1. Introduction
Erythromycin is an important antibiotic in the healthcare system, as it has broadly been applied in clinical treatment over the last years [
1,
2,
3,
4]. In 1949, a team of scientists led by Aguilar first isolated and identified erythromycin from a Philippine soil sample [
3,
4,
5,
6]. Erythromycin was produced by
Saccharopolyspora erythraea – formerly called
Streptomyces erythraeus – a gram–positive bacterium, that produces antibiotic compounds and is isolated from soil [
3,
4,
7,
8,
9]. It was later discovered, that this erythromycin compound could prevent the growth of bacteria and was since used as an antibiotic agent [
3,
10,
11,
12,
13]. Lately, it has been implied, that erythromycin’s modification improves its acidic stability and optimizes this antibiotic for local drug delivery [
12]. It must be highlighted that as of 2023, for instance, a transethosomes – loaded, cinnamon oil – based emulgel, is an effective alternative way to deliver erythromycin for the treatment of topical bacterial infections, culminating in the vindication of erythromycin’s antimicrobial efficiency and potential in the pharmaceutical and medical field [
13].
Erythromycin is synthesized by
Saccharopolyspora erythraea via a multi-step enzymatic process involving the cytochromeP450 enzyme and P450eryF [
3,
14,
15,
16,
17]. P450eryF in fact plays a significant role, as the catalyst in every step of the synthetic reaction of erythromycin. More specifically, eryF is an enzyme that belongs to a group of cytochromes, the P450 enzymes, that is also known as P450eryF [
16,
18,
19,
20,
21]. The catalytic reaction that occurs, proceeds by using eryF to fabricate a group of compounds and ultimately, via many intermediate reactions erythromycin A is formed [
16]. In addition to P450eryF’s role in the production of 6-DEB, the same enzyme is also vital for achieving the proper formation of the macrolide ring, thus making the molecule active as an antibiotic [
17].
In the industrial production of erythromycin, various methods of crystallization are used to ensure a continuous line of production, which are associated with a high yield and an elimination of erythromycin B & C. Via the utilized crystallization technique, erythromycin can be commercially available, and simultaneously remains economically profitable according to the global market’s needs [
22,
23,
24]. It is widely suggested that the production of erythromycin is of great importance, because of erythromycin’s functions in day – to – day life [
25,
26,
27]. Erythromycin displays a significant clinical activity against a variety of mostly bacteria, while it is utilized as a component in bone cement destined for infection prevention purposes [
9,
28]. The group of P450 cytochromes on the contrary, is generally involved in vital functions of the human body including homeostasis, as well as in the ecosystem’s well–being, since for instance, they contribute to the oxidations of xenobiotic chemicals in microorganisms, higher animals and plants [
29]. Cytochromes’ assistance in the metabolism of endogenous substances is indisputable, as such enzymes metabolize hosts of xenobiotics in hepatocytes, while they efficiently remove potentially toxic compounds [
9,
25,
26,
27,
30,
31].
In the year span between 2011 and 2021, the production of erythromycin has mostly increased. However, there were still some continents (Europe and North America) where their total production notably decreased [
31]. Especially during the COVID-19 outbreak, some countries (Asia, South America and Africa) took advantage of this situation, exported erythromycin antibiotics and managed to profit. As a result, their erythromycin production skyrocketed, while many other countries displayed little to no erythromycin import or export activity. Concurrently, hundreds of states, countries, organizations, and different applicants, including pharmaceutical companies, incorporations, universities, colleges and individual inventors from all over the globe (United States of America, China, Japan, the European Patent Office, the Eurasian Patent Organization, the University of California, Novartis, Hoffmann la Roche), have contributed with patents aiming to increase their erythromycin production rates and thus, encourage its wider, worldwide utilization towards combating both infectious diseases and the side effects of many conventional drugs [
9,
31,
32,
33,
34,
35,
36].
Even though the antibiotic of erythromycin has been put aside those five (5) last years, mostly because of the COVID – 19 pandemic outbreak and the global need for its efficient medical treatment, recent studies have implied the crucial capability of erythromycin towards COVID – 19’s treatment. The aim of this study is to elucidate the production of erythromycin, as well as to evaluate its vast potential towards the treatment of globally – spreading diseases including the pandemic of COVID – 19.
2. Historical Retrospective and Recent Applications
2.1. Reactions’ discovery
The dawn of the cytochrome P450 enzymes’ discovery dates back in the 1940s, during experiments that dealt with a class of reactions involving molecular oxygen (O
2) [
1,
2]. Being acquainted that such enzymes further catalyze the erythromycin production, it was ascertained that the antibiotic of erythromycin was a significant invention.
Erythromycin was only later declared as an alternative drug for penicillin – allergic patients. Embarking on the original work organized by a group of scientists guided by Aguilar (1949) [
3], McGuire et al. (1952), named this extraordinary compound “Erythromycin”[
4]. On the other hand, the uncovering of cytochrome P450 enzymes, is attributed to the work of Sato and Omura (1962), who isolated the erythromycin compound produced by the bacterium
Streptomyces erythreus, that was in turn able to restrain the growth of bacteria. Since then, it was confirmed that erythromycin’s production was inhibited by the cytochrome P450 enzymes family. This discovery led to further research, into the role of cytochrome P450 enzymes in drug metabolism and many other biochemical processes that involve mainly antibiotics [
1,
3,
8,
21].
All above discoveries, were considered the milestone of the development of many erythromycin – based remedial products, that predominantly aimed to ameliorate various infectious diseases and conditions and are currently trending in the medical field.
2.2. Reaction’s importance
The relevance of the erythromycin – producing reaction via the catalytic response of cytochrome P450 eryF, has a notable impact on the pharmaceutical field. Cytochrome P450 enzymes form a group of molecules with many different structural variations, that provide an additional defense mechanism to the human body, alongside with the immune system. Digging deeper in those structural variations of the erythromycin group, those originating from bacterial strains, are eryA, B, C, D, E, F & G. Among all different erythromycin molecules, eryA, otherwise known as ilotycin, is the primary constituent, while eryE & F act as erythromycin metabolites, while all the other compounds play the role of mediators, that are formed in the biosynthesis of eryA [
10].
Generally, all cytochrome P450 enzymes, own the power to metabolize hazardous substances, such as benzo[a]pyrene, constituents used in tobacco smoke and many endogenous substances, including steroids and fatty acids. P450 enzymes are located in bacteria and in all types of cells, except those forming red blood and skeletal muscle cells, a state that indicates their great role in a variety of scientific medicinal pathways [
11].
This macrolide antibiotic’s spectrum is the same and maybe moderately wider than that of penicillin. Counting from respiratory tract infections to antibacterial, antifungal and antimicrobial properties, erythromycin combats effectively various atypical organisms, including
mycoplasma and
legionellosis. It was first marketed by Eli Lilly & Company and as of today, it is commonly known as EES (Erythromycin Ethyl – Succinate), an ester – based prodrug, commonly administered in modern treatment [
1,
3,
8,
37].
2.3. Uses of erythromycin – Recent applications
2.3.1. Erythromycins’ profile and applications
Erythromycin participates in both internal and external cures. This drug reportedly encloses vital anti-inflammatory properties, the ability to suspend osteoclasts formation, and as a component in bone cement is able to halt possible infections [
28,
32,
33]. Furthermore, functions as a motilin receptor agonist, is used as effective treatment towards the acceleration of gastric emptying, or against many lung – targeted diseases, like the chronic obstructive pulmonary disease [
32,
35]. Infectious diseases caused by pathogens, such as
Staphylococcus aureus,
Neisseria and many more, have also been considered treatable, under the prescription of erythromycin and/or its derivatives [
32].
Studies have been conducted upon the neuroprotective effects of erythromycin, concerning cerebral ischemia, reperfusion – injury and cell viability, following the oxygen – glucose destitution in cultured neuronal cells [
34]. Additionally, a potent treatment for chest or lung infections (pneumonia, skin conditions such as acne, rosacea, dental abscesses or even Sexually Transmitted Diseases (STDs), like syphilis), is further supported. Regarding children, erythromycin is often considered as a remedy for ear or chest infections [
10,
37]. Erythromycin is in fact a potent threat against cancer, as it hinders proliferation and induces the apoptosis of cancer cells with high HERG K
+ (human ether-a-go-go-related gene potassium) channel expression. A combination of erythromycin with other anticancer agents, leads to a more comprehensive anti – cancer approach [
38].
As of the latest decades, a plethora of scientific projects have been conducted that involve the exploration of erythromycin’s potential. For instance, metoclopramide was lately claimed inferior to erythromycin concerning its ability to lower the need for a second – look gastroscopy in patients suffering from Upper Gastrointestinal Disease (UGID) [
39]. Plus, modified erythromycin has improved local drug delivery [
12,
13], has been successfully impregnated in materials ranging from bone cement [
28] to drug delivery systems like emulgels [
13] and has been proclaimed as a potent candidate for the restoration of osteoblast differentiation and osteogenesis [
40]. Various respiratory, intestinal and skin infections, STDs, or the Pelvic Inflammatory Disease (PID) [
5], as well as serious bacterial infections, are nowadays treated via the use of erythromycin and its derivatives [
10].
Recent scientific studies, have implied erythromycins’ hidden potential, in fighting COVID – 19s’ current negative impact on human health. HCoVs are the main pathogenic viruses, that induced the pandemic of COVID – 19, with its outbreak causing millions of people’s suffering or death, universally. Therefore, scientists all over the globe have collaborated in order to search for and develop effective anti – HCoV drugs. Since this outbreak, drug repurposing has been the main focus in the process of antiviral agents’ development [
37,
41]. Unfortunately, the declared lab biosafety level 3 (BSL – 3) implies, that the needed laboratory facilities that specialize in the conduction of high – risk experimental procedures are inadequate and require a lot of improvement, prior to such development.
Ery-Est, otherwise known as Erythromycin Estolate, is the lauryl sulfate ester of propionyl erythromycin, a macrolide antibiotic, that shows a broad antibacterial activity spectrum. Erythromycin Estolate manages to diffuse inside the bacterial cell membrane and reversibly bind to the 50S subunit of the bacterial ribosome. This ability allows Ery-Est to inhibit infections of ZIKV (Zika Virus) and other flaviviruses, via drug repurposing and implies its hidden potential towards COVID – 19’s treatment [
37,
41].
The constrained activity of Ery – Est against the HCoV – OC43 strain was thoroughly investigated. Later, it was discovered that Ery – Est may efficiently hinder HCoV – OC43 infection in BHK – 21 (Baby Hamster Kidney Fibroblast Cells), as revealed by a plaque reduction assay, and that the viral concentration in supernatant of HCoV – OC43–infected RD and HCT – 8 cells, were crucially reduced using only 2.5 µM of Ery – Est [
37]. This work indicated that Ery – Est was able to accomplish an effective antiviral concentration in many contaminated patients, as it displayed an inhibitory activity against highly pathogenic HCoVs, such as SARS – CoV, MERS – CoV and the newly emerging SARS – CoV – 2, a virus highly connected to COVID – 19’s development and resistance to conventional virus treatment ways [
37]. Ery – Est basically, directly disrupted HoV-OC43 probably by causing damage in its viral lipid envelope, and accelerated the release of viral genomic RNA and the generation of the irreversible loss of HCoV-OC43 infectivity [
37].
Recently, an identification of erythromycin and other drugs as therapeutic agents against COVID – 19’s progression, has been revealed. Not as a single unit, but via a combination of such drugs, a full or close to full effect is now an attainable target of drug therapy [
41]. However, even though Ery – Est is a promising candidate for HCoV – OC43’s infection, more controlled clinical trials and research are needed, so as to further elucidate upon erythromycin’s potential towards COVID – 19’s advancement [
37,
41]. Many clinicians are already focusing on macrolides as “remedies’’ for COVID-19 off – label, yet without sturdy evidence of the appropriate safety measures or effectiveness. Consequently, although the utilization of macrolides in COVID – 19 reception is still questionable, this drug’s reported potential places an urgent need for well – conducted clinical trials, so as to ensure both its safety, treatment validity and effectiveness [
41].
2.3.2. Cytochromes’ P450 profile and applications
Cytochrome P450 Enzymes exhibit a variety of functions both in the human body and in the environment. Specifically, it is worthy to remark their ability to remove foreign chemicals from the body and their role in the metabolism of endogenous substances [
1,
21,
42]. The number of active cytochromes P450 in the human body alters the individual's response to drugs, due to the different side effects individuals may experience when being exposed to a kind of medication. Statistically, 10 % of the population has a deficiency towards these particular enzymes, resulting in the accumulation of the drug.
Great outcomes follow the existence of such enzymes in the human body. For instance, it was discovered that cytochrome P4502D6 was responsible for the riddance of debrisoquine, an antihypertensive drug, from the body [
1,
43]. Moreover, cytochrome P450 reactions participate in the formation of tumors in response to a carcinogen, with either positive or negative effects. The examination of the account of the human cytochrome P4501A2 towards the carcinogenic effects of cigarette smoke and burned foods, is followed by the realization that heterocyclic amines are produced by the burning of these substances, while amino – acids and carbohydrates are being altered. These altered amine – based derivatives’ carcinogenic tendencies are not activated, as long as P450 enzymes do not interfere [
1,
43]. P450 enzymes, also catalyze the synthesis of two alkaloids, morphine and codeine which are produced mainly in the brain and are crucial for our well – being [
1,
28,
43,
44]. Additionally, these enzymes and especially P4450eryF engage in the synthesis of 20 – carbon eicosanoids, that consist of signaling molecules like prostaglandins, thus act as a remedy against neuroinflammatory diseases, while they also take part in the synthesis of poly-eicosatrienes that modify the transport of molecules, Na
+ and K
+ ions, water absorption and the degree of vasoconstriction in the kidney [
1,
45,
46].
