Submitted:
10 July 2024
Posted:
12 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animal Procurement and Care
2.2. Reagents Purchase and Preparation of Solutions
2.3. Experimental Designs
2.4. Tissue Processing
2.5. Immunohistochemistry
2.6. Preparation of Tissue Sample for Biochemical Analyses
2.7. Data Analysis
3. Results
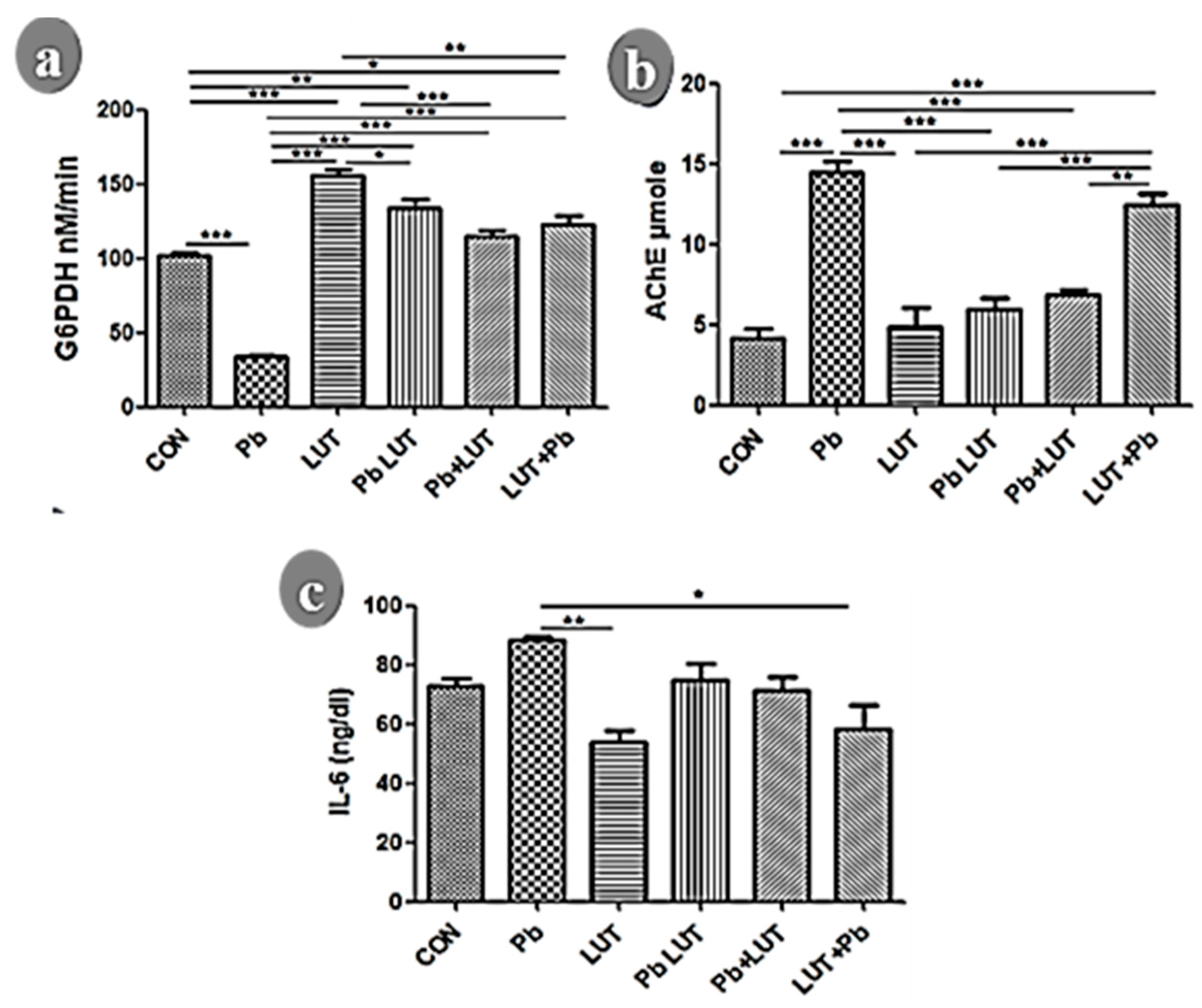
3.1. Luteolin Inhibits Lead-Induced Lipid Peroxidation and Enhances Antioxidative Enzymes
3.2. Luteolin Improves Glucose 6-Phosphate Dehydrogenase Following Lead Intoxication
3.3. Luteolin Prevented Pro-Inflammatory Cytokine Activation Following Lead Intoxication
3.4. Lead Intoxication Increased Acetylcholinesterase (AChE) Activity in the Cerebrum: Treatment with Luteolin Normalizes It
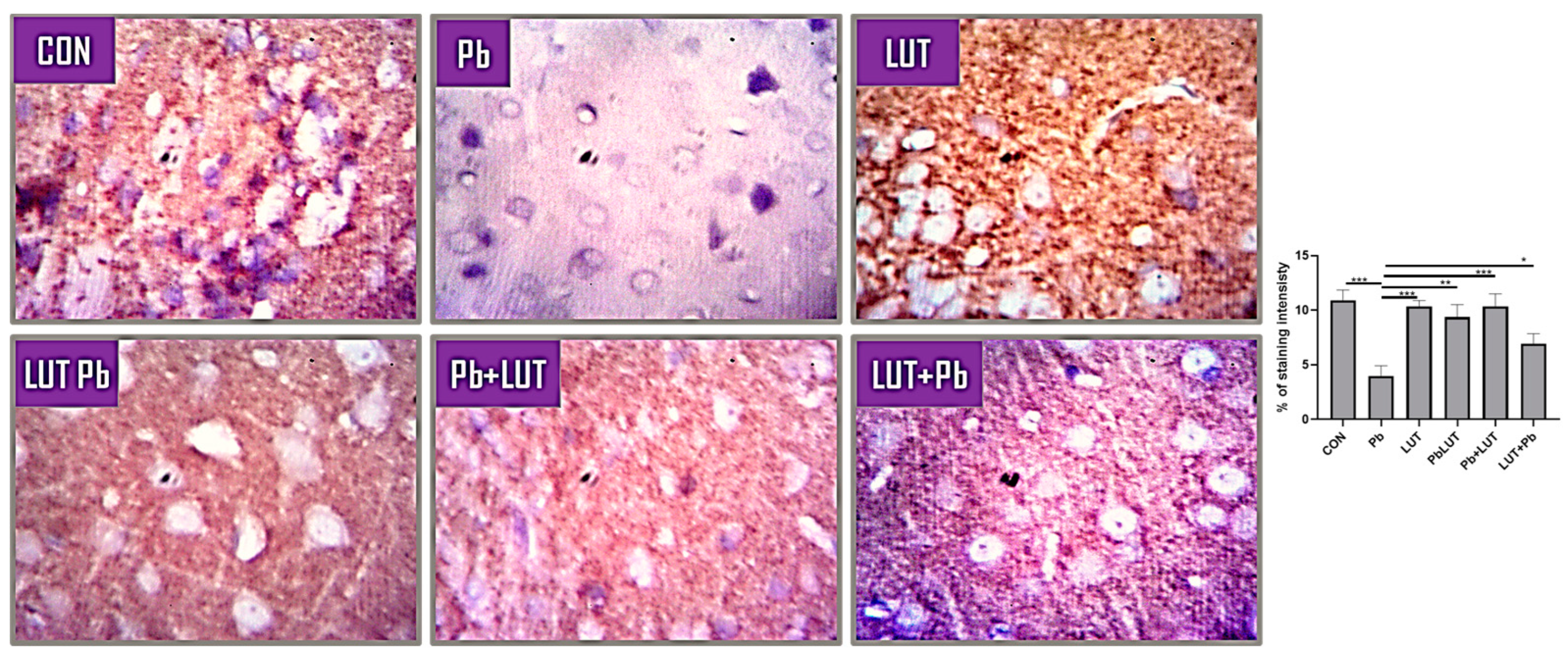
3.5. Luteolin Normalized Histological and Nissl Profiling Changes Caused by Lead Toxicity

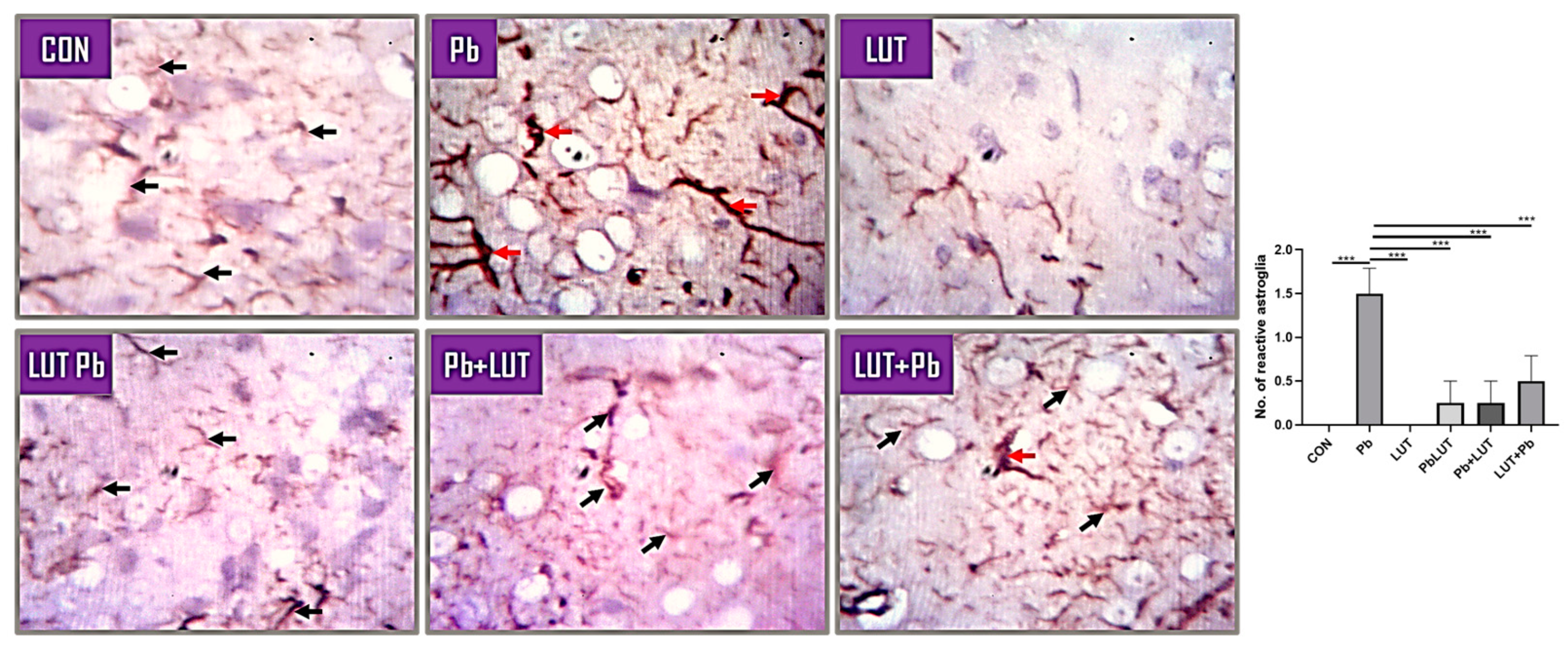
3.6. Lead-Induced Astrogliosis and Luteolin Therapeutic Effects
3.7. Luteolin Improved Long Term Plasticity and Cognition in Lead-Induced Synaptic Alteration
4. Discussion
Author Contributions
Conflicts of Interest
References
- Naranjo, V.I.; Hendricks, M.; Jones, K.S. Lead toxicity in children: An unremitting public health problem. Pediatric Neurology 2020, 113, 51–55. [Google Scholar] [CrossRef]
- Miles-Novelo, A.; Anderson, C.A. Climate Change and Human Behavior: Impacts of a Rapidly Changing Climate on Human Aggression and Violence. Elements in Applied Social Psychology 2022. [Google Scholar]
- Nejad, A.R.; Hatamian, M.; Kafi, M.; Souri, M.K.; Shahbazi, K. Interaction of lead and nitrate on growth characteristics of ornamental judas tree (Cercis siliquastrum L.). Open Agriculture 2018, 3, 670–677. [Google Scholar] [CrossRef]
