1. Introduction
Since “time is myocardium,” rapid reperfusion of an acute coronary occlusion (ACO) is required in order to salvage ischemic myocardium before irreversible infarction leads to increased morbidity and mortality.
For approximately two decades, the patients who were believed to benefit from emergent revascularization have been those presenting with ST Elevation on the ECG, since ST Elevation has been assumed to be an accurate surrogate for ACO. Thus, the dichotomy of ST Elevation Myocardial Infarction (STEMI) vs. Non-STEMI has guided the use of emergency revascularization.
Recently, there has been increasing emphasis on STEMI equivalents, which are associated with high risk. Immediate revascularization in these cases has the potential to significantly improve patient outcomes. [
9,
10]
We know that only 43% acute coronary occlusion MI (OMI) fulfill the STEMI millimeter (mm) criteria [
1]. However NSTEMI patients with delayed treatment of OMI have almost double mortality compared to NSTEMI patients without OMI [
2]. Therefore, we support the idea of expanding the population eligible for emergent reperfusion therapy to any OMI, not only those whose ECGs manifest STEMI mm criteria. Conversely, focusing on the actual outcome of interest will also allow further research aimed at reducing false positive STEMI criteria activations [
1].
The idea that all patients underlying ACO without collateral circulation, regardless of ECG findings, benefit from emergent revascularization has face validity, and is recently acknowledged in the 2022 American College of Cardiology guidelines [
3]. There are ECG patterns beyond ST segment elevation mm criteria which are indicative of ACO, but unfortunately the ”STEMI criteria’’ model restricts our minds and limits us so that we only recognize and emergently intervene on the STEMI subset of OMI [
4].
Therefore, in 2018, the new OMI paradigm was conceived to replace STEMI/NSTEMI [
5]. Blinded physicians with special expertise in OMI ECG findings demonstrated high accuracy in diagnosing OMI using the ECG, with sensitivity more than double the STEMI criteria at equal specificity [
6].
2. Materials and Methods
The study purpose: We sought to evaluate STEMI/NSTEMI cases that result in OMI and the consequences of delayed revascularization immediately among NSTEMI patients who have ACO (NSTEMI-OMI).
Study objectives: 1. Identify the percentage of OMI patients that do not fulfill the STEMI criteria. 2. Compare the total ischemic time in each of the groups according to STEMI criteria and actual OMI outcome. 3. Compare rates of intervention, ejection fraction (EF), complications and hospital day stay in each of these groups.
Study type:The study was performed via retrospective chart review.
Data Collection: A retrospective analysis conducted on 334 patients who underwent coronary angiography for acute coronary syndrome at UHC ‘‘Mother Teresa,’’ Tirana, Albania, during January-May 2023. All patients were enrolled consecutively from cardiology departments I & II and cardiology ICU in UHC ''Mother Teresa'' Tirana, Albania.Data such as patients’ demographic data and comorbidities, total ischemic time (the time from the onset of symptoms to the cath lab), hospital length of stay, ejection fraction, complications, and death cases were obtained from the medical records. Angiographic and relevant treatment data were collected from the standard coronary angiography registry and report, that is currently used in the Cath lab, UHC ''Mother Teresa'' Tirana, Albania. Diagnosis of OMI vs NOMI was determined by angiography ("confirmed OMI"). We separated patiens in four groups: STEMI(+)NOMI, STEMI(-)NOMI, STEMI(-)OMI, and STEMI(+)OMI. STEMI (+) or (-) refers to cases where the criteria for ST-segment elevation myocardial infarction (STEMI) are met(+) or not (-). OMI was defined as an acute culprit lesion with TIMI 0-2 flow, or TIMI 3 flow with highly elevated troponin (cTnI>10.0 ng/mL, hs-cTnI>5000 ng/L) [
