Submitted:
23 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Clinical Reports
2.1. Clinical Phenotypes and Genotypes
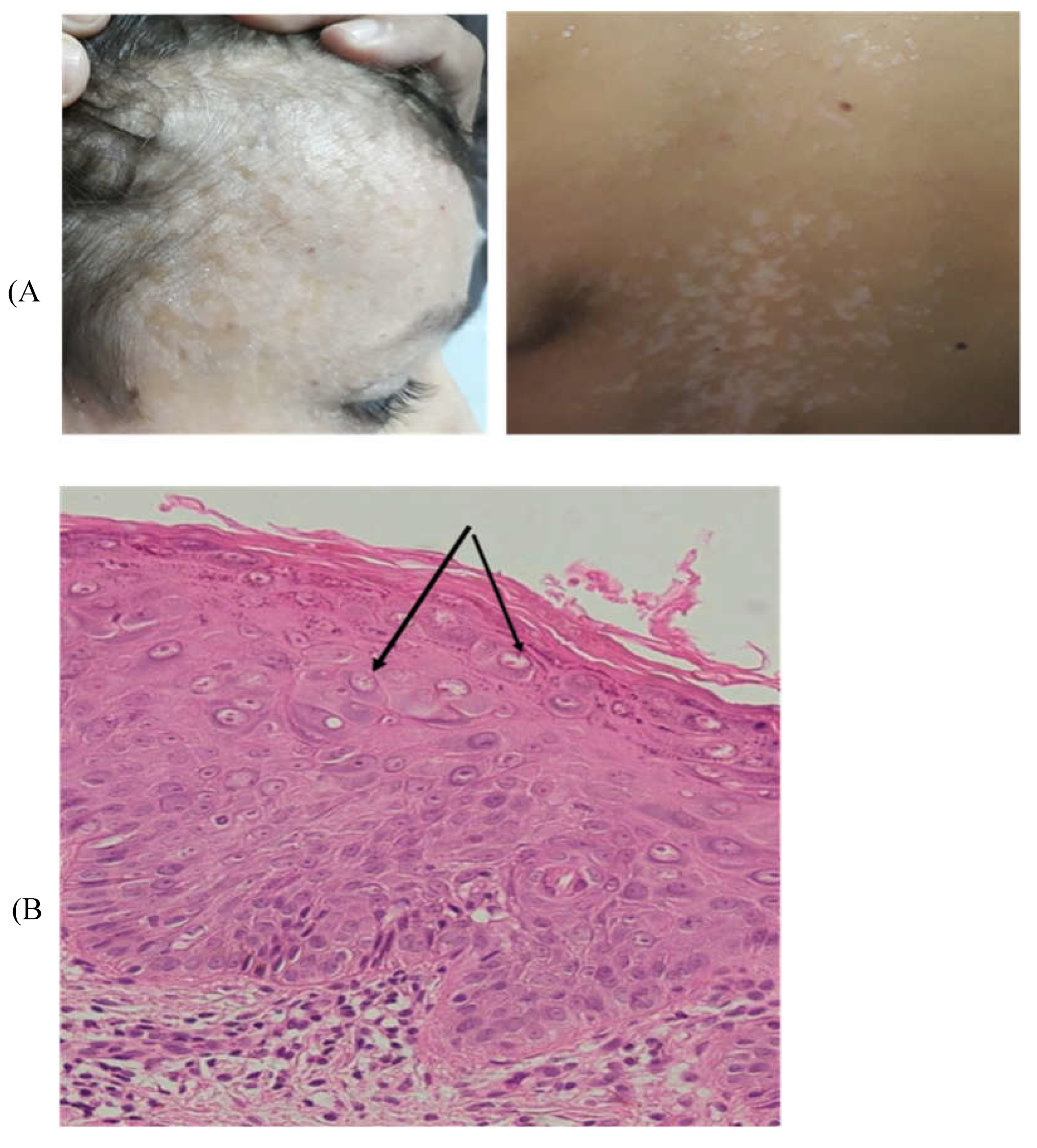
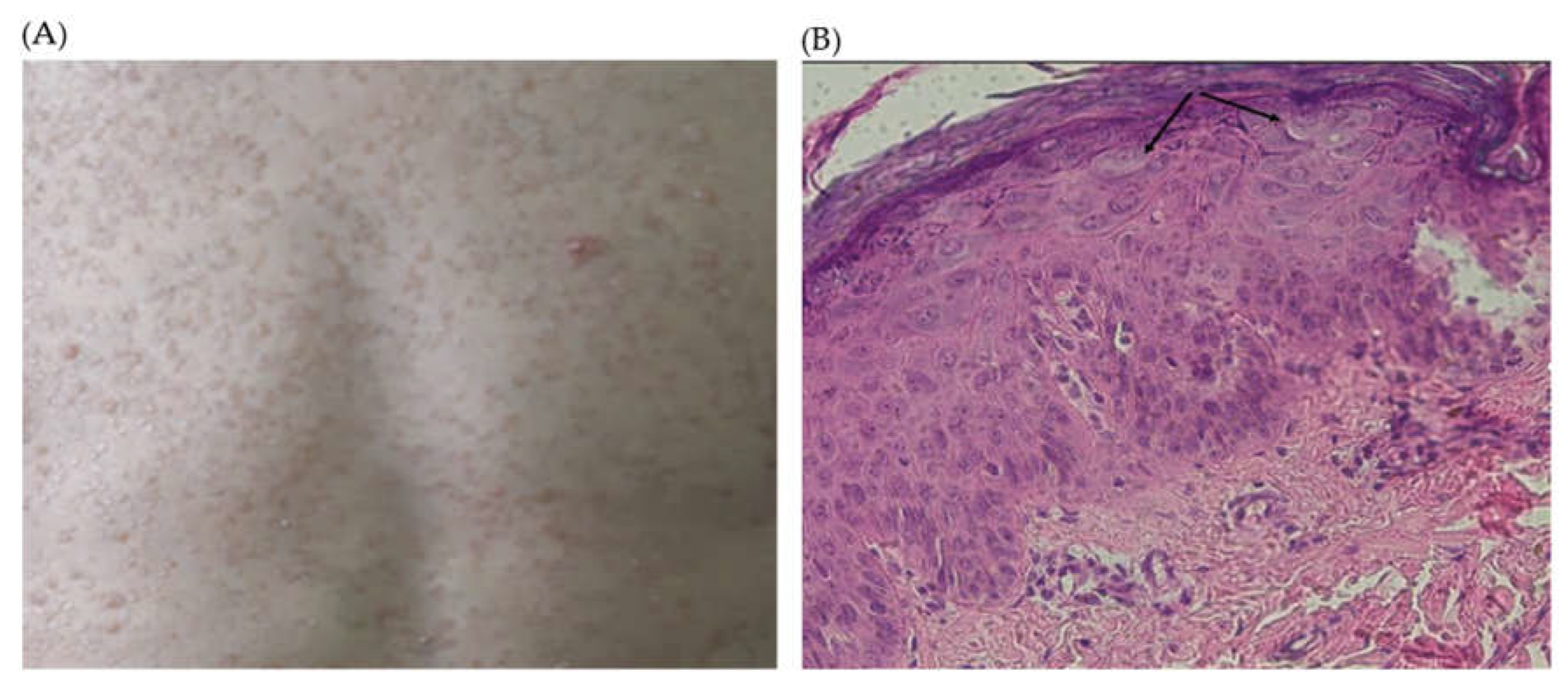
2.1.1. Family 1
2.1.2. Family 2
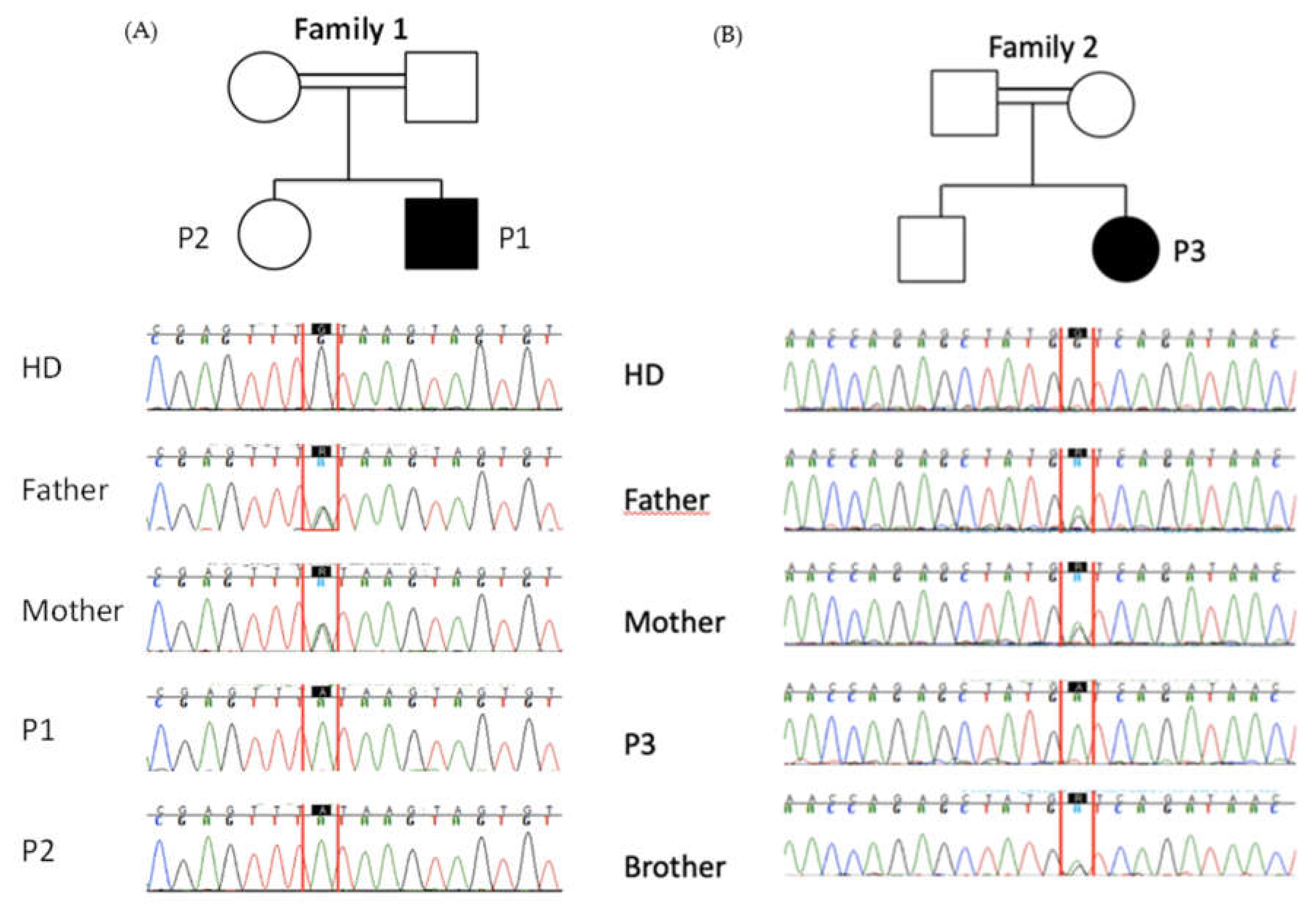
2.1.3. Genotype
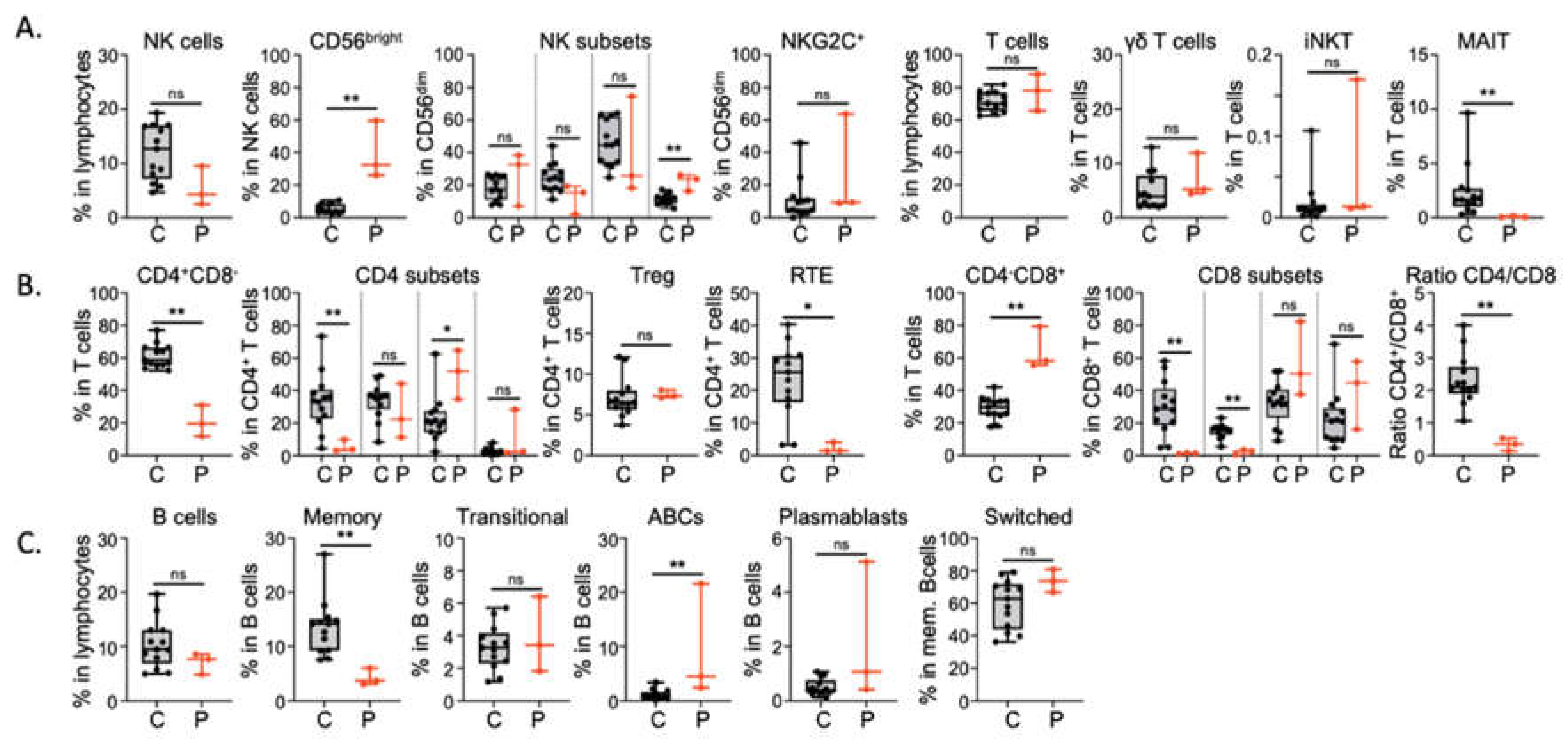
2.2. Immunological Phenotype
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orth, G. Host