Submitted:
20 July 2024
Posted:
23 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
3. Results and Discussion
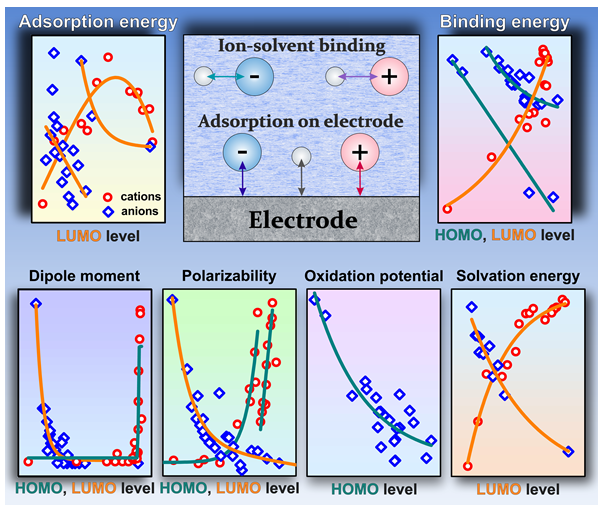
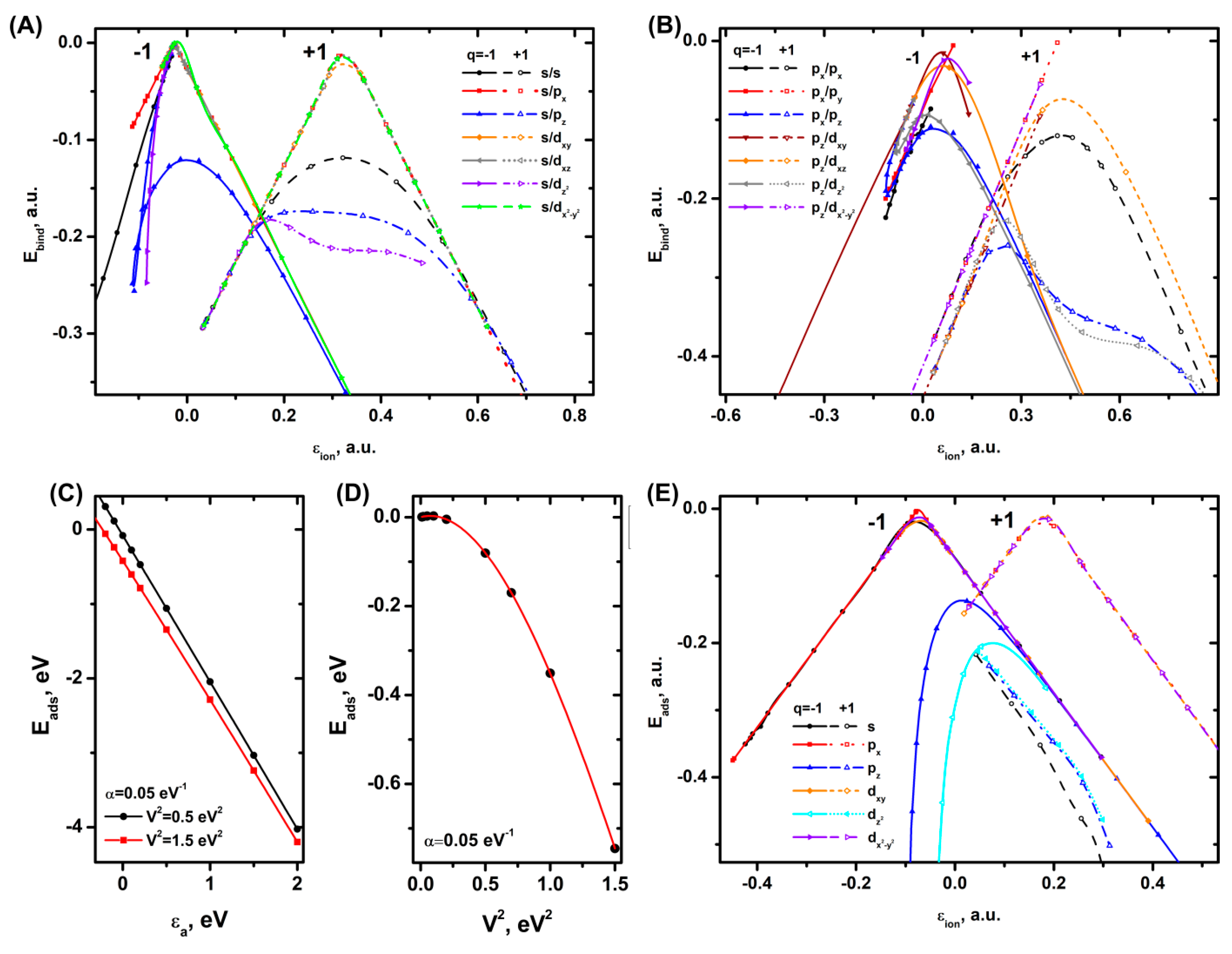
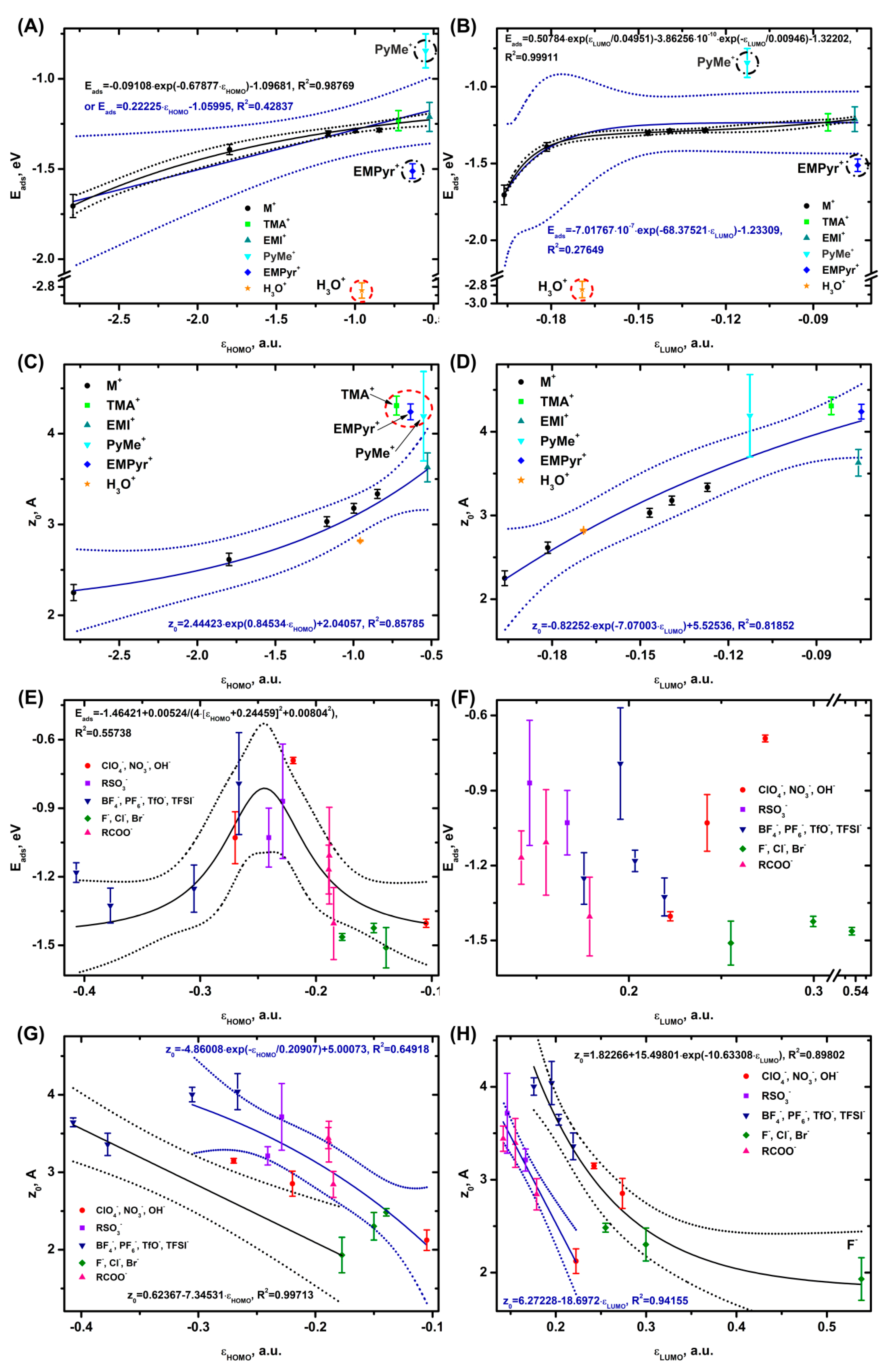
3.1. Relation of Electronic Levels of Ions to Adsorption and Binding Energy
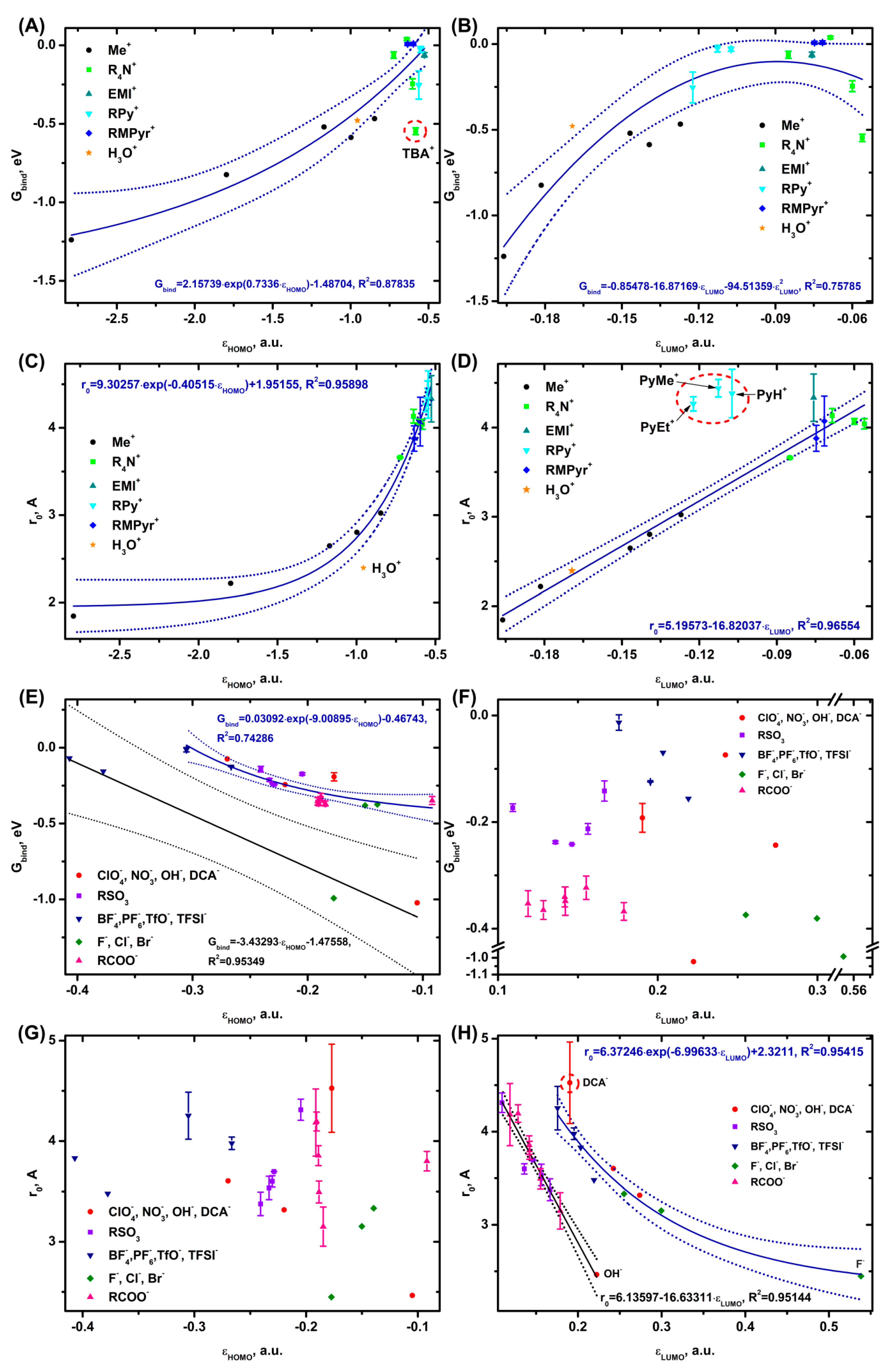
3.2. Trends in Ion Adsorption on α-Al2O3 (0001), Graphene and Au (111)
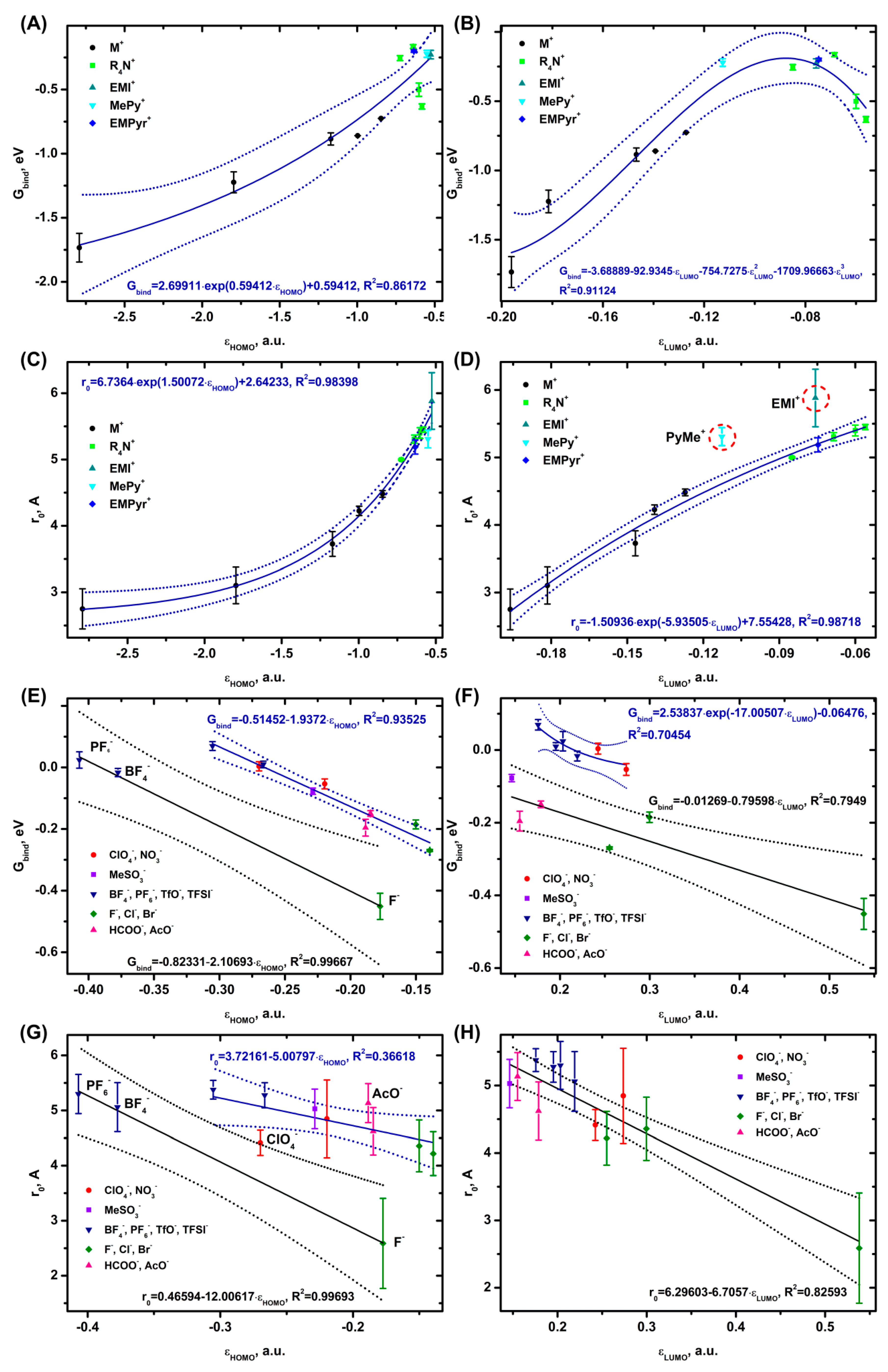
3.3. Ion Binding in Complexes with Water and Dimethyl Carbonate
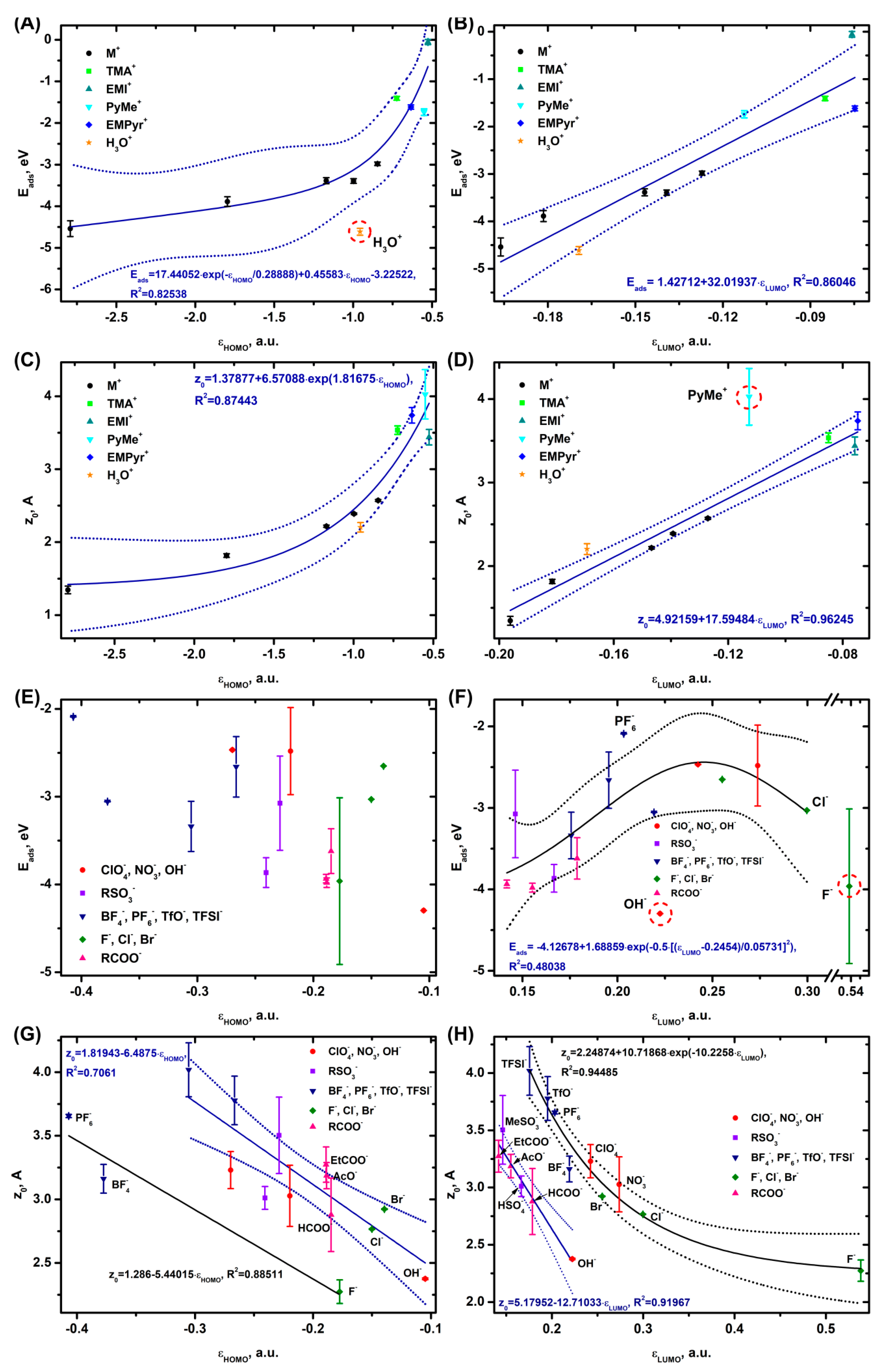
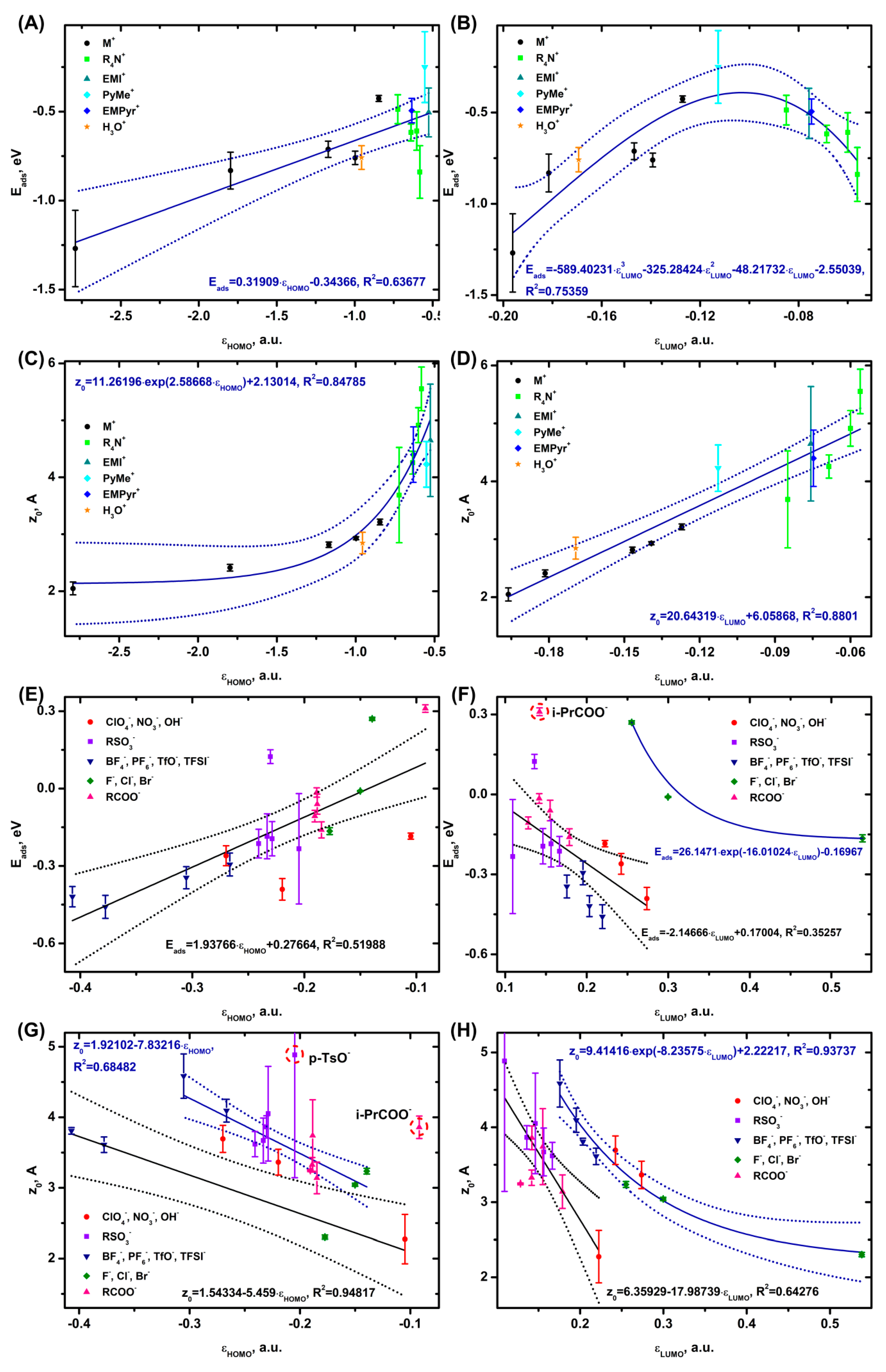
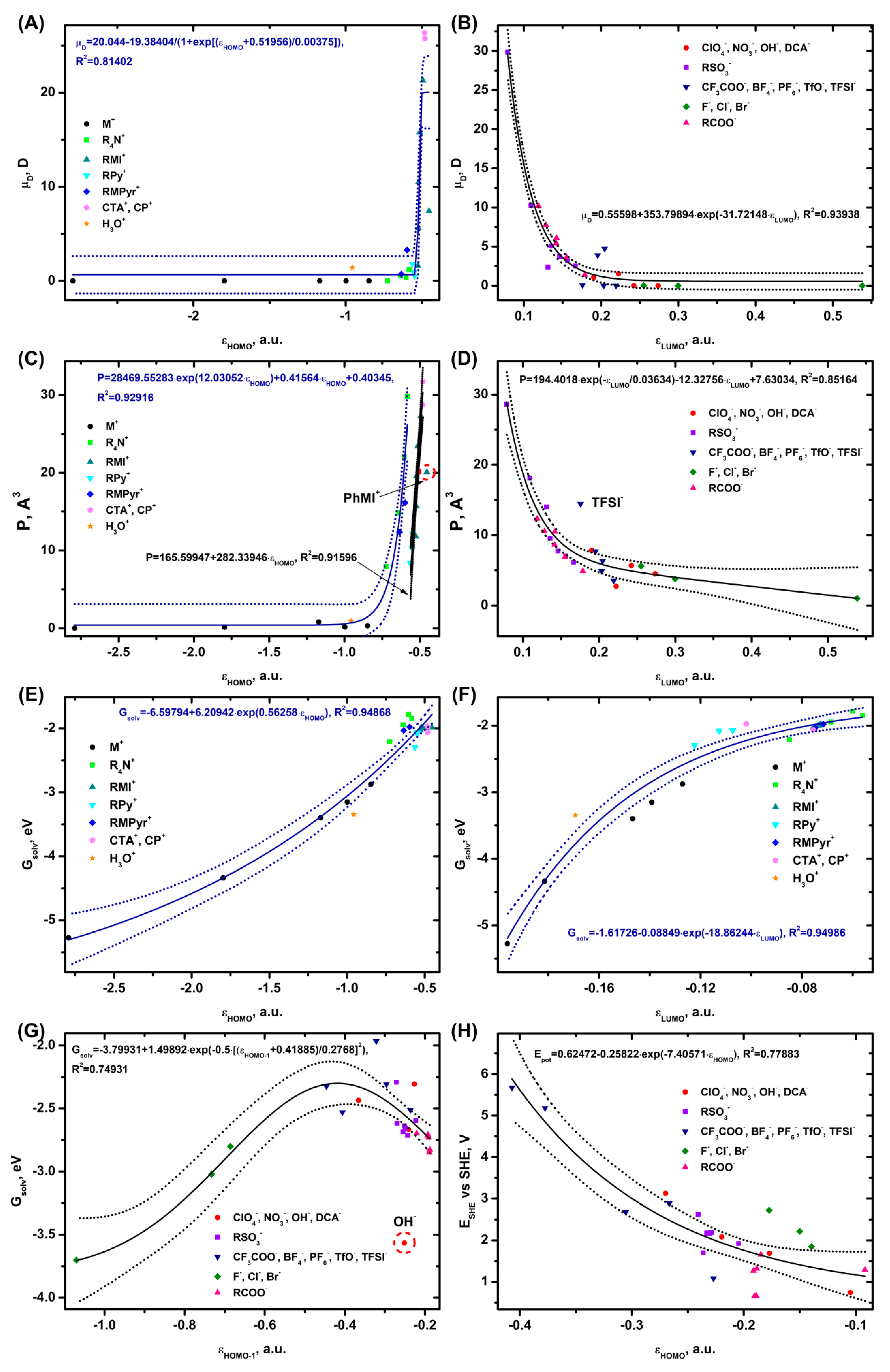
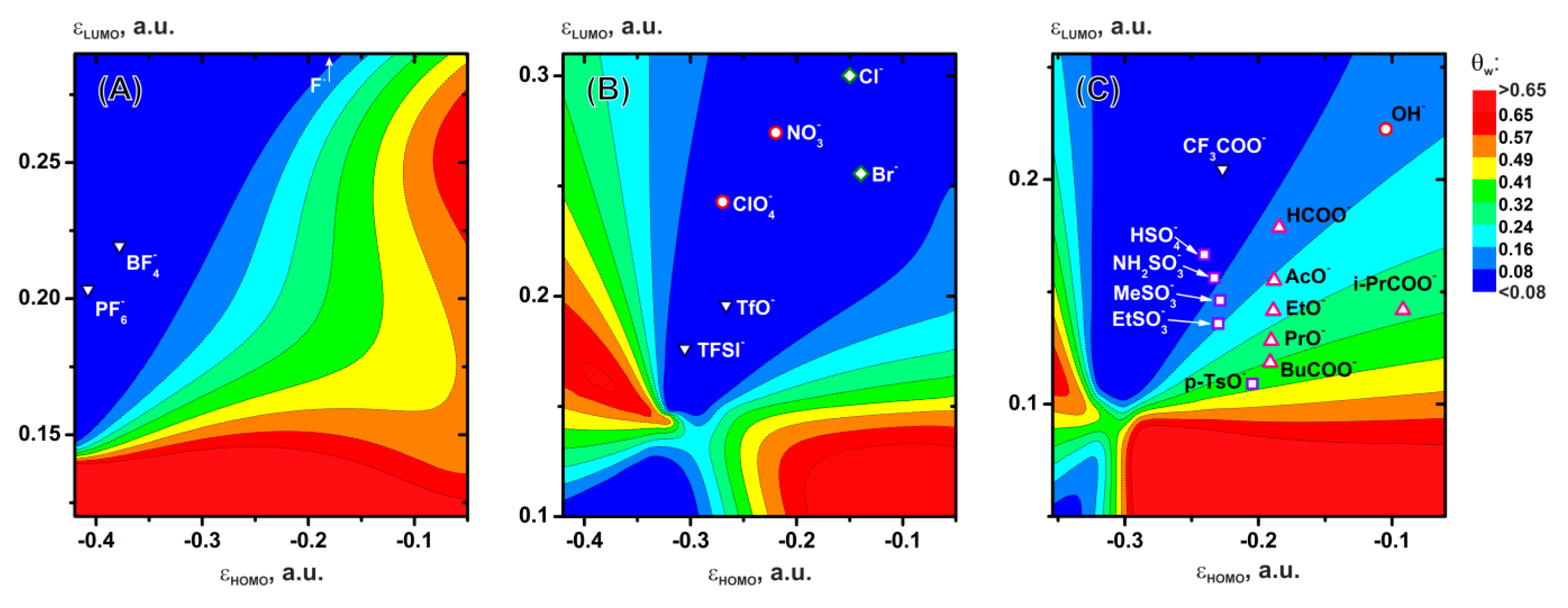
3.4. Correlations with Physicochemical and Electrochemical Parameters of Ions
3.5. Practical Evaluations
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Li, B.; Gao, W.; Jiang, Q. Electronic and Geometric Determinants of Adsorption: Fundamentals and Applications. Journal of Physics: Energy 2021, 3, 022001. [Google Scholar] [CrossRef]
- Zhao, Z.-J.; Liu, S.; Zha, S.; Cheng, D.; Studt, F.; Henkelman, G.; Gong, J. Theory-Guided Design of Catalytic Materials Using Scaling Relationships and Reactivity Descriptors. Nature Reviews Materials 2019, 4, 792–804. [Google Scholar] [CrossRef]
- Newns, D.M. Self-Consistent Model of Hydrogen Chemisorption. Physical Review 1969, 178, 1123–1135. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J.K. (1997). Theory of Adsorption and Surface Reactions. In: Lambert, R.M., Pacchioni, G. (eds) Chemisorption and Reactivity on Supported Clusters and Thin Films. NATO ASI Series, vol 331. Springer, Dordrecht. [CrossRef]
- Hammer, B. Special Sites at Noble and Late Transition Metal Catalysts. Top Catal, 2006, 37, 3–16. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design Principles for Oxygen-Reduction Activity on Perovskite Oxide Catalysts for Fuel Cells and Metal–Air Batteries. Nature Chemistry 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Jaksic, M.M.; Jaksic, J.M. Fermi Dynamics and Some Structural Bonding Aspects of Electrocatalysis for Hydrogen Evolution. Electrochimica Acta 1994, 39, 1695–1714. [Google Scholar] [CrossRef]
- JJakšić, M.; Krstajić, N.V.; Grgur, B.N.; Jakšić, M.M. Hydridic and Electrocatalytic Properties of Hypo-Hyper-d-Electronic Combinations of Transition Metal Intermetallic Phases*1. International Journal of Hydrogen Energy 1998, 23, 667–681. [Google Scholar] [CrossRef]
- Abild-Pedersen, F.; Greeley, J.; Studt, F.; Rossmeisl, J.; Munter, T.R.; Moses, P.G.; Skúlason, E.; Bligaard, T.; Nørskov, J.K. Scaling Properties of Adsorption Energies for Hydrogen-Containing Molecules on Transition-Metal Surfaces. Physical Review Letters 2007, 99. [Google Scholar] [CrossRef] [PubMed]
- Calle-Vallejo, F.; Inoglu, N.G.; Su, H.-Y.; Martínez, J.I.; Man, I.C.; Koper, M.T.M.; Kitchin, J.R.; Rossmeisl, J. Number of Outer Electrons as Descriptor for Adsorption Processes on Transition Metals and Their Oxides. Chemical Science 2013, 4, 1245. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Li, B.; Liu, S.-P.; Liu, X.; Jiang, Q. Determining the Adsorption Energies of Small Molecules with the Intrinsic Properties of Adsorbates and Substrates. Nature Communications 2020, 11. [Google Scholar] [CrossRef]
