1 and Infectivity of Progeny Virions. Nucleic Acids Res. 2010, 38, 1686–1696. [CrossRef]
- Lai, M.-C.; Wang, S.-W.; Cheng, L.; Tarn, W.-Y.; Tsai, S.-J.; Sun, H.S. Human DDX3 Interacts with the HIV-1 Tat Protein to Facilitate Viral mRNA Translation. PloS One 2013, 8, e68665. [CrossRef]
- Jackson, R.J.; Hellen, C.U.T.; Pestova, T.V. The Mechanism of Eukaryotic Translation Initiation and Principles of Its Regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [CrossRef]
- Soto-Rifo, R.; Rubilar, P.S.; Limousin, T.; de Breyne, S.; Décimo, D.; Ohlmann, T. DEAD-Box Protein DDX3 Associates with eIF4F to Promote Translation of Selected mRNAs. EMBO J. 2012, 31, 3745–3756. [CrossRef]
- Soto-Rifo, R.; Rubilar, P.S.; Ohlmann, T. The DEAD-Box Helicase DDX3 Substitutes for the Cap-Binding Protein eIF4E to Promote Compartmentalized Translation Initiation of the HIV-1 Genomic RNA. Nucleic Acids Res. 2013, 41, 6286–6299. [CrossRef]
- Mo, J.; Liang, H.; Su, C.; Li, P.; Chen, J.; Zhang, B. DDX3X: Structure, Physiologic Functions and Cancer. Mol. Cancer 2021, 20, 38. [CrossRef]
- Chen, H.-H.; Yu, H.-I.; Yang, M.-H.; Tarn, W.-Y. DDX3 Activates CBC-eIF3–Mediated Translation of uORF-Containing Oncogenic mRNAs to Promote Metastasis in HNSCC. Cancer Res. 2018, 78, 4512–4523. [CrossRef]
- Ricci, E.P.; Rifo, R.S.; Herbreteau, C.H.; Decimo, D.; Ohlmann, T. Lentiviral RNAs Can Use Different Mechanisms for Translation Initiation. Biochem. Soc. Trans. 2008, 36, 690–693. [CrossRef]
- Brasey, A.; Lopez-Lastra, M.; Ohlmann, T.; Beerens, N.; Berkhout, B.; Darlix, J.-L.; Sonenberg, N. The Leader of Human Immunodeficiency Virus Type 1 Genomic RNA Harbors an Internal Ribosome Entry Segment That Is Active during the G2/M Phase of the Cell Cycle. J. Virol. 2003, 77, 3939–3949. [CrossRef]
- Buck, C.B.; Shen, X.; Egan, M.A.; Pierson, T.C.; Walker, C.M.; Siliciano, R.F. The Human Immunodeficiency Virus Type 1 Gag Gene Encodes an Internal Ribosome Entry Site. J. Virol. 2001, 75, 181–191. [CrossRef]
- Liu, J.; Henao-Mejia, J.; Liu, H.; Zhao, Y.; He, J.J. Translational Regulation of HIV-1 Replication by HIV-1 Rev Cellular Cofactors Sam68, eIF5A, hRIP, and DDX3. J. Neuroimmune Pharmacol. 2011, 6, 308–321. [CrossRef]
- Monette, A.; Ajamian, L.; López-Lastra, M.; Mouland, A.J. Human Immunodeficiency Virus Type 1 (HIV-1) Induces the Cytoplasmic Retention of Heterogeneous Nuclear Ribonucleoprotein A1 by Disrupting Nuclear Import. J. Biol. Chem. 2009, 284, 31350–31362. [CrossRef]
- Vallejos, M.; Deforges, J.; Plank, T.-D.M.; Letelier, A.; Ramdohr, P.; Abraham, C.G.; Valiente-Echeverría, F.; Kieft, J.S.; Sargueil, B.; López-Lastra, M. Activity of the Human Immunodeficiency Virus Type 1 Cell Cycle-Dependent Internal Ribosomal Entry Site Is Modulated by IRES Trans-Acting Factors. Nucleic Acids Res. 2011, 39, 6186–6200. [CrossRef]
- Lee, W.-Y.J.; Fu, R.M.; Liang, C.; Sloan, R.D. IFITM Proteins Inhibit HIV-1 Protein Synthesis. Sci. Rep. 2018, 8, 14551. [CrossRef]
- Shehu-Xhilaga, M.; Crowe, S.M.; Mak, J. Maintenance of the Gag/Gag-Pol Ratio Is Important for Human Immunodeficiency Virus Type 1 RNA Dimerization and Viral Infectivity. J. Virol. 2001, 75, 1834–1841. [CrossRef]
- Jacks, T.; Power, M.D.; Masiarz, F.R.; Luciw, P.A.; Barr, P.J.; Varmus, H.E. Characterization of Ribosomal Frameshifting in HIV-1 Gag-Pol Expression. Nature 1988, 331, 280–283. [CrossRef]
- Wang, X.; Xuan, Y.; Han, Y.; Ding, X.; Ye, K.; Yang, F.; Gao, P.; Goff, S.P.; Gao, G. Regulation of HIV-1 Gag-Pol Expression by Shiftless, an Inhibitor of Programmed −1 Ribosomal Frameshifting. Cell 2019, 176, 625-635.e14. [CrossRef]
- Li, M.; Kao, E.; Gao, X.; Sandig, H.; Limmer, K.; Pavon-Eternod, M.; Jones, T.E.; Landry, S.; Pan, T.; Weitzman, M.D.; et al. Codon-Usage-Based Inhibition of HIV Protein Synthesis by Human Schlafen 11. Nature 2012, 491, 125–128. [CrossRef]
- Kypr, J.; Mrázek, J. Unusual Codon Usage of HIV. Nature 1987, 327, 20–20. [CrossRef]
- Haas, J.; Park, E.C.; Seed, B. Codon Usage Limitation in the Expression of HIV-1 Envelope Glycoprotein. Curr. Biol. CB 1996, 6, 315–324. [CrossRef]
- Kofman, A.; Graf, M.; Bojak, A.; Deml, L.; Bieler, K.; Kharazova, A.; Wolf, H.; Wagner, R. HIV-1 Gag Expression Is Quantitatively Dependent on the Ratio of Native and Optimized Codons. Tsitologiia 2003, 45, 86–93.