defenses against human papillomaviruses: lessons from epidermodysplasia verruciformis. Curr Top Microbiol Immunol. 2008, 321, 59–83. [Google Scholar] [PubMed]
- Przybyszewska, J.; Zlotogorski, A.; Ramot, Y. Re-evaluation of epidermodysplasia verruciformis: Reconciling more than 90 years of debate. J. Am. Acad. Dermatol. 2017, 76, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- de Jong, S.J.; Imahorn, E.; Itin, P.; Uitto, J.; Orth, G.; Jouanguy, E.; Casanova, J.-L.; Burger, B. Epidermodysplasia Verruciformis: Inborn Errors of Immunity to Human Beta-Papillomaviruses. Front. Microbiol. 2018, 9, 1222. [Google Scholar] [CrossRef]
- Rajagopalan, K.; Bahru, J.; Loo, D.S.C.; C, H.T.; Chin, K.N.; Tan, K.K. Familial Epidermodysplasia Verruciformis of Lewandowsky and Lutz. Arch. Dermatol. 1972, 105, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ramoz, N.; Rueda, L.-A.; Bouadjar, B.; Montoya, L.-S.; Orth, G.; Favre, M. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat. Genet. 2002, 32, 579–581. [Google Scholar] [CrossRef] [PubMed]
- de Jong SJ, Créquer A, Matos I, Hum D, Gunasekharan V, Lorenzo L, et al. The human CIB1–EVER1–EVER2 complex governs keratinocyte-intrinsic immunity to β-papillomaviruses. Journal of Experimental Medicine. 1 août 2018;215(9):2289-310.