- Olness, K. Effects on brain development leading to cognitive impairment: A worldwide epidemic. Journal of Developmental & Behavioral Pediatrics 2003, 24, 120–130. [Google Scholar]
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. Heavy metals 2018, 10, 115–132. [Google Scholar]
- Khan, D.A.; Qayyum, S.; Saleem, S.; Khan, F.A. Lead-induced oxidative stress adversely affects health of the occupational workers. Toxicology and Industrial Health 2008, 24, 611–618. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxidative medicine and cellular longevity 2016, 2016. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Reviews on environmental health 2009, 24, 15–46. [Google Scholar] [CrossRef]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. BioMed research international 2014, 2014. [Google Scholar] [CrossRef]
- Shabani, S. A mechanistic view on the neurotoxic effects of air pollution on central nervous system: Risk for autism and neurodegenerative diseases. Environmental Science and Pollution Research 2021, 28, 6349–6373. [Google Scholar] [CrossRef]
- Donno, D.; Boggia, R.; Zunin, P.; Cerutti, A.K.; Guido, M.; Mellano, M.G.; Beccaro, G.L.; et al. Phytochemical fingerprint and chemometrics for natural food preparation pattern recognition: An innovative technique in food supplement quality control. Journal of food science and technology 2016, 53, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.; Vadnere, G.P.; Sharma, V.K.; Usman, M.R. Marrubium vulgare L.: A review on phytochemical and pharmacological aspects. J. Intercult. Ethnopharmacol 2017, 6, 429–452. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Current cancer drug targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: A review. Asian Pacific Journal of Cancer Prevention 2014, 15, 5501–5508. [Google Scholar] [CrossRef] [PubMed]