- Kobayashi-Ishihara, M.; Frazão Smutná, K.; Alonso, F.E.; Argilaguet, J.; Esteve-Codina, A.; Geiger, K.; Genescà, M.; Grau-Expósito, J.; Duran-Castells, C.; Rogenmoser, S.; et al. Schlafen 12 Restricts HIV-1 Latency Reversal by a Codon-Usage Dependent Post-Transcriptional Block in CD4+ T Cells. Commun. Biol. 2023, 6, 1–15. [CrossRef]
- Rivas-Aravena, A.; Ramdohr, P.; Vallejos, M.; Valiente-Echeverría, F.; Dormoy-Raclet, V.; Rodríguez, F.; Pino, K.; Holzmann, C.; Huidobro-Toro, J.P.; Gallouzi, I.-E.; et al. The Elav-like Protein HuR Exerts Translational Control of Viral Internal Ribosome Entry Sites. Virology 2009, 392, 178–185. [CrossRef]
- López-Ulloa, B.; Fuentes, Y.; Pizarro-Ortega, M.S.; López-Lastra, M. RNA-Binding Proteins as Regulators of Internal Initiation of Viral mRNA Translation. Viruses 2022, 14, 188. [CrossRef]
- Wang, Q.; Gao, H.; Clark, K.M.; Mugisha, C.S.; Davis, K.; Tang, J.P.; Harlan, G.H.; DeSelm, C.J.; Presti, R.M.; Kutluay, S.B.; et al. CARD8 Is an Inflammasome Sensor for HIV-1 Protease Activity. Science 2021, 371, eabe1707. [CrossRef]
- Wang, Q.; Clark, K.M.; Tiwari, R.; Raju, N.; Tharp, G.K.; Rogers, J.; Harris, R.A.; Raveendran, M.; Bosinger, S.E.; Burdo, T.H.; et al. The CARD8 Inflammasome Dictates HIV/SIV Pathogenesis and Disease Progression. Cell 2024, 187, 1223-1237.e16. [CrossRef]
- Linder, A.; Bauernfried, S.; Cheng, Y.; Albanese, M.; Jung, C.; Keppler, O.T.; Hornung, V. CARD8 Inflammasome Activation Triggers Pyroptosis in Human T Cells. EMBO J. 2020, 39, e105071. [CrossRef]
- Park, J.; Morrow, C.D. Overexpression of the Gag-Pol Precursor from Human Immunodeficiency Virus Type 1 Proviral Genomes Results in Efficient Proteolytic Processing in the Absence of Virion Production. J. Virol. 1991, 65, 5111–5117. [CrossRef]
- Finkel, T.H.; Tudor-Williams, G.; Banda, N.K.; Cotton, M.F.; Curiel, T.; Monks, C.; Baba, T.W.; Ruprecht, R.M.; Kupfer, A. Apoptosis Occurs Predominantly in Bystander Cells and Not in Productively Infected Cells of HIV- and SIV-Infected Lymph Nodes. Nat. Med. 1995, 1, 129–134. [CrossRef]
- Clerzius, G.; Gélinas, J.-F.; Daher, A.; Bonnet, M.; Meurs, E.F.; Gatignol, A. ADAR1 Interacts with PKR during Human Immunodeficiency Virus Infection of Lymphocytes and Contributes to Viral Replication. J. Virol. 2009, 83, 10119–10128. [CrossRef]
- Clerzius, G.; Gélinas, J.-F.; Gatignol, A. Multiple Levels of PKR Inhibition during HIV-1 Replication. Rev. Med. Virol. 2011, 21, 42–53. [CrossRef]
- Park, H.; Davies, M.V.; Langland, J.O.; Chang, H.W.; Nam, Y.S.; Tartaglia, J.; Paoletti, E.; Jacobs, B.L.; Kaufman, R.J.; Venkatesan, S. TAR RNA-Binding Protein Is an Inhibitor of the Interferon-Induced Protein Kinase PKR. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 4713–4717. [CrossRef]
- Benkirane, M.; Neuveut, C.; Chun, R.F.; Smith, S.M.; Samuel, C.E.; Gatignol, A.; Jeang, K. Oncogenic Potential of TAR RNA Binding Protein TRBP and Its Regulatory Interaction with RNA-dependent Protein Kinase PKR. EMBO J. 1997, 16, 611–624. [CrossRef]
- Sanghvi, V.R.; Steel, L.F. The Cellular TAR RNA Binding Protein, TRBP, Promotes HIV-1 Replication Primarily by Inhibiting the Activation of Double-Stranded RNA-Dependent Kinase PKR. J. Virol. 2011, 85, 12614–12621. [CrossRef]
- Laraki, G.; Clerzius, G.; Daher, A.; Melendez-Peña, C.; Daniels, S.; Gatignol, A. Interactions between the Double-Stranded RNA-Binding Proteins TRBP and PACT Define the Medipal Domain That Mediates Protein-Protein Interactions. RNA Biol. 2008, 5, 92–103. [CrossRef]
- Daher, A.; Laraki, G.; Singh, M.; Melendez-Peña, C.E.; Bannwarth, S.; Peters, A.H.F.M.; Meurs, E.F.; Braun, R.E.; Patel, R.C.; Gatignol, A. TRBP Control of PACT-Induced Phosphorylation of Protein Kinase R Is Reversed by Stress. Mol. Cell. Biol. 2009, 29, 254–265. [CrossRef]
- Peters, G.A.; Dickerman, B.; Sen, G.C. Biochemical Analysis of PKR Activation by PACT. Biochemistry 2009, 48, 7441–7447. [CrossRef]
- Li, S.; Sen, G.C. PACT-Mediated Enhancement of Reporter Gene Expression at the Translational Level. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2003, 23, 689–697. [CrossRef]
- Clerzius, G.; Shaw, E.; Daher, A.; Burugu, S.; Gélinas, J.-F.; Ear, T.; Sinck, L.; Routy, J.-P.; Mouland, A.J.; Patel, R.C.; et al. The PKR Activator, PACT, Becomes a PKR Inhibitor during HIV-1 Replication. Retrovirology 2013, 10, 96. [CrossRef]
- Burugu, S.; Daher, A.; Meurs, E.F.; Gatignol, A. HIV-1 Translation and Its Regulation by Cellular Factors PKR and PACT. Virus Res. 2014, 193, 65–77. [CrossRef]
- Gheysen, D.; Jacobs, E.; Foresta, F. de; Thiriart, C.; Francotte, M.; Thines, D.; Wilde, M.D. Assembly and Release of HIV-1 Precursor Pr55gag Virus-like Particles from Recombinant Baculovirus-Infected Insect Cells. Cell 1989, 59, 103–112. [CrossRef]
- Maréchal, V.; Clavel, F.; Heard, J.M.; Schwartz, O. Cytosolic Gag P24 as an Index of Productive Entry of Human Immunodeficiency Virus Type 1. J. Virol. 1998, 72, 2208–2212. [CrossRef]
- Tedbury, P.R.; Freed, E.O. The Role of Matrix in HIV-1 Envelope Glycoprotein Incorporation. Trends Microbiol. 2014, 22, 372–378. [CrossRef]
- Mu, X.; Fu, Y.; Zhu, Y.; Wang, X.; Xuan, Y.; Shang, H.; Goff, S.P.; Gao, G. HIV-1 Exploits the Host Factor RuvB-like 2 to Balance Viral Protein Expression. Cell Host Microbe 2015, 18, 233–242. [CrossRef]
- Rucevic, M.; Boucau, J.; Dinter, J.; Kourjian, G.; Le Gall, S. Mechanisms of HIV Protein Degradation into Epitopes: Implications for Vaccine Design. Viruses 2014, 6, 3271–3292. [CrossRef]
- Lata, S.; Mishra, R.; Banerjea, A.C. Proteasomal Degradation Machinery: Favorite Target of HIV-1 Proteins. Front. Microbiol. 2018, 9. [CrossRef]
