Submitted:
10 July 2024
Posted:
15 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Paper Review Summary
2.1. Navotas River System
2.2. Anthropogenic Activities
2.3. Water Quality Assessment
2.3.1. pH
2.3.2. Temperature
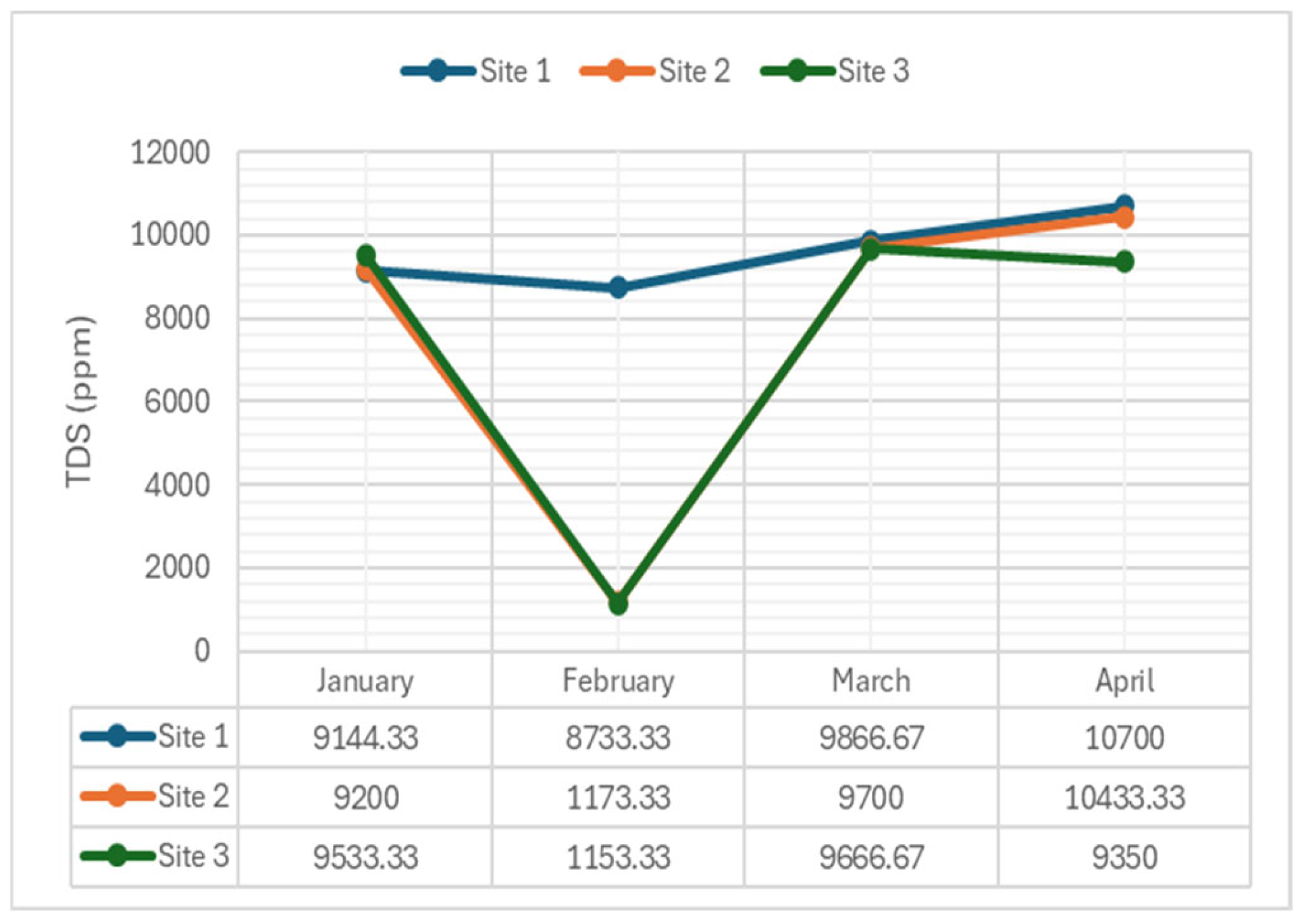
2.3.3. Total Dissolved Solids
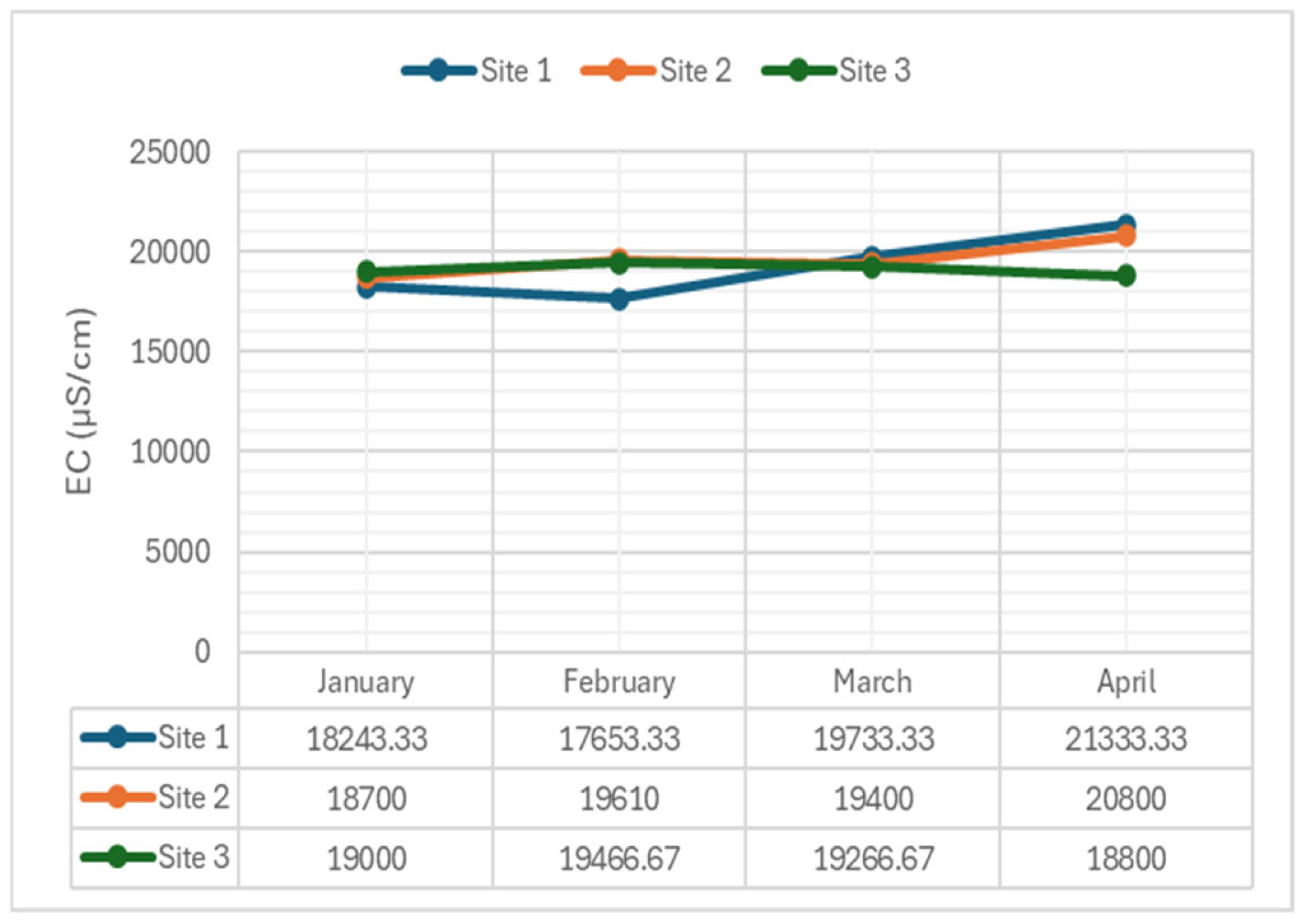
2.3.4. Electrical Conductivity
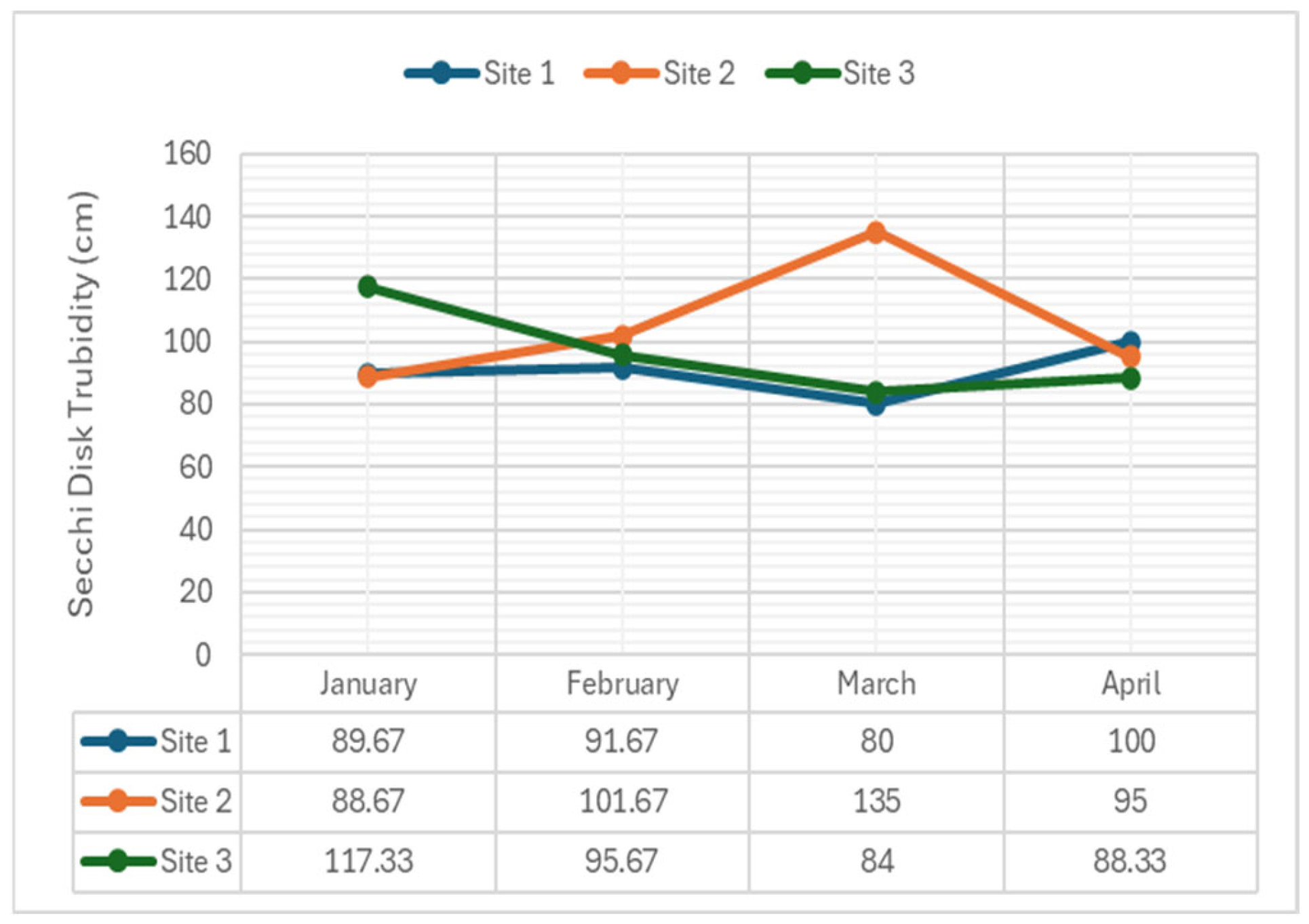
2.3.5. Turbidity