38,
39,
40,
41]. Presence or absence of STEMI criteria was determined in the final diagnosis written on chart by cardiologist according to 3rd Universal Definition of MI [
36].
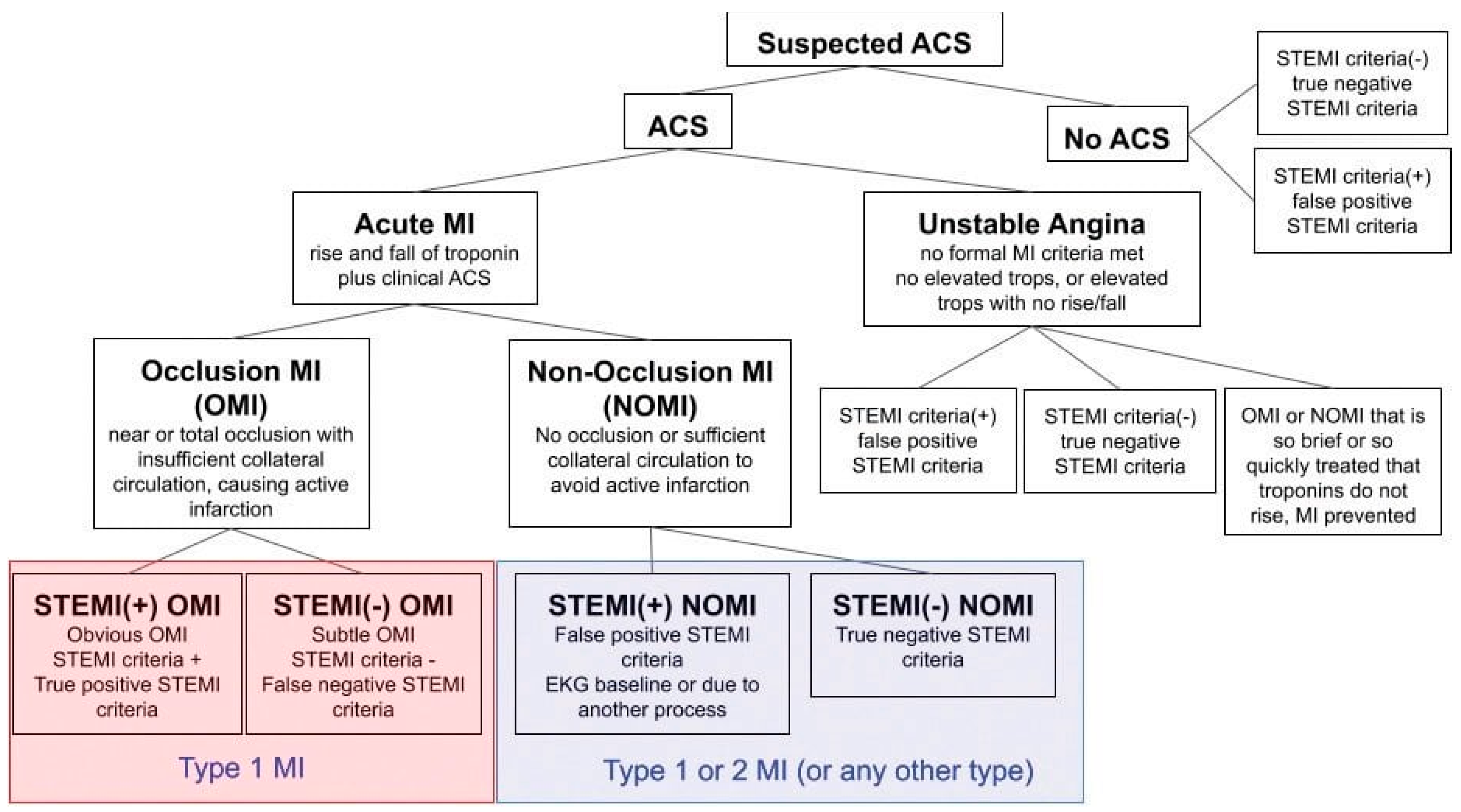
Figure 1.
Adapted from "What Is Occlusion Myocardial Infarction (OMI)?" Powerful Medical[
37]. ACS=acute coronary syndrome, Acute MI= acute Myocardial Infarction, STEMI=ST elevation myocardial infarction, NSTEMI=Non ST elevation myocardial infarction, OMI = occlusion myocardial infarction, NOMI= Non-occlusion myocardial infarction, MI=myocardial infarction, tops=troponins.
Figure 1.
Adapted from "What Is Occlusion Myocardial Infarction (OMI)?" Powerful Medical[
37]. ACS=acute coronary syndrome, Acute MI= acute Myocardial Infarction, STEMI=ST elevation myocardial infarction, NSTEMI=Non ST elevation myocardial infarction, OMI = occlusion myocardial infarction, NOMI= Non-occlusion myocardial infarction, MI=myocardial infarction, tops=troponins.
Inclusion criteria: 1. Patients with a diagnosis of STEMI or NSTEMI. 2. Patients from departaments I & II of cardiology and cardiology ICU who underwent coronary angiography in UHC ''Mother Teresa'' Tirana, Albania.
Exclusionary criteria: 1. Patients who underwent coronary angiography with a diagnosis of unstable angina.2. Patients who refused angiography or passed away before performing angiography.
Statistical analysis methodology: The statistical program SPSS 20.0 (Statistical Package for Social Sciences) was used to analyze the data. The Student’s t-test and the Wilcoxon test were used to compare continuous variables, as appropriate, while the Chi-square test was used to compare categorical variables Categorical variables were presented according to their absolute and relative frequency expressed in percentage. The statistical test of One-way ANOVA was used to determine whether there are any statistically significant differences between the means of three or more independent (unrelated) groups. Continuous data are presented with mean (M) and standard deviation (SD). Statistical significance is defined for p≤0.05. Tables were used to visualize the data.
3. Results
There were 334 patients included, 241 OMI and 93 NOMI. 98 patients (29.3%) were STEMI(-) OMI, 73 patients (21.9%) were STEMI(-)NOMI, 143 (42.8%) were STEMI(+)OMI and 20 (6.0%) were STEMI(+) NOMI (See
Table 2). Only 15 patients (11%) of STEMI(-) OMI had PCI performed within the first 12 hours, vs. 110 patients (77%) of STEMI(+)OMI, p<0.001 (See
Table 6).There was no difference in the percent of patients requiring PCI between the STEMI(+)OMI 133 patients (93%) and STEMI(-)OMI 87 patient(89%) groups (p<0.001) (See
Table 8).
Average EF was: STEMI(+)NOMI 44.70%, STEMI(-)NOMI 50.47%, STEMI(-)OMI 48.70%, STEMI(+)OMI 42.87 %, p<0.001 (See
Table 4). The mortality was 19 patients (5.7 %), 14 (4.2 %) STEMI(+)OMI, 2 (0.6 %) STEMI(+)NOMI, 3 (0.9 %) STEMI(-)OMI, 0% STEMI(-) NOMI, p=0.013 (See
Table 3). The mechanical complications were present in 58 patients (46.8%) STEMI (+)OMI and in 39 patients (46.4%) STEMI(-)OMI. 23 patients (18.5%) of the STEMI(+)OMI and 11 patients (13.1%) of the STEMI(-) OMI developed electrical complications (See
Table 7).
Table 1.
Clinical Characteristics of all Patients in Each Subgroup.
Table 1.