Our specific P450 enzyme group of interest, P450eryF enzymes, are involved in a plethora of reactions. P450eryF is a bacterial P450 enzyme that shows not only excellent solubility, but also great aggregation properties and cooperativity of substrate binding [
47]. Regarding the modification of endogenous substances, P450 enzymes and especially P450eryF [
48] are also involved in the hydroxylation of long – chain fatty acids steroids, bile acids, vitamins A & D and their metabolites. These enzymes assist in the synthesis of NO, which acts as a neurotransmitter, a mediator of blood pressure and a toxin, that kills invading pathogens. Concurrently, P450eryF owns a great structural role, since it catalyzes the 6S-hydroxylation of 6-DEB, in a multistep pathway that leads to the conversion of 6-DEB to erythromycin [
16].
Furthermore, P450eryF’s enlarged substrate binding pocket, enables this enzyme to bind to certain steroid compounds and azole – based steroid hydroxylase inhibitors [
16]. More specifically, in the P450eryF/ketoconazole structure, the azole moiety and the nearby rings of ketoconazole, were placed in the active site in a way that this binding led to unexpected conformational changes in the I – helix, which in turn induced its flexibility and its ability to adopt many conformations [
49]. The azole – based P450eryF inhibitor ketoconazole, is used to treat fungal infections and functions, by blocking ergosterol’s biosynthesis in yeast [
49]. Recently, the ferric P450eryF structure suggested that a well ordered active site water molecule that formed a hydrogen bond with a substrate OH group, could serve as a direct proton donor to the iron – linked dioxygen, since its implications for its participation in O
2 binding and cleavage were confirmed [
50].
3. Catalytic system characterization
Generally, macrolides refer to secondary metabolites regarding the genus
Saccharopolyspora erythraea and are natural products that include a large macrocyclic lactone ring with attached amino – deoxy sugars. Erythromycin (ery), is a macrolide compound consisted of rings, containing 12, 14, or 16 atoms [
9], with a broad clinical activity and crucial antimicrobial role in treating a huge number of respiratory, skin, intestine, bone and other infections originating from different bacteria. This antibiotic’s popularity arose after its discovery in 1952, and stems from its effective therapeutic properties against dangerous pathogens, resistant to many other at the time known and used drugs [
32].
The biosynthesis of erythromycin, involves a complex catalytic system, primarily facilitated by a large enzyme complex widely known as erythromycin polyketide synthetase (PKS), that plays a significant role in the assembly of the erythromycin molecule [
51,
52]. Erythromycin PKS’s key components, are namely modules and enzyme domains, as well as tailoring enzymes including glycotransferases, hydroxylases, methyltransferases and oxidases, which are able to further modify erythromycin’s structure and thus, enhance its pharmacological properties [
51,
52,
53,
54].
In order to obtain a more detailed image of erythromycin’s structure and catalytic system, many characterization techniques are summoned, counting many genetic and molecular biology studies that involve cloning and sequencing the genes encoding erythromycin, biochemical assays, X-ray crystallography and NMR spectroscopy. The characterization of erythromycin’s catalytic system is indeed a multifaceted process, that entails genetic, biochemical and structural techniques, which are crucial in order to fully elucidate upon erythromycin’s biosynthesis [
51,
52,
53,
54].
3.1. Proenzyme
The genome that produces erythromycin is circular.
Saccharopolyspora erythraea “NRRL 2338” with its white and less pigmented form is excessively used, because it results in the production of more erythromycin, than its red counterpart [
14,
22]. The bacteria of the genus
Saccharopolyspora are a member of
Pseudocardiaceae and in general assist in the production of erythromycin and other polyketide macrolide antibiotics [
14,
22]. The specific strain used in the production of erythromycin is called “
Saccharopolyspora erythraea HOE107” [
15]. The first macrolide to be characterized was erythromycin A, that inhibits the protein synthesis inside bacteria cells, thus showing bacteriostatic activity [
14,
55]. Τhe enzymic precursor of the catalytic production of erythromycin is in fact encoded by the bacteria
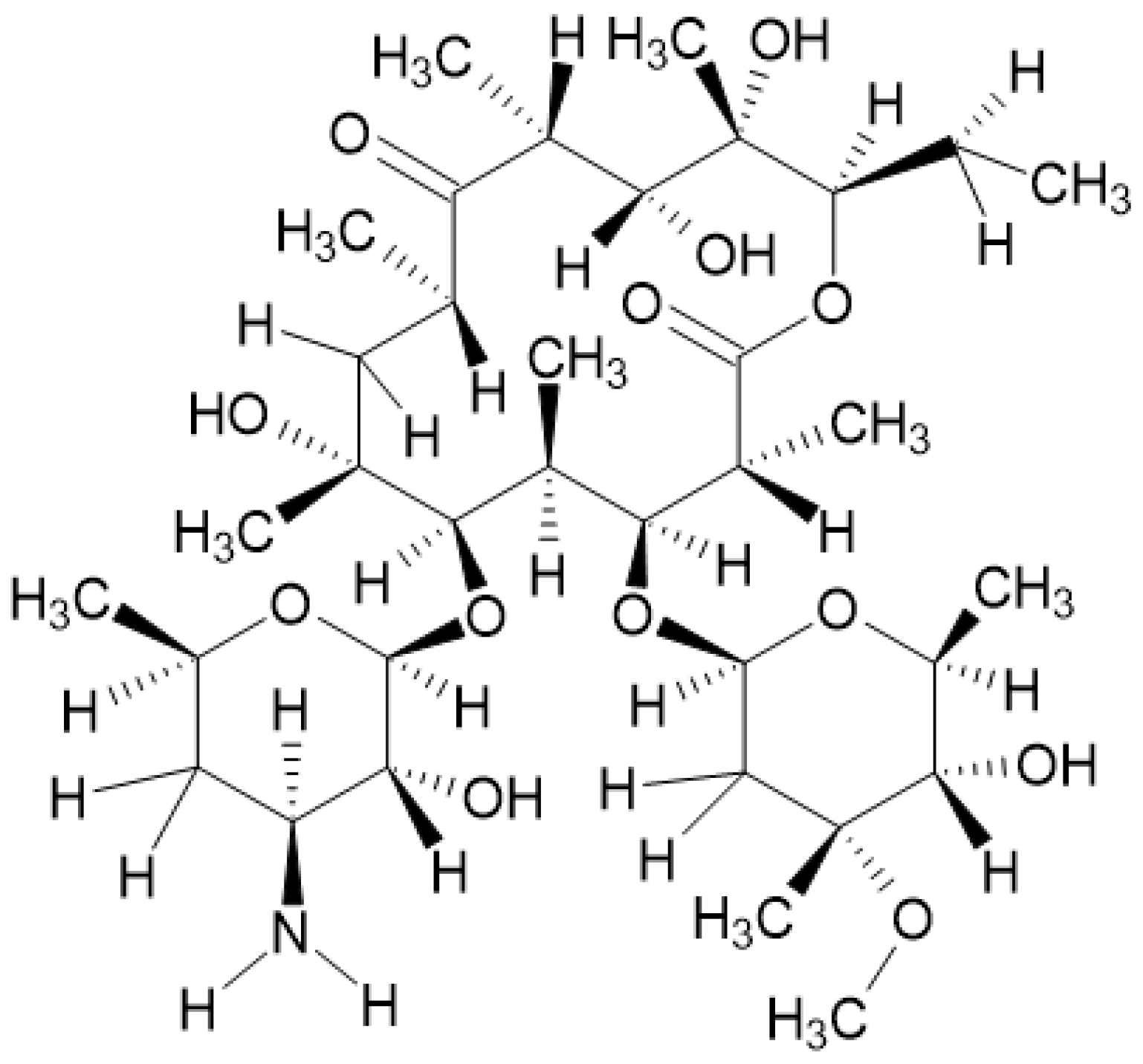
Saccharopolyspora erythraea, namely eryF. In addition, the enzyme P450eryF has experienced neither any modifications nor maturing, so it is used in the production process of erythromycin as is. Erythromycin’s molecular structure is depicted in
Figure 1.
3.2. Catalytic system
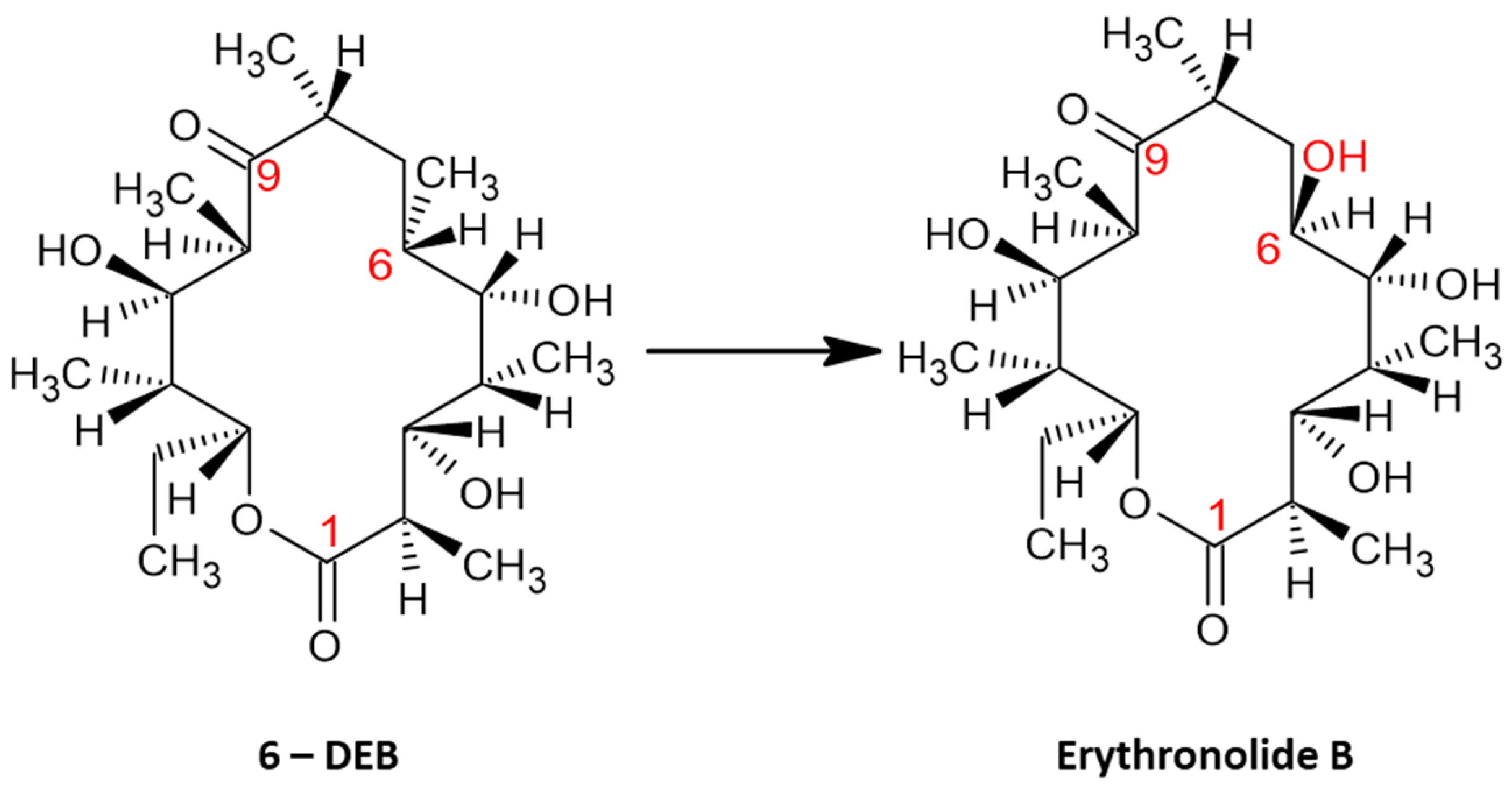
As is widely accepted, cytochrome P450eryF is a type of cytochrome P450 enzyme, that contributes to the convention of 6 – deoxyerythronolide B (6 – DEB) into erythromycin. Explicitly, the conversion of 6-DEB to erythronolide B is catalyzed by P450eryF, that specifically catalyzes the 6S – hydroxylation of 6 – deoxyerythronolide B. Such catalytic process acts as the initial reaction of the convention of 6 – DEB into erythromycin (
Figure 2). Generally, as above – mentioned
Saccharopolyspora erythraea is the bacterium responsible for the production of the desirable product of erythromycin, erythromycin A, by the utilization of a metabolic pathway in which 6 – DEB is converted to erythromycin, as a result of hydroxylation reactions in positions 6 and 12 on the macrolide ring [
16,
17,
22].
3.2.1. Structure
Cytochrome P450eryF, as well as the other cytochromes P450 that belong in this enzyme family, owns an alpha/beta structure with an alpha – helical domain and a domain with coils and beta – sheets [
1,
16,
43]. Most of the residues, 51 % of them specifically, are implicated in an alpha – helical configuration and only a small percentage (17 %) of them is involved in beta – sheets. When a substrate is present, the haem group exists in a high spin state and is penta – coordinated, with the haem iron bound to the sulphur of Cys351. In general, the haem group is found between the I and L helices (
Figure 3) [
1,
16].