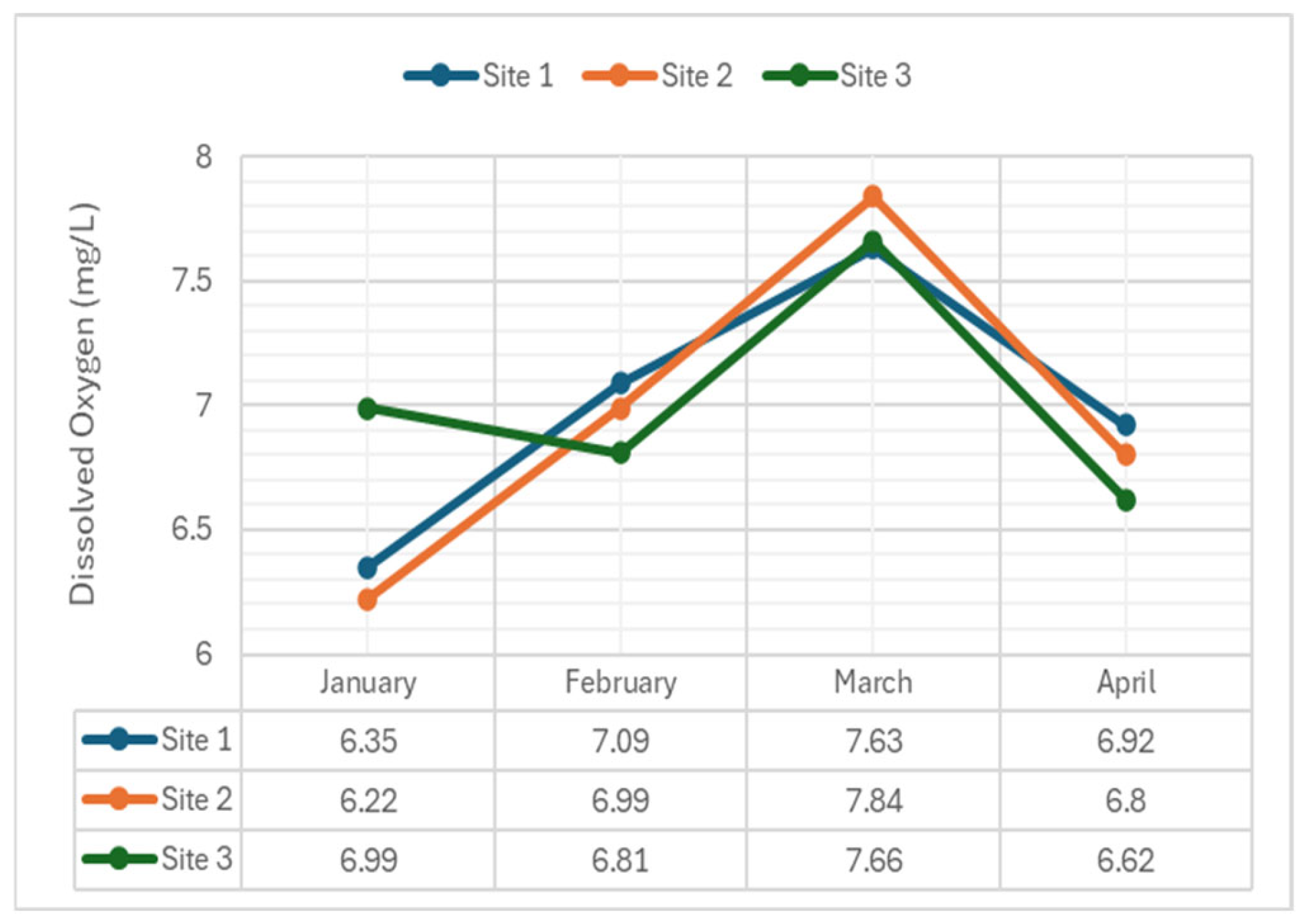
2.3.6. Dissolved Oxygen
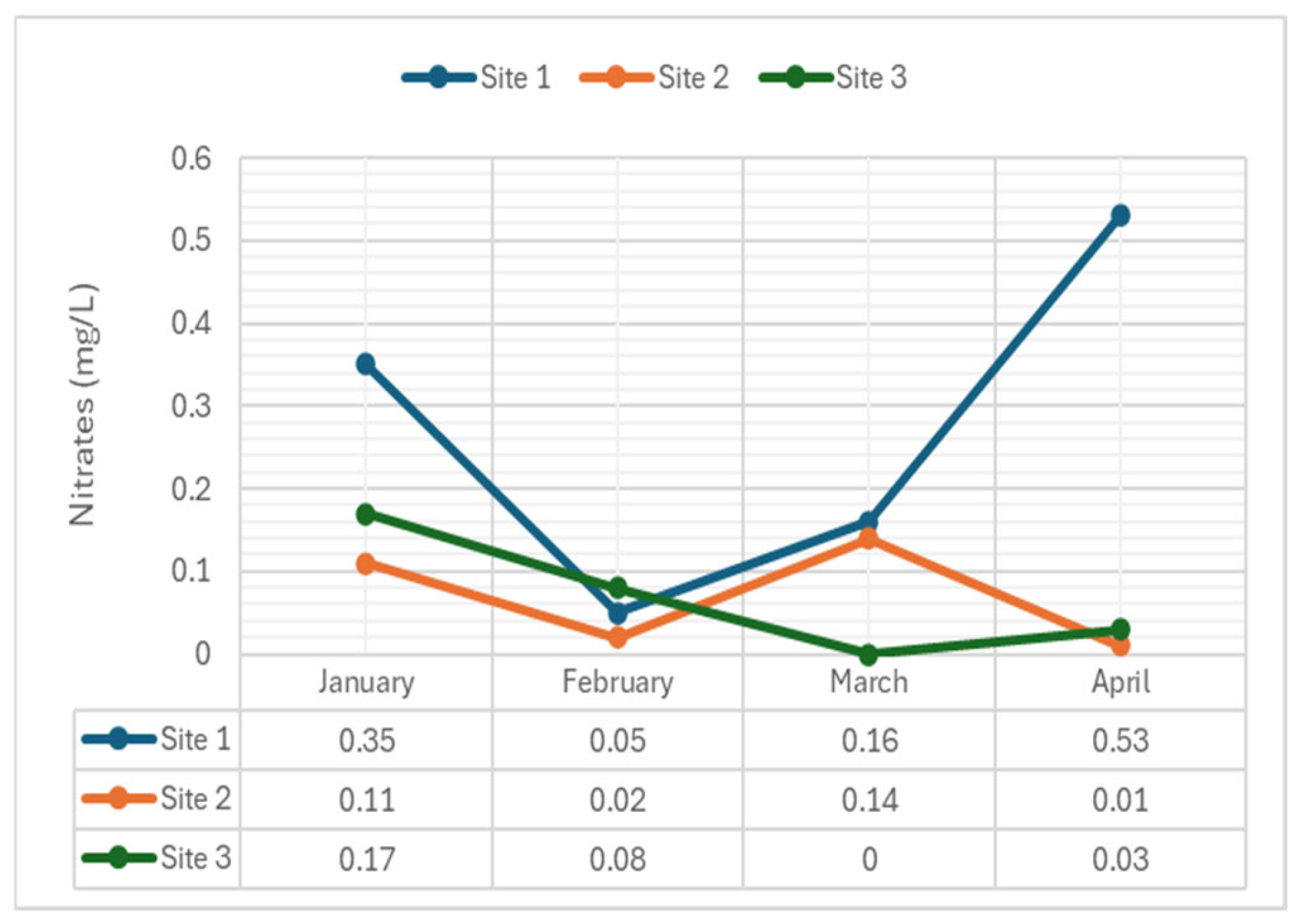
2.3.7. Nitrates
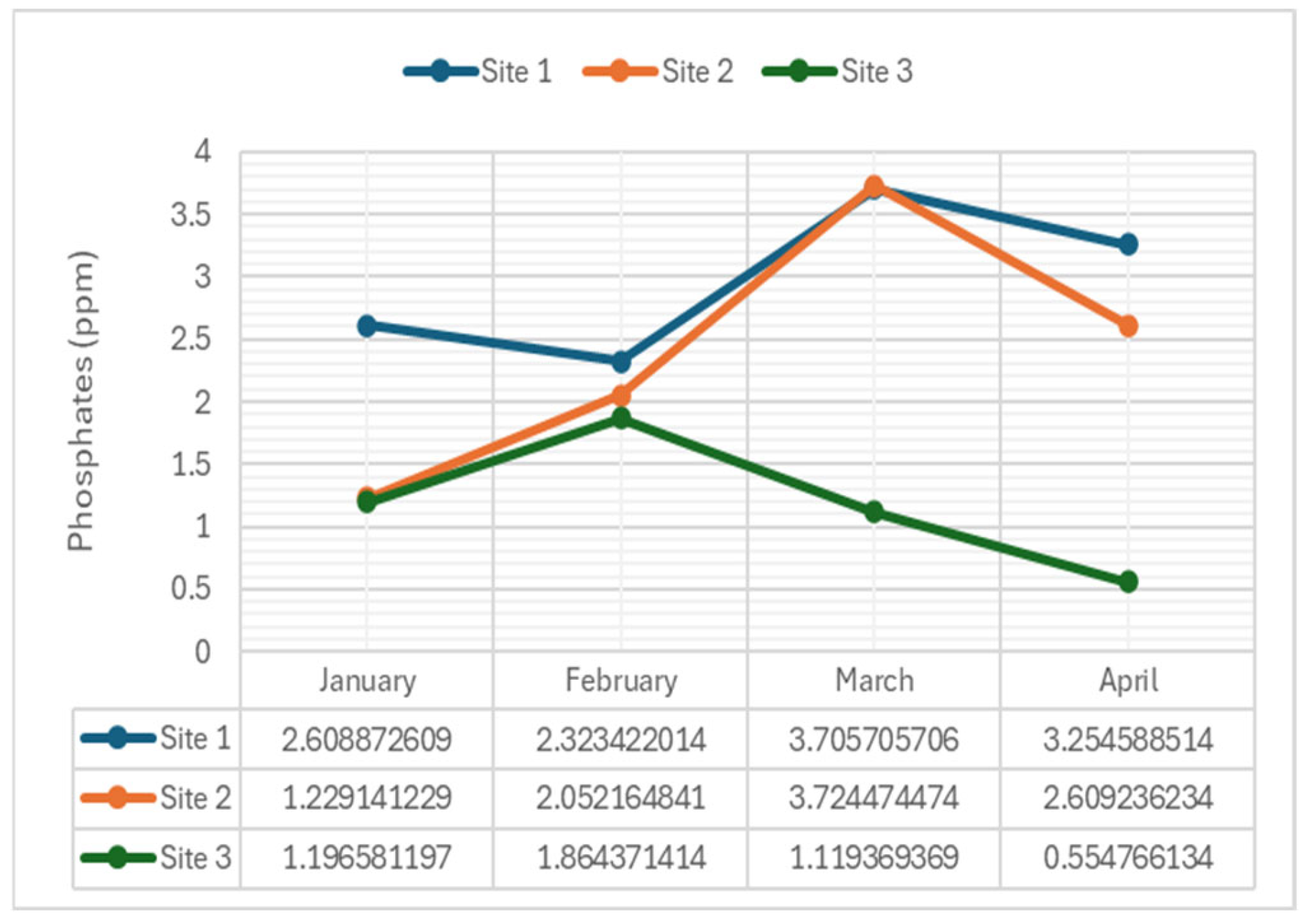
2.3.8. Phosphates
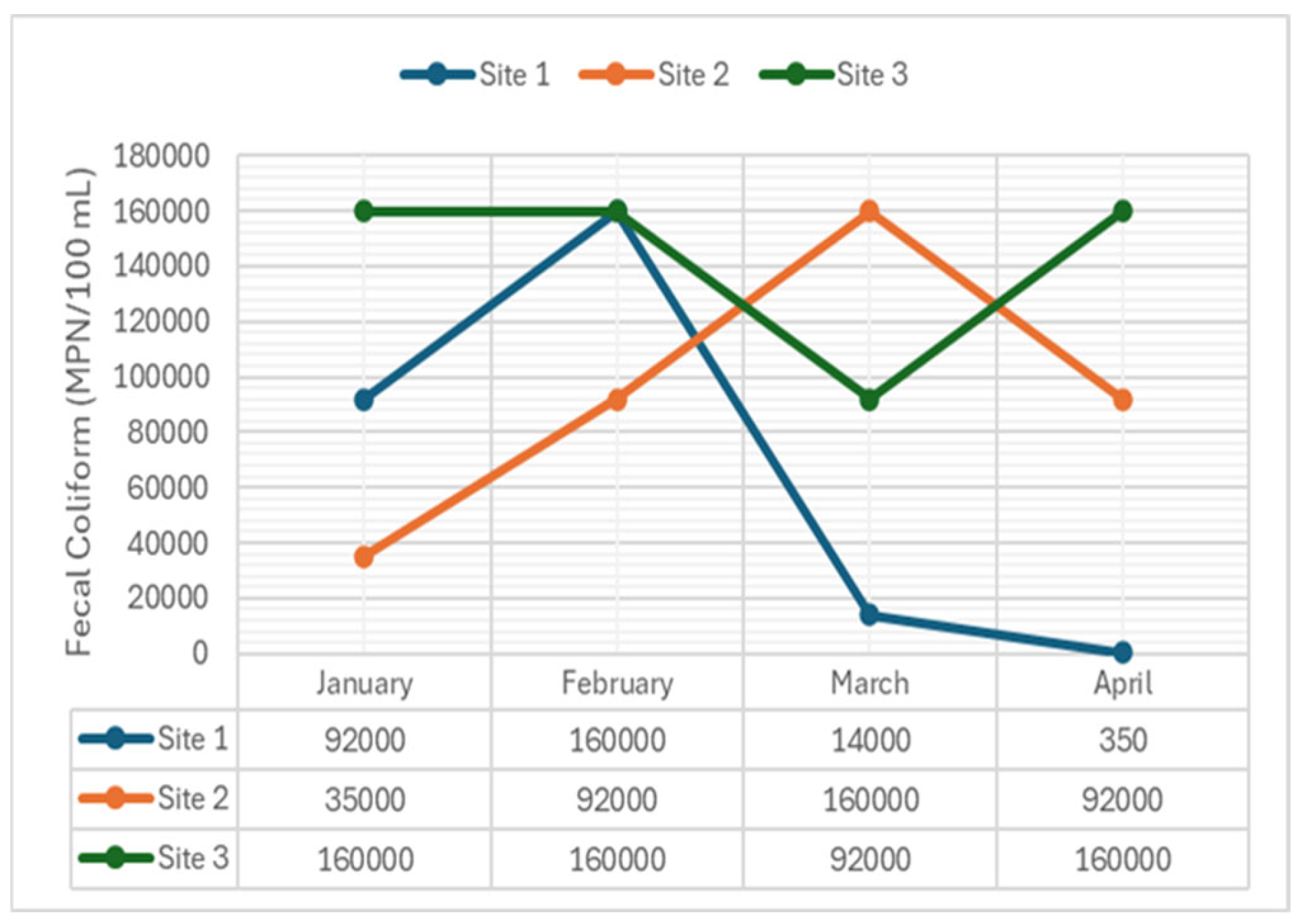
2.3.9. Fecal Coliform
2.4. Philippine Clean Water Act of 2004
2.5. Water Quality Index
2.6. Fish Diversity
2.7. Diversity Index
2.7.1. Species Importance Value Index
2.7.2. Shannon-Wiener Diversity Index