Clinical Characteristics of all Patients in Each Subgroup.
| Characteristic |
STEMI(+) OMI
n =143
|
STEMI(+) NOMI
n =20
|
STEMI(-) OMI
n = 98
|
STEMI(-) NOMI
n = 73
|
| Age, y, mean(SD) |
65.06 (13.33) |
59.16 (12.43) |
64.31 (10.43) |
68.94 (11.78) |
| Female, n (%) |
41 (28.6 %) |
5 (25%) |
22 (22.4% |
29 (39.7%) |
| Known CAD, n (%) |
9 (6,5 %) |
0 (0%) |
9 (9.1%) |
8 (10,9 %) |
| Prior CABG, n (%) |
2 (1.3 %) |
0 (0%) |
5 (5.1%) |
3 (4.1 %) |
| Prior CVA, n (%) |
2 (1.3 %) |
1 (5 %) |
0 (0%) |
3 (4.1 %) |
| CKD, n (%) |
11 (7.7 %) |
1 (5 %) |
5 (5.1%) |
7 (9.5%) |
| CHF, n (%) |
56 (39.1%) |
6 (30%) |
64 (65.9%) |
49 (67.1%) |
| Diabetes, type 2, n (%) |
16 (11.2%) |
6 (30%) |
42 (42.8 %) |
38 (52%) |
| IGT, n (%) |
3 (2%) |
1 (5%) |
5 (5.1%) |
2 (2.7%) |
| HLD, n (%) |
100 (70%) |
8 (40%) |
88 (89.7%) |
54 (73.9%) |
| HTN, n (%) |
108 (75.5%) |
10 (50%) |
88 (89.7%) |
50 (68.4%) |
| PVD, n (%) |
3 (2%) |
0 (0%) |
2 (2%) |
0 (0%) |
Table 2.
The results of the OMI/NOMI classification.
Table 2.
The results of the OMI/NOMI classification.
| |
OMI or NOMI |
Total |
| OMI |
NOMI |
| STEMI |
143 |
20 |
163 |
| 42.8% |
6.0% |
48.8% |
| NSTEMI |
98 |
73 |
171 |
| 29.3% |
21.9% |
51.2% |
| Total |
241 |
93 |
334 |
| 72.2% |
27.8% |
100.0% |
334 patients were included, of whom 241 patients(72.2%) had OMI and 93 patients (27.8 %) had NOMI: 98patients (29.3%) were STEMI(-)OMI, 73 patients (21.9%) were STEMI (-)NOMI, 143patients (42.8%) were STEMI (+)OMI and 20 patients (6.0%) were STEMI (+) NOMI.
Table 3.
In-hospital mortality after PCI in each of the groups.
Table 3.
In-hospital mortality after PCI in each of the groups.
| Exitus |
Groups |
Total |
STEMI +
OMI
|
STEMI + NOMI |
STEMI - OMI |
STEMI-
NOMI
|
| Yes |
14
4.2%
|
2
0.6%
|
3
0.9%
|
0
0%
|
19
5.7%
|
| Total |
143 |
20 |
98 |
73 |
334 |
19 patients (5.7 %) died, 14patients (4.2 %) were STEMI (+) OMI, 2patients (0.6 %) were STEMI(+) NOMI, 3patients (0.9 %) were STEMI(-) OMI, and 0 patient were STEMI(-)NOMI. 14 patients (10%) of patients from STEMI(+)OMI group and 3patients (3%) of patients from STEMI(-) OMI died.
Table 4.
Average hospital length of stay in each groups.
Table 4.
Average hospital length of stay in each groups.
| |
N |
Mean
(day)
|
SD |
95% Confidence Interval for Mean |
Minimum
(day)
|
Maximum
(day)
|
| STEMI (+) OMI |
143 |
5.30 |
3.95 |
4.65 |
5.95 |
1.00 |
30.00 |
| STEMI (+) NOMI |
20 |
9.15 |
13.22 |
2.96 |
15.34 |
1.00 |
64.00 |
| STEMI (-) OMI |
98 |
5.66 |
3.08 |
5.05 |
6.28 |
0.00 |
19.00 |
| STEMI (-) NOMI |
73 |
6.44 |
2.92 |
5.76 |
7.12 |
2.00 |
17.00 |
| Total |
334 |
5.89 |
4.70 |
5.38 |
6.39 |
0.00 |
64.00 |
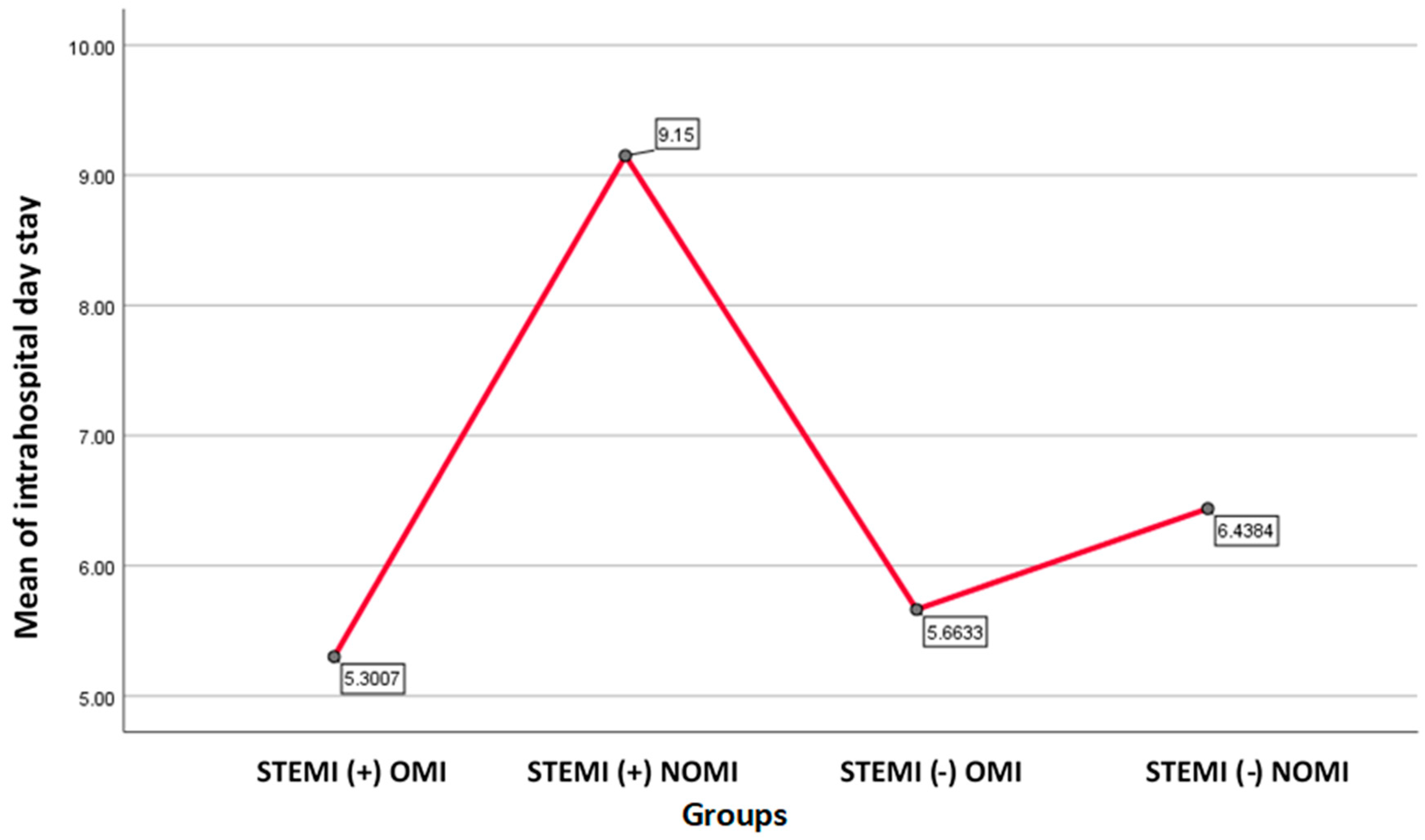
The average of the highest days of stay resulted in the group of STEMI (+) NOMI patients with 9.15 days, followed by STEMI(-) NOMI with 6.44 days, STEMI(-)OMI patients with 5.66 days and those STEMI(+) OMI with 5.30 days (One-way ANOVA F=4.495, p=0.004).