- Kimura, H.; Caturegli, P.; Takahashi, M.; Suzuki, K. New Insights into the Function of the Immunoproteasome in Immune and Nonimmune Cells. J. Immunol. Res. 2015, 2015, 541984. [CrossRef]
- Chen, W.; Norbury, C.C.; Cho, Y.; Yewdell, J.W.; Bennink, J.R. Immunoproteasomes Shape Immunodominance Hierarchies of Antiviral Cd8+ T Cells at the Levels of T Cell Repertoire and Presentation of Viral Antigens. J. Exp. Med. 2001, 193, 1319–1326. [CrossRef]
- Seissler, T.; Marquet, R.; Paillart, J.-C. Hijacking of the Ubiquitin/Proteasome Pathway by the HIV Auxiliary Proteins. Viruses 2017, 9, 322. [CrossRef]
- Li, J.; Chen, C.; Ma, X.; Geng, G.; Liu, B.; Zhang, Y.; Zhang, S.; Zhong, F.; Liu, C.; Yin, Y.; et al. Long Noncoding RNA NRON Contributes to HIV-1 Latency by Specifically Inducing Tat Protein Degradation. Nat. Commun. 2016, 7, 11730. [CrossRef]
- Danielson, C.M.; Cianci, G.C.; Hope, T.J. Recruitment and Dynamics of Proteasome Association with rhTRIM5α Cytoplasmic Complexes during HIV-1 Infection. Traffic Cph. Den. 2012, 13, 1206–1217. [CrossRef]
- Lukic, Z.; Hausmann, S.; Sebastian, S.; Rucci, J.; Sastri, J.; Robia, S.L.; Luban, J.; Campbell, E.M. TRIM5α Associates with Proteasomal Subunits in Cells While in Complex with HIV-1 Virions. Retrovirology 2011, 8, 93. [CrossRef]
- Pyeon, D.; Timani, K.A.; Gulraiz, F.; Park, I.-W. Function of Ubiquitin (Ub) Specific Protease 15 (USP15) in HIV-1 Replication and Viral Protein Degradation. Virus Res. 2016, 223, 161–169. [CrossRef]
- Izumi, T.; Takaori-Kondo, A.; Shirakawa, K.; Higashitsuji, H.; Itoh, K.; Io, K.; Matsui, M.; Iwai, K.; Kondoh, H.; Sato, T.; et al. MDM2 Is a Novel E3 Ligase for HIV-1 Vif. Retrovirology 2009, 6, 1. [CrossRef]
- Fujita, M.; Akari, H.; Sakurai, A.; Yoshida, A.; Chiba, T.; Tanaka, K.; Strebel, K.; Adachi, A. Expression of HIV-1 Accessory Protein Vif Is Controlled Uniquely to Be Low and Optimal by Proteasome Degradation. Microbes Infect. 2004, 6, 791–798. [CrossRef]
- Zhao, L.; Wang, S.; Xu, M.; He, Y.; Zhang, X.; Xiong, Y.; Sun, H.; Ding, H.; Geng, W.; Shang, H.; et al. Vpr Counteracts the Restriction of LAPTM5 to Promote HIV-1 Infection in Macrophages. Nat. Commun. 2021, 12, 3691. [CrossRef]
- Ouyang, J.; Xiong, Y.; Shang, H.; Liang, G. LAPTM5 Restricts HIV-1 Infection in Dendritic Cells and Is Counteracted by Vpr. Microbiol. Spectr. 2022, 10, e0138221. [CrossRef]
- Mashiba, M.; Collins, D.R.; Terry, V.H.; Collins, K.L. Vpr Overcomes Macrophage-Specific Restriction of HIV-1 Env Expression and Virion Production. Cell Host Microbe 2014, 16, 722–735. [CrossRef]
- Collins, D.R.; Lubow, J.; Lukic, Z.; Mashiba, M.; Collins, K.L. Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4+ T Lymphocytes. PLOS Pathog. 2015, 11, e1005054. [CrossRef]
- Lubow, J.; Virgilio, M.C.; Merlino, M.; Collins, D.R.; Mashiba, M.; Peterson, B.G.; Lukic, Z.; Painter, M.M.; Gomez-Rivera, F.; Terry, V.; et al. Mannose Receptor Is an HIV Restriction Factor Counteracted by Vpr in Macrophages. eLife 2020, 9, e51035. [CrossRef]
- MARTíN-OROZCO, N.; ISIBASI, A.; ORTIZ-NAVARRETE, V. Macrophages Present Exogenous Antigens by Class I Major Histocompatibility Complex Molecules via a Secretory Pathway as a Consequence of Interferon-γ Activation. Immunology 2001, 103, 41–48. [CrossRef]
- Reith, W.; LeibundGut-Landmann, S.; Waldburger, J.-M. Regulation of MHC Class II Gene Expression by the Class II Transactivator. Nat. Rev. Immunol. 2005, 5, 793–806. [CrossRef]
- Zhang, N.; Bevan, M.J. CD8+ T Cells: Foot Soldiers of the Immune System. Immunity 2011, 35, 161–168. [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T Cells: Differentiation and Functions. Clin. Dev. Immunol. 2012, 2012, 925135. [CrossRef]
- Perišić Nanut, M.; Sabotič, J.; Jewett, A.; Kos, J. Cysteine Cathepsins as Regulators of the Cytotoxicity of NK and T Cells. Front. Immunol. 2014, 5, 616. [CrossRef]
- Steers, N.J.; Ratto-Kim, S.; Souza, M.S. de; Currier, J.R.; Kim, J.H.; Michael, N.L.; Alving, C.R.; Rao, M. HIV-1 Envelope Resistance to Proteasomal Cleavage: Implications for Vaccine Induced Immune Responses. PLOS ONE 2012, 7, e42579. [CrossRef]
- Tenzer, S.; Wee, E.; Burgevin, A.; Stewart-Jones, G.; Friis, L.; Lamberth, K.; Chang, C.; Harndahl, M.; Weimershaus, M.; Gerstoft, J.; et al. Antigen Processing Influences HIV-Specific Cytotoxic T Lymphocyte Immunodominance. Nat. Immunol. 2009, 10, 636–646. [CrossRef]
- Tenzer, S.; Crawford, H.; Pymm, P.; Gifford, R.; Sreenu, V.B.; Weimershaus, M.; de Oliveira, T.; Burgevin, A.; Gerstoft, J.; Akkad, N.; et al. HIV-1 Adaptation to Antigen Processing Results in Population-Level Immune Evasion and Affects Subtype Diversification. Cell Rep. 2014, 7, 448–463. [CrossRef]
- Hermida-Matsumoto, L.; Resh, M.D. Localization of Human Immunodeficiency Virus Type 1 Gag and Env at the Plasma Membrane by Confocal Imaging. J. Virol. 2000, 74, 8670–8679. [CrossRef]
- Freed, E.O. HIV-1 Assembly, Release and Maturation. Nat. Rev. Microbiol. 2015, 13, 484–496. [CrossRef]
- Ghanam, R.H.; Samal, A.B.; Fernandez, T.F.; Saad, J.S. Role of the HIV-1 Matrix Protein in Gag Intracellular Trafficking and Targeting to the Plasma Membrane for Virus Assembly. Front. Microbiol. 2012, 3. [CrossRef]
- Simonsen, A.; Wurmser, A.E.; Emr, S.D.; Stenmark, H. The Role of Phosphoinositides in Membrane Transport. Curr. Opin. Cell Biol. 2001, 13, 485–492. [CrossRef]
- Ono, A.; Ablan, S.D.; Lockett, S.J.; Nagashima, K.; Freed, E.O. Phosphatidylinositol (4,5) Bisphosphate Regulates HIV-1 Gag Targeting to the Plasma Membrane. Proc. Natl. Acad. Sci. 2004, 101, 14889–14894. [CrossRef]