2.7.3. Simpson’s Diversity Index
2.7.4. Margalef’s Richness Index
2.7.5. Sorensen’s Coefficient Similarity Index
2.7.6. Shannon’s Equitability
3. Methodology
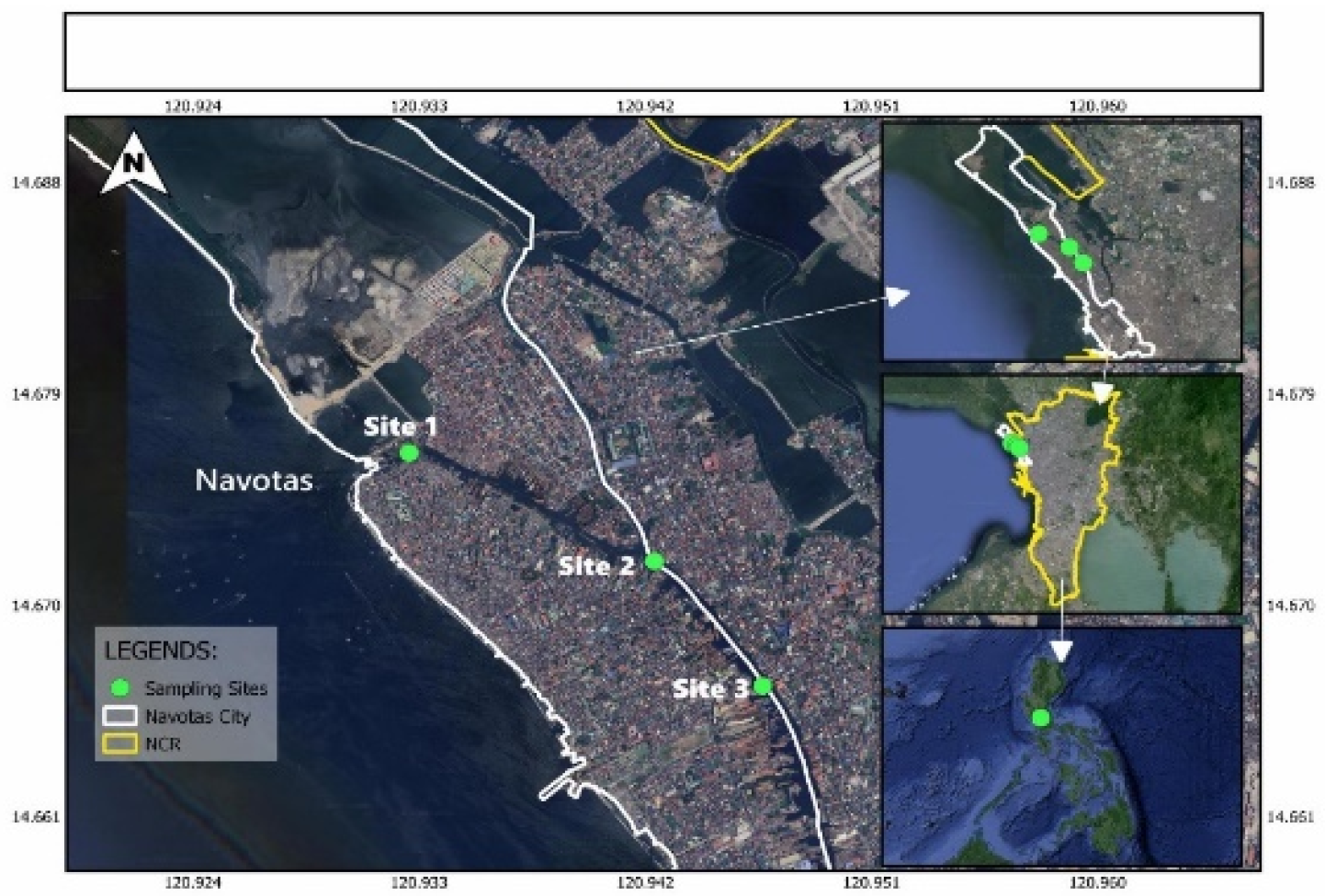
3.1. Study Area
- Site 1: 14°40’35” N, 120°55’57” E, estuary near Manila Bay, populated by fishermen.
- Site 2: 14°40’19” N, 120°56’33” E, diverting channel of Tanza and Tangos Rivers.
- Site 3: 14°39’60” N, 120°56’48” E, downstream leading to Tullahan River.