- Doronin, S.V.; Dokhlikova, N.V.; Grishin, M.V. Descriptor of Catalytic Activity Nanoparticles Surface: Atomic and Molecular Hydrogen on Gold. Molecular Catalysis 2022, 529, 112534. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Davey, K.; Qiao, S.-Z. . Activity Origin and Catalyst Design Principles for Electrocatalytic Hydrogen Evolution on Heteroatom-Doped Graphene. Nature Energy 2016, 1. [Google Scholar] [CrossRef]
- Doronin, S.V.; Volykhov, A.A.; Inozemtseva, A.I.; Usachov, D.Y.; Yashina, L.V. Comparative Catalytic Activity of Graphene Imperfections in Oxygen Reduction Reaction. The Journal of Physical Chemistry C 2020, 124, 6038–6053. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chemical Reviews 2018, 118, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Tian, Y.; An, Y.; Liu, R.; Shaik, F. Electrocatalytic Activity Analysis of Vinegar Residue-Based Heteroatom-Doped Carbon Quantum Dots Integrated on Vertically Aligned Graphene Arrays for Hydrogen Evolution Reaction. International Journal of Hydrogen Energy 2023, 48, 38686–38698. [Google Scholar] [CrossRef]
- Cui, X.; Tang, C.; Zhang, Q. . A Review of Electrocatalytic Reduction of Dinitrogen to Ammonia under Ambient Conditions. Advanced Energy Materials 2018, 8. [Google Scholar] [CrossRef]
- Manjunatha, R.; Karajić, A.; Liu, M.; Zhai, Z.; Dong, L.; Yan, W.; Wilkinson, D.P.; Zhang, J. A Review of Composite/Hybrid Electrocatalysts and Photocatalysts for Nitrogen Reduction Reactions: Advanced Materials, Mechanisms, Challenges and Perspectives. Electrochemical Energy Reviews 2020, 3, 506–540. [Google Scholar] [CrossRef]
- Masoumi, Z.; Tayebi, M.; Tayebi, M.; Lari, S.A.M.; Sewwandi, N.; Seo, B.; Lim, C.-S.; Kim, H.-G.; Kyung, D. Electrocatalytic Reactions for Converting CO2 to Value-Added Products: Recent Progress and Emerging Trends. International Journal of Molecular Sciences 2023, 24, 9952. [Google Scholar] [CrossRef]
- Sajna, M.S.; Zavahir, S.; Popelka, A.; Kasak, P.; Al-Sharshani, A.; Onwusogh, U.; Wang, M.; Park, H.; Han, D.S. Electrochemical System Design for CO2 Conversion: A Comprehensive Review. Journal of Environmental Chemical Engineering 2023, 11, 110467. [Google Scholar] [CrossRef]
- Cardenas, C.; Rabi, N.; Ayers, P.W.; Morell, C.; Jaramillo, P.; Fuentealba, P. Chemical Reactivity Descriptors for Ambiphilic Reagents: Dual Descriptor, Local Hypersoftness, and Electrostatic Potential. The Journal of Physical Chemistry A 2009, 113, 8660–8667. [Google Scholar] [CrossRef]
- Pearson, R.G. The Electronic Chemical Potential and Chemical Hardness. Journal of Molecular Structure: THEOCHEM 1992, 255, 261–270. [Google Scholar] [CrossRef]
- Choudhary, V.K.; Bhatt, A.K.; Dash, D.; Sharma, N. DFT Calculations on Molecular Structures, HOMO–LUMO Study, Reactivity Descriptors and Spectral Analyses of Newly Synthesized Diorganotin(IV) 2-chloridophenylacetohydroxamate Complexes. Journal of Computational Chemistry 2019, 40, 2354–2363. [Google Scholar] [CrossRef]