- Graham, D.R.M.; Chertova, E.; Hilburn, J.M.; Arthur, L.O.; Hildreth, J.E.K. Cholesterol Depletion of Human Immunodeficiency Virus Type 1 and Simian Immunodeficiency Virus with β-Cyclodextrin Inactivates and Permeabilizes the Virions: Evidence for Virion-Associated Lipid Rafts. J. Virol. 2003, 77, 8237–8248. [CrossRef]
- Alfadhli, A.; Still, A.; Barklis, E. Analysis of Human Immunodeficiency Virus Type 1 Matrix Binding to Membranes and Nucleic Acids. J. Virol. 2009, 83, 12196–12203. [CrossRef]
- Murphy, R.E.; Saad, J.S. The Interplay between HIV-1 Gag Binding to the Plasma Membrane and Env Incorporation. Viruses 2020, 12, 548. [CrossRef]
- Udenwobele, D.I.; Su, R.-C.; Good, S.V.; Ball, T.B.; Varma Shrivastav, S.; Shrivastav, A. Myristoylation: An Important Protein Modification in the Immune Response. Front. Immunol. 2017, 8. [CrossRef]
- Göttlinger, H.G.; Sodroski, J.G.; Haseltine, W.A. Role of Capsid Precursor Processing and Myristoylation in Morphogenesis and Infectivity of Human Immunodeficiency Virus Type 1. Proc. Natl. Acad. Sci. U. S. A. 1989, 86, 5781–5785. [CrossRef]
- Bryant, M.; Ratner, L. Myristoylation-Dependent Replication and Assembly of Human Immunodeficiency Virus 1. Proc. Natl. Acad. Sci. U. S. A. 1990, 87, 523–527. [CrossRef]
- Ono, A.; Freed, E.O. Binding of Human Immunodeficiency Virus Type 1 Gag to Membrane: Role of the Matrix Amino Terminus. J. Virol. 1999, 73, 4136–4144. [CrossRef]
- Li, H.; Dou, J.; Ding, L.; Spearman, P. Myristoylation Is Required for Human Immunodeficiency Virus Type 1 Gag-Gag Multimerization in Mammalian Cells. J. Virol. 2007, 81, 12899–12910. [CrossRef]
- Paillart, J.C.; Göttlinger, H.G. Opposing Effects of Human Immunodeficiency Virus Type 1 Matrix Mutations Support a Myristyl Switch Model of Gag Membrane Targeting. J. Virol. 1999, 73, 2604–2612. [CrossRef]
- Saad, J.S.; Miller, J.; Tai, J.; Kim, A.; Ghanam, R.H.; Summers, M.F. Structural Basis for Targeting HIV-1 Gag Proteins to the Plasma Membrane for Virus Assembly. Proc. Natl. Acad. Sci. 2006, 103, 11364–11369. [CrossRef]
- Dalton, A.K.; Ako-Adjei, D.; Murray, P.S.; Murray, D.; Vogt, V.M. Electrostatic Interactions Drive Membrane Association of the Human Immunodeficiency Virus Type 1 Gag MA Domain. J. Virol. 2007, 81, 6434–6445. [CrossRef]
- Alfadhli, A.; Huseby, D.; Kapit, E.; Colman, D.; Barklis, E. Human Immunodeficiency Virus Type 1 Matrix Protein Assembles on Membranes as a Hexamer. J. Virol. 2007, 81, 1472–1478. [CrossRef]
- Willey, R.L.; Bonifacino, J.S.; Potts, B.J.; Martin, M.A.; Klausner, R.D. Biosynthesis, Cleavage, and Degradation of the Human Immunodeficiency Virus 1 Envelope Glycoprotein Gp160. Proc. Natl. Acad. Sci. 1988, 85, 9580–9584. [CrossRef]
- Qi, M.; Williams, J.A.; Chu, H.; Chen, X.; Wang, J.-J.; Ding, L.; Akhirome, E.; Wen, X.; Lapierre, L.A.; Goldenring, J.R.; et al. Rab11-FIP1C and Rab14 Direct Plasma Membrane Sorting and Particle Incorporation of the HIV-1 Envelope Glycoprotein Complex. PLoS Pathog. 2013, 9, e1003278. [CrossRef]
- Postler, T.S.; Desrosiers, R.C. The Tale of the Long Tail: The Cytoplasmic Domain of HIV-1 Gp41. J. Virol. 2013, 87, 2–15. [CrossRef]
- Wyss, S.; Berlioz-Torrent, C.; Boge, M.; Blot, G.; Höning, S.; Benarous, R.; Thali, M. The Highly Conserved C-Terminal Dileucine Motif in the Cytosolic Domain of the Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Is Critical for Its Association with the AP-1 Clathrin Adapter. J. Virol. 2001, 75, 2982–2992. [CrossRef]
- Checkley, M.A.; Luttge, B.G.; Freed, E.O. HIV-1 Envelope Glycoprotein Biosynthesis, Trafficking, and Incorporation. J. Mol. Biol. 2011, 410, 582–608. [CrossRef]
- Murakami, T.; Freed, E.O. Genetic Evidence for an Interaction between Human Immunodeficiency Virus Type 1 Matrix and Alpha-Helix 2 of the Gp41 Cytoplasmic Tail. J. Virol. 2000, 74, 3548–3554. [CrossRef]
- Gousset, K.; Ablan, S.D.; Coren, L.V.; Ono, A.; Soheilian, F.; Nagashima, K.; Ott, D.E.; Freed, E.O. Real-Time Visualization of HIV-1 GAG Trafficking in Infected Macrophages. PLOS Pathog. 2008, 4, e1000015. [CrossRef]
- Raposo, G.; Moore, M.; Innes, D.; Leijendekker, R.; Leigh-Brown, A.; Benaroch, P.; Geuze, H. Human Macrophages Accumulate HIV-1 Particles in MHC II Compartments. Traffic Cph. Den. 2002, 3, 718–729. [CrossRef]
- Pelchen-Matthews, A.; Kramer, B.; Marsh, M. Infectious HIV-1 Assembles in Late Endosomes in Primary Macrophages. J. Cell Biol. 2003, 162, 443–455. [CrossRef]
- Ono, A.; Freed, E.O. Cell-Type-Dependent Targeting of Human Immunodeficiency Virus Type 1 Assembly to the Plasma Membrane and the Multivesicular Body. J. Virol. 2004, 78, 1552–1563. [CrossRef]
- Deneka, M.; Pelchen-Matthews, A.; Byland, R.; Ruiz-Mateos, E.; Marsh, M. In Macrophages, HIV-1 Assembles into an Intracellular Plasma Membrane Domain Containing the Tetraspanins CD81, CD9, and CD53. J. Cell Biol. 2007, 177, 329–341. [CrossRef]
- Tan, J.; Sattentau, Q.J. The HIV-1-Containing Macrophage Compartment: A Perfect Cellular Niche? Trends Microbiol. 2013, 21, 405–412. [CrossRef]
- Lerner, G.; Weaver, N.; Anokhin, B.; Spearman, P. Advances in HIV-1 Assembly. Viruses 2022, 14, 478. [CrossRef]
- Bennett, A.E.; Narayan, K.; Shi, D.; Hartnell, L.M.; Gousset, K.; He, H.; Lowekamp, B.C.; Yoo, T.S.; Bliss, D.; Freed, E.O.; et al. Ion-Abrasion Scanning Electron Microscopy Reveals Surface-Connected Tubular Conduits in HIV-Infected Macrophages. PLOS Pathog. 2009, 5, e1000591. [CrossRef]
- Hammonds, J.E.; Beeman, N.; Ding, L.; Takushi, S.; Francis, A.C.; Wang, J.-J.; Melikyan, G.B.; Spearman, P. Siglec-1 Initiates Formation of the Virus-Containing Compartment and Enhances Macrophage-to-T Cell Transmission of HIV-1. PLOS Pathog. 2017, 13, e1006181. [CrossRef]
- Groot, F.; Welsch, S.; Sattentau, Q.J. Efficient HIV-1 Transmission from Macrophages to T Cells across Transient Virological Synapses. Blood 2008, 111, 4660–4663. [CrossRef]