3.2.2. Substrate-binding site
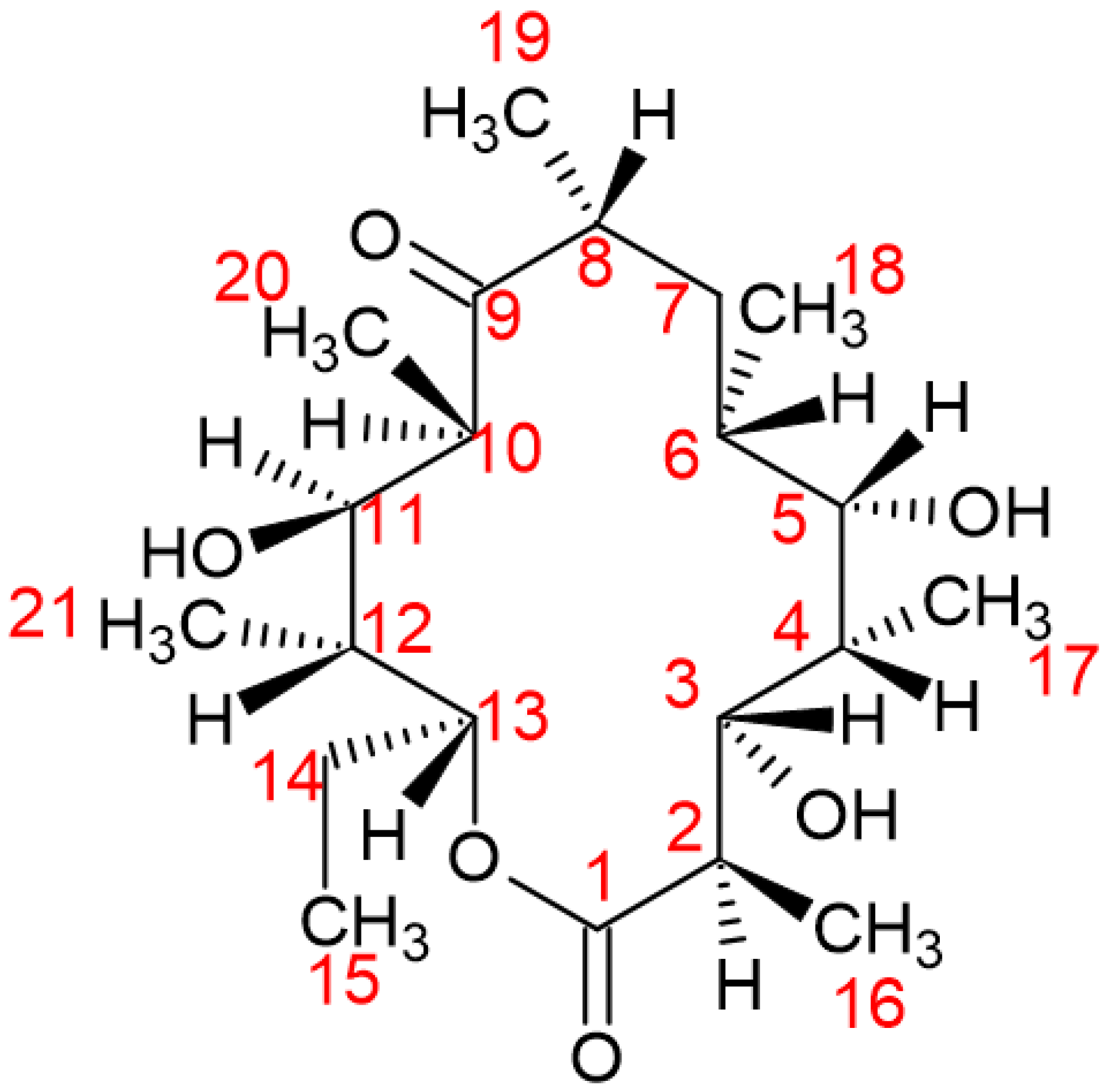
The substrate of P450eryF 6 – DEB, is placed over the A and D pyrrole rings, while the macrolide plane is oriented around the haem plane. The upper part of the F helix and the haem group form altogether the “floor” of the substrate. Concurrently, the binding pocket and its sides are formed by the synergistic work of the B helix, the I helix and the beta – structure with residues of 290 – 296 [
17,
56,
57].
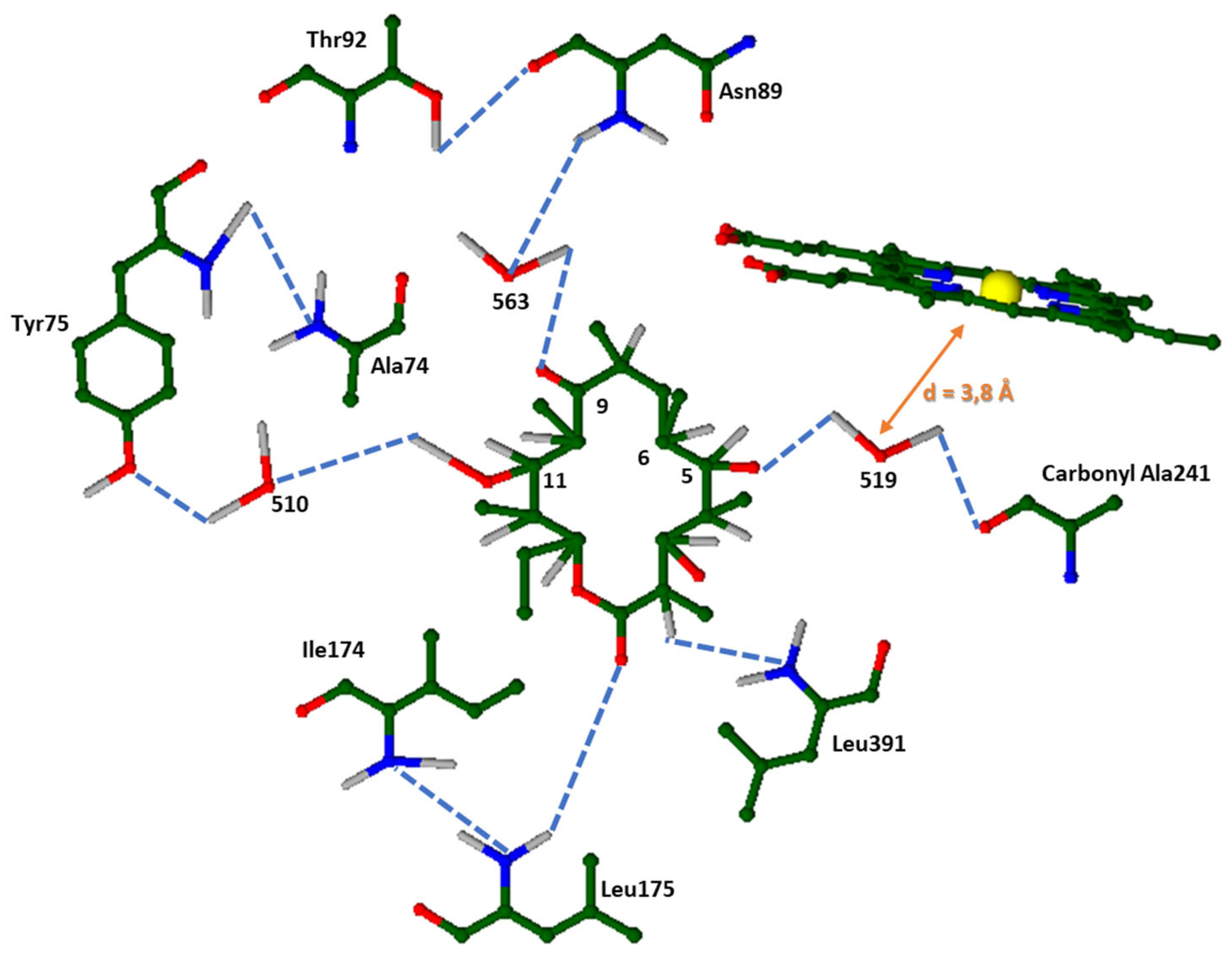
In the substrate – binding pocket of P450eryF, several ordered water molecules exist, which form a hydrogen – bonding network and interact with the peptide backbone, amino – acid side chains, the substrate and the haem prosthetic group. As a result, three ordered water molecules create hydrogen – bonding bridges between the protein and the substrate. More specifically, the C15 and C16 of 6 – DEB contact the side chains of the Ile174 and Leu175 of the F helix. With the aim of placing the previous residues near the substrate - binding pocket, the F helix is repositioned [
56,
57].
Different hydrophobic interactions occur between Ala 74 and C14, Thr 92 and C20, Val237 and C19, Ala241 and C19 and lastly Leu391 and C21. The substrate – binding pocket consists of more hydrophobic residues, that are greater than 4Å in size and therefore aid in isolating and binding the substrate. Moreover, the keto group C9 of 6-DEB and hydroxyl groups of C5 and C11 are within the radius of solvent molecules, making hydrogen bond formation possible with the peptide backbone and amino – acid chains of Tyr75 and Asn89 (
Figure 4) [
17,
56,
57].
3.2.3. Helix I
The keto group C9 of 6 – DEB and hydroxyl groups of C5 and C11 are within the radius of solvent molecules, making hydrogen bond formation possible with the peptide backbone and amino – acid chains of Tyr75 and Asn89. P450 cytochromes bear a unique structure of an extended I helix, whose residues around the haem group play a crucial role in this catalytic system [
16,
17]. This helix displays a different hydrogen – bonding sequence alongside a gap between the aforementioned region and the iron protoporphyrin IX [
16,
17,
56]. Under normal circumstances, the hydrogen bond system of alpha – helix would consist of bonds formed between the carbonyl oxygen of Ala241 and Gly242 with the amide nitrogens of Ala245 and Ser246. Poulos et al. (1995) [
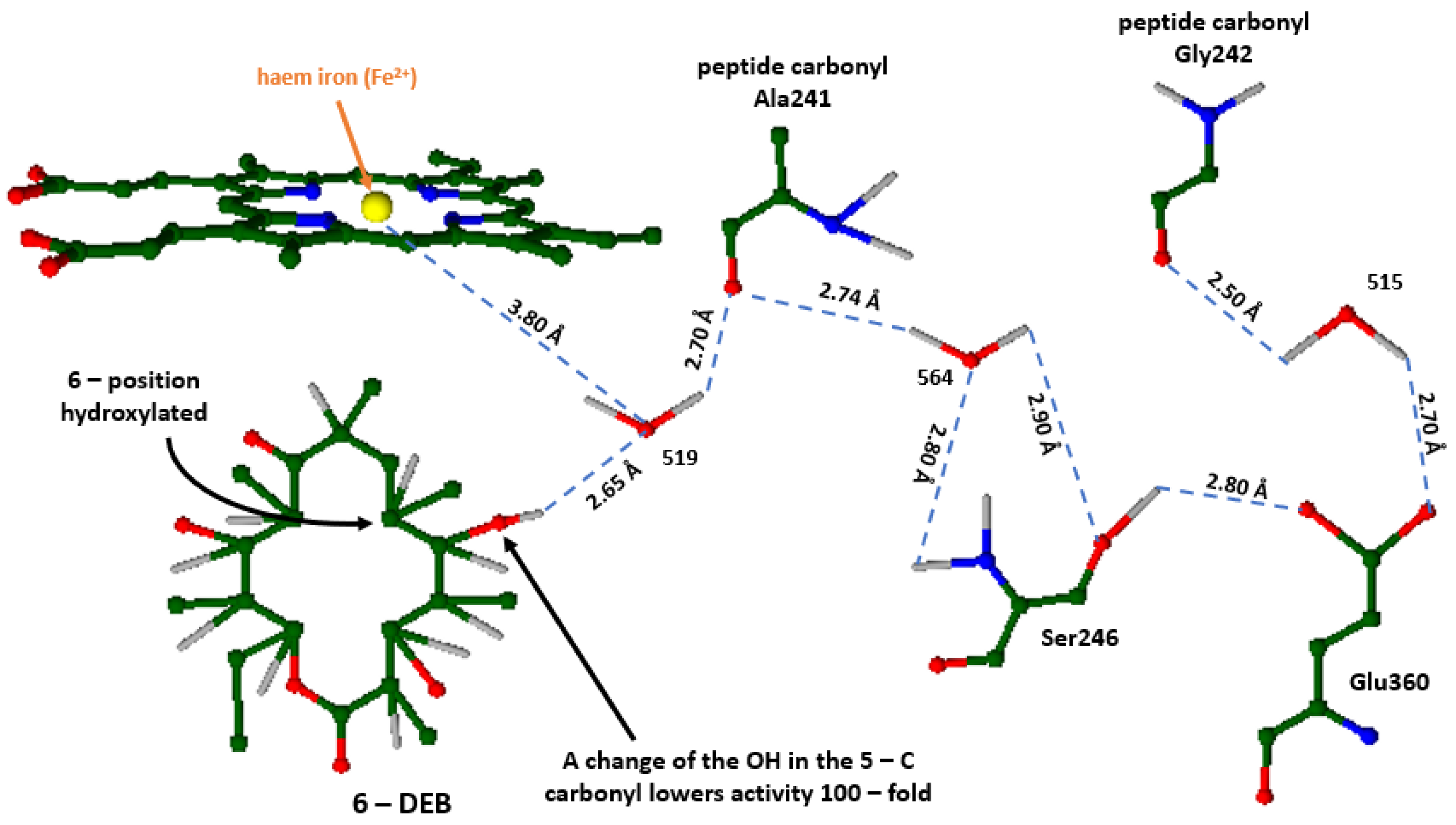
16], suggest that Ser246 forms hydrogen bonds through the amine group with Wat564, which is also bonded similarly with the -NH of the peptide group of Ser246. Likewise, hydrogen bonds are formed between Glu360 and Wat515. Therefore, this hydrogen bond system stabilizes the I Helix's local distortion, as it substitutes the missing alpha – helical hydrogen bonds. In actuality, the water molecule Wat519 is close to the iron of the haem group by 3.8 Å and it is also suggested that it may be the proton donor of the O
2 cleavage reaction, hence its position is vital for the haem’s functionality (
Figure 5) [
16,
56].