- Karelson, M.; Lobanov, V.S.; Katritzky, A.R. Quantum-Chemical Descriptors in QSAR/QSPR Studies. Chemical Reviews 1996, 96, 1027–1044. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, M. On the Alleged Importance of the Molecular Electron-Donating Ability and the HOMO–LUMO Gap in Corrosion Inhibition Studies. Corrosion Science 2021, 180, 109016. [Google Scholar] [CrossRef]
- Abraham, M.H.; Acree, W.E. The Transfer of Neutral Molecules, Ions and Ionic Species from Water to Wet Octanol. Physical Chemistry Chemical Physics 2010, 12, 13182. [Google Scholar] [CrossRef]
- Abraham, M.H.; Acree, W.E. Descriptors for Ions and Ion-Pairs for Use in Linear Free Energy Relationships. Journal of Chromatography A 2016, 1430, 2–14. [Google Scholar] [CrossRef]
- Doronin, S.V.; Nazarov, M.A. Superconcentrated Electrolytes for Aqueous Batteries Based on Alkali Metal Formates and Propionates. The Journal of Physical Chemistry C 2022, 126, 14611–14625. [Google Scholar] [CrossRef]
- Bailey, E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A Review of Potentially Low-Cost Sorbents for Heavy Metals. Water Research 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Burakov, E.; Galunin, V.; Burakova, V.; Kucherova, E.; Agarwal, S.; Tkachev, G.; Gupta, K. Adsorption of Heavy Metals on Conventional and Nanostructured Materials for Wastewater Treatment Purposes: A Review. Ecotoxicology and Environmental Safety 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. Journal of Cleaner Production 2021, 296, 126589. [Google Scholar] [CrossRef]
- Kamachi, T.; Tatsumi, T.; Toyao, T.; Hinuma, Y.; Maeno, Z.; Takakusagi, S.; Furukawa, S.; Takigawa, I.; Shimizu, K. Linear Correlations between Adsorption Energies and HOMO Levels for the Adsorption of Small Molecules on TiO2 Surfaces. The Journal of Physical Chemistry C 2019, 123, 20988–20997. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Takao, M.; Toyao, T.; Maeno, Z.; Kamachi, T.; Hinuma, Y.; Takigawa, I.; Shimizu, K. Frontier Molecular Orbital Based Analysis of Solid–Adsorbate Interactions over Group 13 Metal Oxide Surfaces. The Journal of Physical Chemistry C 2020, 124, 15355–15365. [Google Scholar] [CrossRef]
- Hamamoto, N.; Tatsumi, T.; Takao, M.; Toyao, T.; Hinuma, Y.; Shimizu, K.; Kamachi, T. Effect of Oxygen Vacancies on Adsorption of Small Molecules on Anatase and Rutile TiO2 Surfaces: A Frontier Orbital Approach. The Journal of Physical Chemistry C 2021, 125, 3827–3844. [Google Scholar] [CrossRef]
- Pérez, P.; Contreras, R.; Aizman, A. Relationship between Solvation Energy, Chemical Potential and Hardness Variations. Journal of Molecular Structure: THEOCHEM 1997, 390, 169–175. [Google Scholar] [CrossRef]
- Miranda-Quintana, R.A.; Smiatek, J. Theoretical Insights into Specific Ion Effects and Strong-Weak Acid-Base Rules for Ions in Solution: Deriving the Law of Matching Solvent Affinities from First Principles. ChemPhysChem 2020, 21, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Doronin, S.V.; Manzhos, R.A.; Krivenko, A.G.; Manzhos, A.P. Electron Transfer Kinetics of the Ferrous/Ferric Redox System on the Platinum Deposits on Gold. Journal of Electroanalytical Chemistry 2017, 784, 140–144. [Google Scholar] [CrossRef]
