Submitted:
22 July 2024
Posted:
23 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
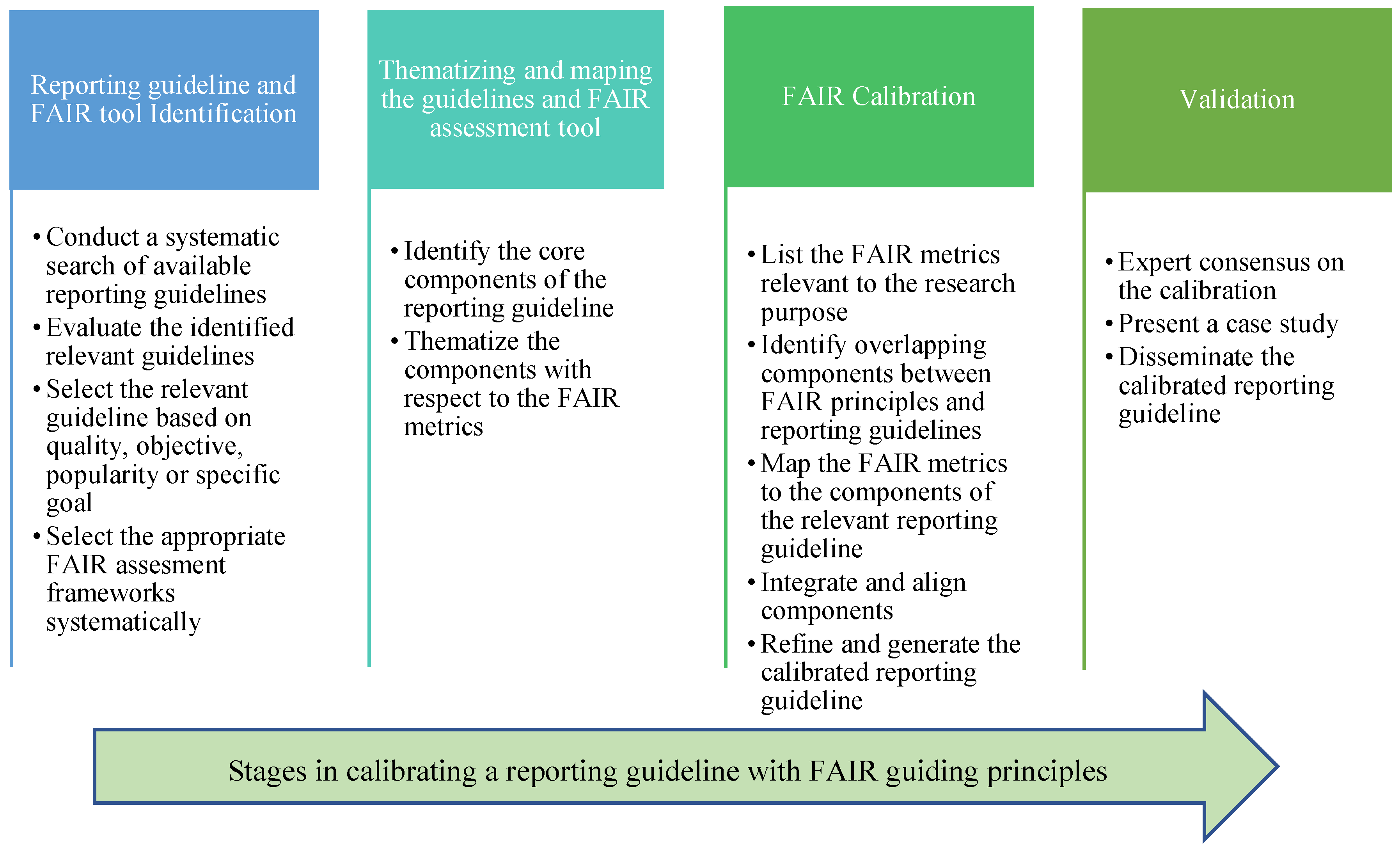
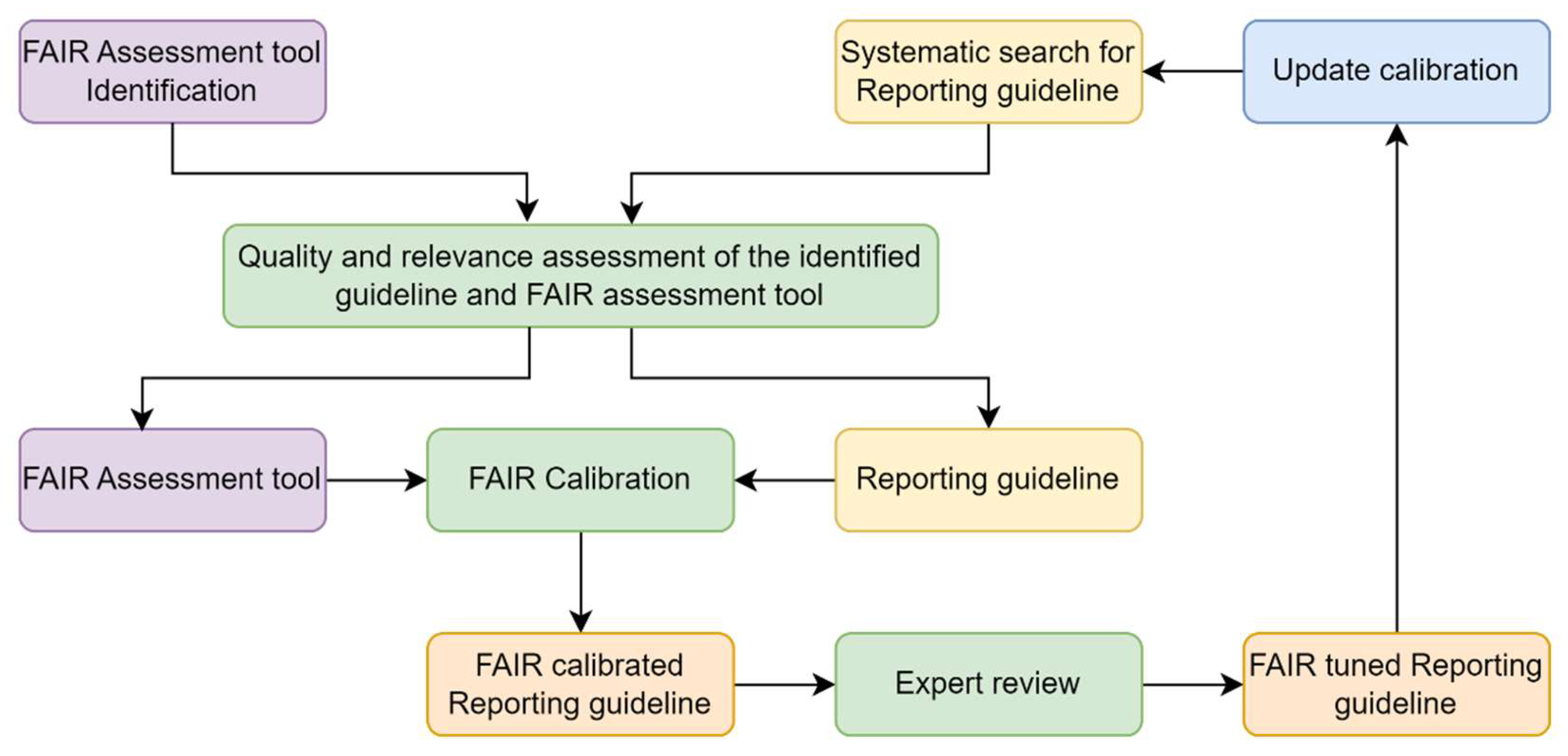
Methods
Results
Discussion
Conclusions
Supplementary Materials
References
- Samuel, S.; Löffler, F.; König-Ries, B. Machine Learning Pipelines: Provenance, Reproducibility and FAIR Data Principles. Cham; pp. 226–230.
- Hutson, M. Artificial intelligence faces reproducibility crisis. American Association for the Advancement of Science: 2018.