3.2.4. Active site
The substrate 6 – DEB, connects with the active site in the P450eryF, by the aid of seven hydrophobic residues and three water molecules that provide hydrogen – bonding bridges between the protein and the substrate. To estimate the significance of the hydrophobic and hydrophilic interactions to the substrate – binding’s specificity and hydroxylation site’s stereospecificity, it is essential to examine the binding and hydroxylation of altered substrates in this condition [
5,
16,
17,
22].
Andersen et al. (1993) [
16], have revealed that the nature of the C14 side chain and the differences of the oxidation state of C5 and C9 may affect the P450eryF enzyme. While the crystal structure of both the C9 keto and the C5 hydroxyl groups was examined, it was found that they existed within the hydrogen – bonding distance of water molecules. Normally, altering the oxidation state of the previously mentioned groups should not be able to modify the hydrogen bonds [
16,
17], due to the fact that water molecules act both as an H – bond donor, and as an acceptor. However, it is possible that the alternation of the binding affinity observed after the change of the oxidation state, is likely due to the transformation of the hybrid state of a carbon in the macrolide ring from sp
3 to sp
2 hybridization, or vice versa, that led to the alternation of the ring’s geometry. Furthermore, because of this change, the hydrogen – bonding of 6 – DEB with the protein through the water molecules, may alter as a result of the reposition of the oxygen substituents of C5 and C9.
Adding up to his previous work, Andersen et al (1993) [
16] examined an extra derivative in which a hydrogen took the place of the methyl substituent in C14. As a result, the binding affinity was decreased by 50 – fold. The fact that the Ile174 is positioned in such a way, so that it could interact with the C14 and the shortage in hydrophobic interactions, justifies the binding affinity decrease. Lastly, 6-DEB becomes more symmetrical by losing the C14 methyl group and might reorientate the active site [
16,
17,
56].
3.3. Catalytic system mechanism
The substrate 6 – DEB is catalytically synthesized by 1 equivalent of propionyl CoA (coenzyme A) and 6 equivalents of methylmalonyl CoA, with the assistance of eryAI, eryAII and eryAIII. Propanol and glucose consumption leads to the accumulation of methylmalonyl – CoA and propionyl – CoA, whereas propanol conversion into propionate and transformation into propionyl – CoA is catalyzed by propionate kinase, otherwise propionyl – CoA synthase. In addition, glucose is converted to succinyl – CoA, which also participates into the TCA (Tri – Carboxylic Acid) metabolic cycle [
18,
19,
20]. The catalytic mechanism of erythromycin A consists of several modifying enzymes, such as eryBV (eryB5), eryCIII (eryC3), eryK, and eryG [
18]. Lee et al. (2004) [
18,
19] reported that the gene cluster of
S. erythraea consists of 20 genes – translated into the aforementioned enzymes, a state that allowed the catalytic biosynthesis of the polyketide ring and mycarose that was attached to the macrolide ring alongside desosamine [
19].
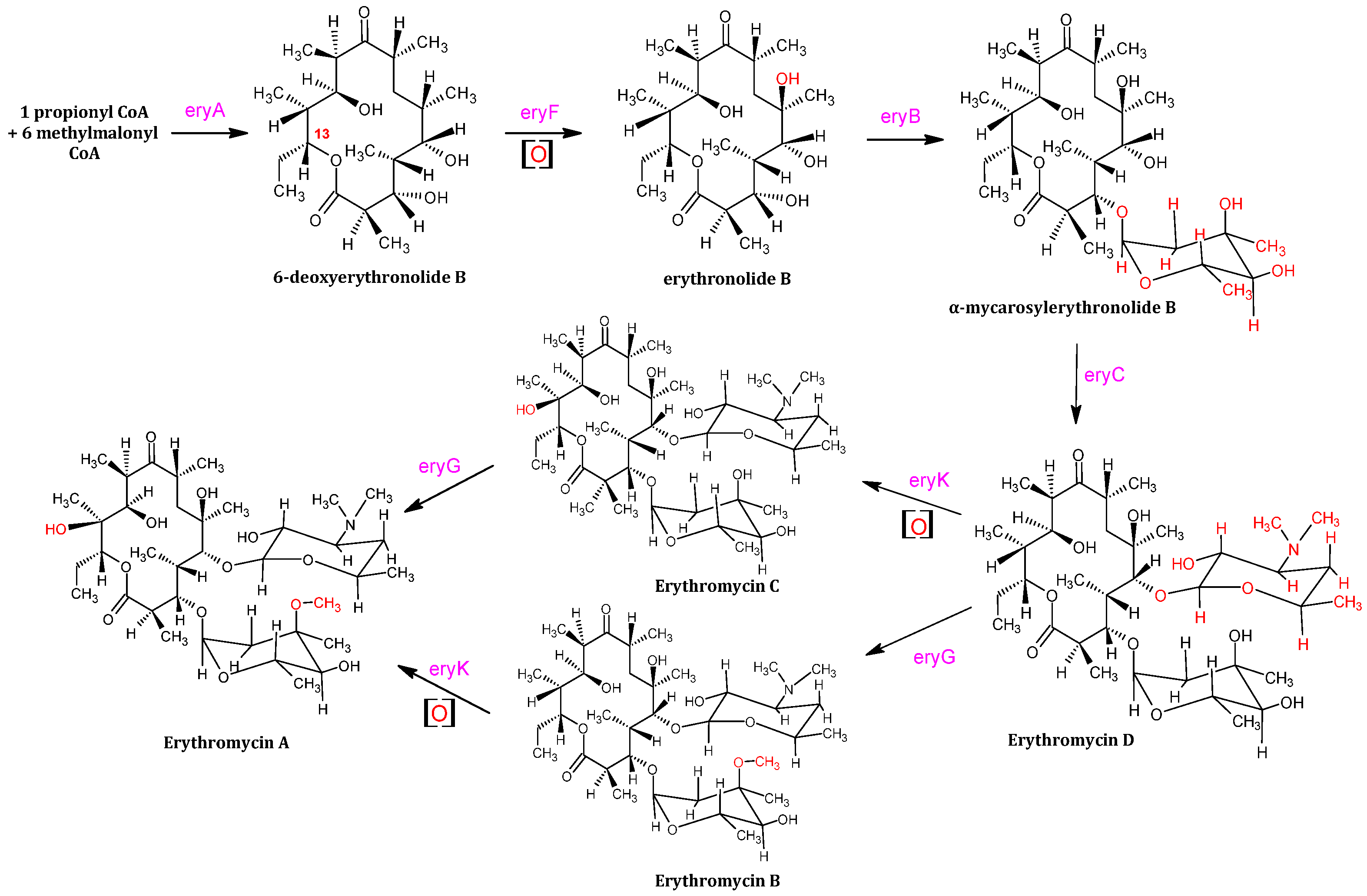
However, it should be noted that succinyl – CoA is utilized in both the synthesis of methylmalonyl – CoA and the TCA metabolic flux, which mostly indirectly decreases the overall yield of erythromycin. The enzyme eryF modifies firstly the substrate 6 – DEB, by adding a hydroxyl group to C6 [
21]; hence, erythronolide B is produced (
Figure 2). Following erythronolide B’s production, the addition of mycarose to the C3 hydroxyl of erythronolide B is catalyzed by eryB5. The alpha – mycarosyl – erythronolide B is then modified by the eryC3 enzyme, that glycosylates the C5 hydroxyl, leading to the formation of erythromycin D [
22]. In other words, eryB5 and eryC3 transfer two deoxysugar units on C5 and C3 of 6 – deoxyerythronolide B [
23]. EryK and eryG, both catalyze erythromycin D into erythromycin B and erythromycin C. EryK catalyzes the hydroxylation of C12 of erythromycin D in which erythromycin C is produced, whereas eryG catalyzes the methylation of erythromycin D; thus, erythromycin B is synthesized [
24,
25]. Erythromycin A is generated by erythromycin C’s methylation and erythromycin B’s C12 hydroxylation. It is also reported [
23], that eryG acts competitively on the erythromycin D substrate, as it cannot be catalyzed into erythromycin A effectively; thus it accumulates into the final product of erythromycin. Erythromycin B is not as toxic as the C counterpart, however both substrates show low antibacterial activity and are less effective antibiotics, unlike erythromycin A which on the contrary is vastly effective (
Figure 6) [
26].
During the industrial production process, the broth emerging from the bioreactor is impure, due to the presence of bacterial cells and other waste, like the by-products consisting mainly of erythromycin B and erythromycin C that derive from the aforementioned catalytic mechanism. Erythromycin C is highly toxic [
26], thus the isolation process of erythromycin A must be arranged, so that the purity of the final product does not exceed specific limits, according to Pharmacopeia specifications of each continent, but also so as to limit the potential damage of the product caused by the high temperatures during all crystallization stages. There are three different purification pathways erythromycin A can be obtained from: antisolvent, evaporative and reactive crystallization [
27], following a pre – treatment plan consisted of isolation processes, that include product extraction by filtration from resins, plate and membrane filters. The industrial production process, will be further analyzed in
Section 3.4 below.
3.4. Industrial production of erythromycin
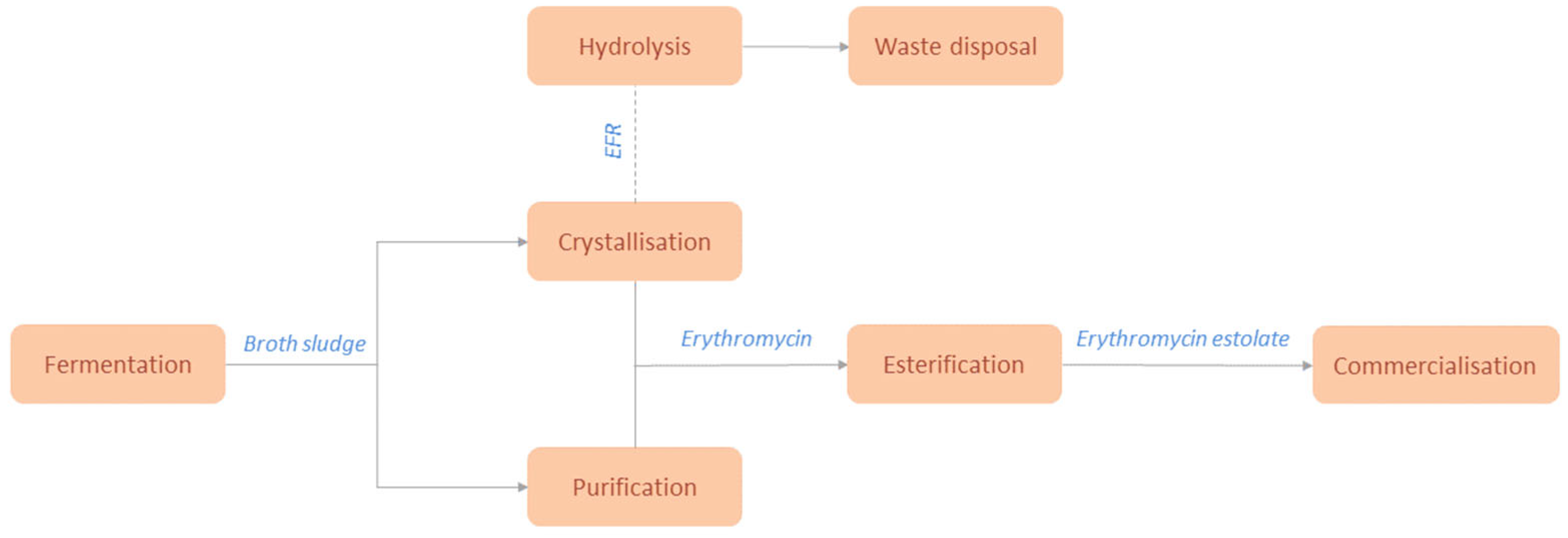
The fermentation broth of the industrial strain of
Saccharopolyspora Erythraea HOE107 is added adjacent to glucose, which is considered a standard carbon source for the industrialized synthesis of erythromycin (
Figure 7) [
58]. The aforementioned method, produces Erythromycin A but also its by–products of Erythromycin B and Erythromycin C. Nevertheless, it is important to mention that other bacterial colonies have been introduced to genetic tailoring in order to produce erythromycin in a higher yield or purity.
E. coli can be used as a host due to its ability to convert D–glucose-1’-phosphate to TDP and thus introducing an alternative heterologous pathway, by altering its genome accordingly through plasmid insertion [
59].