- Doronin, S.V. Energy of the Surface Segregation of Ag Atoms in Ag–Au Alloys in an Aqueous Solution. Mendeleev Communications 2020, 30, 288–290. [Google Scholar] [CrossRef]
- Doronin, S.V.; Manzhos, R.A.; Krivenko, A.G. EDL Structure and Peculiarities of Ferricyanide Cyclic Voltammetry for Silver Deposits on Gold. Electrochemistry Communications 2015, 57, 35–38. [Google Scholar] [CrossRef]
- Fuchigami, T.; Atobe, M.; Inagi, S. Fundamentals and Applications of Organic Electrochemistry: Synthesis, Materials, Devices; John Wiley & Sons, 2014, p.
- Volke, J.; Liška, F. Electrochemistry in Organic Synthesis; Springer Science & Business Media, 2012, p.153.
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic Liquids as Electrolytes. Electrochimica Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical Properties of Ionic Liquids: Database and Evaluation. Journal of Physical and Chemical Reference Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.; Qing, T.; Fan, X.; Sun, W.; von Cresce, A.; Ding, M.S.; Borodin, O.; Vatamanu, J.; Schroeder, M.A.; Eidson, N.; Wang, C.; Xu, K. 4.0 V Aqueous Li-Ion Batteries. Joule 2017, 1, 122–132. [Google Scholar] [CrossRef]
- Vatamanu, J.; Borodin, O. Ramifications of Water-in-Salt Interfacial Structure at Charged Electrodes for Electrolyte Electrochemical Stability. The Journal of Physical Chemistry Letters 2017, 8, 4362–4367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, M.; Ta, K.; Madsen, K.E.; Chen, X.; Zhang, X.; Espinosa-Marzal, R.M.; Gewirth, A.A. Potential-Dependent Layering in the Electrochemical Double Layer of Water-in-Salt Electrolytes. ACS Applied Energy Materials 2020, 3, 8086–8094. [Google Scholar] [CrossRef]
- Han, J.; Mariani, A.; Passerini, S.; Varzi, A. A Perspective on the Role of Anions in Highly Concentrated Aqueous Electrolytes. Energy & Environmental Science 2023, 16, 1480–1501. [Google Scholar] [CrossRef]
- Jow, T.R.; Xu, K.; Borodin, O.; Ue, M. Electrolytes for Lithium and Lithium-Ion Batteries; Springer, 2014, 476. [CrossRef]
- Jonsson, E.; Johansson, P. Electrochemical Oxidation Stability of Anions for Modern Battery Electrolytes: A CBS and DFT Study. Physical Chemistry Chemical Physics 2015, 17, 3697–3703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lewis, N.H.C.; Mars, J.; Wan, G.; Weadock, N.J.; Takacs, C.J.; Lukatskaya, M.R.; Steinrück, H.-G.; Toney, M.F.; Tokmakoff, A.; Maginn, E.J. Water-in-Salt LiTFSI Aqueous Electrolytes. 1. Liquid Structure from Combined Molecular Dynamics Simulation and Experimental Studies. The Journal of Physical Chemistry B 2021, 125, 4501–4513. [Google Scholar] [CrossRef]
- Vazquez, D.G.; Pollard, T.P.; Mars, J.; Yoo, J.M.; Steinrück, H.-G.; Bone, S.E.; Safonova, O.V.; Toney, M.F.; Borodin, O.; Lukatskaya, M.R. Creating Water-in-Salt-like Environment Using Coordinating Anions in Non-Concentrated Aqueous Electrolytes for Efficient Zn Batteries. Energy & Environmental Science 2023, 16, 1982–1991. [CrossRef]