- Béziat, V.; Casanova, J.-L.; Jouanguy, E. Human genetic and immunological dissection of papillomavirus-driven diseases: new insights into their pathogenesis. Curr. Opin. Virol. 2021, 51, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lazarczyk, M.; Dalard, C.; Hayder, M.; Dupre, L.; Pignolet, B.; Majewski, S.; Vuillier, F.; Favre, M.; Liblau, R.S. EVER Proteins, Key Elements of the Natural Anti-Human Papillomavirus Barrier, Are Regulated upon T-Cell Activation. PLOS ONE 2012, 7, e39995. [Google Scholar] [CrossRef] [PubMed]
- Crequer, A.; Troeger, A.; Patin, E.; Ma, C.S.; Picard, C.; Pedergnana, V.; Fieschi, C.; Lim, A.; Abhyankar, A.; Gineau, L.; et al. Human RHOH deficiency causes T cell defects and susceptibility to EV-HPV infections. J. Clin. Investig. 2012, 122, 3239–3247. [Google Scholar] [CrossRef] [PubMed]
- Crequer, A.; Picard, C.; Patin, E.; D’amico, A.; Abhyankar, A.; Munzer, M.; Debré, M.; Zhang, S.-Y.; de Saint-Basile, G.; Fischer, A.; et al. Inherited MST1 Deficiency Underlies Susceptibility to EV-HPV Infections. PLOS ONE 2012, 7, e44010. [Google Scholar] [CrossRef]
- Sharafian, S.; Ziaee, V.; Shahrooei, M.; Ahadi, M.; Parvaneh, N. A Novel STK4 Mutation Presenting with Juvenile Idiopathic Arthritis and Epidermodysplasia Verruciformis. J. Clin. Immunol. 2019, 39, 11–14. [Google Scholar] [CrossRef]
- Gutierrez-Marin, P.A.; Castano-Jaramillo, L.M.; Velez-Tirado, N.; Villamil-Osorio, M.; Patiño, E.; Reina, M.F.; Hernandez, M.T. STK4 deficiency and epidermodysplasia verruciformis-like lesions: A case report. Pediatr. Dermatol. 2023, 41, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Stray-Pedersen, A.; Jouanguy, E.; Crequer, A.; Bertuch, A.A.; Brown, B.S.; Jhangiani, S.N.; Muzny, D.M.; Gambin, T.; Sorte, H.; Sasa, G.; et al. Compound Heterozygous CORO1A Mutations in Siblings with a Mucocutaneous-Immunodeficiency Syndrome of Epidermodysplasia Verruciformis-HPV, Molluscum Contagiosum and Granulomatous Tuberculoid Leprosy. J. Clin. Immunol. 2014, 34, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Momenilandi M, Lévy R, Sobrino S, et al. FLT3L governs the development of partially overlapping hematopoietic lineages in humans and mice. Cell. 2024;187(11):2817-2837.
- Rawat, A.; Singh, A.; Dobbs, K.; Pala, F.; Delmonte, O.M.; Vignesh, P.; Jindal, A.K.; Gupta, A.; Suri, D.; Kaur, A.; et al. Skewed TCR Alpha, but not Beta, Gene Rearrangements and Lymphoma Associated with a Pathogenic TRAC Variant. J. Clin. Immunol. 2021, 41, 1395–1399. [Google Scholar] [CrossRef]
- Tahiat, A.; Badran, Y.R.; Chou, J.; Cangemi, B.; Lefranc, G.; Labgaa, Z.-M.; Oussalam, S.; Kaddouri-Slimani, A.; Belarbi, A.; Bendissari-Bouzid, K.; et al. Epidermodysplasia verruciformis as a manifestation of ARTEMIS deficiency in a young adult. J. Allergy Clin. Immunol. 2016, 139, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Sanal, O.; Jing, H.; Ozgur, T.; Ayvaz, D.; Strauss-Albee, D.M.; Ersoy-Evans, S.; Tezcan, I.; Turkkani, G.; Matthews, H.F.; Haliloglu, G.; et al. Additional Diverse Findings Expand the Clinical Presentation of DOCK8 Deficiency. J. Clin. Immunol. 2012, 32, 698–708. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, G.; Mo, X.; Wang, B.; Wu, F.; Chen, J.; Luo, H.; Zhu, L.; Xu, M.; Zhou, Q.; et al. A novel homozygous DOCK8 mutation associated with unusual coexistence of gross molluscum contagiosum and epidermodysplasia verruciformis in a DOCK8 deficiency patient. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e504–e505. [Google Scholar] [CrossRef]
- Platt, C.D.; Fried, A.J.; Hoyos-Bachiloglu, R.; Usmani, G.N.; Schmidt, B.; Whangbo, J.; Chiarle, R.; Chou, J.; Geha, R.S. Combined immunodeficiency with EBV positive B cell lymphoma and epidermodysplasia verruciformis due to a novel homozygous mutation in RASGRPClin. Immunol. 2017, 183, 142–144. [Google Scholar] [CrossRef]
- Li SL, Duo LN, Wang HJ, Dai W, Zhou EYH, Xu YN, et al. Identification of LCK mutation in a family with atypical epidermodysplasia verruciformis with T-cell defects and virus-induced squamous cell carcinoma. Br J Dermatol. déc 2016;175(6):1204-9.