Saccharopolyspora Erythraea’s genome contains many regulatory factors that provide surviving mechanisms to inhibit the expression of several genes so as to conserve energy and secure its survival in a hostile environment. Many of these genes attribute to the lesser production of erythromycin, even in nutrient rich environments and thus genetic tailoring takes place in some industrial colonies to deactivate these inhibiting factors [
60]. Other carbon sources were also investigated, such as galactose, arabinose, fucose and starches and molasses with the latter showing promise, as an increase of the erythromycin concertation in the broth and a decrease of the possible economic costs by utilizing cheaper carbon source alternatives were observed. Other factors that contribute to erythromycin’s production and cell growth, except the pH and temperature conditions inside the bioreactor, are the concertation of nitrogen and its supplementation form, n-propanol and the ratio of carbon and nitrogen sources [
61].
There is a variety of purification and isolation pathways from which EryA can be obtained with Chen et al suggesting the antisolvent crystallization, evaporative crystallization and reactive crystallization methods [
27]. The antisolvent method, consists of several pre-treatment steps of the broth so that the Erythromycin batch would be separated from any solid impurities by plate filters. Butyl acetate is added to the mixture once the purified broth enters the extraction chamber. Once the extraction of EryA during the broth filtration is completed, the pre-product is mixed with lactic acid or HSCN and it is moved to the precipitation tank. The precipitate is moved to a double-wall crystallizer in which the antisolvent (water) is added to the alkaline solution and so crystallization takes place since erythromycin is less soluble in water than in acetone [
35]. The target compound, enters the mixing section where it interacts with the antisolvent in a microchannel by diffusive mixing in a laminar flow, to ensure decent mixing between the solvent and the final product [
62]. Finally, the erythromycin thiocyanate salt is conversed in an alkaline environment and obtained in an erythromycin free base form. The evaporative and reactive crystallization processes provide erythromycin and erythromycin thiocyanate respectively. The broth is initially isolated by solid impurities such as mycelium, proteins, and other particles with the method of microfiltration. Resin filtration is necessary for the removal of un–ionized organic particles and pigment. Butyl acetate is also used in this method during the elution phase of the filtered broth to isolate erythromycin from the rest of the broth’s impurities [
27]. EryC shows high toxicity thus the isolation process of EryA must be arranged so that the purity of the final product can be maintained within acceptable limits. A separation process of EryA from the broth and EryC, was provided by Jin et al by obtaining the absorbent from SP825 macroporous resin and preparing it accordingly so as to experiment on the absorption selectivity of EryA and EryC to various aqueous solutions [
26].
The erythromycin fermentation residue (EFR) from the bioreactor is also an important part of the production waste, which is later hydrolyzed in order to prevent environmental damage from occurring during waste disposal, since they show high chemical oxygen demand (COD) values. Erythromycin can be hydrolyzed under 85
oC in one hour, after the residues have been diluted and pretreated in anaerobic bioreactors. This process also reduces the risk of bacteria developing resistance to antibiotics found in wastes [
63,
64]. Erythromycin, however, is introduced to the general public in its derivatives such as a base in tablets or capsules, salts or ester in oral suspension, since these forms provide increased absorbability of the drug by the human body. The form of erythromycin estolate has been found to be the most easily absorbed by its counterparts and can be synthesised by the esterification of erythromycin. This drug is synthesized in the esterification of the hydroxyl group of amino sugar of the erythromycin molecule and the carboxyl group of propionic acid [
65]. Moreover, its bitterness is reduced while it is easily absorbed orally and gets released as free erythromycin in the bloodstream. Nevertheless, this form shows a number of serious side effects such as the development of cholestatic hepatitis, especially to pregnant patients [
66].
4. Industrial application
4.1. Production per country
The production of the antibiotic erythromycin and its derivatives (salts thereof) worldwide keeps expanding. More and more countries partake in the production of erythromycin. However, as expected, some countries’ production ”blossomed”, while other countries’ rates dropped back. With a view to provide a more rounded overview, the comparison will include the years 2011 and 2021, a decade of fluctuations [
31]. The website “OEC” (Observatory of Economic Complexity) [
31] provides the annual exports and imports of erythromycin by country. Over the 2011 – 2021 decade any fluctuations in the countries’ imports and exports percentages, will be thoroughly examined.
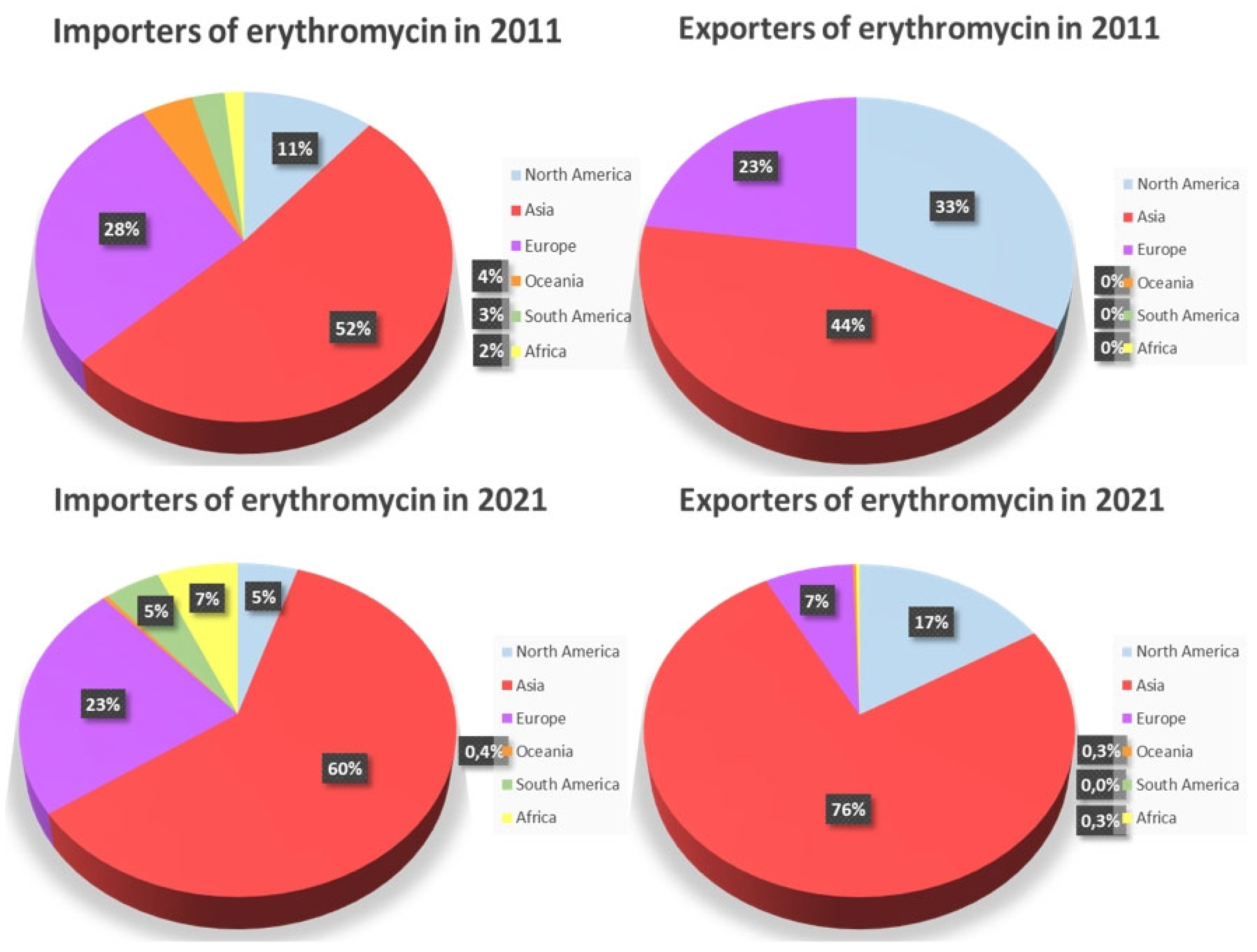
From the results shown in
Figure 8, a comparison for the years 2011 and 2021 is displayed. During the ten – year span between 2011 to 2021 Asia, South America and Africa exhibited an increase in the imports of erythromycin, while all the other continents experienced a decrease. As for the exports of erythromycin in the same time interval, Europe and North America experienced a decrease in the export rates, while the rest of the continents’ export percentages increased. Lastly, South America remained constant at 0 %.
As illustrated in
Figure 8, in 2021, the top exporters of erythromycin were China (
$185 M), India (
$77.9 M), United States (
$63.1 M), South Korea (
$8.86 M), and Spain (
$7.23 M). Meanwhile in 2021, the top importers of erythromycin were India (
$104 M), Japan (
$32.3 M), Croatia (
$31.8 M), Pakistan (
$11.5 M), and France (
$10.7 M) [
31].
5. Statistics
According to the listed patents that derived from “Espacenet” [
67] by searching the key words: “erythromycin”, “production”, “2011 – 2021”, a full access to those 10 – year span data was achieved. Statistical analysis that took place for the evaluation of all obtained information, allowed the comparison [
67] of erythromycin production patents’ data between 2011 – 2021, that were categorized according to countries, organizations, applicants and inventors of erythromycin patents. A comparison of the publication and earliest priority patent dates, also took place.
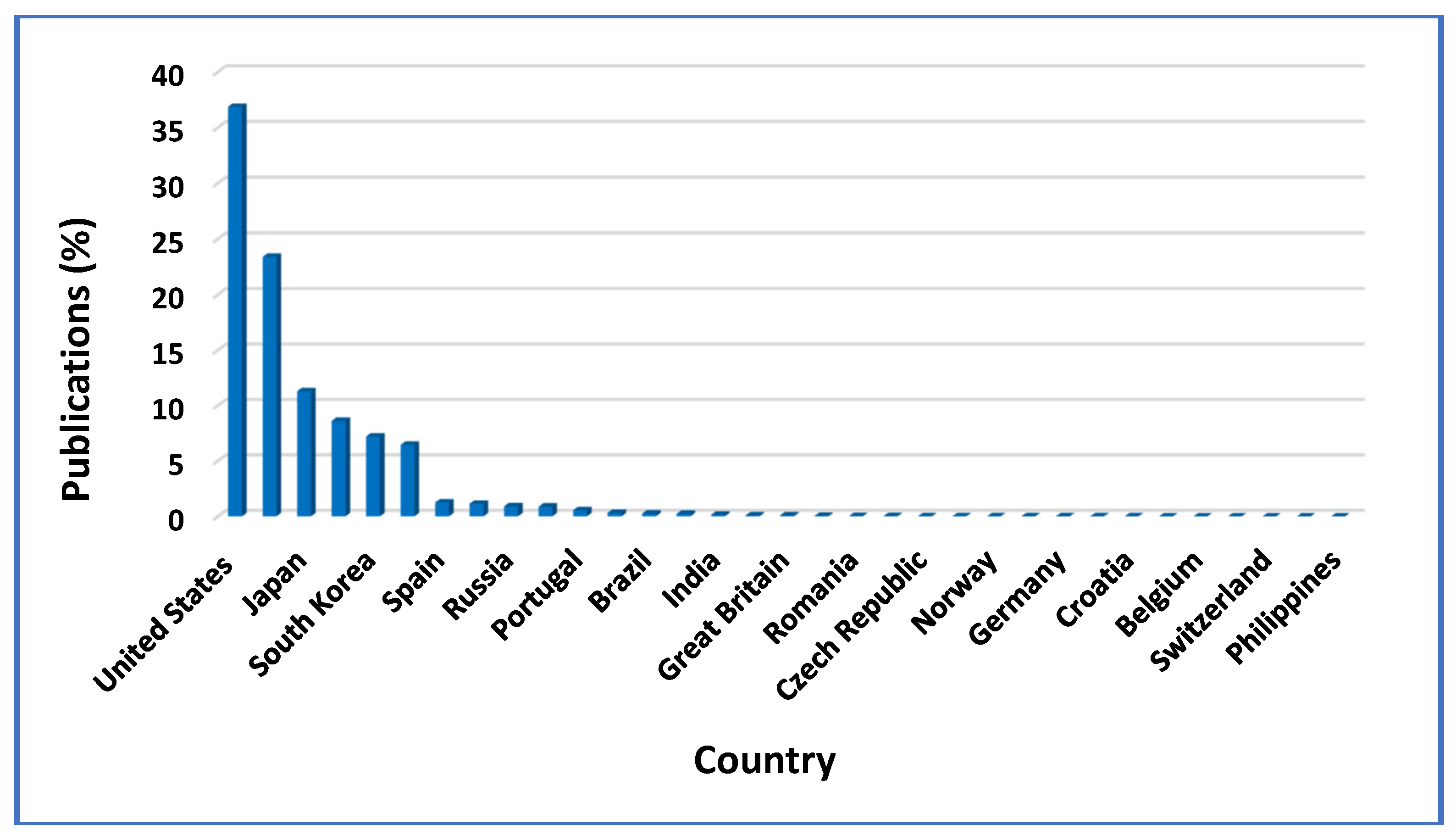
Figure 9 illustrates 17 countries that published patents about erythromycin, in the year span 2011 – 2021. Total patents, filled out by countries and organizations in this 10-year period, were 60,046 worldwide, from which 49,744 (82.84 %) were published by individual countries and 10,302 (17.16 %), by some well – known global organizations. Out of all countries, the United States of America, placed first considering the erythromycin patents’ publication, with 36.88 % of the 49,744 overall patents being issued by countries. China (23.40 %) and Japan (11.30 %) followed 2
nd and 3
rd respectively, (
Figure 9), while Australia, South Korea, Canada, Spain and Singapore came in this order, with their corresponding patent’s publication percentages being a little above 1 % of the total publications. All the other countries participated in less than 1 % of the total publications. Out of the total 10,302 patents published by universal organizations, the World Intellectual Property Organization (WO) filled out 61.68 %, whilst the European Patent Office (EP) and the Eurasian Patent Organization (EA) published 36.72 % and 1.60 % of the total patents, respectively, as the statistical analysis results revealed [
67].