- P. Giannozzi et al., J. Phys. Condens. Matter 2009, 21, 395502. [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Physical Review Letters 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Physical Review B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Physical Review B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Physical Review B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Methfessel, M.; Paxton, A.T. High-Precision Sampling for Brillouin-Zone Integration in Metals. Physical Review B 1989, 40, 3616–3621. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurateab Initioparametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. The Journal of Chemical Physics 2010, 132. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Computational Molecular Science 2011, 2, 73–78. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. The Journal of Chemical Physics 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. The Journal of Physical Chemistry 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. The Journal of Physical Chemistry A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab Initio Study of Solvated Molecules: A New Implementation of the Polarizable Continuum Model. Chemical Physics Letters 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chemical Reviews 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Tundo, P.; Selva, M. The Chemistry of Dimethyl Carbonate. Accounts of Chemical Research 2002, 35, 706–716. [Google Scholar] [CrossRef]
- Comelli, F.; Francesconi, R. Isothermal Vapor-Liquid Equilibria, Densities, Refractive Indices, Excess Molar Volumes, and Excess Molar Enthalpies of Dimethyl Carbonate + 1,2-Dichloroethane and + 1,1,1-Trichloroethane. Journal of Chemical & Engineering Data 1994, 39, 560–564. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Banerjee, K. Density, Viscosity, Refractive Index, and Speed of Sound in Binary Mixtures of Dimethyl Carbonate with Methanol, Chloroform, Carbon Tetrachloride, Cyclohexane, and Dichloromethane in the Temperature Interval (298.15−308.15) K. Journal of Chemical & Engineering Data 1998, 43, 1096–1101. [Google Scholar]
- Cramer, C.J.; Truhlar, D.G. A Universal Approach to Solvation Modeling. Accounts of Chemical Research 2008, 41, 760–768. [Google Scholar] [CrossRef]
- Trasatti, S. The Absolute Electrode Potential: An Explanatory Note (Recommendations 1986). Pure and Applied Chemistry 1986, 58, 955–966. [Google Scholar] [CrossRef]
- A.H. Larsen et al, The Atomic Simulation Environment—a Python Library for Working with Atoms. Journal of Physics: Condensed Matter 2017, 29, 273002. [CrossRef]
- Gao, Y.Q.; Georgievskii, Y.; Marcus, R.A. On the Theory of Electron Transfer Reactions at Semiconductor Electrode/Liquid Interfaces. The Journal of Chemical Physics 2000, 112, 3358–3369. [Google Scholar] [CrossRef]
- Doronin, S.V.; Budkov, Y.A.; Itkis, D.M. Electrocatalytic Activity of Doped Graphene: Quantum-Mechanical Theory View. Carbon 2021, 175, 202–214. [Google Scholar] [CrossRef]
- Anderson, P.W. Localized Magnetic States in Metals. Physical Review 1961, 124, 41–53. [Google Scholar] [CrossRef]