Figure 2.
Graph showing mean of hospital day stay for each group.STEMI(+)NOMI had the highest hospitalday stay, followed by STEMI(-)NOMI.
Figure 2.
Graph showing mean of hospital day stay for each group.STEMI(+)NOMI had the highest hospitalday stay, followed by STEMI(-)NOMI.
Table 5.
Ejection fraction in each of the groups.
Table 5.
Ejection fraction in each of the groups.
| |
N |
Mean |
SD |
95% Confidence Interval for Mean |
| STEMI (+) OMI |
143 |
42.9 |
9.52 |
42.87 +/- 1.58 |
| STEMI (+) NOMI |
20 |
44.7 |
11.11 |
44.70 +/- 5.2 |
| STEMI (-) OMI |
98 |
48.7 |
12.00 |
48.70 +/- 2.4 |
| STEMI (-) NOMI |
73 |
50.5 |
10.77 |
50.47 +/- 2.51 |
| Total |
334 |
46.4 |
11.13 |
46.355 +/- 1.22 |
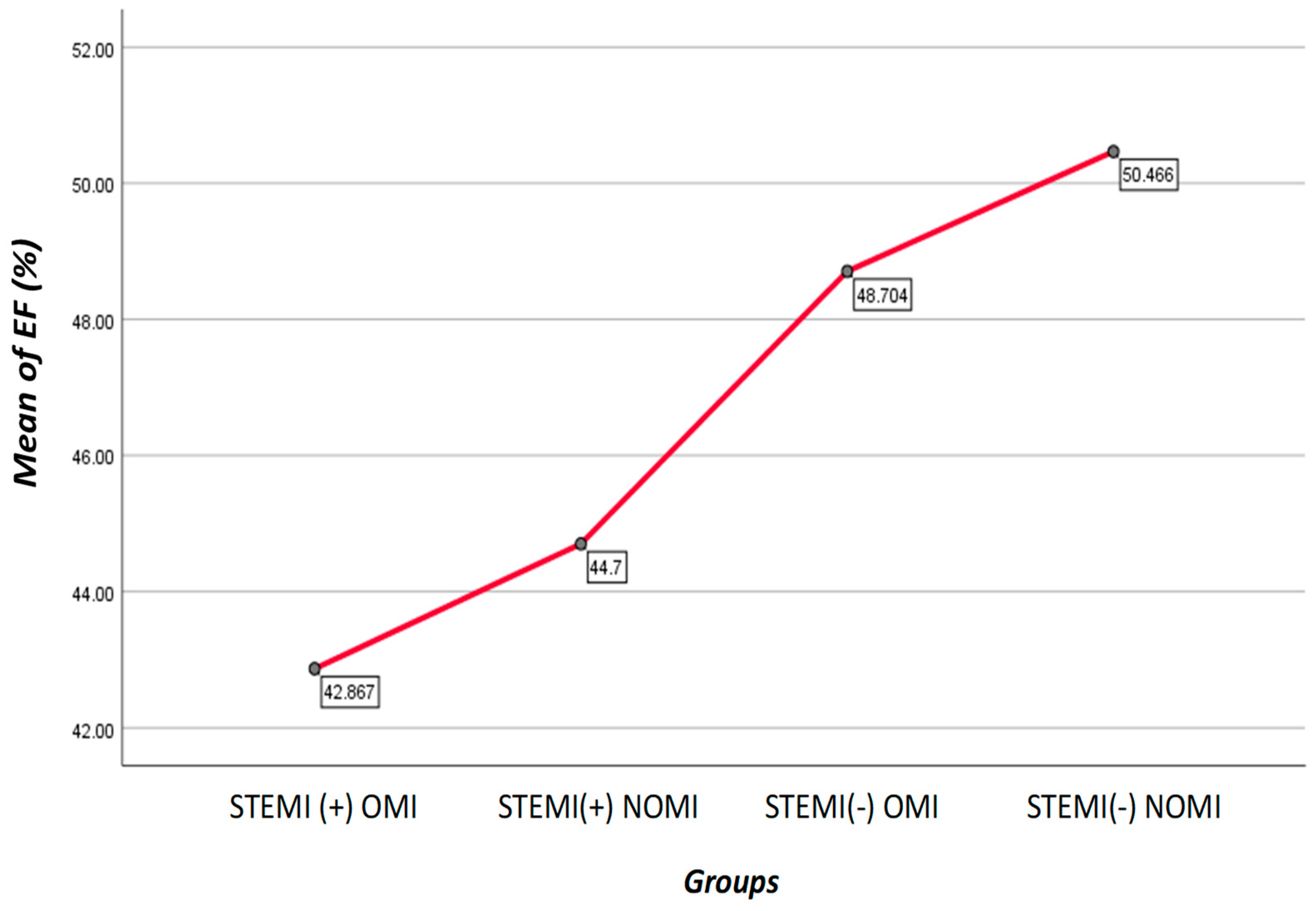
Average EF was: STEMI(+)NOMI 44.70%, STEMI(-)NOMI 50.47%, STEMI(-)OMI 48.70%, STEMI(+)OMI 42.87 %, p<0.001
Figure 3.
