Submitted:
24 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
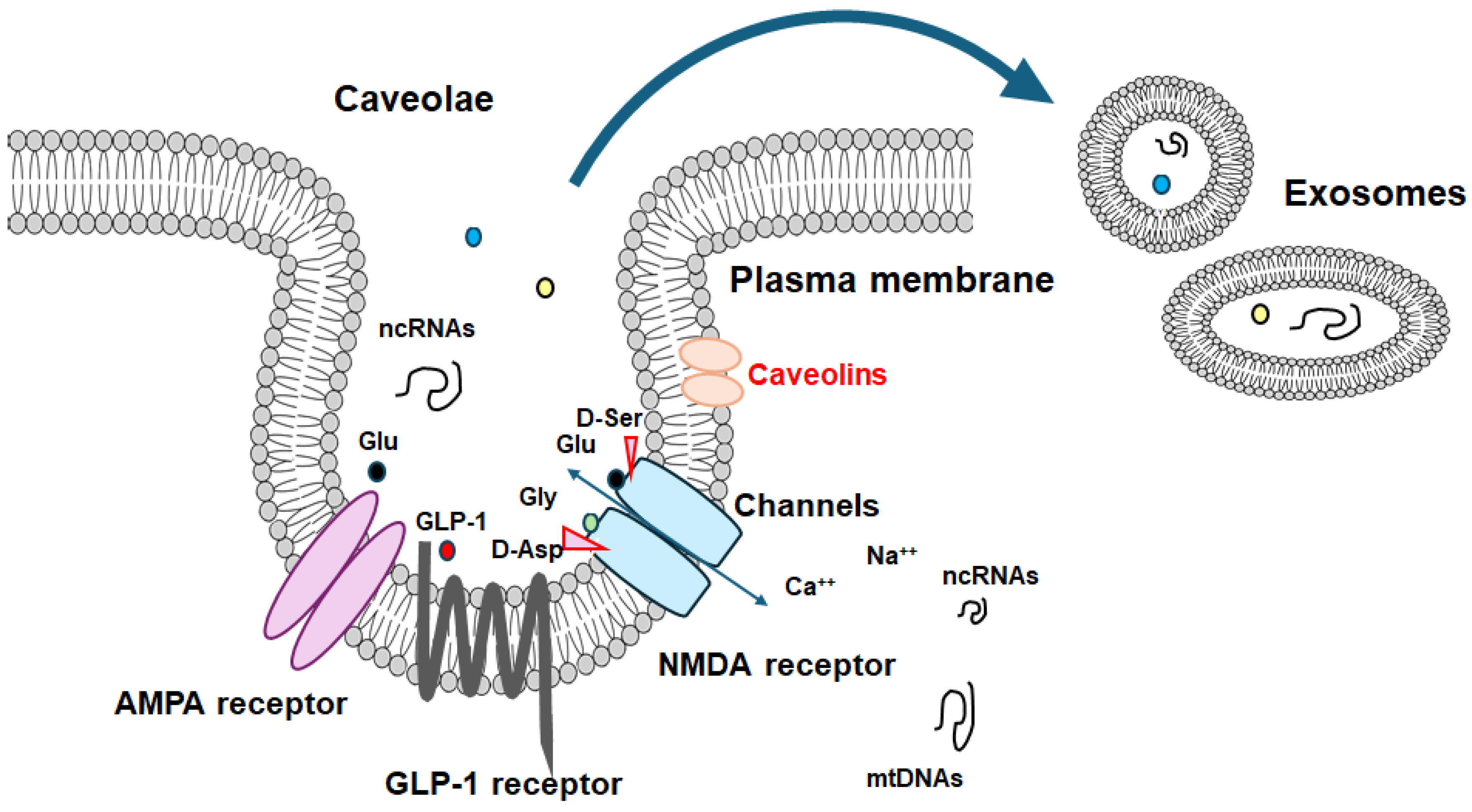
2. Caveolae
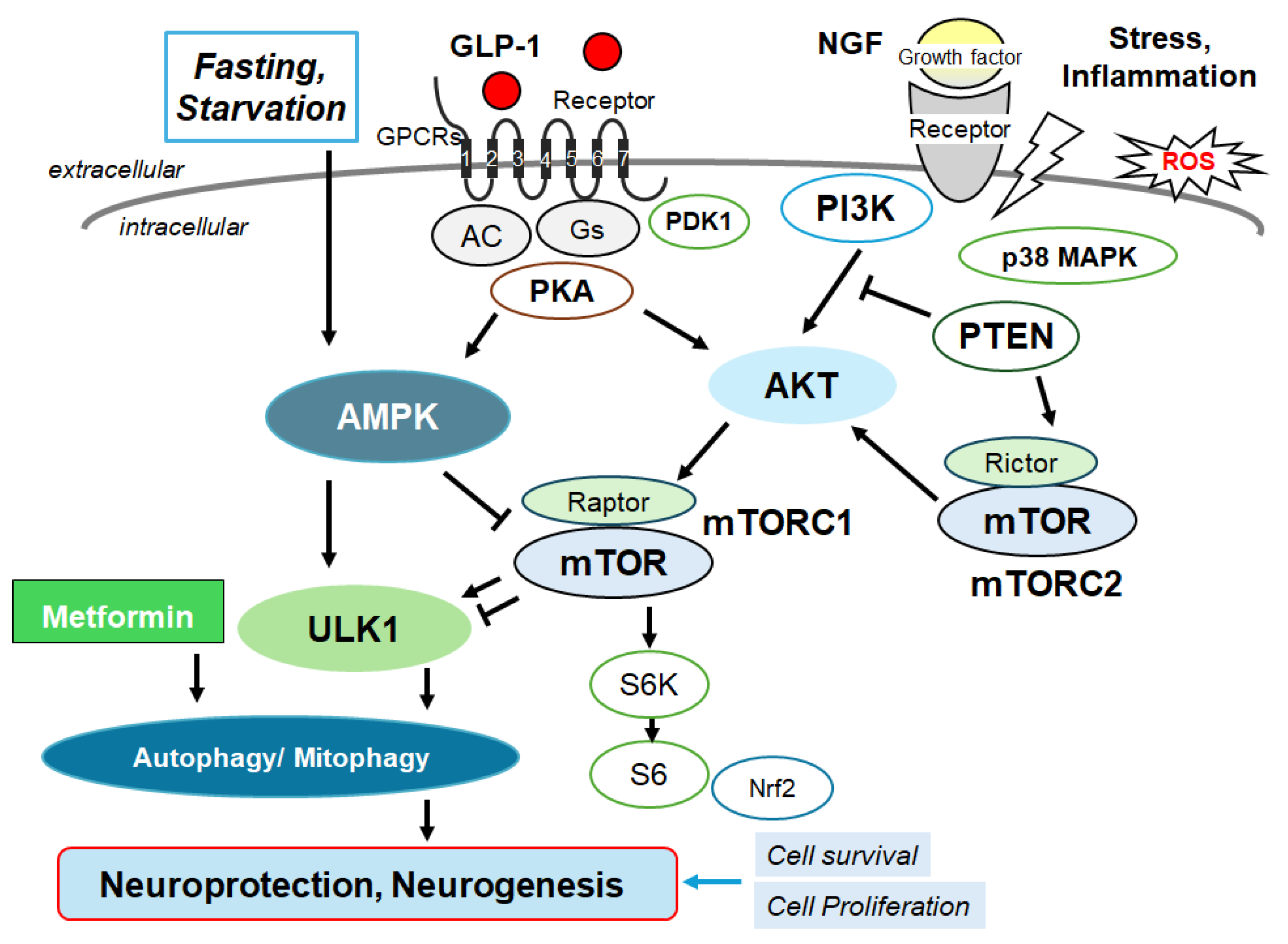
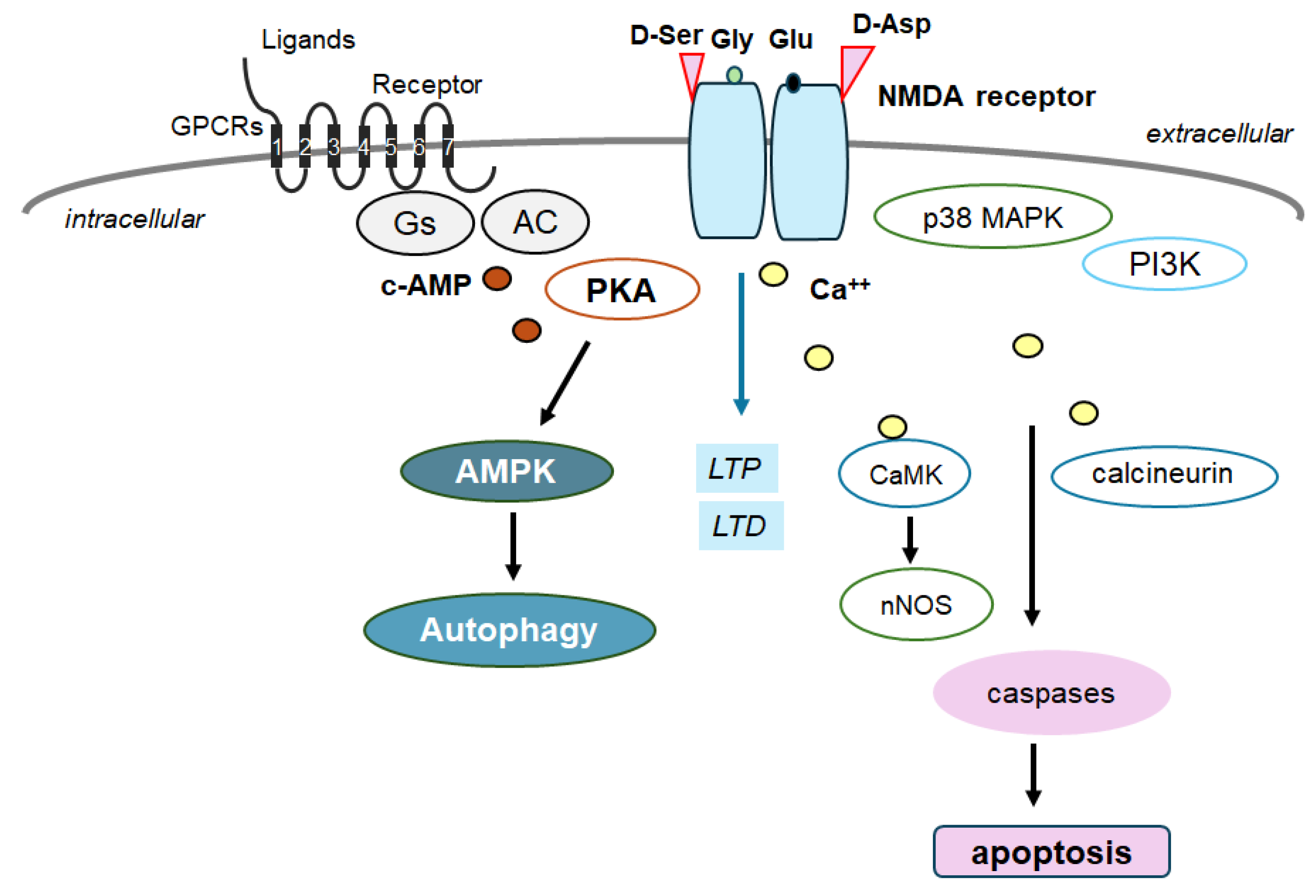
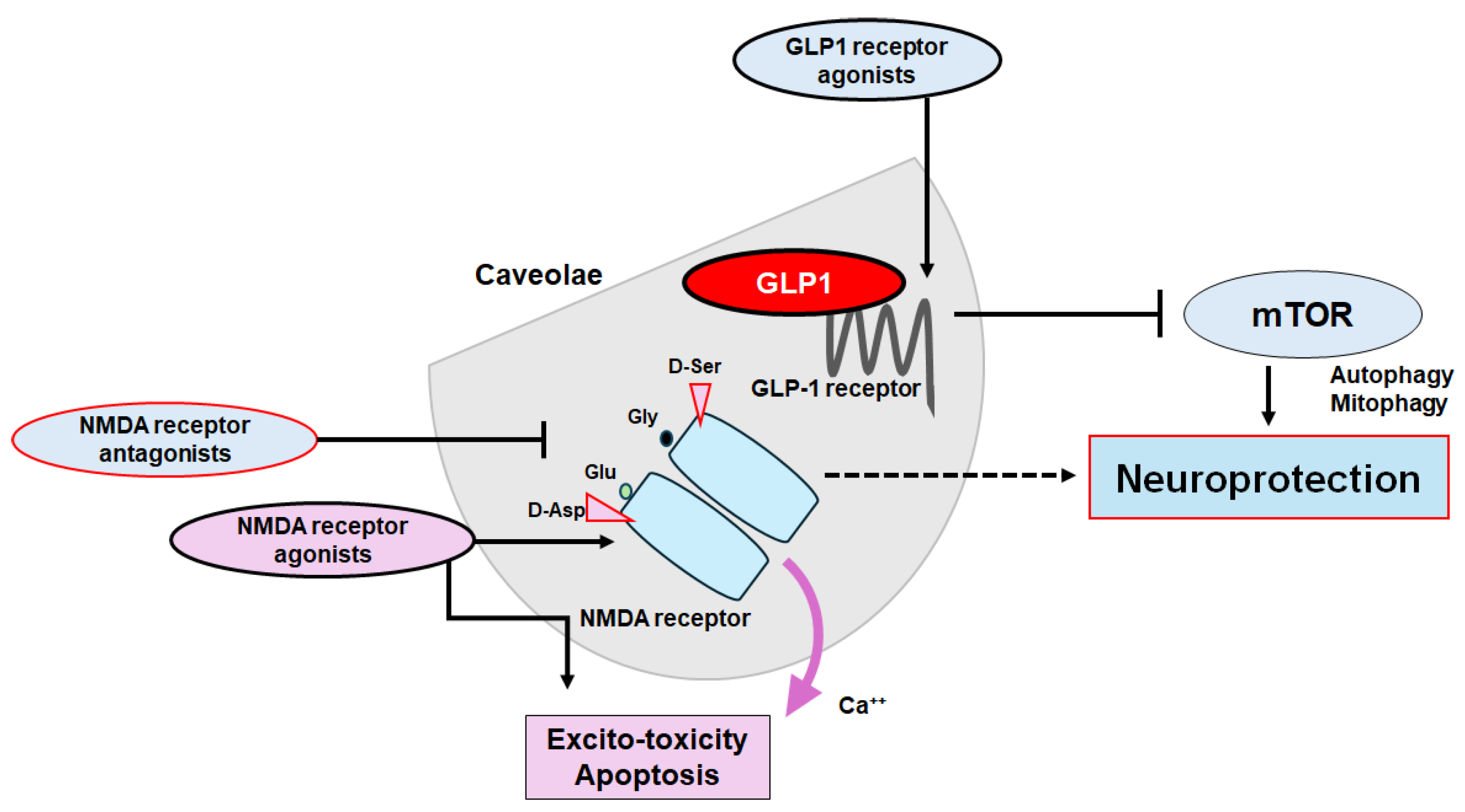
3. Two Relevant Key Receptors to Cognition in Caveolae
4. Autophagy/Mitophagy
5. Clinical Translation
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | adenosine monophosphate-activated protein kinase |
| BDNF | brain-derived neurotrophic factor |
| CSF | cerebrospinal fluid |
| CNS | central nervous system |
| GLS1 | glutaminase 1 |
| GLP-1 | glucagon-like peptide-1 |
| CREB | cAMP-response element binding protein |
| LTD | long-term depression |
| LTP | long-term potentiation |
| mTOR | mammalian/mechanistic target of rapamycin |
| NMDA | N-methyl-d-aspartate |
| ROS | reactive oxygen species |
| SSRI | selective serotonin reuptake inhibitor |
References
- Rong L, et al. Effects of ketogenic diet on cognitive function of patients with Alzheimer's disease: a systematic review and meta-analysis. J Nutr Health Aging. 2024;28(8):100306.