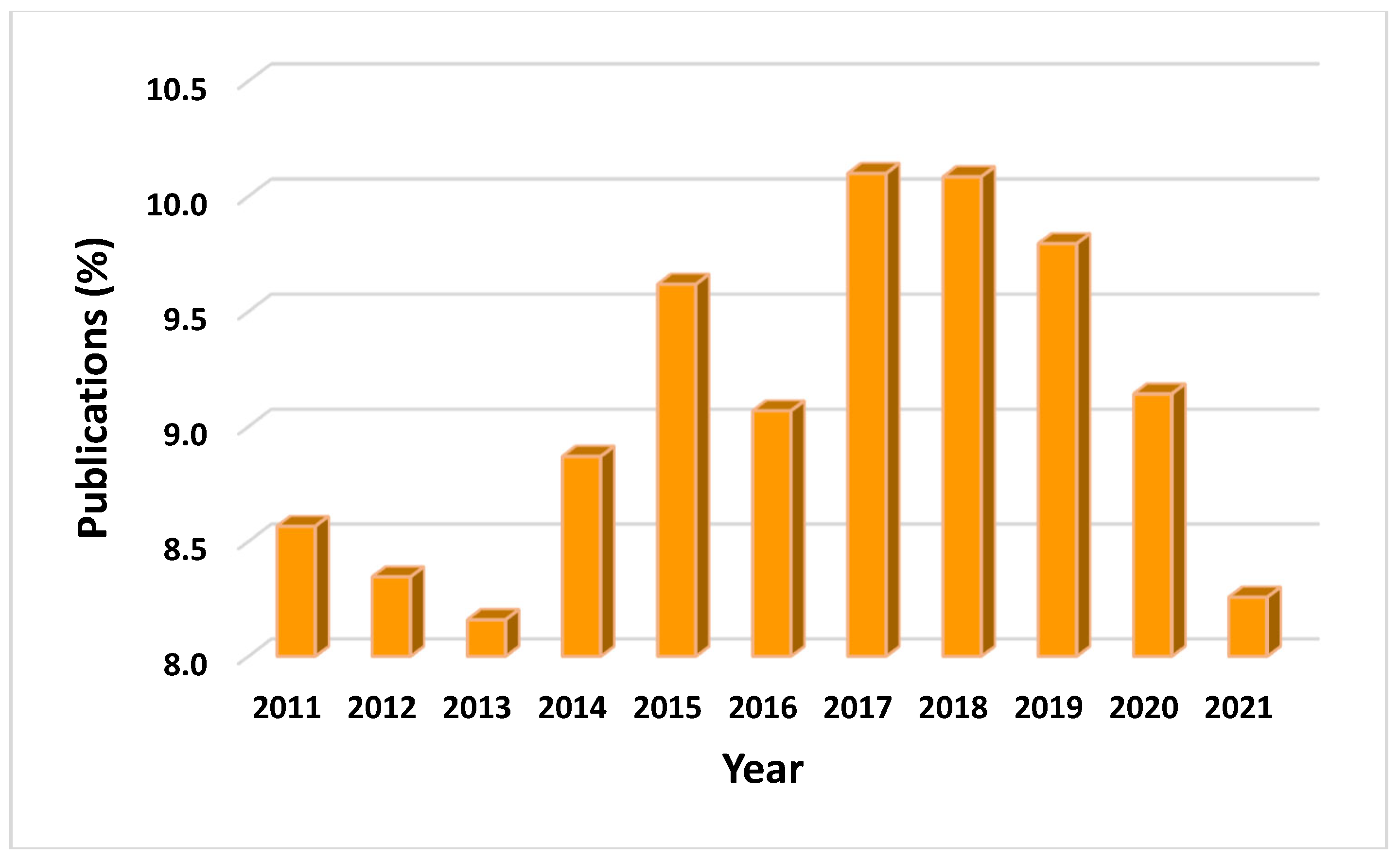
According to the patent’s publication, in each year between 2011 – 2021, in the total 60,046 documents, during every year significant alterations in the number of published patents was observed. The most erythromycin published patents were recorded in 2017 (10.10 %) and 2018 (10.08 %), numbers that later dropped, due to the shifting of global focus upon COVID – 19’s outbreak and the urgent need for its treatment (
Figure 10) [
67]. However, as apparent in the graph listed below, the drop seen from 2015 to 2016, in comparison to this of 2019 to 2020, reveals that all data are comparable. Specifically, numbers may have dropped in both cases, but the percentage of listed publications in the year span of 2019 – 2020 is still greater than that of 2015 – 2016. Similarly, the percentage of patents filled during the peak of the pandemic (2020 – 2021), is still higher than that of patents filled during 2011 – 2013 and nearly of those filled in 2014.
Statistically in the year span of 2011 – 2021, patents were published in many different languages with the vast majority of them being published in the English language (68.22 %), followed by the Chinese (Mandarin) and the Japanese language (13.91 % and 6.70 %). It must be highlighted that, the onset of erythromycin patent’s publication was reported back in 1983, with only one document listed. Most patents were filled out between the years 2015–2018, numbers that later on dropped, but are expected to rise in the next decade [
67]. Topical antibiotics market report attributes, claimed that the market size of antibiotics like erythromycin in 2023 reached 6.4 billion USD, which in the forecast period of 2024 – 2032 is expected to own a value projection of 10.4 billion USD [
9,
55,
68].
Many applicants and of course inventors all over the globe, in 2011 – 2021, dealt with erythromycin patents and managed to fill out such documents. A huge number of applicants in this year span, including famous pharmaceutical companies, incorporations, universities, institutes and colleges worked upon erythromycin production – related publications. The University of California placed first in this “race”, by exhibiting a percentage of 4.70 % out of the total patents, followed by Novartis AG, Genentech INC and Hoffmann la Roche. Moreover, we must refer to the applicant’s and inventor’s origin, were reports in the time period of 2011 – 2021, showed that more than half of them – as expected – derived from the United States of America, while less than 5 % were obtained from Germany, Japan and less than 3 % was shared between the rest of the listed countries [
67]. Greece also marginally contributed with erythromycin patents in 2011 – 2021, with 4 published erythromycin patents. All of these patents were published in the Greek language, while 23 Greek applicants and 65 Greek inventors participated in those publications.
As of 2024, erythromycin thiocyanate is currently trending in the global market as recent insights claim its important role in eliminating cyanobacterial blooms [
69]. This derivative is thought to be responsible for the hindrance of
M. aeruginosa’s photosynthesis and possibly, for the low promotion and high inhibition of soluble proteins’ synthesis in synergy with enrofloxacin [
70].
The development of semi – synthetic macrolides such as roxithromycin, dirithromycin, spiramycin, clarithromycin, josamycin and especially, azithromycin, greatly altered the anti – infective drug picture and the medical practice procedures of the 20
th century [
9,
71]. Yet, delving into the 21
st century, telithromycin and solithromycin, as well as azithromycin and other macrolide erythromycin – derived antibiotics, reveal favorable activity [
9,
71], including an excellent antimicrobial potency towards some key erythromycin – resistant pathogens [
9,
72,
73]. At this point, it is important to note that erythromycin, as well as clarithromycin and azithromycin, which are namely the main erythromycin – derived compounds, are substantially different in terms of properties and drug – drug interaction abilities [
74]. Destined predominantly for human and veterinary medication, erythromycin is expected to thrive in the global market, since this molecule either as a single or mainly in synergy with other drugs like levofloxacin [
75] and retapamulin [
76], or nano – particles (NPs) such as Ag – ZnO NPs [
77], is able to effectively battle multi – drug resistant gram – positive bacteria [
10].
6. Conclusions
The current study demonstrated the importance of the antibiotic erythromycin A and illustrated in detail the catalytic production process. Saccharopolyspora erythraea - HOE107 strain, is used industrially to produce erythromycin A, by synthesizing the catalytic system of the metabolic path to the final production. There is certainly room for development, considering the TCA metabolic pathway, in which succinyl – CoA, the precursor to methylmalonyl – CoA, poses a limiting factor to the synthesis of DEB – 6. Therefore, newer studies recommend the use of mutant strains, in which the gene cluster responsible for the activation of the TCA cycle is suppressed. A plethora of methods and patents has been proposed for the crystallization process, regarding the solubility of erythromycin and its derivatives to less harmful, economical and more environmentally friendly solutes; so that, erythromycin A can be obtained in both higher yield and purity.
Despite the fact that, erythromycin is currently placed in the shadow of its derivatives namely azithromycin and clarithromycin, this molecule’s excellent drug profile and antimicrobial, antibiotic and antifungal properties are believed to be the main reason of its expected vast exploitation in the near future. Either in synergy with many other drugs, or as a drug – loading molecule in emulgels or nanoparticles, erythromycin is expected to play a significant role in drug delivery systems and hence, aid in the amelioration of many pestering global diseases and conditions like COVID – 19.
Author Contributions
Conceptualization, T.A., E.P., E.S., P.T. and N.C.K.; methodology, N.C.K.; validation, N.C.K.; formal analysis, N.C.K.; investigation, T.A., E.P., E.S. and P.T.; resources, T.A., E.P., E.S. and P.T.; data curation, T.A., E.P., E.S. and P.T.; writing—original draft preparation, T.A., E.P., E.S., P.T. and N.C.K.; writing—review and editing, N.C.K.; visualization, T.A., E.P., E.S., P.T. and N.C.K.; supervision, N.C.K.; project administration, N.C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The Industrial Catalysis Group (catalysi.eu) is gratefully acknowledged for its administrative and technical support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Guengerich, F.P. Cytochrome P450 Enzymes. American Scientist 1993, 81(5), 440–447. [Google Scholar]
- OMURA, T. Recollection of the Early Years of the Research on Cytochrome P450. Proc Jpn Acad Ser B Phys Biol Sci 2011, 87, 617–640. [Google Scholar] [CrossRef] [PubMed]
- Robertsen, H.L.; Musiol-Kroll, E.M. Actinomycete-Derived Polyketides as a Source of Antibiotics and Lead Structures for the Development of New Antimicrobial Drugs. Antibiotics 2019, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Grela, A.; Kuc, J.; Klimek, A.; Matusik, J.; Pamuła, J.; Franus, W.; Urbański, K.; Bajda, T. Erythromycin Scavenging from Aqueous Solutions by Zeolitic Materials Derived from Fly Ash. Molecules 2023, 28, 798. [Google Scholar] [CrossRef] [PubMed]
- Farzam, K.; Nessel, T.A.; Quick, J. Erythromycin. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Ettler, P.; Votruba, J. Determination of the Optimal Feeding Regime during Biosynthesis of Erythromycin. Folia Microbiol 1980, 25, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.M.; Wierman, C.K.; Hutchinson, C.R. Genetic Analysis of Erythromycin Production in Streptomyces Erythreus. J Bacteriol 1985, 164, 425–433. [Google Scholar] [CrossRef]
- De Simeis, D.; Serra, S. Actinomycetes: A Never-Ending Source of Bioactive Compounds—An Overview on Antibiotics Production. Antibiotics 2021, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Jelić, D.; Antolović, R. From Erythromycin to Azithromycin and New Potential Ribosome-Binding Antimicrobials. Antibiotics 2016, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Platon, V.-M.; Dragoi, B.; Marin, L. Erythromycin Formulations—A Journey to Advanced Drug Delivery. Pharmaceutics 2022, 14, 2180. [Google Scholar] [CrossRef] [PubMed]
- Peter Guengerich, F.; Waterman, M.R.; Egli, M. Recent Structural Insights into Cytochrome P450 Function. Trends Pharmacol Sci 2016, 37, 625–640. [Google Scholar] [CrossRef]
- Cyphert, E.L.; Wallat, J.D.; Pokorski, J.K.; Von Recum, H.A. Erythromycin Modification That Improves Its Acidic Stability While Optimizing It for Local Drug Delivery. Antibiotics 2017, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Elghamry, H.A.; Khalifa, N.E.; Khojali, W.M.A.; Khafagy, E.-S.; Shawky, S.; El-Horany, H.E.-S.; El-Housiny, S. Development and Optimization of Erythromycin Loaded Transethosomes Cinnamon Oil Based Emulgel for Antimicrobial Efficiency. Gels 2023, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A. m.; Abdel-Wahab, N. m.; Hassan, H. m.; Abdelmohsen, U. r. Saccharopolyspora: An Underexplored Source for Bioactive Natural Products. Journal of Applied Microbiology 2020, 128, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Long, Q.; Zhao, Z.; Chen, L.; He, W.; Hong, J.; Liu, K.; Wang, Y.; Pang, X.; Deng, Z.; et al. Engineering the Erythromycin-Producing Strain Saccharopolyspora Erythraea HOE107 for the Heterologous Production of Polyketide Antibiotics. Frontiers in Microbiology 2020, 11. [Google Scholar] [CrossRef]
- Cupp-Vickery, J.R.; Poulos, T.L. Structure of Cytochrome P450eryF Involved in Erythromycin Biosynthesis. Nat Struct Mol Biol 1995, 2, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, J.; Liu, H.; Wang, Y. Engineered EryF Hydroxylase Improving Heterologous Polyketide Erythronolide B Production in Escherichia Coli. Microb Biotechnol 2022, 15, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Basnet, D.; Choi, C.; Sohng, J.K.; Ahn, J.S.; Yoon, Y. The Role of Erythromycin C-12 Hydroxylase, EryK, as a Substitute for PikC Hydroxylase in Pikromycin Biosynthesis. Bioorganic chemistry 2005, 32, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.R.; English, R.S.; Lampel, J.S.; Post, D.A.; Vanden Boom, T.J. Transcriptional Organization of the Erythromycin Biosynthetic Gene Cluster of Saccharopolyspora Erythraea. J Bacteriol 1999, 181, 7098–7106. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Lu, J.; Ke, X.; Shao, M.; Huang, M.; Chu, J. Reconstruction of the Genome-Scale Metabolic Model of Saccharopolyspora Erythraea and Its Application in the Overproduction of Erythromycin. Metabolites 2022, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Guengerich, F.P.; Ma, L.; Li, S.; Zhang, W. Engineering Cytochrome P450 Enzyme Systems for Biomedical and Biotechnological Applications. Journal of Biological Chemistry 2020, 295, 833–849. [Google Scholar] [CrossRef]
- Carreras, C.; Frykman, S.; Ou, S.; Cadapan, L.; Zavala, S.; Woo, E.; Leaf, T.; Carney, J.; Burlingame, M.; Patel, S.; et al. Saccharopolyspora Erythraea-Catalyzed Bioconversion of 6-Deoxyerythronolide B Analogs for Production of Novel Erythromycins. Journal of Biotechnology 2002, 92, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Savino, C.; Montemiglio, L.C.; Sciara, G.; Miele, A.E.; Kendrew, S.G.; Jemth, P.; Gianni, S.; Vallone, B. Investigating the Structural Plasticity of a Cytochrome P450: THREE-DIMENSIONAL STRUCTURES OF P450 EryK AND BINDING TO ITS PHYSIOLOGICAL SUBSTRATE*. Journal of Biological Chemistry 2009, 284, 29170–29179. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Basnet, D.; Choi, C.; Sohng, J.K.; Ahn, J.S.; Yoon, Y. The Role of Erythromycin C-12 Hydroxylase, EryK, as a Substitute for PikC Hydroxylase in Pikromycin Biosynthesis. Bioorganic chemistry 2005, 32, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Q.; Lei, H.-M.; Hu, Q.-Y.; Li, G.-H.; Zhao, P.-J. Recent Advances in the Synthetic Biology of Natural Drugs. Frontiers in Bioengineering and Biotechnology 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, K.; Zhu, J.; Wu, Y. Effect of Solution Polarity and Temperature on Adsorption Separation of Erythromycin A and C onto Macroporous Resin SP825. Separation Science and Technology 2014, 49, 898–906. [Google Scholar] [CrossRef]
- Chen, K.; Ji, L.-J.; Wu, Y.-Y.; Chen, K.; Ji, L.-J.; Wu, Y.-Y. Purification of Erythromycin by Antisolvent Crystallization or Azeotropic Evaporative Crystallization. In Advanced Topics on Crystal Growth; IntechOpen, 2013 ISBN 978-953-51-1010-1.