- Site 1 to Site 2: 1.18 km
- Site 2 to Site 3: 0.80 km
3.2. Administration of Structured Questionnaire
3.3. Water Sample Collection
3.3.1. Water Quality Parameters
- pH and Temperature: Digital pH Pen Meter and laboratory thermometer.
- Conductivity and TDS: Digital Multi-function LCD Monitor.
- Turbidity: Modified Secchi Disk.
- Dissolved Oxygen: DO9100 Dissolved Oxygen Meter.
- Nitrates: Tested using 352.1 Brucine – Colorimetric Method.
- Phosphates: Tested using Hitachi UH5300 UV-Vis Spectrophotometer.
- Fecal Coliforms: Tested using 9221 E. Multiple Tube Fermentation Technique
3.4. Determination of Water Quality
3.5. Collection of Fish Samples
3.6. Computation of Diversity Index
- Species Importance Value (SIV): Dominance of species.
- Shannon-Wiener Index (H'): Species diversity.
- Simpson’s Diversity Index (D): Species diversity.
- Margalef’s Richness Index (R): Species richness.
- Sorensen’s Coefficient (IS): Community similarity.
- Shannon’s Equitability (EH): Species evenness.
3.7. Statistical Treatment of Data
- Kruskal-Wallis Test: Differences in water quality among sites.
- Friedman Test: Differences in water quality over time.
- Pearson R Correlation: Relationships among water quality parameters and between water quality and fish species.