- Carotenuto, A.; Andreone, V.; Amenta, F.; Traini, E. Effect of Treatment of the Cholinergic Precursor Choline Alphoscerate in Mild Cognitive Dysfunction: A Randomized Controlled Trial. Medicina 2024, 60, 925. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, T.; Chouliaras, L.; Morrell, R.; Rubio, D.; Radford, D.; Marchant, N.L.; Walker, Z. The criteria used to rule out mild cognitive impairment impact dementia incidence rates in subjective cognitive decline. Alzheimer's Res. Ther. 2024, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Self, W.K.; Holtzman, D.M. Emerging diagnostics and therapeutics for Alzheimer disease. Nat. Med. 2023, 29, 2187–2199. [Google Scholar] [CrossRef] [PubMed]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.-C.; Otto, A.; Pfrieger, F.W. CNS Synaptogenesis Promoted by Glia-Derived Cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Yang, B.; Wang, H.; Lu, C.; Chang, A.K.; Huang, X.; Zhang, X.; Lu, Z.; Lu, X.; et al. Suppression of neuronal cholesterol biosynthesis impairs brain functions through insulin-like growth factor I-Akt signaling. Int. J. Biol. Sci. 2021, 17, 3702–3716. [Google Scholar] [CrossRef] [PubMed]
- Lentini, D.; Guzzi, F.; Pimpinelli, F.; Zaninetti, R.; Cassetti, A.; Coco, S.; Maggi, R.; Parenti, M. Polarization of caveolins and caveolae during migration of immortalized neurons. J. Neurochem. 2007, 104, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Shin JW, Lee JC. Roles of microglial membranes in Alzheimer's disease. Curr Top Membr. 2020;86:301-314.
- Mattera, V.; Occhiuzzi, F.; Correale, J.; Pasquini, J.M. Remyelinating effect driven by transferrin-loaded extracellular vesicles. Glia 2023, 72, 338–361. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.W.; Hnasko, R.; Schubert, W.; Lisanti, M.P. Role of Caveolae and Caveolins in Health and Disease. Physiol. Rev. 2004, 84, 1341–1379. [Google Scholar] [CrossRef]
- Parton, R.G.; Simons, K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Head, B.P.; Hu, Y.; Finley, J.C.; Saldana, M.D.; Bonds, J.A.; Miyanohara, A.; Niesman, I.R.; Ali, S.S.; Murray, F.; Insel, P.A.; et al. Neuron-targeted Caveolin-1 Protein Enhances Signaling and Promotes Arborization of Primary Neurons. J. Biol. Chem. 2011, 286, 33310–33321. [Google Scholar] [CrossRef] [PubMed]
- Mandyam, C.D.; Schilling, J.M.; Cui, W.; Egawa, J.; Niesman, I.R.; Kellerhals, S.E.; Staples, M.C.; Busija, A.R.; Risbrough, V.B.; Posadas, E.; et al. Neuron-Targeted Caveolin-1 Improves Molecular Signaling, Plasticity, and Behavior Dependent on the Hippocampus in Adult and Aged Mice. Biol. Psychiatry 2015, 81, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, A.; Kumari, R.; Zukin, R.S. Regulation of Group I Metabotropic Glutamate Receptor Trafficking and Signaling by the Caveolar/Lipid Raft Pathway. J. Neurosci. 2009, 29, 3590–3602. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, L.; Zhang, K.; Guo, Y.; Liu, S.; Wu, Y.; Li, X.; Song, Q.; Zhuo, M.; Zhao, M. Increased coupling of caveolin-1 and estrogen receptor α contributes to the fragile X syndrome. Ann. Neurol. 2015, 77, 618–636. [Google Scholar] [CrossRef] [PubMed]
- Luo L, et al. Caveolin-1-Mediated Cholesterol Accumulation Contributes to Exaggerated mGluR-Dependent Long-Term Depression and Impaired Cognition in Fmr1 Knockout Mice. Mol Neurobiol. 2023;60(6):3379-3395.