- Ruzaimi, M.Y.; Shahril, Y.; Masbah, O.; Salasawati, H. Antimicrobial Properties of Erythromycin and Colistin Impregnated Bone Cement. An in Vitro Analysis. Med J Malaysia 2006, 61 Suppl A, 21–26. [Google Scholar]
- Guengerich, F.P. Cytochrome P450 Proteins and Potential Utilization in Biodegradation. Environ Health Perspect 1995, 103 Suppl 5, 25–28. [Google Scholar] [CrossRef]
- Gilani, B.; Cassagnol, M. Biochemistry, Cytochrome P450. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2024. [Google Scholar]
- Erythromycin, Derivatives, in Bulk, Salts | OEC - The Observatory of Economic Complexity. Available online: https://oec.world/en/profile/hs/erythromycin-derivatives-in-bulk-salts?redirect=true#trade (accessed on 7 July 2023).
- Platon, V.-M.; Dragoi, B.; Marin, L. Erythromycin Formulations—A Journey to Advanced Drug Delivery. Pharmaceutics 2022, 14, 2180. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shan-Guang, W.; Yu-Fang, P.; Feng-Lan, S.; Tao, L. Preparation and Characteristics of Erythromycin Microspheres for Lung Targeting. Drug Development and Industrial Pharmacy 2009, 35, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Inaba, T.; Nito, C.; Ueda, M.; Katsura, K. Neuroprotective Effects of Erythromycin on Cerebral Ischemia Reperfusion-Injury and Cell Viability after Oxygen-Glucose Deprivation in Cultured Neuronal Cells. Brain Res 2014, 1588, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Zhang, M.; Dang, L. Solubility of Erythromycin A Dihydrate in Different Pure Solvents and Acetone + Water Binary Mixtures between 293 K and 323 K. J. Chem. Eng. Data 2006, 51, 1062–1065. [Google Scholar] [CrossRef]
- Baranauskaite-Fedorova, I.; Dvarioniene, J. Management of Macrolide Antibiotics (Erythromycin, Clarithromycin and Azithromycin) in the Environment: A Case Study of Environmental Pollution in Lithuania. Water 2023, 15, 10. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Shi, H.; Zou, P. Erythromycin Estolate Is a Potent Inhibitor Against HCoV-OC43 by Directly Inactivating the Virus Particle. Frontiers in Cellular and Infection Microbiology 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, S.; Gong, J.; Zhen, Y. [Erythromycin inhibits the proliferation of HERG K+ channel highly expressing cancer cells and shows synergy with anticancer drugs]. Zhonghua Yi Xue Za Zhi 2006, 86, 3353–3357. [Google Scholar] [PubMed]
- Ayoub, M.; Faris, C.; Tomanguillo, J.; Anwar, N.; Chela, H.; Daglilar, E. The Use of Pre-Endoscopic Metoclopramide Does Not Prevent the Need for Repeat Endoscopy: A U.S. Based Retrospective Cohort Study. Life 2024, 14, 526. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Maekawa, T.; Domon, H.; Sirisereephap, K.; Isono, T.; Hirayama, S.; Hiyoshi, T.; Sasagawa, K.; Takizawa, F.; Maeda, T.; et al. Erythromycin Restores Osteoblast Differentiation and Osteogenesis Suppressed by Porphyromonas Gingivalis Lipopolysaccharide. Pharmaceuticals 2023, 16, 303. [Google Scholar] [CrossRef] [PubMed]
- Bello, S.O.; Imam, M.U.; Bello, M.B.; Yunusa, A.; Ahmed Adamu, A.; Shuaibu, A.; Igumbor, E.U.; Habib, Z.G.; Popoola, M.A.; Ochu, C.L.; et al. Erythromycin, Retapamulin, Pyridoxine, Folic Acid, and Ivermectin Inhibit Cytopathic Effect, Papain-like Protease, and MPRO Enzymes of SARS-CoV-2. Front Cell Infect Microbiol 2023, 13, 1273982. [Google Scholar] [CrossRef] [PubMed]
- Girvan, H.M.; Munro, A.W. Applications of Microbial Cytochrome P450 Enzymes in Biotechnology and Synthetic Biology. Curr Opin Chem Biol 2016, 31, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, R.; Urlacher, V.B. Cytochromes P450 as Promising Catalysts for Biotechnological Application: Chances and Limitations. Appl Microbiol Biotechnol 2014, 98, 6185–6203. [Google Scholar] [CrossRef] [PubMed]
- Davydov, D.R.; Davydova, N.Y.; Halpert, J.R. Allosteric Transitions in Cytochrome P450eryF Explored with Pressure-Perturbation Spectroscopy, Lifetime FRET, and a Novel Fluorescent Substrate, Fluorol-7GA. Biochemistry 2008, 47, 11348–11359. [Google Scholar] [CrossRef]
- Kompanowska-Jezierska, E.; Walkowska, A.; Sadowski, J. Role of Prostaglandin Cyclooxygenase and Cytochrome P450 Pathways in the Mechanism of Natriuresis Which Follows Hypertonic Saline Infusion in the Rat. Acta Physiol Scand 2003, 177, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Atone, J.; Wagner, K.; Hashimoto, K.; Hammock, B.D. Prostaglandins and Other Lipid Mediators Cytochrome P450 Derived Epoxidized Fatty Acids as a Therapeutic Tool against Neuroinflammatory Diseases. Prostaglandins Other Lipid Mediat 2020, 147, 106385. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.G.; Díaz, M.D.; Lampe, J.N.; Shireman, L.M.; Grinstead, J.S.; Dabrowski, M.J.; Pearson, J.T.; Bowman, M.K.; Atkins, W.M.; Campbell, A.P. NMR Studies of Ligand Binding to P450eryF Provides Insight into the Mechanism of Cooperativity. Biochemistry 2006, 45, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Q. Harnessing P450 Enzyme for Biotechnology and Synthetic Biology. ChemBioChem 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Cupp-Vickery, J.R.; Garcia, C.; Hofacre, A.; McGee-Estrada, K. Ketoconazole-Induced Conformational Changes in the Active Site of Cytochrome P450eryF1. Journal of Molecular Biology 2001, 311, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Nagano, S.; Cupp-Vickery, J.R.; Poulos, T.L. Crystal Structures of the Ferrous Dioxygen Complex of Wild-Type Cytochrome P450eryF and Its Mutants, A245S and A245T: Investigation of the Proton Transfer System in P450eryF. J Biol Chem 2005, 280, 22102–22107. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Haydock, S.F.; Roberts, G.A.; Bevitt, D.J.; Leadlay, P.F. An Unusually Large Multifunctional Polypeptide in the Erythromycin-Producing Polyketide Synthase of Saccharopolyspora Erythraea. Nature 1990, 348, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Menzella, H.G.; Reid, R.; Carney, J.R.; Chandran, S.S.; Reisinger, S.J.; Patel, K.G.; Hopwood, D.A.; Santi, D.V. Combinatorial Polyketide Biosynthesis by de Novo Design and Rearrangement of Modular Polyketide Synthase Genes. Nat Biotechnol 2005, 23, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Staunton, J.; Wilkinson, B. Biosynthesis of Erythromycin and Rapamycin. Chem. Rev. 1997, 97, 2611–2630. [Google Scholar] [CrossRef] [PubMed]
- Khosla, C.; Tang, Y.; Chen, A.Y.; Schnarr, N.A.; Cane, D.E. Structure and Mechanism of the 6-Deoxyerythronolide B Synthase. Annu Rev Biochem 2007, 76, 195–221. [Google Scholar] [CrossRef]
- Baranauskaitė-Fedorova, I.; Dvarioniene, J. Management of Macrolide Antibiotics (Erythromycin, Clarithromycin and Azithromycin) in the Environment: A Case Study of Environmental Pollution in Lithuania. Water 2022, 15, 10. [Google Scholar] [CrossRef]
- Bell, S.G.; Orton, E.; Boyd, H.; Stevenson, J.-A.; Riddle, A.; Campbell, S.; Wong, L.-L. Engineering Cytochrome P450cam into an Alkane Hydroxylase. Dalton Trans. 2003, 2133–2140. [Google Scholar] [CrossRef]
- Ma, L.; Li, F.; Zhang, X.; Chen, H.; Huang, Q.; Su, J.; Liu, X.; Sun, T.; Fang, B.; Liu, K.; et al. Development of MEMS Directed Evolution Strategy for Multiplied Throughput and Convergent Evolution of Cytochrome P450 Enzymes. Sci. China Life Sci. 2022, 65, 550–560. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, M.; Wang, Z.; Chu, J.; Zhuang, Y.; Zhang, S. Controlling the Feed Rate of Glucose and Propanol for the Enhancement of Erythromycin Production and Exploration of Propanol Metabolism Fate by Quantitative Metabolic Flux Analysis. Bioprocess Biosyst Eng 2013, 36, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Y.; Fang, L.; Pfeifer, B.A. Tailoring Pathway Modularity in the Biosynthesis of Erythromycin Analogs Heterologously Engineered in E. Coli. Science Advances 2015, 1, e1500077. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Khosla, C. Antibiotic Production from the Ground Up. Nat Biotechnol 2007, 25, 428–429. [Google Scholar] [CrossRef] [PubMed]
- El-Enshasy, H.A.; Mohamed, N.A.; Farid, M.A.; El-Diwany, A.I. Improvement of Erythromycin Production by Saccharopolyspora Erythraea in Molasses Based Medium through Cultivation Medium Optimization. Bioresource Technology 2008, 99, 4263–4268. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Yang, P.; Gao, Z.; Li, Z.; Fang, C.; Gong, J. Recent Progress in Antisolvent Crystallization. CrystEngComm 2022, 24, 3122–3135. [Google Scholar] [CrossRef]
- Tang, M.; Gu, Y.; Wei, D.; Tian, Z.; Tian, Y.; Yang, M.; Zhang, Y. Enhanced Hydrolysis of Fermentative Antibiotics in Production Wastewater: Hydrolysis Potential Prediction and Engineering Application. Chemical Engineering Journal 2020, 391, 123626. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, M.; Li, S.; Zhang, S.; Han, G. The Removal of Erythromycin and Its Effects on Anaerobic Fermentation. Int J Environ Res Public Health 2022, 19, 7256. [Google Scholar] [CrossRef] [PubMed]
- Parise Filho, R.; Polli, M.; Barberato-Filho, S.; Garcia, M.; Ferreira, E. Prodrugs Available on the Brazilian Pharmaceutical Market and Their Corresponding Bioactivation Pathways. Brazilian Journal of Pharmaceutica Science 2010, 46. [Google Scholar] [CrossRef]
- Scholar, E. Erythromycin. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, 2007; pp. 1–9. ISBN 978-0-08-055232-3. [Google Scholar]
- Espacenet – Search Results. Available online: https://worldwide.espacenet.com/patent/search?q=nftxt%20%3D%20%22erythromycin%22%20AND%20nftxt%20%3D%20%22production%22%20AND%20pd%20%3D%20%222011-2021%22 (accessed on 7 July 2023).