4. Results and Discussion
4.1. Anthropogenic Activities in the Navotas River
4.2. Water Quality of Navotas River during the Four-month Period
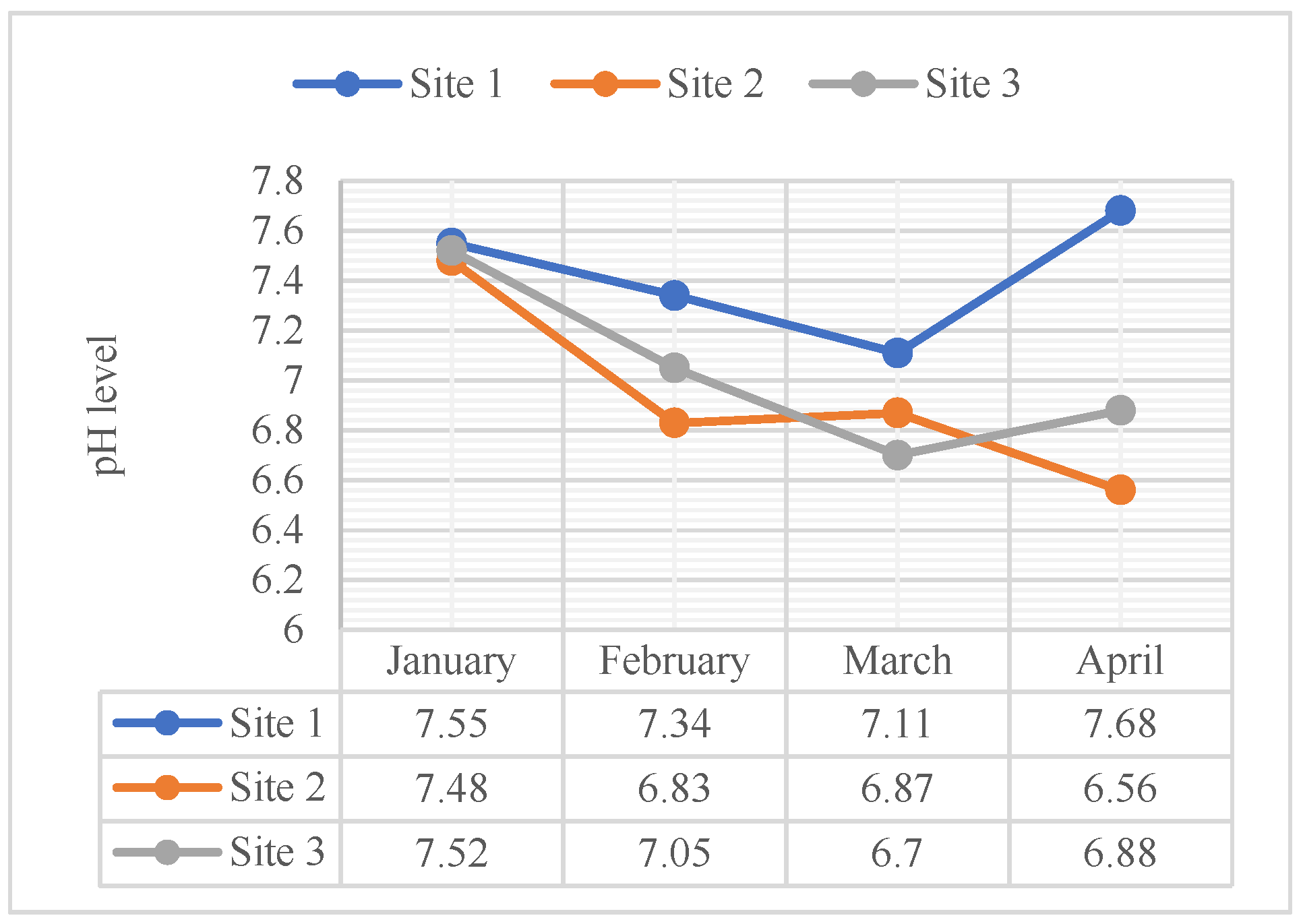
4.2.1. pH
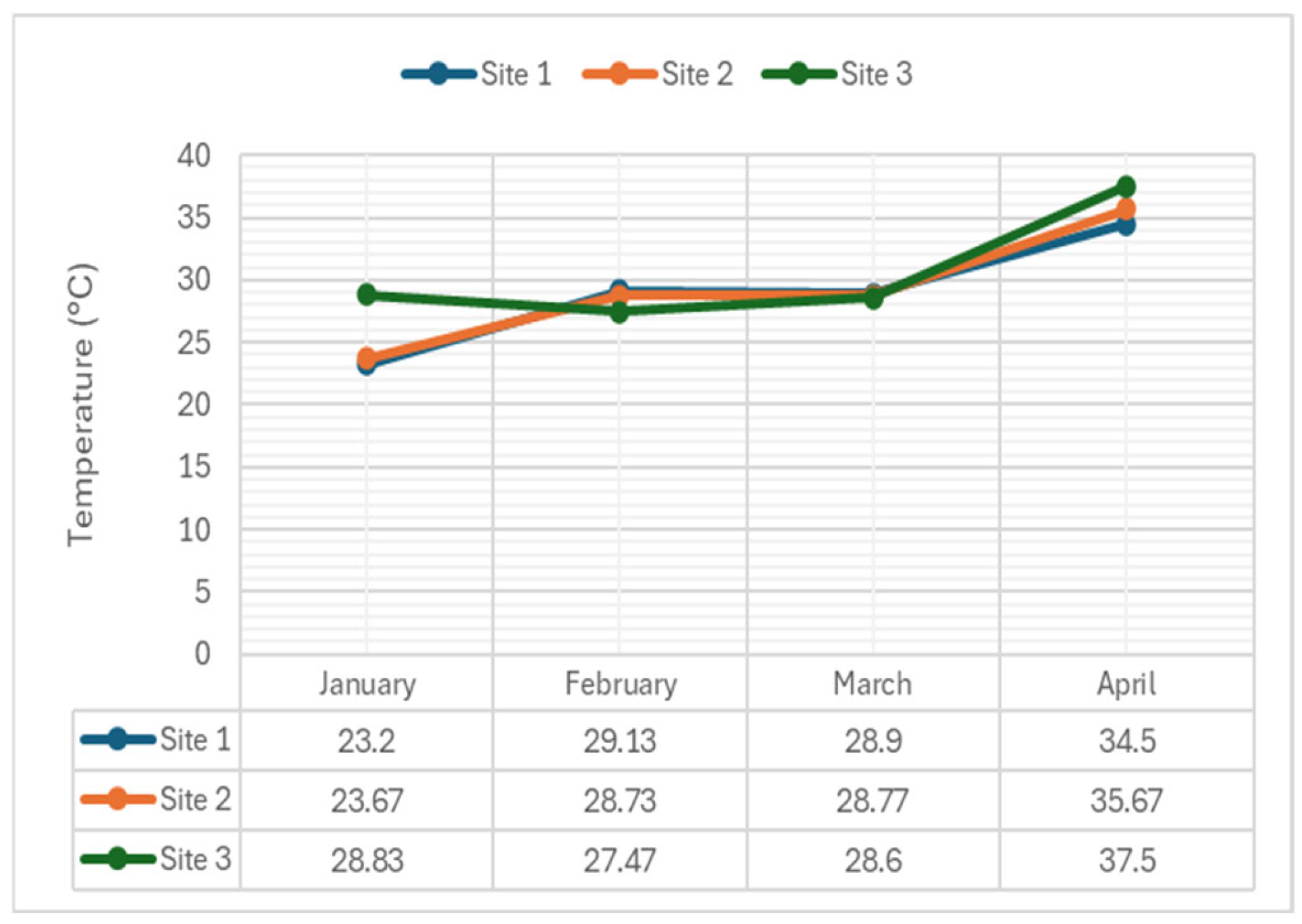
4.2.2. Temperature
4.2.3. Total Dissolved Solids
4.2.4. Electrical Conductivity
4.2.5. Turbidity
4.2.6. Dissolved Oxygen
4.2.7. Nitrates
4.2.8. Phosphates
4.2.9. Fecal Coliform
4.3. Kruskal-Wallis Test
- pH: Significant variations were observed in February, March, and April (p-value = 0.027), while January (p-value = 0.925) showed no significant difference. This indicates changes in water chemistry likely influenced by pollution sources or natural processes, with stability in January possibly due to balanced buffering capacity or reduced pollutant input.
- Temperature: Displayed significant changes in February (p-value = 0.026), March (p-value = 0.020), and April (p-value = 0.021), while January (p-value = 0.058) was non-significant. These fluctuations could be attributed to seasonal warming and increased industrial or domestic discharges, impacting aquatic life and ecosystem health.
- Total Dissolved Solids (TDS): Showed non-significant differences in January (p-value = 0.575), February (p-value = 0.065), and March (p-value = 0.517), with a significant difference in April (p-value = 0.027). This suggests relatively stable dissolved solid levels, indicating consistent water quality regarding mineral content, with potential pollution events in April.
- Electrical Conductivity (EC): Was non-significant in January (p-value = 0.924), February (p-value = 0.190), and March (p-value = 0.295), but significant in April (p-value = 0.035). This reflects stability in ionic concentrations, with variations in April possibly due to increased pollutant inputs.
- Turbidity: Was significant in February (p-value = 0.026), indicating increased sediment or runoff disturbance, but non-significant in January (p-value = 0.066), March (p-value = 0.066), and April (p-value = 0.148), suggesting minimal disturbance or effective sediment settling. The water in Navotas River was turbid, containing a higher concentration of suspended particles, including clay, silt, fine organic and inorganic materials, soluble colored organic compounds, and other microscopic organisms.