- Chu, H.; Wang, J.-J.; Qi, M.; Yoon, J.-J.; Chen, X.; Wen, X.; Hammonds, J.; Ding, L.; Spearman, P. Tetherin/BST-2 Is Essential for the Formation of the Intracellular Virus-Containing Compartment in HIV-Infected Macrophages. Cell Host Microbe 2012, 12, 360–372. [CrossRef]
- Mlcochova, P.; Pelchen-Matthews, A.; Marsh, M. Organization and Regulation of Intracellular Plasma Membrane-Connected HIV-1 Assembly Compartments in Macrophages. BMC Biol. 2013, 11, 89. [CrossRef]
- Mariani, C.; Desdouits, M.; Favard, C.; Benaroch, P.; Muriaux, D.M. Role of Gag and Lipids during HIV-1 Assembly in CD4+ T Cells and Macrophages. Front. Microbiol. 2014, 5. [CrossRef]
- Hogue, I.B.; Grover, J.R.; Soheilian, F.; Nagashima, K.; Ono, A. Gag Induces the Coalescence of Clustered Lipid Rafts and Tetraspanin-Enriched Microdomains at HIV-1 Assembly Sites on the Plasma Membrane. J. Virol. 2011, 85, 9749–9766. [CrossRef]
- Ono, A.; Freed, E.O. Plasma Membrane Rafts Play a Critical Role in HIV-1 Assembly and Release. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 13925–13930. [CrossRef]
- Zimmerman, C.; Klein, K.C.; Kiser, P.K.; Singh, A.R.; Firestein, B.L.; Riba, S.C.; Lingappa, J.R. Identification of a Host Protein Essential for Assembly of Immature HIV-1 Capsids. Nature 2002, 415, 88–92. [CrossRef]
- Lingappa, J.R.; Dooher, J.E.; Newman, M.A.; Kiser, P.K.; Klein, K.C. Basic Residues in the Nucleocapsid Domain of Gag Are Required for Interaction of HIV-1 Gag with ABCE1 (HP68), a Cellular Protein Important for HIV-1 Capsid Assembly. J. Biol. Chem. 2006, 281, 3773–3784. [CrossRef]
- Dooher, J.E.; Schneider, B.L.; Reed, J.C.; Lingappa, J.R. Host ABCE1 Is at Plasma Membrane HIV Assembly Sites and Its Dissociation from Gag Is Linked to Subsequent Events of Virus Production. Traffic Cph. Den. 2007, 8, 195–211. [CrossRef]
- Chatel-Chaix, L.; Abrahamyan, L.; Fréchina, C.; Mouland, A.J.; DesGroseillers, L. The Host Protein Staufen1 Participates in Human Immunodeficiency Virus Type 1 Assembly in Live Cells by Influencing pr55Gag Multimerization. J. Virol. 2007, 81, 6216–6230. [CrossRef]
- Chatel-Chaix, L.; Boulay, K.; Mouland, A.J.; DesGroseillers, L. The Host Protein Staufen1 Interacts with the Pr55Gagzinc Fingers and Regulates HIV-1 Assembly via Its N-Terminus. Retrovirology 2008, 5, 41. [CrossRef]
- Mouland, A.J.; Mercier, J.; Luo, M.; Bernier, L.; DesGroseillers, L.; Cohen, E.A. The Double-Stranded RNA-Binding Protein Staufen Is Incorporated in Human Immunodeficiency Virus Type 1: Evidence for a Role in Genomic RNA Encapsidation. J. Virol. 2000, 74, 5441–5451. [CrossRef]
- Dick, R.A.; Xu, C.; Morado, D.R.; Kravchuk, V.; Ricana, C.L.; Lyddon, T.D.; Broad, A.M.; Feathers, J.R.; Johnson, M.C.; Vogt, V.M.; et al. Structures of Immature EIAV Gag Lattices Reveal a Conserved Role for IP6 in Lentivirus Assembly. PLoS Pathog. 2020, 16, e1008277. [CrossRef]
- Dick, R.A.; Zadrozny, K.K.; Xu, C.; Schur, F.K.M.; Lyddon, T.D.; Ricana, C.L.; Wagner, J.M.; Perilla, J.R.; Ganser-Pornillos, B.K.; Johnson, M.C.; et al. Inositol Phosphates Are Assembly Co-Factors for HIV-1. Nature 2018, 560, 509–512. [CrossRef]
- Nikolaitchik, O.A.; Dilley, K.A.; Fu, W.; Gorelick, R.J.; Tai, S.-H.S.; Soheilian, F.; Ptak, R.G.; Nagashima, K.; Pathak, V.K.; Hu, W.-S. Dimeric RNA Recognition Regulates HIV-1 Genome Packaging. PLoS Pathog. 2013, 9, e1003249. [CrossRef]
- Levin, J.G.; Guo, J.; Rouzina, I.; Musier-Forsyth, K. Nucleic Acid Chaperone Activity of HIV-1 Nucleocapsid Protein: Critical Role in Reverse Transcription and Molecular Mechanism. Prog. Nucleic Acid Res. Mol. Biol. 2005, 80, 217–286. [CrossRef]
- Dorfman, T.; Luban, J.; Goff, S.P.; Haseltine, W.A.; Göttlinger, H.G. Mapping of Functionally Important Residues of a Cysteine-Histidine Box in the Human Immunodeficiency Virus Type 1 Nucleocapsid Protein. J. Virol. 1993, 67, 6159–6169. [CrossRef]
- Muriaux, D.; Darlix, J.-L. Properties and Functions of the Nucleocapsid Protein in Virus Assembly. RNA Biol. 2010, 7, 744–753. [CrossRef]
- Bacharach, E.; Gonsky, J.; Alin, K.; Orlova, M.; Goff, S.P. The Carboxy-Terminal Fragment of Nucleolin Interacts with the Nucleocapsid Domain of Retroviral Gag Proteins and Inhibits Virion Assembly. J. Virol. 2000, 74, 11027–11039. [CrossRef]
- Ueno, T.; Tokunaga, K.; Sawa, H.; Maeda, M.; Chiba, J.; Kojima, A.; Hasegawa, H.; Shoya, Y.; Sata, T.; Kurata, T.; et al. Nucleolin and the Packaging Signal, ψ, Promote the Budding of Human Immunodeficiency Virus Type-1 (HIV-1). Microbiol. Immunol. 2004, 48, 111–118. [CrossRef]
- Cohen, E.A.; Dehni, G.; Sodroski, J.G.; Haseltine, W.A. Human Immunodeficiency Virus Vpr Product Is a Virion-Associated Regulatory Protein. J. Virol. 1990, 64, 3097–3099. [CrossRef]
- Kao, S.; Akari, H.; Khan, M.A.; Dettenhofer, M.; Yu, X.-F.; Strebel, K. Human Immunodeficiency Virus Type 1 Vif Is Efficiently Packaged into Virions during Productive but Not Chronic Infection. J. Virol. 2003, 77, 1131–1140. [CrossRef]
- Khan, M.A.; Akari, H.; Kao, S.; Aberham, C.; Davis, D.; Buckler-White, A.; Strebel, K. Intravirion Processing of the Human Immunodeficiency Virus Type 1 Vif Protein by the Viral Protease May Be Correlated with Vif Function. J. Virol. 2002, 76, 9112–9123. [CrossRef]
- Bukovsky, A.A.; Dorfman, T.; Weimann, A.; Göttlinger, H.G. Nef Association with Human Immunodeficiency Virus Type 1 Virions and Cleavage by the Viral Protease. J. Virol. 1997, 71, 1013–1018. [CrossRef]
- Forshey, B.M.; Aiken, C. Disassembly of Human Immunodeficiency Virus Type 1 Cores in Vitro Reveals Association of Nef with the Subviral Ribonucleoprotein Complex. J. Virol. 2003, 77, 4409–4414. [CrossRef]