Graph shows mean of EF(%) for each group Patients with STEMI(-)NOMI had the highest EF, while the patients with STEMI(+)OMI had the lowest EF.
Figure 3.
Graph shows mean of EF(%) for each group Patients with STEMI(-)NOMI had the highest EF, while the patients with STEMI(+)OMI had the lowest EF.
Table 6.
Total ischemic time in each of the groups.
Table 6.
Total ischemic time in each of the groups.
| Time interval (hours) |
Groups |
Total |
| STEMI (+) OMI |
STEMI (+) NOMI |
STEMI (-) OMI |
STEMI (-) NOMI |
| 0-2 |
7 |
0 |
1 |
0 |
8 |
| |
4.9% |
0.0% |
1.0% |
0.0% |
2.4% |
| 2-6 |
52 |
5 |
2 |
2 |
61 |
| |
36.4% |
25.0% |
2.1% |
2.8% |
18.4% |
| 6-12 |
51 |
7 |
12 |
6 |
76 |
| |
35.7% |
35.0% |
12.5% |
8.3% |
23.0% |
| 12-24 |
15 |
2 |
26 |
12 |
55 |
| |
10.5% |
10.0% |
27.1% |
16.7% |
16.6% |
| 24-48 |
3 |
1 |
21 |
15 |
40 |
| |
2.1% |
5.0% |
21.9% |
19.4% |
11.8% |
| > 48 |
15 |
5 |
36 |
38 |
94 |
| 10.5% |
25.0% |
35.4% |
52.8% |
27.8% |
| Total |
143 |
20 |
98 |
73 |
334 |
| 100.0% |
100.0% |
100.0% |
100.0% |
100.0% |
| Median ischemic time |
8.0000 |
10.0000 |
48.0000 |
72.0000 |
|
| IQR |
7.00 |
113.75 |
48.00 |
96.00 |
|
110 patients (77%) of STEMI (+) OMI patients had PCI performed PCI within the first 12 hours, vs. 15 patients (11%) of STEMI (-) OMI. 125 patients (87.4%) of STEMI (+) OMI patients performed PCI within 24 hours vs. 41 patients (42.7%) in the STEMI (-) OMI group. The majority of patients belonging to the STEMI (-) OMI group, 55 patients (57.3%) underwent PCI after 24 hours, 34 patients of which (61.7%) underwent PCI after 48 hours.
Table 7.
The balance of intrahospital complications in each of the groups.
Table 7.
The balance of intrahospital complications in each of the groups.
| Complications |
Groups |
Total |
| STEMI (+) OMI |
STEMI (+) NOMI |
STEMI(-) OMI |
STEMI (-)
NOMI
|
| None |
36 |
3 |
33 |
29 |
101 |
| 29.0% |
15.8% |
39.3% |
44.6% |
34.6% |
| * Mechanical complications |
58 |
11 |
39 |
26 |
134 |
| 46.8% |
57.9% |
46.4% |
40.0% |
45.9% |
| * Electrical complications |
23 |
3 |
11 |
7 |
44 |
| 18.5% |
15.8% |
13.1% |
10.8% |
15.1% |
| Mechanical & electrical complications |
7 |
2 |
1 |
3 |
13 |
| 5.6% |
10.5% |
1.2% |
4.6% |
4.5% |
| Total |
124 |
19 |
84 |
65 |
292 |
| 100.0% |
100.0% |
100.0% |
100.0% |
100.0% |
The group with fewer complications were STEMI (-) NOMI, 29 patients (44.6%). In total, 101 patients (34.6% of patients) had no complications. 134 patients (45.9%) had only mechanical complications, whereas STEMI(+) OMI and STEMI(-) OMI had a similar frequency of complications. The mechanical complications were present in 58 patients (46.8%) STEMI (+)OMI and in 39 patients (46.4%) STEMI(-)OMI. 23 patients (18.5%) of the STEMI(+)OMI and 11 patients (13.1%) of the STEMI(-) OMI developed electrical complications. Patients who had electrical and mechanical complications make up the smallest group, only 13 patients (4.5% of the total).
Table 8.
STEMI(+) OMI vs. STEMI(−) OMI interventions.
Table 8.
STEMI(+) OMI vs. STEMI(−) OMI interventions.
| Interventions |
STEMI (+) OMI |
STEMI (-) OMI |
STEMI (+) NOMI |
STEMI (-) NOMI |
| Angiogram |
143 (100%) |
98(100%) |
20 (100%) |
73(100%) |
| PCI |
134 (93%) |
87 (88,7%) |
0 (0%) |
61 (83.6%) |
| CABG * |
5 (3,4%) |
6 (6,2%) |
0 (0%) |
0 (0%) |
| PCI + CABG * |
4 (2,6%) |
5 (5,1%) |
0 (0%) |
9 (12.4%) |
| Total |
143 (100%) |
98 (100%) |
20 (100%) |
73 (100%) |
There is no difference in the percent of patients requiring PCI between the STEMI(+)OMI 133 patients (93%) and STEMI(-)OMI 87 patients (89%) groups.