- Topical Antibiotics Market Size & Share, Forecasts Report 2032. Available online: https://www.gminsights.com/industry-analysis/topical-antibiotics-market (accessed on 21 June 2024).
- Ren, X.; Wang, Y.; Zhang, K.; Ding, Y.; Zhang, W.; Wu, M.; Xiao, B.; Gu, P. Transmission of Microcystins in Natural Systems and Resource Processes: A Review of Potential Risks to Humans Health. Toxins 2023, 15, 448. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xie, Q.-M.; Chu, T.-J. Effects of Enrofloxacin and Ciprofloxacin on Growth and Toxin Production of Microcystis Aeruginosa. Water 2023, 15, 3580. [Google Scholar] [CrossRef]
- Jednačak, T.; Mikulandra, I.; Novak, P. Advanced Methods for Studying Structure and Interactions of Macrolide Antibiotics. International Journal of Molecular Sciences 2020, 21, 7799. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Pham, D.L.; O’Brien, E.P.; Li, M.S. Erythromycin Leads to Differential Protein Expression through Differences in Electrostatic and Dispersion Interactions with Nascent Proteins. Sci Rep 2018, 8, 6460. [Google Scholar] [CrossRef] [PubMed]
- Siibak, T.; Peil, L.; Xiong, L.; Mankin, A.; Remme, J.; Tenson, T. Erythromycin- and Chloramphenicol-Induced Ribosomal Assembly Defects Are Secondary Effects of Protein Synthesis Inhibition. Antimicrob Agents Chemother 2009, 53, 563–571. [Google Scholar] [CrossRef]
- Cicali, B.; Schmidt, S.; Zeitlinger, M.; Brown, J.D. Macrolide Treatment Failure Due to Drug–Drug Interactions: Real-World Evidence to Evaluate a Pharmacological Hypothesis. Pharmaceutics 2022, 14, 704. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ahmed, W.; Mehmood, S.; Ou, W.; Li, J.; Xu, W.; Wang, L.; Mahmood, M.; Li, W. Evaluating the Combined Effects of Erythromycin and Levofloxacin on the Growth of Navicula Sp. and Understanding the Underlying Mechanisms. Plants 2023, 12, 2547. [Google Scholar] [CrossRef]
- Park, B.; Min, Y.-H. In Vitro Synergistic Effect of Retapamulin with Erythromycin and Quinupristin against Enterococcus Faecalis. J Antibiot 2020, 73, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Lee, S.; Lee, Y.; Kim, S.; Kim, K. A New Nano-Platform of Erythromycin Combined with Ag Nano-Particle ZnO Nano-Structure against Methicillin-Resistant Staphylococcus Aureus. Pharmaceutics 2020, 12, 841. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Erythromycin molecule structure.
Figure 1.
Erythromycin molecule structure.
Figure 2.
The conversion of 6-DEB to erythronolide B, which is catalyzed by P450eryF. P450eryF catalyzes the 6S – hydroxylation of 6 – deoxyerythronolide B, that finally leads to the production of erythromycin. The transformation – hydroxylation that took place in the 6S is marked in red.
Figure 2.
The conversion of 6-DEB to erythronolide B, which is catalyzed by P450eryF. P450eryF catalyzes the 6S – hydroxylation of 6 – deoxyerythronolide B, that finally leads to the production of erythromycin. The transformation – hydroxylation that took place in the 6S is marked in red.
Figure 3.
Above: Cytochrome P450eryF general 3D structure (downloaded by the RCSB PDB (Protein Data Bank)). Below: The detailed overall structure of Cytochrome P450eryF. In the general structure above, alpha – Helices are represented by ribbons of different colors on the right side of the molecule, while beta – structures with coils are located on the left side and depicted with orange arrows. In the overall alpha/beta structure depicted below, alpha helical domains are represented by ribbons of different colors, all coils are represented by green lines and beta – structures (beta – sheets), are depicted as purple – fuchsia broad arrows. The color coding consists of red as oxygen, blue as nitrogen, grey as hydrogen, green as carbon (as well as carbon/carbon bonds), sulfur as deep purple and yellow as iron (II). The cyan dashed lines correspond to all hydrogen bonds formed between amino acids, water molecules etc., while the red dash line correspond to the electrostatic interaction (repulsion) formed between the iron of the haem group and the sulfur of Cys351. The water molecule Wat519 is close to the iron of the haem group by 3.8Å. The haem prosthetic group is in a high spin state, as well as penta–coordinate, with the haem iron bound to the tethered to the sulfur of Cys351 and embedded between the I and L helices. .
Figure 3.
Above: Cytochrome P450eryF general 3D structure (downloaded by the RCSB PDB (Protein Data Bank)). Below: The detailed overall structure of Cytochrome P450eryF. In the general structure above, alpha – Helices are represented by ribbons of different colors on the right side of the molecule, while beta – structures with coils are located on the left side and depicted with orange arrows. In the overall alpha/beta structure depicted below, alpha helical domains are represented by ribbons of different colors, all coils are represented by green lines and beta – structures (beta – sheets), are depicted as purple – fuchsia broad arrows. The color coding consists of red as oxygen, blue as nitrogen, grey as hydrogen, green as carbon (as well as carbon/carbon bonds), sulfur as deep purple and yellow as iron (II). The cyan dashed lines correspond to all hydrogen bonds formed between amino acids, water molecules etc., while the red dash line correspond to the electrostatic interaction (repulsion) formed between the iron of the haem group and the sulfur of Cys351. The water molecule Wat519 is close to the iron of the haem group by 3.8Å. The haem prosthetic group is in a high spin state, as well as penta–coordinate, with the haem iron bound to the tethered to the sulfur of Cys351 and embedded between the I and L helices. .
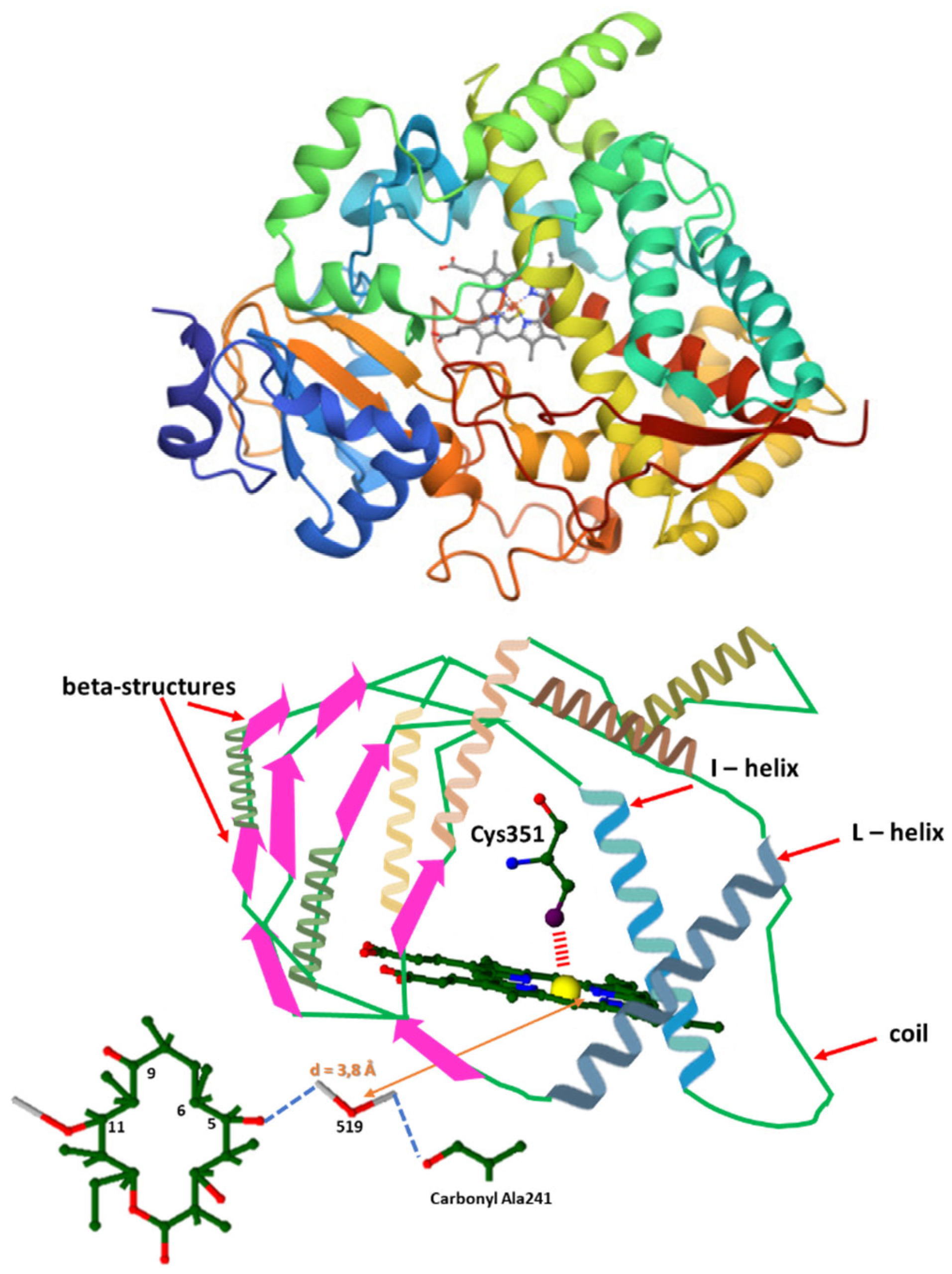
Figure 4.
Above: The substrate of P450eryF, 6 – deoxyerythronolide and the numbering system used. Below: 6-DEB in the active site of P450eryF. The color coding consists of red as oxygen, blue as nitrogen, grey as hydrogen, green as carbon (as well as carbon/carbon bonds) and yellow as iron (II). The cyan dashed lines correspond to all hydrogen bonds formed between amino acids, water molecules etc. Not connected to the actual structure but vital for P450eryF’s functionality, is the haem structure. All water molecules that participate in the reaction are named as 510, 519 and 563. In actuality, the water molecule Wat519 is close to the iron of the haem group by 3.8Å and it is also suggested that it may be the proton donor of the O2 cleavage reaction (orange arrow that shows this distance).
Figure 4.
Above: The substrate of P450eryF, 6 – deoxyerythronolide and the numbering system used. Below: 6-DEB in the active site of P450eryF. The color coding consists of red as oxygen, blue as nitrogen, grey as hydrogen, green as carbon (as well as carbon/carbon bonds) and yellow as iron (II). The cyan dashed lines correspond to all hydrogen bonds formed between amino acids, water molecules etc. Not connected to the actual structure but vital for P450eryF’s functionality, is the haem structure. All water molecules that participate in the reaction are named as 510, 519 and 563. In actuality, the water molecule Wat519 is close to the iron of the haem group by 3.8Å and it is also suggested that it may be the proton donor of the O2 cleavage reaction (orange arrow that shows this distance).
Figure 5.
Schematic representation of the hydrogen bonded system – The deformation of the I – helix in the P450eryF enzyme. The color coding consists of red as oxygen, blue as nitrogen, grey as hydrogen, green as carbon (as well as carbon/carbon bonds) and yellow as iron (II) (is depicted via an orange arrow). The cyan dashed lines correspond to all hydrogen bonds formed between amino acids, water molecules etc. The bonds’ length is measured in Angstroms. All water molecules that participate in the reaction are named as 515, 519, and 564.
Figure 5.
Schematic representation of the hydrogen bonded system – The deformation of the I – helix in the P450eryF enzyme. The color coding consists of red as oxygen, blue as nitrogen, grey as hydrogen, green as carbon (as well as carbon/carbon bonds) and yellow as iron (II) (is depicted via an orange arrow). The cyan dashed lines correspond to all hydrogen bonds formed between amino acids, water molecules etc. The bonds’ length is measured in Angstroms. All water molecules that participate in the reaction are named as 515, 519, and 564.
Figure 6.
Catalytic process of erythromycin A via two different ways (the oxidation reaction is displayed using [O]). The catalysts of all reactions are depicted in pink and all transformations are marked in red.
Figure 6.
Catalytic process of erythromycin A via two different ways (the oxidation reaction is displayed using [O]). The catalysts of all reactions are depicted in pink and all transformations are marked in red.
Figure 7.
Flow chart of Erythromycin production.
Figure 7.
Flow chart of Erythromycin production.
Figure 8.
Importers and exporters of erythromycin throughout the years 2011 – 2021.
Figure 8.
Importers and exporters of erythromycin throughout the years 2011 – 2021.
Figure 9.
Publication of erythromycin patents per country.
Figure 9.
Publication of erythromycin patents per country.
Figure 10.
Publication of erythromycin patents per year during the years 2011-2021.
Figure 10.
Publication of erythromycin patents per year during the years 2011-2021.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).