- Gaudreault, S.B.; Chabot, C.; Gratton, J.-P.; Poirier, J. The Caveolin Scaffolding Domain Modifies 2-Amino-3-hydroxy-5-methyl-4-isoxazole Propionate Receptor Binding Properties by Inhibiting Phospholipase A2 Activity. J. Biol. Chem. 2004, 279, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ramsakha, N.; Ojha, P.; Pal, S.; Routh, S.; Citri, A.; Bhattacharyya, S. A vital role for PICK1 in the differential regulation of metabotropic glutamate receptor internalization and synaptic AMPA receptor endocytosis. J. Biol. Chem. 2023, 299, 104837. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.M.; Lisanti, M.P. The caveolin proteins. Genome Biol. 2004, 5, 214–214. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.M.; Bastiani, M.; Luetterforst, R.; Kirkham, M.; Kirkham, A.; Nixon, S.J.; Walser, P.; Abankwa, D.; Oorschot, V.M.; Martin, S.; et al. PTRF-Cavin, a Conserved Cytoplasmic Protein Required for Caveola Formation and Function. Cell 2008, 132, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Carman, C.V.; Lisanti, M.P.; Benovic, J.L. Regulation of G Protein-coupled Receptor Kinases by Caveolin. J. Biol. Chem. 1999, 274, 8858–8864. [Google Scholar] [CrossRef] [PubMed]
- Panetta, D.; Biedi, C.; Repetto, S.; Cordera, R.; Maggi, D. IGF-I regulates caveolin 1 and IRS1 interaction in caveolae. Biochem. Biophys. Res. Commun. 2004, 316, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Tang W, Li Y, Li Y, Wang Q. Caveolin-1, a novel player in cognitive decline. Neurosci Biobehav Rev. 2021;129:95-106.
- Wu, J.; Zhou, S.-L.; Pi, L.-H.; Shi, X.-J.; Ma, L.-R.; Chen, Z.; Qu, M.-L.; Li, X.; Nie, S.-D.; Liao, D.-F.; et al. High glucose induces formation of tau hyperphosphorylation via Cav-1-mTOR pathway: A potential molecular mechanism for diabetes-induced cognitive dysfunction. Oncotarget 2017, 8, 40843–40856. [Google Scholar] [CrossRef] [PubMed]
- Gupta A, Sharma A, Kumar A, Goyal R. Alteration in memory cognition due to activation of caveolin-1 and oxidative damage in a model of dementia of Alzheimer's type. Indian J Pharmacol. 2019;51(3):173-180.
- Ikezu, T.; Trapp, B.D.; Song, K.S.; Schlegel, A.; Lisanti, M.P.; Okamoto, T. Caveolae, Plasma Membrane Microdomains for α-Secretase-mediated Processing of the Amyloid Precursor Protein. J. Biol. Chem. 1998, 273, 10485–10495. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, M.; Sharma, P.L. Ameliorative effect of daidzein: a caveolin-1 inhibitor in vascular endothelium dysfunction induced by ovariectomy. . 2012, 50, 28–34. [Google Scholar] [PubMed]
- Ajmani, P.; Yadav, H.N.; Singh, M.; Sharma, P.L. Possible involvement of caveolin in attenuation of cardioprotective effect of ischemic preconditioning in diabetic rat heart. BMC Cardiovasc. Disord. 2011, 11, 43–43. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, Y.; He, S.; Jiang, T.; Wang, N.; Du, M.; Cheng, B.; Gao, W.; Li, Y.; Wang, Q. Caveolin-1 Alleviates Diabetes-Associated Cognitive Dysfunction Through Modulating Neuronal Ferroptosis-Mediated Mitochondrial Homeostasis. Antioxidants Redox Signal. 2022, 37, 867–886. [Google Scholar] [CrossRef] [PubMed]
- Puddu, A.; Maggi, D. Emerging Role of Caveolin-1 in GLP-1 Action. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Brubaker PL, Drucker DJ. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Recept Channels. 2002;8(3-4):179–188.
- Syme, C.A.; Zhang, L.; Bisello, A. Caveolin-1 Regulates Cellular Trafficking and Function of the Glucagon-Like Peptide 1 Receptor. Mol. Endocrinol. 2006, 20, 3400–3411. [Google Scholar] [CrossRef] [PubMed]
- Del Galdo F, Lisanti MP, Jimenez SA. Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol. 2008;20(6):713–719.
- Hamoudane M, Maffioli S, Cordera R, Maggi D, Salani B. Caveolin-1 and polymerase I and transcript release factor: new players in insulin-like growth factor-I receptor signaling. J Endocrinol Invest. 2013;36(3):204–208.
- Lee H, et al. Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: caveolin-1 (P132L) behaves in a dominant-negative manner and caveolin-1 (-/-) null mice show mammary epithelial cell hyperplasia. Am J Pathol. 2002;161(4):1357–1369.