- Manju, V.; Nalini, N. Protective role of luteolin in 1, 2-dimethylhydrazine induced experimental colon carcinogenesis. Cell Biochemistry and Function: Cellular biochemistry and its modulation by active agents or disease 2007, 25, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, P.; Sudhandiran, G. Protective role of luteolin on the status of lipid peroxidation and antioxidant defense against azoxymethane-induced experimental colon carcinogenesis. Biomedicine & Pharmacotherapy 2008, 62, 590–597. [Google Scholar]
- Damani, J.J.; De Souza, M.J.; VanEvery, H.L.; Strock, N.C.; Rogers, C.J. The Role of Prunes in Modulating Inflammatory Pathways to Improve Bone Health in Postmenopausal Women. Advances in Nutrition 2022. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative stress and neurotoxicity. Chemical research in toxicology 2008, 21, 172–188. [Google Scholar] [CrossRef]
- Wszelaki, N.; Melzig, M.F. (2013). Additive protective effects of luteolin and pyruvate against 6-hydroxydopamine and 3-hydroxykynurenine induced neurotoxicity in SH-SY5Y cells.
- Olajide, O.J.; Fatoye, J.O.; Idowu, O.F.; Ilekoya, D.; Gbadamosi, I.T.; Gbadamosi, M.T.; Asogwa, N.T. Reversal of behavioral decline and neuropathology by a complex vitamin supplement involves modulation of key neurochemical stressors. Environmental Toxicology and Pharmacology 2018, 62, 120–131. [Google Scholar] [CrossRef]
- Lidsky, T.I.; Schneider, J.S. Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain 2003, 126, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L. Lead Toxicity, a review of the literature. Part I: Exposure, Evaluation, and treatment. Alternative medicine review 2006, 11. [Google Scholar]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdisciplinary toxicology 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, D.; Chen, J.; Qin, Y.Y.; Sheng, R.; Feng, X.; Qin, Z.H.; et