4. Discussion
Contemporary articles are displaying the same essence: "The STEMI criteria underestimates many patients with the same risk as STEMI ones " [
1,
2,
3,
4,
5,
6,
7,
8]. Our results support the idea that the STEMI criteria fail to diagnose a large proportion of OMI and fail to identify patients who require emergent reperfusion and who will have complications. STEMI(-) OMI patients with the same pathology and risk as STEMI(+) OMI patients.
For years, authors have attempted to compensate this discrepancy with terminologies such as “STEMI equivalents,” “subtle STEMI” or “semi-STEMI.” Nevertheless, only 11 patients (11%) of patients with STEMI(-) OMI received reperfusion therapy within 12 hours in our cohort. The terminology "STEMI" suggests that what is important is ST elevation when in reality it is the acute coronary occlusion that is important [
1,
5,
6].
ESC 2023 Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation emphasize early risk stratification, dual antiplatelet therapy (DAPT), and optimal timing for invasive strategies. Key updates include guidance on P2Y12 inhibitors, routine coronary angiography, and tailored approaches for high-risk groups, including the elderly and those with renal impairment [
12].
In their trial Mehta SR, Yusuf S, Peters RJG, et investigate the optimal timing for coronary intervention in patients with NSTEMI. It indicates that early intervention within 24 hours of symptom onset can benefit high-risk patients, while a delayed approach may be appropriate for those at lower risk [
13].
The ESC guidelines provide specific recommendations for revascularization in patients presenting with STEMI [
14]. Firstly, reperfusion therapy is recommended for all patients diagnosed with STEMI, characterized by persistent ST-segment elevation or its equivalents, and with symptoms lasting less than 12 hours (Class IA)[
14]. For patients with STEMI symptoms lasting more than 12 hours, primary PCI is recommended if symptoms persist, if there is hemodynamic instability, or if life-threatening arrhythmias occur (Class IC)[
14]. Additionally, primary PCI should be considered for patients who present late, within 12 to 48 hours after symptom onset (Class IIaB)[
14]. However, routine PCI of an occluded infarct-related artery is not recommended for patients who present more than 48 hours after symptom onset and who do not have persistent symptoms (Class IIIA)[
14].
2017 ESC guidelines on STEMI also highlight that in certain situations, patients may have coronary artery occlusion or global ischemia without the characteristic ST-segment elevation [
15]. Such situations include left bundle branch block, hyperacute T-waves, isolated ST-segment depressions in anterior leads, ventricular pacing, and universal ST-segment depressions with ST elevation in aVR. In patients with these ECG changes and a clinical presentation suggestive of myocardial ischemia, a strategy of primary PCI (urgent angiography and PCI if indicated) should be implemented [
15].
Furthermore, a meta-analysis fromde Alencar Neto JN, Scheffer MK, Correia BP, et al. (2024) focuses on the diagnostic accuracy of ST-segment elevation in identifying acute coronary occlusion, differentiating true STEMI cases from mimics. It emphasizes the importance of distinguishing STEMI-like ECG patterns, such as early repolarization, pericarditis, and hyperkalemia, which can present similarly but require different management [
16].
Khan AR, Golwala H, Tripathi A, et al. (2023) in their study evaluate the use of high-sensitivity troponins to improve the diagnostic accuracy in patients presenting with STEMI-like ECG patterns. The study found that elevated troponin levels in the absence of true coronary occlusion were often associated with conditions like myocarditis, which necessitate different therapeutic approaches [
17].
Thus, in our cohort, “STEMI” and “ST Elevation” were obstacles for improving the management of AMI in 96 patients (40% of our OMI patients). When we see an ECG in the setting of potential ischemia, we should first look for any pattern that reliably predicts OMI, because these are the patients who benefit from emergency reperfusion [
1,
2,
3,
4,
5,
6,
7]. In addition to ischemic ST elevation, we must consider: minimal ST elevations that do not meet the classic criteria, hyperacute T waves (including the de Winter pattern), reciprocal ST depressions and/or hyperacute negative T waves, depressions of ST in V1-V4 indicative of posterior AMI, acute pathological Q waves (Q waves associated with minimal ST elevation, not attributable to a previous myocardial infarction), terminal QRS distortion (loss of the preceding S wave in the context of minimal ST elevation), any inferior ST elevation with any ST depression or T wave inversion in aVL, and modified Sgarbossa criteria for patients with LBBB or paced rhythm [
1,
2,
3,
4,
5,
6,
7,
8].
Table 9 presents ECG patterns that do not meet the criteria for STEMI but are found to be OMI.
Table 9.
STEMI(−) OMI patterns.
Table 9.
STEMI(−) OMI patterns.
| STEMI(-) OMI patterns |
| 1. LBBB or paced rhythm meeting Sgarbossa criteria [17,18]. |
| 2. Terminal QRS distortion (Subtle STE) [19]. |
| 3. De Winter / hyperacute T-waves [20,21,22]. |
| 4. ST depressions in V1-4, without changes in V5-6 and without ST-segment elevation in V7-9 (Isolated posterior MI) [34,35]. |
| 5. Diffuse ST-segment depressions with ST-segment elevation in aVR [25]. |
| 6. New bifascicular block (RBBB + LAFB) or new LBBB [26,27,28,29]. |
| 7. Wellens syndrome [30,31,32,33]. |
| 8. Aslanger pattern: ST-segment elevation in lead III with reciprocal depression in aVL [23,24] |
Lindow et al. in their article included 623 patients, among which 441 (71%) had LBBB and 182 (29%) had VPR. 82 (13%) of these patients were diagnosed with AMI, and an OMI was identified in 15 (2.4%) cases. Sensitivity/specificity of the original unweighted Sgarbossa criteria were 26.7/86.2%, for Barcelona criteria 53.3/82.2%, for MSC 60.0/86.0%, and for Selvester criteria 46.7/88.3%. In this setting with low prevalence of OMI, positive predictive values were low (Sgarbossa: 4.6%; MSC: 9.4%; Barcelona criteria: 6.9%; Selvester criteria: 9.0%) and negative predictive values were high (all >98.0%). Their results suggest that ECG criteria alone are insufficient in predicting presence of OMI in an ED setting with low prevalence of OMI, and the search for better rapid diagnostic instruments in this setting should continue [
8]. Recently, a deep neural network has been shown to diagnose OMI with twice the sensitivity of STEMI criteria at the same specificity [
7].