- Jones, B.; Buenaventura, T.; Kanda, N.; Chabosseau, P.; Owen, B.M.; Scott, R.; Goldin, R.; Angkathunyakul, N.; Jr, I.R.C.; Bosco, D.; et al. Targeting GLP-1 receptor trafficking to improve agonist efficacy. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Thompson A, Kanamarlapudi V. Agonist-induced internalisation of the glucagon-like peptide-1 receptor is mediated by the Galphaq pathway. Biochem Pharmacol. 2015;93(1):72–84.
- Kutlu MD, Kose S, Akillioglu K.GLP-1 agonist Liraglutide prevents MK-801-induced schizophrenia-like behaviors and BDNF, CREB, p-CREB, Trk-B expressions in the hippocampus and prefrontal cortex in Balb/c mice. Behav Brain Res. 2023;445:114386.
- Abubakar MD, et al. GLP-1/GIP Agonist as an Intriguing and Ultimate Remedy for Combating Alzheimer's Disease through its Supporting DPP4 Inhibitors: A Review. Curr Top Med Chem. 2024.
- Ikeda, Y.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Neuroprotection by dipeptidyl-peptidase-4 inhibitors and glucagon-like peptide-1 analogs via the modulation of AKT-signaling pathway in Alzheimer’s disease. World J. Biol. Chem. 2021, 12, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.M.; E Noble, E.; Liu, C.M.; Cortella, A.M.; Konanur, V.R.; Suarez, A.N.; Reiner, D.J.; Hahn, J.D.; Hayes, M.R.; E Kanoski, S. A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Mol. Psychiatry 2017, 23, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Abraham WC, Jones OD, Glanzman DL. Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci Learn. 2019;4:9.
- Li, W.; Kutas, M.; Gray, J.A.; Hagerman, R.H.; Olichney, J.M. The Role of Glutamate in Language and Language Disorders - Evidence from ERP and Pharmacologic Studies. Neurosci. Biobehav. Rev. 2020, 119, 217–241. [Google Scholar] [CrossRef]
- Wu E, Zhang J, Zhang J, Zhu S. Structural Insights into Gating Mechanism and Allosteric Regulation of NMDA Receptors. Curr. Opin. Neurobiol. 2023;83:102806.
- Tsai, Y.-C.; Huang, S.-M.; Peng, H.-H.; Lin, S.-R.; Chin, T.-Y.; Huang, S.-M. Imbalance of synaptic and extrasynaptic NMDA receptors induced by the deletion of CRMP1 accelerates age-related cognitive decline in mice. Neurobiol. Aging 2024, 135, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tajima, N. Structural insights into NMDA receptor pharmacology. Biochem. Soc. Trans. 2023, 51, 1713–1731. [Google Scholar] [CrossRef] [PubMed]
- Mony, L.; Paoletti, P. Mechanisms of NMDA receptor regulation. Curr. Opin. Neurobiol. 2023, 83, 102815. [Google Scholar] [CrossRef]
- Morris, P.G.; Mishina, M.; Jones, S. Altered Synaptic and Extrasynaptic NMDA Receptor Properties in Substantia Nigra Dopaminergic Neurons From Mice Lacking the GluN2D Subunit. Front. Cell. Neurosci. 2018, 12, 354. [Google Scholar] [CrossRef]
- Olivero, G.; Grilli, M.; Marchi, M.; Pittaluga, A. Metamodulation of presynaptic NMDA receptors: New perspectives for pharmacological interventions. Neuropharmacology 2023, 234, 109570. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Barone, E.; Butterfield, D. mTOR in Alzheimer disease and its earlier stages: Links to oxidative damage in the progression of this dementing disorder. Free. Radic. Biol. Med. 2021, 169, 382–396. [Google Scholar] [CrossRef]
- Su, H.; Wang, X. Autophagy and p62 in cardiac protein quality control. Autophagy 2011, 7, 1382–1383. [Google Scholar] [CrossRef]
- Mary, A.; Eysert, F.; Checler, F.; Chami, M. Mitophagy in Alzheimer’s disease: Molecular defects and therapeutic approaches. Mol. Psychiatry 2022, 28, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Babygirija, R.; Sonsalla, M.M.; Mill, J.; James, I.; Han, J.H.; Green, C.L.; Calubag, M.F.; Wade, G.; Tobon, A.; Michael, J.; et al. Protein restriction slows the development and progression of pathology in a mouse model of Alzheimer’s disease. Nat. Commun. 2024, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, M.; Ibrahim, N.; Yap, K.H.; Azmin, S.; Makpol, S.; Damanhuri, H.; Hamzah, J. Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases: a systematic review. Neural Regen. Res. 2023, 18, 1179–1185. [Google Scholar] [CrossRef]
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Vezzoli, G.; Tedesco, B.; Meroni, M.; Messi, E.; et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2018, 15, 631–651. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, M.; Read, M.I.; Barreto, G.E.; Sahebkar, A. Trehalose against Alzheimer's Disease: Insights into a Potential Therapy. BioEssays 2020, 42, e1900195. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Barkhordarian, H.; Emadi, S.; Park, C.B.; Sierks, M.R. Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol. Dis. 2005, 20, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.T.; Karaca, I.; Tamboli, I.Y.; Walter, J. Trehalose Alters Subcellular Trafficking and the Metabolism of the Alzheimer-associated Amyloid Precursor Protein. J. Biol. Chem. 2016, 291, 10528–10540. [Google Scholar] [CrossRef] [PubMed]
- Pupyshev AB, et al. Combined induction of mTOR-dependent and mTOR-independent pathways of autophagy activation as an experimental therapy for Alzheimer’s disease-like pathology in a mouse model. Pharmacol. Biochem. Behav. 2022;217:173406.