- Rosen, A.S.; Vijay, S.; Persson, K.A. Free-Atom-like d States beyond the Dilute Limit of Single-Atom Alloys. Chemical Science 2023, 14, 1503–1511. [Google Scholar] [CrossRef]
- Vijay, S.; Kastlunger, G.; Chan, K.; Nørskov, J.K. Limits to Scaling Relations between Adsorption Energies? The Journal of Chemical Physics 2022, 156. [Google Scholar] [CrossRef]
- Schmickler, W.; Santos, E.; Bronshtein, M.; Nazmutdinov, R. Adiabatic Electron-Transfer Reactions on Semiconducting Electrodes. ChemPhysChem 2016, 18, 111–116. [Google Scholar] [CrossRef]
- Santos, E.; Nazmutdinov, R.; Schmickler, W. Electron Transfer at Different Electrode Materials: Metals, Semiconductors, and Graphene. Current Opinion in Electrochemistry 2020, 19, 106–112. [Google Scholar] [CrossRef]
- Schmickler, W.; Henderson, D. New Models for the Structure of the Electrochemical Interface. Progress in Surface Science 1986, 22, 323–419. [Google Scholar] [CrossRef]
- Guidelli, R.; Schmickler, W. Recent Developments in Models for the Interface between a Metal and an Aqueous Solution. Electrochimica Acta 2000, 45, (15–16). [Google Scholar] [CrossRef]
- Nazmutdinov, R.R.; Berezin, A.S.; Soldano, G.; Schmickler, W. Orbital Overlap Effects in Electron Transfer Reactions across a Metal Nanowire/Electrolyte Solution Interface. The Journal of Physical Chemistry C 2013, 117, 13021–13027. [Google Scholar] [CrossRef]
- Kornyshev, A.A.; Kuznetsov, A.M.; Ulstrup, J. Effect of Overpotential on the Electronic Tunnel Factor in Diabatic Electrochemical Processes. The Journal of Physical Chemistry 1994, 98, 3832–3837. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Georgievskii, Y.; Marcus, R.A. On the Theory of Electron Transfer Reactions at Semiconductor Electrode/Liquid Interfaces. The Journal of Chemical Physics 2000, 112, 3358–3369. [Google Scholar] [CrossRef]
- Nazmutdinov, R.R.; Tsirlina, G.A.; Manyurov, I.R.; Bronshtein, M.D.; Titova, N.V.; Kuzminova, Z.V. Misleading Aspects of the Viscosity Effect on the Heterogeneous Electron Transfer Reactions. Chemical Physics 2006, 326, 123–137. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Marcus, R.A. On the Theory of Electron Transfer Reactions at Semiconductor/Liquid Interfaces. II. A Free Electron Model. The Journal of Chemical Physics 2000, 113, 6351–6360. [Google Scholar] [CrossRef]
- Medvedev, G. Non-Local Effects in the Kinetics of Heterogeneous Charge Transfer Reactions. Journal of Electroanalytical Chemistry 2000, 481, 215–221. [Google Scholar] [CrossRef]
- Schmickler, W. The Surface Dipole Moment of Species Adsorbed from a Solution. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry 1988, 249, (1–2). [Google Scholar] [CrossRef]
- Krylov, V.S.; Damaskin, B.B.; Kir, V.A. The Present State and Problems of the Theory of the Kinetics of Electrode Reactions Accompanied by the Adsorption of Inactive Substances and Reagents. Russian Chemical Reviews 1986, 55, 706–720. [Google Scholar] [CrossRef]
- Brown, W.B.; Chang, T.Y. Exact Formula for the Dipole Moment of a One-Electron Diatomic Molecule. Proceedings of the Royal Society of London. A. Mathematical and Physical Sciences 1985, 401, 373–392. [Google Scholar] [CrossRef]