Recently, a review article has thoroughly examined the preclinical and clinical evidence regarding the use of SGLT2 inhibitors as adjuncts to standard care in acute coronary syndrome (ACS) patients. The article highlights that preclinical studies suggest these inhibitors may reduce ischemia-reperfusion injury and myocardial infarct size, especially with prior treatment. Clinical trials and real-world data also indicate potential benefits in acute ischemic settings, including improved left ventricular function, decongestion, and cardiometabolic parameters. However, further studies are needed to assess the effectiveness of SGLT2 inhibitors in STEMI (+)/(-) and OMI/NOMI populations [
11].
It is important to highlight that the new AMI classification will face implementation challenges. Paradigm shift requires changes on the AMI management protocols and this may lead to potential confusion to the involved medical staff. Therefore, in such conditions multidisciplinary trainings, seminars or lectures will be continuously required. In this way these protocols will be understood and implemented easily.
On the other hand, since the current paradigm excludes 40% of OMI from immediate revascularization, we will need greater capacities and more human resources to cope with 40% more immediate revascularizations.
5. Study’s Limitations
First, the study is a retrospective analysis conducted on 334 patients and all cases were obtained from a single tertiary center that offers emergency coronary angiography service (24/7). Therefore, despite the small sample size and the length of follow-up, this study suffers from the basic limitations of a retrospective registry, and these conclusions should therefore be confirmed in a randomised study.
Second, data on chest pain onset times were obtained from the patient's cardiology referral note on the chart. Third, of 375 patients who were initially identified, 41 patients were excluded because they refused PCI or passed away before performing PCI. Fourth, among 334 included patients, 42 patients datas were missing displaying potential complications.Thus not fulfilling at the end the wished statistical significance due to the restricted number of events.
6. Conclusions
STEMI(-) OMI patients had significant delays in catheterization, and had complications similar to STEMI(+) OMI. These data add further support to refocusing the paradigm of acute MI to improve recognition and rapid reperfusion of all OMIs, rather than only those with STEMI criteria. Clinicians should keep in mind that OMI is a clinical diagnosis, which includes OMI ECG findings but also clinical parameters, and is not defined entirely by the ECG or angiogram. Critical next steps of this paradigm shift include finding reliable methods to quickly identify all OMI patients for emergent reperfusion.
Author Contributions
“Conceptualization, M.K. N.SH,P.M,S.S; methodology, M.K, N.SH, E.P, S.S.P.M; software, M.K.; validation.M.K,N.SH. and E.P.; formal analysis, MK,EP.; investigation, MK.; resources, M.K,N.SH,EP.; data curation, M.K,N.SH, E.P.; writing—original draft preparation, M.K, N.SH, P.M,S.S.; writing—review and editing, M.K, N.SH, P.M, S.S.; visualization, M.K, N.SH, P.M, S.S.; supervision, M.K, N.SH.; project administration, M.K, N.SH.
Funding
This research received no external funding.
Institutional Review Board Statement
“The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of UMT (protocol code 1560 /Date 6, 12, 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
Martiola Kola and Naltin Shuka contributed equally to the work and should be considered co-first authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de Alencar Neto JN, Scheffer MK, Correia BP, Franchini KG, Felicioni SP, De Marchi MF: Systematic review and meta-analysis of diagnostic test accuracy of ST-segment elevation for acute coronary occlusion. Int J Cardiol. 2024. [CrossRef]
- de Silva R, Steg PG: Identifying patients with acute total coronary occlusion in NSTEACS: finding the high-risk needle in the haystack. Eur Heart J. 2017, 38:3090-3. [CrossRef]
- Kontos MC, de Lemos JA, Deitelzweig SB, et al.: 2022 ACC Expert Consensus Decision Pathway on the Evaluation and Disposition of Acute Chest Pain in the Emergency Department: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022, 80:1925-60. [CrossRef]
- H Pendell Meyers, Alexander Bracey, Daniel Lee, et al.: Accuracy of Expert Electrocardiography versus ST-Segment Elevation Myocardial Infarction Criteria for Diagnosis of Acute Coronary Occlusion Myocardial Infarction.. Circulation. 2020, 142. [CrossRef]
- The OMI Manifesto. (2018). https://hqmeded-ecg.blogspot.com/2018/04/the-omi-manifesto.html.