- Santos, S.; Obukhov, Y.; Nekhai, S.; Bukrinsky, M.; Iordanskiy, S. Virus-Producing Cells Determine the Host Protein Profiles of HIV-1 Virion Cores. Retrovirology 2012, 9, 65. [CrossRef]
- Burnie, J.; Guzzo, C. The Incorporation of Host Proteins into the External HIV-1 Envelope. Viruses 2019, 11, 85. [CrossRef]
- Orecchini, E.; Federico, M.; Doria, M.; Arenaccio, C.; Giuliani, E.; Ciafrè, S.A.; Michienzi, A. The ADAR1 Editing Enzyme Is Encapsidated into HIV-1 Virions. Virology 2015, 485, 475–480. [CrossRef]
- Göttlinger, H.G.; Dorfman, T.; Sodroski, J.G.; Haseltine, W.A. Effect of Mutations Affecting the P6 Gag Protein on Human Immunodeficiency Virus Particle Release. Proc. Natl. Acad. Sci. U. S. A. 1991, 88, 3195–3199. [CrossRef]
- Huang, M.; Orenstein, J.M.; Martin, M.A.; Freed, E.O. p6Gag Is Required for Particle Production from Full-Length Human Immunodeficiency Virus Type 1 Molecular Clones Expressing Protease. J. Virol. 1995, 69, 6810–6818. [CrossRef]
- Nguyen, D.G.; Booth, A.; Gould, S.J.; Hildreth, J.E.K. Evidence That HIV Budding in Primary Macrophages Occurs through the Exosome Release Pathway. J. Biol. Chem. 2003, 278, 52347–52354. [CrossRef]
- Votteler, J.; Sundquist, W.I. Virus Budding and the ESCRT Pathway. Cell Host Microbe 2013, 14, 10.1016/j.chom.2013.08.012. [CrossRef]
- Raiborg, C.; Stenmark, H. The ESCRT Machinery in Endosomal Sorting of Ubiquitylated Membrane Proteins. Nature 2009, 458, 445–452. [CrossRef]
- VerPlank, L.; Bouamr, F.; LaGrassa, T.J.; Agresta, B.; Kikonyogo, A.; Leis, J.; Carter, C.A. Tsg101, a Homologue of Ubiquitin-Conjugating (E2) Enzymes, Binds the L Domain in HIV Type 1 Pr55(Gag). Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 7724–7729. [CrossRef]
- Garrus, J.E.; von Schwedler, U.K.; Pornillos, O.W.; Morham, S.G.; Zavitz, K.H.; Wang, H.E.; Wettstein, D.A.; Stray, K.M.; Côté, M.; Rich, R.L.; et al. Tsg101 and the Vacuolar Protein Sorting Pathway Are Essential for HIV-1 Budding. Cell 2001, 107, 55–65. [CrossRef]
- Demirov, D.G.; Ono, A.; Orenstein, J.M.; Freed, E.O. Overexpression of the N-Terminal Domain of TSG101 Inhibits HIV-1 Budding by Blocking Late Domain Function. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 955–960. [CrossRef]
- Martin-Serrano, J.; Zang, T.; Bieniasz, P.D. HIV-1 and Ebola Virus Encode Small Peptide Motifs That Recruit Tsg101 to Sites of Particle Assembly to Facilitate Egress. Nat. Med. 2001, 7, 1313–1319. [CrossRef]
- Joshi, A.; Munshi, U.; Ablan, S.D.; Nagashima, K.; Freed, E.O. Functional Replacement of a Retroviral Late Domain by Ubiquitin Fusion. Traffic Cph. Den. 2008, 9, 1972–1983. [CrossRef]
- Bleck, M.; Itano, M.S.; Johnson, D.S.; Thomas, V.K.; North, A.J.; Bieniasz, P.D.; Simon, S.M. Temporal and Spatial Organization of ESCRT Protein Recruitment during HIV-1 Budding. Proc. Natl. Acad. Sci. 2014, 111, 12211–12216. [CrossRef]
- Sette, P.; Nagashima, K.; Piper, R.C.; Bouamr, F. Ubiquitin Conjugation to Gag Is Essential for ESCRT-Mediated HIV-1 Budding. Retrovirology 2013, 10, 79. [CrossRef]
- Martin-Serrano, J.; Neil, S.J.D. Host Factors Involved in Retroviral Budding and Release. Nat. Rev. Microbiol. 2011, 9, 519–531. [CrossRef]
- Schwedler, U.K. von; Stuchell, M.; Müller, B.; Ward, D.M.; Chung, H.-Y.; Morita, E.; Wang, H.E.; Davis, T.; He, G.-P.; Cimbora, D.M.; et al. The Protein Network of HIV Budding. Cell 2003, 114, 701–713. [CrossRef]
- Pineda-Molina, E.; Belrhali, H.; Piefer, A.J.; Akula, I.; Bates, P.; Weissenhorn, W. The Crystal Structure of the C-Terminal Domain of Vps28 Reveals a Conserved Surface Required for Vps20 Recruitment. Traffic 2006, 7, 1007–1016. [CrossRef]
- Langelier, C.; von Schwedler, U.K.; Fisher, R.D.; De Domenico, I.; White, P.L.; Hill, C.P.; Kaplan, J.; Ward, D.; Sundquist, W.I. Human ESCRT-II Complex and Its Role in Human Immunodeficiency Virus Type 1 Release. J. Virol. 2006, 80, 9465–9480. [CrossRef]
- Strack, B.; Calistri, A.; Craig, S.; Popova, E.; Göttlinger, H.G. AIP1/ALIX Is a Binding Partner for HIV-1 P6 and EIAV P9 Functioning in Virus Budding. Cell 2003, 114, 689–699. [CrossRef]
- Fisher, R.D.; Chung, H.-Y.; Zhai, Q.; Robinson, H.; Sundquist, W.I.; Hill, C.P. Structural and Biochemical Studies of ALIX/AIP1 and Its Role in Retrovirus Budding. Cell 2007, 128, 841–852. [CrossRef]
- Fujii, K.; Munshi, U.M.; Ablan, S.D.; Demirov, D.G.; Soheilian, F.; Nagashima, K.; Stephen, A.G.; Fisher, R.J.; Freed, E.O. Functional Role of Alix in Hiv-1 Replication. Virology 2009, 391, 284–292. [CrossRef]
- Gupta, S.; Bendjennat, M.; Saffarian, S. Abrogating ALIX Interactions Results in Stuttering of the ESCRT Machinery. Viruses 2020, 12, 1032. [CrossRef]
- Popov, S.; Popova, E.; Inoue, M.; Göttlinger, H.G. Human Immunodeficiency Virus Type 1 Gag Engages the Bro1 Domain of ALIX/AIP1 through the Nucleocapsid. J. Virol. 2008, 82, 1389–1398. [CrossRef]
- Dussupt, V.; Javid, M.P.; Abou-Jaoudé, G.; Jadwin, J.A.; Cruz, J. de L.; Nagashima, K.; Bouamr, F. The Nucleocapsid Region of HIV-1 Gag Cooperates with the PTAP and LYPXnL Late Domains to Recruit the Cellular Machinery Necessary for Viral Budding. PLOS Pathog. 2009, 5, e1000339. [CrossRef]
- Chung, H.-Y.; Morita, E.; von Schwedler, U.; Müller, B.; Kräusslich, H.-G.; Sundquist, W.I. NEDD4L Overexpression Rescues the Release and Infectivity of Human Immunodeficiency Virus Type 1 Constructs Lacking PTAP and YPXL Late Domains. J. Virol. 2008, 82, 4884–4897. [CrossRef]
- Mercenne, G.; Alam, S.L.; Arii, J.; Lalonde, M.S.; Sundquist, W.I. Angiomotin Functions in HIV-1 Assembly and Budding. eLife 2015, 4, e03778. [CrossRef]