al. G6PD plays a neuroprotective role in brain ischemia through promoting pentose phosphate pathway. Free Radical Biology and Medicine 2017, 112, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.R.; Bates, K.A.; Porter, T.; Teimouri, E.; Perry, G.; Steele, J.W.; Verdile, G.; et al. Latrepirdine: Molecular mechanisms underlying potential therapeutic roles in Alzheimer’s and other neurodegenerative diseases. Translational psychiatry 2013, 3, e332. [Google Scholar] [CrossRef] [PubMed]
- Qiusheng, Z.; Yuntao, Z.; Rongliang, Z.; Dean, G.; Changling, L. Effects of verbascoside and luteolin on oxidative damage in brain of heroin treated mice. Die Pharmazie-An International Journal of Pharmaceutical Sciences 2005, 60, 539–543. [Google Scholar]
- Metryka, E.; Chibowska, K.; Gutowska, I.; Falkowska, A.; Kupnicka, P.; Barczak, K.; Baranowska-Bosiacka, I.; et al. Lead (Pb) exposure enhances expression of factors associated with inflammation. International journal of molecular sciences 2018, 19, 1813. [Google Scholar] [CrossRef] [PubMed]
- Bourgognon, J.M.; Cavanagh, J. The role of cytokines in modulating learning and memory and brain plasticity. Brain and Neuroscience Advances 2020, 4, 2398212820979802. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Li, Q.Z. Prediction of apoptosis protein subcellular location using improved hybrid approach and pseudo-amino acid composition. Journal of Theoretical Biology 2007, 248, 377–381. [Google Scholar] [CrossRef]
- Ziyan, L.; Yongmei, Z.; Nan, Z.; Ning, T.; Baolin, L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta medica 2007, 73, 221–226. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain research bulletin 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Amjad, S.; Umesalma, S. (2015). Journal of Molecular Biomarkers & Diagnosis.