- Mitroy, J.; Safronova, M.S.; Clark, C.W. Theory and Applications of Atomic and Ionic Polarizabilities. Journal of Physics B: Atomic, Molecular and Optical Physics 2010, 43, 202001. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. A Universal Approach to Solvation Modeling. Accounts of Chemical Research 2008, 41, 760–768. [Google Scholar] [CrossRef]
- Bryantsev, V.S.; Diallo, M.S.; Goddard, W.A., III. Calculation of Solvation Free Energies of Charged Solutes Using Mixed Cluster/Continuum Models. The Journal of Physical Chemistry B 2008, 112, 9709–9719. [Google Scholar] [CrossRef]
- Kelly, C.P.; Cramer, C.J.; Truhlar, D.G. Aqueous Solvation Free Energies of Ions and Ion−Water Clusters Based on an Accurate Value for the Absolute Aqueous Solvation Free Energy of the Proton. The Journal of Physical Chemistry B 2006, 110, 16066–16081. [Google Scholar] [CrossRef] [PubMed]
- Parker, V.D. Energetics of Electrode Reactions. II. The Relationship between Redox Potentials, Ionization Potentials, Electron Affinities, and Solvation Energies of Aromatic Hydrocarbons. Journal of the American Chemical Society 1976, 98, 98–103. [Google Scholar] [CrossRef]
- Marenich, A.V.; Ho, J.; Coote, M.L.; Cramer, C.J.; Truhlar, D.G. Computational Electrochemistry: Prediction of Liquid-Phase Reduction Potentials. Phys. Chem. Chem. Phys. 2014, 16, 15068–15106. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon Nanomaterials for High-Performance Supercapacitors. Materials Today 2013, 16, (7–8). [Google Scholar] [CrossRef]
- Lemine, A.S.; Zagho, M.M.; Altahtamouni, T.M.; Bensalah, N. Graphene a Promising Electrode Material for Supercapacitors-A Review. International Journal of Energy Research 2018, 42, 4284–4300. [Google Scholar] [CrossRef]
- Iamprasertkun, P.; Ejigu, A.; Dryfe, R.A.W. Understanding the Electrochemistry of “Water-in-Salt” Electrolytes: Basal Plane Highly Ordered Pyrolytic Graphite as a Model System. Chemical Science 2020, 11, 6978–6989. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent Progress in the Synthesis of Graphene and Derived Materials for next Generation Electrodes of High Performance Lithium Ion Batteries. Progress in Energy and Combustion Science 2019, 75, 100786. [Google Scholar] [CrossRef]
- Nikitas, P. Theory of Electrochemically Modulated Liquid Chromatography. Journal of Electroanalytical Chemistry 2000, 484, 137–143. [Google Scholar] [CrossRef]
- Nikitas, P.; Pappa-Louisi, A. Adsorption Isotherms for Coadsorption Studies from Solution. Canadian Journal of Chemistry 1986, 64, 328–332. [Google Scholar] [CrossRef]
- Nikitas, P. A Unified Treatment of the Equilibrium Properties of Electrosorbed Layers Composed of Neutral and Ionic Species. Electrochimica Acta 1996, 41, 2159–2170. [Google Scholar] [CrossRef]
- Grahame, D.C. The Electrical Double Layer and the Theory of Electrocapillarity. Chemical Reviews 1947, 41, 441–501. [Google Scholar] [CrossRef] [PubMed]
- CRC Handbook of Chemistry and Physics, 95th Edition by William M. Haynes. CRC Press. 2014.