- Rheinemann, L.; Thompson, T.; Mercenne, G.; Paine, E.L.; Peterson, F.C.; Volkman, B.F.; Alam, S.L.; Alian, A.; Sundquist, W.I. Interactions between AMOT PPxY Motifs and NEDD4L WW Domains Function in HIV-1 Release. J. Biol. Chem. 2021, 297, 100975. [CrossRef]
- Hurley, J.H.; Hanson, P.I. Membrane Budding and Scission by the ESCRT Machinery: It’s All in the Neck. Nat. Rev. Mol. Cell Biol. 2010, 11, 556–566. [CrossRef]
- Morita, E.; Sandrin, V.; McCullough, J.; Katsuyama, A.; Hamilton, I.B.; Sundquist, W.I. ESCRT-III Protein Requirements for HIV-1 Budding. Cell Host Microbe 2011, 9, 235–242. [CrossRef]
- Babst, M.; Wendland, B.; Estepa, E.J.; Emr, S.D. The Vps4p AAA ATPase Regulates Membrane Association of a Vps Protein Complex Required for Normal Endosome Function. EMBO J. 1998, 17, 2982–2993. [CrossRef]
- Lata, S.; Schoehn, G.; Jain, A.; Pires, R.; Piehler, J.; Gottlinger, H.G.; Weissenhorn, W. Helical Structures of ESCRT-III Are Disassembled by VPS4. Science 2008, 321, 1354–1357. [CrossRef]
- Wollert, T.; Wunder, C.; Lippincott-Schwartz, J.; Hurley, J.H. Membrane Scission by the ESCRT-III Complex. Nature 2009, 458, 172–177. [CrossRef]
- Watanabe, T.; Wang, S.; Kaibuchi, K. IQGAPs as Key Regulators of Actin-Cytoskeleton Dynamics. Cell Struct. Funct. 2015, 40, 69–77. [CrossRef]
- Abel, A.M.; Schuldt, K.M.; Rajasekaran, K.; Hwang, D.; Riese, M.J.; Rao, S.; Thakar, M.S.; Malarkannan, S. IQGAP1: Insights into the Function of a Molecular Puppeteer. Mol. Immunol. 2015, 65, 336–349. [CrossRef]
- Sabo, Y.; Santos, K. de los; Goff, S.P. IQGAP1 Negatively Regulates HIV-1 Gag Trafficking and Virion Production. Cell Rep. 2020, 30, 4065-4081.e4. [CrossRef]
- Barr, S.D.; Smiley, J.R.; Bushman, F.D. The Interferon Response Inhibits HIV Particle Production by Induction of TRIM22. PLoS Pathog. 2008, 4, e1000007. [CrossRef]
- Okumura, A.; Lu, G.; Pitha-Rowe, I.; Pitha, P.M. Innate Antiviral Response Targets HIV-1 Release by the Induction of Ubiquitin-like Protein ISG15. Proc. Natl. Acad. Sci. 2006, 103, 1440–1445. [CrossRef]
- Pincetic, A.; Kuang, Z.; Seo, E.J.; Leis, J. The Interferon-Induced Gene ISG15 Blocks Retrovirus Release from Cells Late in the Budding Process. J. Virol. 2010, 84, 4725–4736. [CrossRef]
- Woods, M.W.; Kelly, J.N.; Hattlmann, C.J.; Tong, J.G.; Xu, L.S.; Coleman, M.D.; Quest, G.R.; Smiley, J.R.; Barr, S.D. Human HERC5 Restricts an Early Stage of HIV-1 Assembly by a Mechanism Correlating with the ISGylation of Gag. Retrovirology 2011, 8, 95. [CrossRef]
- Umetsu, S.E.; Lee, W.-L.; McIntire, J.J.; Downey, L.; Sanjanwala, B.; Akbari, O.; Berry, G.J.; Nagumo, H.; Freeman, G.J.; Umetsu, D.T.; et al. TIM-1 Induces T Cell Activation and Inhibits the Development of Peripheral Tolerance. Nat. Immunol. 2005, 6, 447–454. [CrossRef]
- Miyanishi, M.; Tada, K.; Koike, M.; Uchiyama, Y.; Kitamura, T.; Nagata, S. Identification of Tim4 as a Phosphatidylserine Receptor. Nature 2007, 450, 435–439. [CrossRef]
- Kobayashi, N.; Karisola, P.; Peña-Cruz, V.; Dorfman, D.M.; Jinushi, M.; Umetsu, S.E.; Butte, M.J.; Nagumo, H.; Chernova, I.; Zhu, B.; et al. TIM-1 and TIM-4 Glycoproteins Bind Phosphatidylserine and Mediate Uptake of Apoptotic Cells. Immunity 2007, 27, 927–940. [CrossRef]
- Li, M.; Ablan, S.D.; Miao, C.; Zheng, Y.-M.; Fuller, M.S.; Rennert, P.D.; Maury, W.; Johnson, M.C.; Freed, E.O.; Liu, S.-L. TIM-Family Proteins Inhibit HIV-1 Release. Proc. Natl. Acad. Sci. 2014, 111, E3699–E3707. [CrossRef]
- Li, M.; Waheed, A.A.; Yu, J.; Zeng, C.; Chen, H.-Y.; Zheng, Y.-M.; Feizpour, A.; Reinhard, B.M.; Gummuluru, S.; Lin, S.; et al. TIM-Mediated Inhibition of HIV-1 Release Is Antagonized by Nef but Potentiated by SERINC Proteins. Proc. Natl. Acad. Sci. 2019, 116, 5705–5714. [CrossRef]
- Nasr, N.; Maddocks, S.; Turville, S.G.; Harman, A.N.; Woolger, N.; Helbig, K.J.; Wilkinson, J.; Bye, C.R.; Wright, T.K.; Rambukwelle, D.; et al. HIV-1 Infection of Human Macrophages Directly Induces Viperin Which Inhibits Viral Production. Blood 2012, 120, 778–788. [CrossRef]
- Wang, X.; Hinson, E.R.; Cresswell, P. The Interferon-Inducible Protein Viperin Inhibits Influenza Virus Release by Perturbing Lipid Rafts. Cell Host Microbe 2007, 2, 96–105. [CrossRef]
- Lim, E.S.; Wu, L.I.; Malik, H.S.; Emerman, M. The Function and Evolution of the Restriction Factor Viperin in Primates Was Not Driven by Lentiviruses. Retrovirology 2012, 9, 55. [CrossRef]
- McLaren, P.J.; Gawanbacht, A.; Pyndiah, N.; Krapp, C.; Hotter, D.; Kluge, S.F.; Götz, N.; Heilmann, J.; Mack, K.; Sauter, D.; et al. Identification of Potential HIV Restriction Factors by Combining Evolutionary Genomic Signatures with Functional Analyses. Retrovirology 2015, 12, 41. [CrossRef]
- Krapp, C.; Hotter, D.; Gawanbacht, A.; McLaren, P.J.; Kluge, S.F.; Stürzel, C.M.; Mack, K.; Reith, E.; Engelhart, S.; Ciuffi, A.; et al. Guanylate Binding Protein (GBP) 5 Is an Interferon-Inducible Inhibitor of HIV-1 Infectivity. Cell Host Microbe 2016, 19, 504–514. [CrossRef]
- Braun, E.; Hotter, D.; Koepke, L.; Zech, F.; Groß, R.; Sparrer, K.M.J.; Müller, J.A.; Pfaller, C.K.; Heusinger, E.; Wombacher, R.; et al. Guanylate-Binding Proteins 2 and 5 Exert Broad Antiviral Activity by Inhibiting Furin-Mediated Processing of Viral Envelope Proteins. Cell Rep. 2019, 27, 2092-2104.e10. [CrossRef]
- Sauter, D.; Hotter, D.; Van Driessche, B.; Stürzel, C.M.; Kluge, S.F.; Wildum, S.; Yu, H.; Baumann, B.; Wirth, T.; Plantier, J.-C.; et al. Differential Regulation of NF-κB-Mediated Proviral and Antiviral Host Gene Expression by Primate Lentiviral Nef and Vpu Proteins. Cell Rep. 2015, 10, 586–599. [CrossRef]
- Schubert, U.; Bour, S.; Willey, R.L.; Strebel, K. Regulation of Virus Release by the Macrophage-Tropic Human Immunodeficiency Virus Type 1 AD8 Isolate Is Redundant and Can Be Controlled by Either Vpu or Env. J. Virol. 1999, 73, 887–896. [CrossRef]