- Stepensky, P.; Rensing-Ehl, A.; Gather, R.; Revel-Vilk, S.; Fischer, U.; Nabhani, S.; Beier, F.; Brümmendorf, T.H.; Fuchs, S.; Zenke, S.; et al. Early-onset Evans syndrome, immunodeficiency, and premature immunosenescence associated with tripeptidyl-peptidase II deficiency. Blood 2015, 125, 753–761. [Google Scholar] [CrossRef]
- Youssefian, L.; Vahidnezhad, H.; Yousefi, M.; Saeidian, A.H.; Azizpour, A.; Touati, A.; Nikbakht, N.; Hesari, K.K.; Adib-Sereshki, M.M.; Zeinali, S.; et al. Inherited Interleukin 2–Inducible T-Cell (ITK) Kinase Deficiency in Siblings With Epidermodysplasia Verruciformis and Hodgkin Lymphoma. Clin. Infect. Dis. 2019, 68, 1938–1941. [Google Scholar] [CrossRef]
- Invitae Primary Immunodeficiency Panel | Test catalog | Invitae [Internet]. [cité 3 juill 2023]. Disponible sur: https://www.invitae.com/en/providers/test-catalog/test-08100.
- Nehme, N.T.; Schmid, J.P.; Debeurme, F.; André-Schmutz, I.; Lim, A.; Nitschke, P.; Rieux-Laucat, F.; Lutz, P.; Picard, C.; Mahlaoui, N.; et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 2012, 119, 3458–3468. [Google Scholar] [CrossRef]
- Abdollahpour, H.; Appaswamy, G.; Kotlarz, D.; Diestelhorst, J.; Beier, R.; Schäffer, A.A.; Gertz, E.M.; Schambach, A.; Kreipe, H.H.; Pfeifer, D.; et al. The phenotype of human STK4 deficiency. Blood 2012, 119, 3450–3457. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.E.; Al-Mousawi, A.; Assing, K.; Hartling, U.; Grosen, D.; Fisker, N.; Nielsen, C.; Jakobsen, M.A.; Mogensen, T.H. STK4 Deficiency Impairs Innate Immunity and Interferon Production Through Negative Regulation of TBK1-IRF3 Signaling. J. Clin. Immunol. 2021, 41, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Radwan, N.; El-Owaidy, R.; El-Sayed, Z.A.; Abdel-Baky, A.; El-Haddad, A.; Rashad, H.; Khorshed, E.N.; Platt, C.D.; Wallace, J.G.; Chou, J.; et al. A Case of STK4 Deficiency with Complications Evoking Mycobacterial Infection. J. Clin. Immunol. 2020, 40, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, F.; Klein, C.; Poorpooneh, M.; Sherkat, R.; Khoshnevisan, R. A case report of sinusoidal diffuse large B-cell lymphoma in a STK4 deficient patient. Medicine 2020, 99, e18601. [Google Scholar] [CrossRef] [PubMed]
- Abolnezhadian F, Iranparast S, Ahmadpour F. Identical Twins with a Mutation in the STK4 Gene Showing Clinical Manifestations of the Mutation at Different Ages: A Case Report. Iran J Immunol. 2020;17(4):333-340.
- Halacli, S.O.; Ayvaz, D.C.; Sun-Tan, C.; Erman, B.; Uz, E.; Yilmaz, D.Y.; Ozgul, K.; Tezcan, I.; Sanal, O. STK4 (MST1) deficiency in two siblings with autoimmune cytopenias: A novel mutation. Clin. Immunol. 2015, 161, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Schipp, C.; Schlütermann, D.; Hönscheid, A.; Nabhani, S.; Höll, J.; Oommen, P.T.; Ginzel, S.; Fleckenstein, B.; Stork, B.; Borkhardt, A.; et al. EBV Negative Lymphoma and Autoimmune Lymphoproliferative Syndrome Like Phenotype Extend the Clinical Spectrum of Primary Immunodeficiency Caused by STK4 Deficiency. Front. Immunol. 2018, 9, 2400. [Google Scholar] [CrossRef] [PubMed]
- Sherkat R, Sabri MR, Dehghan B, Bigdelian H, Reisi N, Afsharmoghadam N, et al. EBV lymphoproliferative-associated disease and primary cardiac T-cell lymphoma in a STK4 deficient patient: A case report. Medicine (Baltimore). déc 2017;96(48):e8852.