- Meyers HP, Bracey A, Lee D, Lichtenheld A, et al.: Accuracy of OMI ECG findings versus STEMI criteria for diagnosis of acute coronary occlusion myocardial infarction. IJC Heart & Vasculature. 2021, 33. [CrossRef]
- Herman R, Meyers HP, Smith SW, et al.: International evaluation of an artificial intelligence-powered electrocardiogram model detecting acute coronary occlusion myocardial infarction. Eur Heart J Digit Health. 2024, 5:123-33. [CrossRef]
- Lindow T, Mokhtari A, Nyström A, Koul S, Smith SW, Ekelund U: Comparison of diagnostic accuracy of current left bundle branch block and ventricular pacing ECG criteria for detection of occlusion myocardial infarction. Int J Cardiol. 2024, 395:131569. [CrossRef]
- O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4). [CrossRef]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119-177. [CrossRef]
- Karakasis P, Fragakis N, Kouskouras K, Karamitsos T, Patoulias D, Rizzo M: Sodium-glucose cotransporter-2 inhibitors in patients with acute coronary syndrome: A modern Cinderella? Clin Ther. 2024, Online ahead of print. [CrossRef]
- Collet JP, Thiele H, Barbato E, et al. (2023). 2023 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2023;44(2):361-372. [CrossRef]
- Mehta SR, Yusuf S, Peters RJG, et al. (2023). Timing of coronary intervention in patients with non-ST-elevation acute coronary syndromes: A randomized controlled trial. Lancet. 2023;401(10376):753-762. [CrossRef]
- Ibanez B, James S, Agewall S, et al. (2021). 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting with ST-segment elevation. Eur Heart J. 2021;42(14):1289-1367. [CrossRef]
- Ibanez B, James S, Agewall S, et al. (2018). 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 39(2):119-177. [CrossRef]
- de Alencar Neto JN, Scheffer MK, Correia BP, et al. (2024). Systematic review and meta-analysis of diagnostic test accuracy of ST-segment elevation for acute coronary occlusion. Int J Cardiol. 2024. [CrossRef]
- Sgarbossa EB, Pinski SL, Barbagelata A, et al. (1996). Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. N Engl J Med. 1996;334(8):481-487. [CrossRef]
- Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. (2012). Diagnosis of STEMI in the presence of left bundle branch block using the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012;60(6):766-776. [CrossRef]
- Birnbaum Y, Bayés de Luna A, Fiol M, et al. (2014). Common pitfalls in the interpretation of electrocardiograms from patients with acute coronary syndromes with narrow QRS: a consensus report. J Electrocardiol. 2014;47(4):435-439. [CrossRef]
- De Winter RJ, Verouden NJW, Wellens HJ, Wilde AA. (2008). A new ECG sign of proximal LAD occlusion. N Engl J Med. 2008;359(19):2071-2073. [CrossRef]
- Wellens HJ, Conover MB. (2005). The Electrocardiogram in Acute Myocardial Infarction and Unstable Angina. Kluwer Academic Publishers.
- Hwang J, Kim K, Jo YH, et al. (2012). Hyperacute T wave as a predictor of myocardial salvage in patients with anterior ST-segment elevation myocardial infarction: a cardiac magnetic resonance imaging study. Clin Cardiol. 2012;35(7):420-425. [CrossRef]
- Aslanger E, Yalin K, Turkoglu C, et al. (2021). The Aslanger pattern: A reflection of the reciprocal changes in patients with inferior STEMI. J Electrocardiol. 2021;64:129-134. [CrossRef]
- Wellens HJ, Conover MB. (2013). Clinical Electrocardiography: A Simplified Approach. Elsevier.
- Kosuge M, Kimura K. (2020). Clinical implications of electrocardiograms with prominent ST-segment depression, but ST-segment elevation limited to lead aVR. J Cardiol. 2020;75(3):233-239. [CrossRef]
- Mehta N, Legato MJ, Lucas AR. (2014). Bifascicular block in acute myocardial infarction: clinical implications and management strategies. Am Heart J. 2014;168(6):797-804. [CrossRef]
- Elikowski W, Małek K, Krauze T, et al. (2019). The prognostic significance of new-onset bifascicular block in patients with acute myocardial infarction. Pol Arch Intern Med. 2019;129(7-8):456-463. [CrossRef]
- Gorgels AP, Vos MA, Mulleneers R, et al. (1993). Value of the electrocardiogram in diagnosing the number of severely narrowed coronary arteries in rest angina and non-ST elevation ischemia. Am J Cardiol. 1993;72(13):999-1003. [CrossRef]
- Steg PG, James SK, Atar D, et al. (2012). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569-2619. [CrossRef]
- de Zwaan C, Bar FW, Wellens HJ. (1982). Characteristic electrocardiographic pattern indicating a critical stenosis high in the left anterior descending artery in patients admitted with impending myocardial infarction. Am Heart J. 1982;103(4):730-736. [CrossRef]
- Tandy TK, Bottomy DP, Lewis JG. (1999). Wellens' syndrome. Ann Emerg Med. 1999;33(3):347-351. [CrossRef]
- Farkouh ME, Boden WE, Bittner V, et al. (2021). Contemporary review on Wellens syndrome: Pathophysiology, diagnostic approach, and clinical management. J Am Coll Cardiol. 2021;77(12):1477-1485. [CrossRef]
- Nagurney JT, Hennings JR, Brown DF, et al. (2017). Wellens syndrome: ECG recognition and outcomes in the era of high-sensitivity troponins. Ann Emerg Med. 2017;69(5):640-648. [CrossRef]
- Goldberg A, Southern DA, Galbraith PD, et al. (2011). Misdiagnosis of posterior-wall myocardial infarction. Am J Cardiol. 2011;107(5):745-750. [CrossRef]
- Wagner GS, Macfarlane PW, Wellens HJ, et al. (2009). AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part VI: Acute ischemia/infarction: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. J Am Coll Cardiol. 2009;53(11):1003-1011. [CrossRef]
- Thygesen, K., Alpert, J. S., Jaffe, A. S., et al. (2012). "Third Universal Definition of Myocardial Infarction." Journal of the American College of Cardiology, 60(16), 1581-1598. [CrossRef]
- Herman R, MD: What Is Occlusion Myocardial Infarction (OMI)?. Powerful Medical. 2024. https://www.powerfulmedical.com/blog/what-is-occlusion-myocardial-infarction-omi/. Accessed 17 Aug 2024.
- Karwowski J, D'Ascenzo F, De Ferrari GM, et al.: The impact of TIMI flow on outcomes in patients with ST-elevation myocardial infarction. J Am Coll Cardiol. 2017;70(14):1729-1738. [CrossRef]
- Stone GW, Ellis SG, Cox DA, et al.: Comparison of angiographic findings in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Circulation. 2001;104(17):2122-2129. [CrossRef]
- Cox DA, Stone GW, Lincoff AM, et al.: Incidence of TIMI 3 flow in patients with ST-elevation myocardial infarction and its relationship with outcomes. Am J Cardiol. 2006;97(3):353-359. [CrossRef]
- Verheugt FW, de Boer MJ, de Groot H, et al.: Spontaneous reperfusion and clinical outcomes in patients with acute myocardial infarction. Eur Heart J. 1998;19(2):263-270. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).