- Taïr, K.; Kharoubi, O.; Taïr, O.A.; Hellal, N.; Benyettou, I.; Aoues, A. Aluminium-induced acute neurotoxicity in rats: Treatment with aqueous extract of Arthrophytum (Hammada scoparia). Journal of acute disease 2016, 5, 470–482. [Google Scholar] [CrossRef]
- Nwobi, N.L.; Nwobi, J.C.; Akinosun, M.O.; Atulomah, N.O.; Nwazuoke, I.A.; Anetor, J.I. Impaired Antioxidant-Defence Status in Nigerian Children with Elevated Blood Lead Levels: A Possible Predisposing Factor to Chronic Diseases. Journal of Krishna Institute of Medical Sciences (JKIMSU) 2020, 9. [Google Scholar]
- Ademuyiwa OU, R.N.; Ugbaja, R.N.; Rotimi, S.O.; Abam, E.; Okediran, B.S.; Dosumu, O.A.; Onunkwor, B.O. Erythrocyte acetylcholinesterase activity as a surrogate indicator of lead-induced neurotoxicity in occupational lead exposure in Abeokuta, Nigeria. Environmental Toxicology and Pharmacology 2007, 24, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, M.; Tian, Y.; Liu, J.; Shang, J. Luteolin inhibits ROS-activated MAPK pathway in myocardial ischemia/reperfusion injury. Life Sciences 2015, 122, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.; Shady, N.H.; Ahmed, S.R.; Alnusaire, T.S.; Sayed, A.M.; Alowaiesh, B.F.; Abdelmohsen, U.R.; et al. Antiulcer potential of olea europea l. Cv. arbequina leaf extract supported by metabolic profiling and molecular docking. Antioxidants 2021, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Monturiol-Gross, L.; Flores-Díaz, M.; Pineda-Padilla, M.J.; Castro-Castro, A.C.; Alape-Giron, A. Clostridium perfringens phospholipase C induced ROS production and cytotoxicity require PKC, MEK1 and NFκB activation. PLoS ONE 2014, 9, e86475. [Google Scholar] [CrossRef] [PubMed]
- Vinters, H.V.; Kleinschmidt-DeMasters, B.K. (2018). General pathology of the central nervous system. In Greenfield’s Neuropathology-Two Volume Set (pp. 25-82). CRC Press.
- Hussein, M.T.; Nemah, M.N. (2015, October). Modeling and control of quadrotor systems. In 2015 3rd RSI International Conference on Robotics and Mechatronics (ICROM) (pp. 725-730). IEEE.
- Sattler, R.; Rothstein, J.D. Regulation and dysregulation of glutamate transporters. Neurotransmitter Transporters 2006, 277–303. [Google Scholar]
- Schousboe, A.; Waagepetersen, H.S. Role of astrocytes in glutamate homeostasis: Implications for excitotoxicity. Neurotoxicity research 2005, 8, 221–225. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Reviews on environmental health 2009, 24, 15–46. [Google Scholar] [CrossRef]
- Halassa, M.M.; Haydon, P.G. Integrated brain circuits: Astrocytic networks modulate neuronal activity and behavior. Annual review of physiology 2010, 72, 335–355. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