- Pupyshev, A.B.; Akopyan, A.A.; Tenditnik, M.V.; Ovsyukova, M.V.; Dubrovina, N.I.; Belichenko, V.M.; Korolenko, T.A.; Zozulya, S.A.; Klyushnik, T.P.; Tikhonova, M.A. Alimentary Treatment with Trehalose in a Pharmacological Model of Alzheimer’s Disease in Mice: Effects of Different Dosages and Treatment Regimens. Pharmaceutics 2024, 16, 813. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Li, M.; Tian, L.; Yang, Z.; Yu, Z.; Zhou, Z. Enhancing lysosomal biogenesis and autophagic flux by activating the transcription factor EB protects against cadmium-induced neurotoxicity. Sci. Rep. 2017, 7, srep43466. [Google Scholar] [CrossRef]
- Querfurth, H.; Lee, H.-K. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol. Neurodegener. 2021, 16, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Złotek M, Kurowska A, Herbet M, Piątkowska-Chmiel I. GLP-1 analogs, SGLT-2, and DPP-4 inhibitors: a triad of Hope for Alzheimer's disease therapy. Biomedicine. 2023;11(11):3035.
- Xia W, Yu H, Wen P. Meta-analysis on GLP-1 mediated modulation of autophagy in islet beta-cells: Prospectus for improved wound healing in type 2 diabetes. Int Wound J. 2024;21(4):e14841.
- Shankar, G.M.; Bloodgood, B.L.; Townsend, M.; Walsh, D.M.; Selkoe, D.J.; Sabatini, B.L. Natural Oligomers of the Alzheimer Amyloid-β Protein Induce Reversible Synapse Loss by Modulating an NMDA-Type Glutamate Receptor-Dependent Signaling Pathway. J. Neurosci. 2007, 27, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Skeberdis, V.A.; Chevaleyre, V.; Lau, C.G.; Goldberg, J.H.; Pettit, D.L.; O Suadicani, S.; Lin, Y.; Bennett, M.V.L.; Yuste, R.; E Castillo, P.; et al. Protein kinase A regulates calcium permeability of NMDA receptors. Nat. Neurosci. 2006, 9, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, B.; Luo, W. Memantine ameliorates oxaliplatin-induced neurotoxicity via mitochondrial protection. Bioengineered 2022, 13, 6688–6697. [Google Scholar] [CrossRef] [PubMed]
- Lee JR, Jeong KW. N-retinylidene-N-retinylethanolamine degradation in human retinal pigment epithelial cells via memantine- and ifenprodil-mediated autophagy. Korean J Physiol Pharmacol. 2023;27(5):449-456.
- Johnson, J.W.; Kotermanski, S. Mechanism of action of memantine. Curr. Opin. Pharmacol. 2005, 6, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Winkler D, Leyhe T. [Alzheimer's disease - State of the art, and emerging diagnostics and therapeutics]. Ther Umsch. 2018;75:432–437.
- Companys-Alemany J, et al. NMDA receptor antagonists reduce amyloid-β deposition by modulating calpain-1 signaling and autophagy, rescuing cognitive impairment in 5XFAD mice. Cell Mol Life Sci. 2022;79:408.