- Al-Saud, B.; Alajlan, H.; Sabar, H.; Anwar, S.; Alruwaili, H.; Al-Hussain, T.; Alamri, N.; Alazami, A.M. STK4 Deficiency in a Patient with Immune Complex Glomerulonephritis, Salt-Losing Tubulopathy, and Castleman’s-Like Disease. J. Clin. Immunol. 2019, 39, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, A.; Bougarn, S.; Khan, T.; Mackeh, R.; Rahman, M.; Al-Ali, F.; Ata, M.; Aamer, W.; Prosser, D.; Habib, T.; et al. A Novel STK4 Mutation Impairs T Cell Immunity Through Dysregulation of Cytokine-Induced Adhesion and Chemotaxis Genes. J. Clin. Immunol. 2021, 41, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Saglam, A.; Cagdas, D.; Aydin, B.; Keles, S.; Reisli, I.; Arslankoz, S.; Katipoglu, K.; Uner, A. STK4 deficiency and EBV-associated lymphoproliferative disorders, emphasis on histomorphology, and review of literature. Virchows Arch. 2022, 480, 393–401. [Google Scholar] [CrossRef]
- Vignesh, P.; Rawat, A.; Kumrah, R.; Singh, A.; Gummadi, A.; Sharma, M.; Kaur, A.; Nameirakpam, J.; Jindal, A.; Suri, D.; et al. Clinical, Immunological, and Molecular Features of Severe Combined Immune Deficiency: A Multi-Institutional Experience From India. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef]
- Uygun, V.; Keleş, S.; Daloğlu, H.; Öztürkmen, S.; Yalçın, K.; Karasu, G.; Yeşilipek, A. Hematopoietic stem cell transplantation in serine/threonine kinase 4 (STK4) deficiency: Report of two cases and literature review. Pediatr. Transplant. 2022, 27, e14439. [Google Scholar] [CrossRef] [PubMed]
- Cagdas, D.; Halacli, S.O.; Tan, C.; Esenboga, S.; Karaatmaca, B.; Cetinkaya, P.G.; Balcı-Hayta, B.; Ayhan, A.; Uner, A.; Orhan, D.; et al. Diversity in Serine/Threonine Protein Kinase-4 Deficiency and Review of the Literature. J. Allergy Clin. Immunol. Pr. 2021, 9, 3752–3766. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.S.; Willet, J.D.; Griffin, H.R.; Morgan, N.V.; O’boyle, G.; Arkwright, P.D.; Hughes, S.M.; Abinun, M.; Tee, L.J.; Barge, D.; et al. Defective Leukocyte Adhesion and Chemotaxis Contributes to Combined Immunodeficiency in Humans with Autosomal Recessive MST1 Deficiency. J. Clin. Immunol. 2016, 36, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Dotta, L.; Badolato, R. Primary immunodeficiencies appearing as combined lymphopenia, neutropenia, and monocytopenia. Immunol. Lett. 2014, 161, 222–225. [Google Scholar] [CrossRef]
| Lymphocyte subpopulations (N/mm3) |
P1 (12 years) |
P2 (8 years) |
P3 (6 years) |
Normal range (age matched) |
|---|---|---|---|---|
| T cells | ||||
| CD3+ | 771 | 2990 | 1489 | 1200-2600 |
| CD4+ | 253 | 470 | 243 | 650-1500 |
| CD8+ | 450 | 2330 | 1057 | 404-826 |
| B cells | ||||
| CD19+ | 128 | 430 | 131 | 270-860 |
| NK cells | ||||
| CD16+/CD56+ | 74 | 110 | 123 | 100-480 |
| Immunoglobulin levels (g/L) |
P1 (12 years) |
P2 (8 years) |
P3 (6 years) |
Normal range (age matched) |
|---|---|---|---|---|
| IgG | 11.62 | 5.17 | 17,3 | 6,10-16,16 |
| IgM | 0.49 | 0.46 | 2,09 | 0,22-2,40 |
| IgA | 1.99 | 3.21 | 2,17 | 0,84-4,99 |
| IgE (UI/mL) | 10 | 5.18 | 175,68 | <100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