- Levinson, M.A.; Niestroy, J.; Al Manir, S.; Fairchild, K.; Lake, D.E.; Moorman, J.R.; Clark, T. FAIRSCAPE: A Framework for FAIR and Reproducible Biomedical Analytics. Neuroinformatics 2022, 20, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.S.; Waite, L.K.; Wierzba, M.; Hoffstaedter, F.; Waite, A.Q.; Poldrack, B.; Eickhoff, S.B.; Hanke, M. FAIRly big: A framework for computationally reproducible processing of large-scale data. Scientific Data 2022, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Narayanan, A. Leakage and the reproducibility crisis in ML-based science. arXiv 2022, arXiv:2207.07048. [Google Scholar]
- Baker, M. Reproducibility crisis. Nature 2016, 533, 353–366. [Google Scholar]
- Thibeau-Sutre, E.; Díaz, M.; Hassanaly, R.; Routier, A.; Dormont, D.; Colliot, O.; Burgos, N. ClinicaDL: An open-source deep learning software for reproducible neuroimaging processing. Computer Methods and Programs in Biomedicine 2022, 220, 106818. [Google Scholar] [CrossRef] [PubMed]
- Hutson, M. Artificial intelligence faces reproducibility crisis. Science 2018, 359, 725–726. [Google Scholar] [CrossRef]
- Shelmerdine, S.C.; Arthurs, O.J.; Denniston, A.; Sebire, N.J. Review of study reporting guidelines for clinical studies using artificial intelligence in healthcare. BMJ Health & Care Informatics 2021, 28. [Google Scholar] [CrossRef]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for scientific data management and stewardship. Scientific Data 2016, 3, 160018. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.; Chaturvedi, P.; Huerta, E.; Liu, Z.; Chard, R.; Scourtas, A.; Schmidt, K.; Chard, K.; Blaiszik, B.; Foster, I. FAIR principles for AI models with a practical application for accelerated high energy diffraction microscopy. Scientific Data 2022, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Huerta, E.; Blaiszik, B.; Brinson, L.C.; Bouchard, K.E.; Diaz, D.; Doglioni, C.; Duarte, J.M.; Emani, M.; Foster, I.; Fox, G. FAIR for AI: An interdisciplinary and international community building perspective. Scientific Data 2023, 10, 487. [Google Scholar] [CrossRef]
- Bahim, C.; Casorrán-Amilburu, C.; Dekkers, M.; Herczog, E.; Loozen, N.; Repanas, K.; Russell, K.; Stall, S. The FAIR Data Maturity Model: An Approach to Harmonise FAIR Assessments. 2020.
- Carroll, C.; Booth, A.; Cooper, K. A worked example of" best fit" framework synthesis: A systematic review of views concerning the taking of some potential chemopreventive agents. BMC medical research methodology 2011, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Network, E. Reporting guidelines. United Kingdom 2019.
- Shiferaw, K.B.; Roloff, M.; Waltemath, D.; Zeleke, A.A. Guidelines and Standard Frameworks for AI in Medicine: Protocol for a Systematic Literature Review. JMIR Res Protoc 2023, 12, e47105. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Chen, L.; Wu, M.; Meng, S.; Dai, Z.; Zhang, Y.; Clarke, M. Guidelines, Consensus Statements, and Standards for the Use of Artificial Intelligence in Medicine: Systematic Review. J Med Internet Res 2023, 25, e46089. [Google Scholar] [CrossRef]
- Brouwers, M.C.; Kho, M.E.; Browman, G.P.; Burgers, J.S.; Cluzeau, F.; Feder, G.; Fervers, B.; Graham, I.D.; Grimshaw, J.; Hanna, S.E. AGREE II: Advancing guideline development, reporting and evaluation in health care. Cmaj 2010, 182, E839–E842. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. International journal of surgery 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rivera, S.C.; Moher, D.; Calvert, M.J.; Denniston, A.K.; Ashrafian, H.; Beam, A.L.; Chan, A.-W.; Collins, G.S.; Deeks, A.D.J. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI extension. The Lancet Digital Health 2020, 2, e537–e548. [Google Scholar] [CrossRef] [PubMed]
- RDA FAIR Data Maturity Model Working Group, B. FAIR Data Maturity Model: Specification and guidelines. Res. Data Alliance 2020, 10. [Google Scholar]
- Carroll, C.; Booth, A.; Leaviss, J.; Rick, J. “Best fit” framework synthesis: Refining the method. BMC medical research methodology 2013, 13, 1–16. [Google Scholar] [CrossRef]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E. The FAIR Guiding Principles for scientific data management and stewardship. Scientific data 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Goble, C.; Cohen-Boulakia, S.; Soiland-Reyes, S.; Garijo, D.; Gil, Y.; Crusoe, M.R.; Peters, K.; Schober, D. FAIR Computational Workflows. Data Intelligence 2020, 2, 108–121. [Google Scholar] [CrossRef]
- Hong, N.P.C.; Katz, D.S.; Barker, M.; Lamprecht, A.-L.; Martinez, C.; Psomopoulos, F.E.; Harrow, J.; Castro, L.J.; Gruenpeter, M.; Martinez, P.A. FAIR principles for research software (FAIR4RS principles). 2022.
- Vandenbroucke, J.P.; Elm, E.v.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Initiative, S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Annals of internal medicine 2007, 147, W–163. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, W.; Foster, I.; Fraser, S.; Kesselman, C. Sharing begins at home: How continuous and ubiquitous FAIRness can enhance research productivity and data reuse. Harvard data science review 2022, 4. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