- Song, X.; Jensen, M.; Jogini, V.; Stein, R.A.; Lee, C.-H.; Mchaourab, H.S.; Shaw, D.E.; Gouaux, E. Mechanism of NMDA receptor channel block by MK-801 and memantine. Nature 2018, 556, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimer's Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.D.; Regan, M.C.; Myers, S.J.; Nocilla, K.A.; Akins, N.S.; Tahirovic, Y.A.; Wilson, L.J.; Dingledine, R.; Furukawa, H.; Traynelis, S.F.; et al. Novel GluN2B-Selective NMDA Receptor Negative Allosteric Modulator Possesses Intrinsic Analgesic Properties and Enhances Analgesia of Morphine in a Rodent Tail Flick Pain Model. ACS Chem. Neurosci. 2023, 14, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Lavretsky, H.; Laird, K.T.; Krause-Sorio, B.; Heimberg, B.F.; Yeargin, J.; Grzenda, A.; Wu, P.; Thana-Udom, K.; Ercoli, L.M.; Siddarth, P. A Randomized Double-Blind Placebo-Controlled Trial of Combined Escitalopram and Memantine for Older Adults With Major Depression and Subjective Memory Complaints. Am. J. Geriatr. Psychiatry 2019, 28, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.P.; Sawant, N.; Morton, H.; Kshirsagar, S.; E Bunquin, L.; Yin, X.; Reddy, P.H. Selective serotonin reuptake inhibitor citalopram ameliorates cognitive decline and protects against amyloid beta-induced mitochondrial dynamics, biogenesis, autophagy, mitophagy and synaptic toxicities in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2021, 30, 789–810. [Google Scholar] [CrossRef] [PubMed]
- Koychev, I.; I Adler, A.; Edison, P.; Tom, B.; E Milton, J.; Butchart, J.; Hampshire, A.; Marshall, C.; Coulthard, E.; Zetterberg, H.; et al. Protocol for a double-blind placebo-controlled randomised controlled trial assessing the impact of oral semaglutide in amyloid positivity (ISAP) in community dwelling UK adults. BMJ Open 2024, 14, e081401. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.D.; Larsen, M.O.; Jelic, K.; Lindgren, O.; Vikman, J.; Holst, J.J.; Deacon, C.F.; Ahrén, B. Secretion and Dipeptidyl Peptidase-4-Mediated Metabolism of Incretin Hormones after a Mixed Meal or Glucose Ingestion in Obese Compared to Lean, Nondiabetic Men. J. Clin. Endocrinol. Metab. 2010, 95, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yan, R.; Zhang, C.; Bai, X.; Yang, X.; Yang, Y.; Feng, T.; Liu, X. Dipeptidyl peptidase-4 inhibitors alleviate cognitive dysfunction in type 2 diabetes mellitus. Lipids Heal. Dis. 2023, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pekar T, et al. The positive effect of spermidine in older adults suffering from dementia : First results of a 3-month trial. Wien Klin Wochenschr. 2021;133(9-10):484-491.
- Rosli, H.; Shahar, S.; Rajab, N.F.; Din, N.C.; Haron, H. The effects of polyphenols-rich tropical fruit juice on cognitive function and metabolomics profile – a randomized controlled trial in middle-aged women. Nutr. Neurosci. 2021, 25, 1577–1593. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, N.; Tarabra, E.; Toledo, M.; Garcia-Macia, M.; Sahu, S.; Coletto, L.; Batista-Gonzalez, A.; Barzilai, N.; Pessin, J.E.; Schwartz, G.J.; et al. System-wide Benefits of Intermeal Fasting by Autophagy. Cell Metab. 2017, 26, 856–871. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G.; Neilson, L.E.; McHill, A.W.; Hiller, A.L. Dietary fasting and time-restricted eating in Huntington’s disease: therapeutic potential and underlying mechanisms. Transl. Neurodegener. 2024, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Peng, J.; Tang, W.; Xia, Y.; Song, P. A circadian rhythm-restricted diet regulates autophagy to improve cognitive function and prolong lifespan. Biosci. Trends 2023, 17, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Rivas, C.; Tovar, L.M.; Zúñiga, N.; Pinto-Borguero, I.; Retamal, C.; Yévenes, G.E.; Moraga-Cid, G.; Fuentealba, J.; Guzmán, L.; Coddou, C.; et al. Altered Glutaminase 1 Activity During Neurulation and Its Potential Implications in Neural Tube Defects. Front. Pharmacol. 2020, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Petersen J, et al. GLP-1 directed NMDA receptor antagonism for obesity treatment. Nature. 2024;629(8014):1133-1141.
- Mishra, D.; Richard, J.E.; Maric, I.; Shevchouk, O.T.; Börchers, S.; Eerola, K.; Krieger, J.-P.; Skibicka, K.P.; B, S. Lateral parabrachial nucleus astrocytes control food intake. Front. Endocrinol. 2024, 15, 1389589. [Google Scholar] [CrossRef] [PubMed]
- Caruso Bavisotto C, et al. Extracellular vesicle-mediated cell⁻cell communication in the nervous system: focus on neurological diseases. Int J Mol Sci. 2019;20(2):434.
- Mendes-Silva, A.P.; Nikolova, Y.S.; Rajji, T.K.; Kennedy, J.L.; Diniz, B.S.; Gonçalves, V.F.; Vieira, E.L. Exosome-associated mitochondrial DNA in late-life depression: Implications for cognitive decline in older adults. J. Affect. Disord. 2024, 362, 217–224. [Google Scholar] [CrossRef]
- Sun, M.; Chen, Z. Unveiling the Complex Role of Exosomes in Alzheimer’s Disease. J. Inflamm. Res. 2024, ume 17, 3921–3948. [Google Scholar] [CrossRef]
- Ebrahim N, et al. Exploring the molecular mechanisms of MSC-derived exosomes in Alzheimer's disease: Autophagy, insulin and the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2024;176:116836.
- Micci, M.-A.; Krishnan, B.; Bishop, E.; Zhang, W.-R.; Guptarak, J.; Grant, A.; Zolochevska, O.; Tumurbaatar, B.; Franklin, W.; Marino, C.; et al. Hippocampal stem cells promotes synaptic resistance to the dysfunctional impact of amyloid beta oligomers via secreted exosomes. Mol. Neurodegener. 2019, 14, 25. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