- Macdonald, J.R.; Barlow, C.A. Work Function Change on Monolayer Adsorption. The Journal of Chemical Physics 1963, 39, 412–422. [Google Scholar] [CrossRef]
- Di Muzio, S.; Palumbo, O.; Brutti, S.; Paolone, A. Thermodynamic Analysis of the Hydrolysis of Borate-Based Lithium Salts by Density Functional Theory. Journal of The Electrochemical Society 2022, 169, 070523. [Google Scholar] [CrossRef]
- Younesi, R.; Veith, G.M.; Johansson, P.; Edstrom, K.; Vegge, T. Lithium Salts for Advanced Lithium Batteries: Li–Metal, Li–O2, and Li–S. Energy & Environmental Science 2015, 8, 1905–1922. [Google Scholar] [CrossRef]
- Kawamura, T.; Sonoda, T.; Okada, S.; Yamaki, J. Improvement of the Stability of LiPF6 Electrolytes toward Water by the Addition of LiCl. Electrochemistry 2003, 71, 1139–1141. [Google Scholar] [CrossRef]
- Nagayama, K.; Kamioka, K.; Iwata, E.; Oka, H.; Tokunaga, Y.; Okada, T. The Reaction of Lithium-Manganese Oxides for the Cathode Materials of Rechargeable Lithium Batteries with Nonaqueous Electrolyte. Electrochemistry 2001, 69, 6–9. [Google Scholar] [CrossRef]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Chiang, C.H.; Tateyama, Y.; Yamada, A. Superconcentrated Electrolytes for a High-Voltage Lithium-Ion Battery. Nature Communications 2016, 7, 12032. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Wang, Y.; Sun, Y.; Lang, J.; Yang, S.; Yan, X. “Cation/Anion with Co-Solvation” Type High-Voltage Aqueous Electrolyte Enabled by Strong Hydrogen Bonding. Nano Energy 2022, 99, 107377. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Sun, W.; Fan, X.; Yang, C.; Wang, F.; Gao, T.; Ma, Z.; Schroeder, M.; von Cresce, A.; Russell, S.M.; Armand, M.; Angell, A.; Xu, K.; Wang, C. Advanced High-Voltage Aqueous Lithium-Ion Battery Enabled by “Water-in-Bisalt” Electrolyte. Angewandte Chemie International Edition 2016, 55, 7136–7141. [Google Scholar] [CrossRef]
- Wu, S.L.; Su, B.Z.; Sun, M.Z.; Gu, S.; Lu, Z.G.; Zhang, K.L.; Yu, D.Y.W.; Huang, B.L.; Wang, P.F.; Lee, C.-S.; Zhang, W.J. Dilute Aqueous-Aprotic Hybrid Electrolyte Enabling a Wide Electrochemical Window through Solvation Structure Engineering. Advanced Materials 2021, 33. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, H.; Shikata, H.; Takao, K.; Asanuma, N.; Taruta, S.; Park, Y.-Y. An Aqueous Electrolyte of the Widest Potential Window and Its Superior Capability for Capacitors. Scientific Reports 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tan, G.; Shan, P.; Liu, T.; Hu, J.; Feng, Y.; Yang, L.; Zhang, M.; Chen, Z.; Lin, Y.; Lu, J.; Neuefeind, J.C.; Ren, Y.; Amine, K.; Wang, L.-W.; Xu, K.; Pan, F. Understanding Thermodynamic and Kinetic Contributions in Expanding the Stability Window of Aqueous Electrolytes. Chem 2018, 4, 2872–2882. [Google Scholar] [CrossRef]
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable Lithium Batteries with Aqueous Electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, M.I.; Cheng, F.; Lu, W. A Review on Selection Criteria of Aqueous Electrolytes Performance Evaluation for Advanced Asymmetric Supercapacitors. Journal of Energy Storage 2021, 40, 102729. [Google Scholar] [CrossRef]
- Tomiyasu, H.; Shikata, H.; Takao, K.; Asanuma, N.; Taruta, S.; Park, Y.-Y. An Aqueous Electrolyte of the Widest Potential Window and Its Superior Capability for Capacitors. Scientific Reports 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Redondo, E.; Goikolea, E.; Mysyk, R. The Decisive Role of Electrolyte Concentration in the Performance of Aqueous Chloride-Based Carbon/Carbon Supercapacitors with Extended Voltage Window. Electrochimica Acta 2016, 221, 177–183. [Google Scholar] [CrossRef]
- Li, C.; Zhang, S.; Wang, Y.; Liu, H.; Xing, T.; Lin, Y.; Rong, X.; Ren, H.; Wu, M.; Abbas, Q.; Li, Z. Dual Breaking of Ionic Association in Water-in-LiTFSI Electrolyte for Low Temperature Battery Applications. Journal of Power Sources 2022, 544, 231874. [Google Scholar] [CrossRef]
- Yang, B.; Tang, X.; She, W.; Zhang, D.; He, Y.; Wang, B.; Xia, X.; Li, Y.; Han, Z.; Wang, K. Exploring Potassium Trifluoroacetate as a Novel Electrolyte for Enhancing Energy Density of Biomass-Derived Carbon-Based Supercapacitors and Improving Electrolyte-Electrode Adaptability. Journal of Energy Storage 2023, 73, 108805. [Google Scholar] [CrossRef]
- Li, M.; Su, H.; Zheng, G.; Kuhn, U.; Kim, N.; Li, G.; Ma, N.; Pöschl, U.; Cheng, Y. Aerosol pH and Ion Activities of HSO4– and SO4 [2]– in Supersaturated Single Droplets. Environmental Science & Technology 2022, 56, 12863–12872. [Google Scholar] [CrossRef]
- A. Guha; Sahoo, M.; Alam, K.; Rao, D.K.; Sen, P.; Narayanan, T.N. Role of Water Structure in Alkaline Water Electrolysis. iScience 2022, 25, 104835. [Google Scholar] [CrossRef]
- He, Z.-H.; Gao, J.-F.; Kong, L.-B. Electrolyte Effect on Electrochemical Behaviors of Manganese Fluoride Material for Aqueous Asymmetric and Symmetric Supercapacitors. Rare Metals 2023, 43, 1048–1061. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Methanesulfonic Acid (MSA) in Hydrometallurgy. Journal of Sustainable Metallurgy 2022, 9, 26–45. [Google Scholar] [CrossRef]
- Amini, K.; Pritzker, M.D. Improvement of Zinc-Cerium Redox Flow Batteries Using Mixed Methanesulfonate-Chloride Negative Electrolyte. Applied Energy 2019, 255, 113894. [Google Scholar] [CrossRef]
- Li, Z.; Robertson, A.W. Electrolyte Engineering Strategies for Regulation of the Zn Metal Anode in Aqueous Zn-ion Batteries. Battery Energy 2022, 2. [Google Scholar] [CrossRef]
- Liu, T.; Tang, L.; Luo, H.; Cheng, S.; Liu, M. A Promising Water-in-Salt Electrolyte for Aqueous Based Electrochemical Energy Storage Cells with a Wide Potential Window: Highly Concentrated HCOOK. Chemical Communications 2019, 55, 12817–12820. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Wang, X.; Liu, C.; Li, C.; Chen, J.; Zhu, N.; Li, R.; Xue, M. Li/K Mixed Superconcentrated Aqueous Electrolyte Enables High-Performance Hybrid Aqueous Supercapacitors. Energy Storage Materials 2019, 20, 373–379. [Google Scholar] [CrossRef]
- Han, J.; Zhang, H.; Varzi, A.; Passerini, S. Fluorine-Free Water-in-Salt Electrolyte for Green and Low-Cost Aqueous Sodium-Ion Batteries. ChemSusChem 2018, 11, 3704–3707. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Feldblyum, J.I.; Mackanic, D.G.; Lissel, F.; Michels, D.L.; Cui, Y.; Bao, Z. Concentrated Mixed Cation Acetate “Water-in-Salt” Solutions as Green and Low-Cost High Voltage Electrolytes for Aqueous Batteries. Energy & Environmental Science 2018, 11, 2876–2883. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