- Tada, T.; Zhang, Y.; Koyama, T.; Tobiume, M.; Tsunetsugu-Yokota, Y.; Yamaoka, S.; Fujita, H.; Tokunaga, K. MARCH8 Inhibits HIV-1 Infection by Reducing Virion Incorporation of Envelope Glycoproteins. Nat. Med. 2015, 21, 1502–1507. [CrossRef]
- Eyster, C.A.; Cole, N.B.; Petersen, S.; Viswanathan, K.; Früh, K.; Donaldson, J.G. MARCH Ubiquitin Ligases Alter the Itinerary of Clathrin-Independent Cargo from Recycling to Degradation. Mol. Biol. Cell 2011, 22, 3218–3230. [CrossRef]
- Chen, R.; Li, M.; Zhang, Y.; Zhou, Q.; Shu, H.-B. The E3 Ubiquitin Ligase MARCH8 Negatively Regulates IL-1β-Induced NF-κB Activation by Targeting the IL1RAP Coreceptor for Ubiquitination and Degradation. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 14128–14133. [CrossRef]
- Tada, T.; Zhang, Y.; Fujita, H.; Tokunaga, K. MARCH8: The Tie That Binds to Viruses. FEBS J. 2022, 289, 3642–3654. [CrossRef]
- Zhang, Y.; Tada, T.; Ozono, S.; Yao, W.; Tanaka, M.; Yamaoka, S.; Kishigami, S.; Fujita, H.; Tokunaga, K. Membrane-Associated RING-CH (MARCH) 1 and 2 Are MARCH Family Members That Inhibit HIV-1 Infection. J. Biol. Chem. 2019, 294, 3397–3405. [CrossRef]
- Zhang, Y.; Lu, J.; Liu, X. MARCH2 Is Upregulated in HIV-1 Infection and Inhibits HIV-1 Production through Envelope Protein Translocation or Degradation. Virology 2018, 518, 293–300. [CrossRef]
- Van Damme, N.; Goff, D.; Katsura, C.; Jorgenson, R.L.; Mitchell, R.; Johnson, M.; Stephens, E.B.; Guatelli, J. The Interferon-Induced Protein BST-2/CD317 Restricts Release of Virions from Infected Cells and Is down-Regulated from the Cell Surface by HIV-1 Vpu. Cell Host Microbe 2008, 3, 245–252. [CrossRef]
- Neil, S.J.D.; Zang, T.; Bieniasz, P.D. Tetherin Inhibits Retrovirus Release and Is Antagonized by HIV-1 Vpu. Nature 2008, 451, 425–430. [CrossRef]
- Fitzpatrick, K.; Skasko, M.; Deerinck, T.J.; Crum, J.; Ellisman, M.H.; Guatelli, J. Direct Restriction of Virus Release and Incorporation of the Interferon-Induced Protein BST-2 into HIV-1 Particles. PLoS Pathog. 2010, 6, e1000701. [CrossRef]
- Perez-Caballero, D.; Zang, T.; Ebrahimi, A.; McNatt, M.W.; Gregory, D.A.; Johnson, M.C.; Bieniasz, P.D. Tetherin Inhibits HIV-1 Release by Directly Tethering Virions to Cells. Cell 2009, 139, 499–511. [CrossRef]
- Kupzig, S.; Korolchuk, V.; Rollason, R.; Sugden, A.; Wilde, A.; Banting, G. Bst-2/HM1.24 Is a Raft-Associated Apical Membrane Protein with an Unusual Topology. Traffic Cph. Den. 2003, 4, 694–709. [CrossRef]
- Hinz, A.; Miguet, N.; Natrajan, G.; Usami, Y.; Yamanaka, H.; Renesto, P.; Hartlieb, B.; McCarthy, A.A.; Simorre, J.-P.; Göttlinger, H.; et al. Structural Basis of HIV-1 Tethering to Membranes by the BST-2/Tetherin Ectodomain. Cell Host Microbe 2010, 7, 314–323. [CrossRef]
- Venkatesh, S.; Bieniasz, P.D. Mechanism of HIV-1 Virion Entrapment by Tetherin. PLOS Pathog. 2013, 9, e1003483. [CrossRef]
- Jolly, C.; Booth, N.J.; Neil, S.J.D. Cell-Cell Spread of Human Immunodeficiency Virus Type 1 Overcomes Tetherin/BST-2-Mediated Restriction in T Cells. J. Virol. 2010, 84, 12185–12199. [CrossRef]
- Galão, R.P.; Le Tortorec, A.; Pickering, S.; Kueck, T.; Neil, S.J.D. Innate Sensing of HIV-1 Assembly by Tetherin Induces NFκB-Dependent Proinflammatory Responses. Cell Host Microbe 2012, 12, 633–644. [CrossRef]
- Hotter, D.; Sauter, D.; Kirchhoff, F. Emerging Role of the Host Restriction Factor Tetherin in Viral Immune Sensing. J. Mol. Biol. 2013, 425, 4956–4964. [CrossRef]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS Pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [CrossRef]
- Todd, S.; Laboissière, M.C.; Craik, C.S. Yeast Two-Hybrid Assay for Examining Human Immunodeficiency Virus Protease Heterodimer Formation with Dominant-Negative Inhibitors and Multidrug-Resistant Variants. Anal. Biochem. 2000, 277, 247–253. [CrossRef]
- Danielson, C.M.; Hope, T.J. Imaging of HIV/Host Protein Interactions. Curr. Top. Microbiol. Immunol. 2009, 339, 103–123. [CrossRef]
- Saffarian, S. Application of Advanced Light Microscopy to the Study of HIV and Its Interactions with the Host. Viruses 2021, 13, 223. [CrossRef]
- Luo, Y.; Muesing, M.A. Mass Spectrometry-Based Proteomic Approaches for Discovery of HIV–Host Interactions. Future Virol. 2014, 9, 979–992. [CrossRef]
- Knoener, R.A.; Becker, J.T.; Scalf, M.; Sherer, N.M.; Smith, L.M. Elucidating the in Vivo Interactome of HIV-1 RNA by Hybridization Capture and Mass Spectrometry. Sci. Rep. 2017, 7, 16965. [CrossRef]
- van Manen, D.; van ‘t Wout, A.B.; Schuitemaker, H. Genome-Wide Association Studies on HIV Susceptibility, Pathogenesis and Pharmacogenomics. Retrovirology 2012, 9, 70. [CrossRef]
- Montoya, V.R.; Ready, T.M.; Felton, A.; Fine, S.R.; OhAinle, M.; Emerman, M. A Virus-Packageable CRISPR System Identifies Host Dependency Factors Co-Opted by Multiple HIV-1 Strains. mBio 2023, 14, e0000923. [CrossRef]
- Deshiere, A.; Joly-Beauparlant, C.; Breton, Y.; Ouellet, M.; Raymond, F.; Lodge, R.; Barat, C.; Roy, M.-A.; Corbeil, J.; Tremblay, M.J. Global Mapping of the Macrophage-HIV-1 Transcriptome Reveals That Productive Infection Induces Remodeling of Host Cell DNA and Chromatin. Sci. Rep. 2017, 7, 5238. [CrossRef]
- Zhou, H.; Xu, M.; Huang, Q.; Gates, A.T.; Zhang, X.D.; Castle, J.C.; Stec, E.; Ferrer, M.; Strulovici, B.; Hazuda, D.J.; et al. Genome-Scale RNAi Screen for Host Factors Required for HIV Replication. Cell Host Microbe 2008, 4, 495–504. [CrossRef]
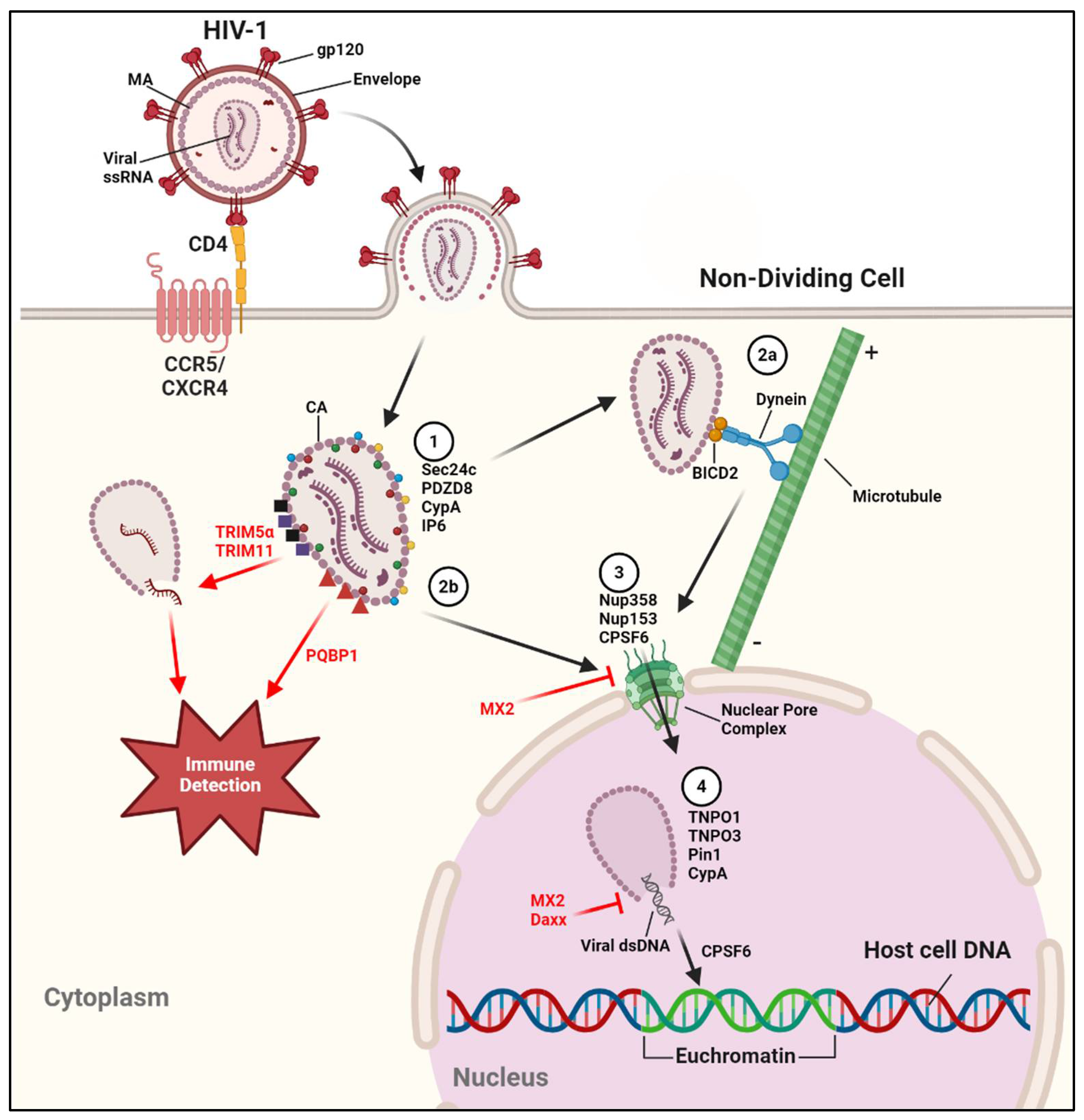
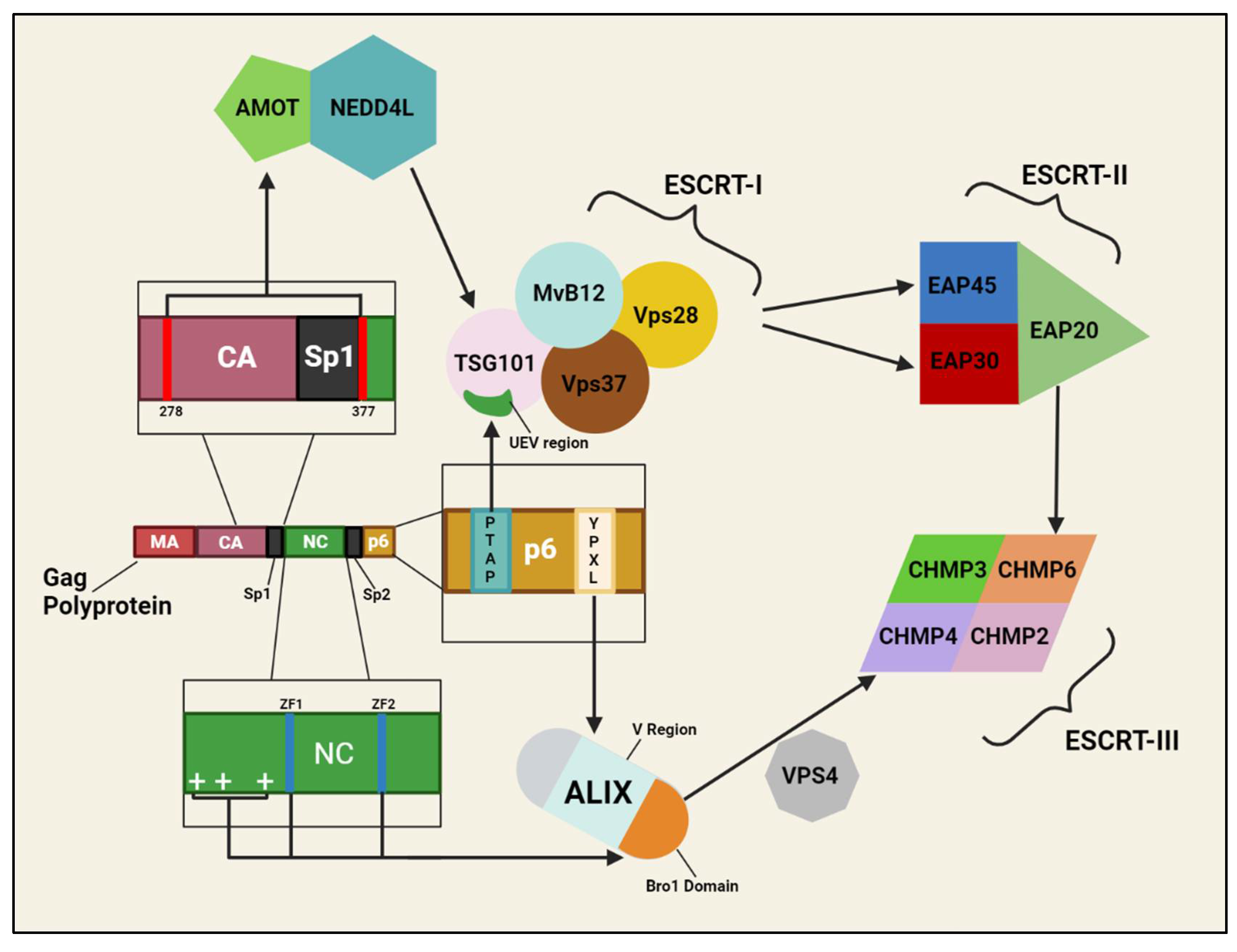
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