- Dissolved Oxygen (DO): Exhibited significant differences in January, February, and April (p-value = 0.027), but was non-significant in March (p-value = 0.295). These variations reflected changes in temperature, organic load, or water flow, impacting aquatic life.
- Nitrates: Consistently showed significant variations across all months (p-value = 0.018). The consistent significance in nitrate levels pointed to ongoing nitrate pollution, likely from agricultural runoff or sewage discharge, which could lead to eutrophication and affect water quality.
- Phosphates: Were non-significant in January (p-value = 0.066), February (p-value = 0.050), and March (p-value = 0.061), suggesting stable levels initially but increased pollution in April.
- Fecal Coliform: Consistently exhibited significant differences across all months (p-value = 0.018), indicating continuous contamination from human or animal waste, highlighting poor sanitation or waste management practices.
4.4. Friedman Test
- pH: The water collected over four months showed significantly different pH levels at each site. Variations in pH levels among the sampling sites indicate potential differences in acidity or alkalinity, influenced by factors such as agricultural runoff, industrial discharge, or natural processes. Variations in pH can have significant implications for aquatic life and ecosystem health, as they directly affect the solubility of nutrients and the availability of toxic substances.
- Temperature: The test revealed significant differences in temperature across all sites, indicating statistically significant variations at each site. Monitoring temperature is important when evaluating water quality. Significant differences in temperature suggest varying thermal conditions across the sampling sites, influencing biological processes, metabolism rates, growth patterns, and reproductive behaviors of aquatic organisms. Variations in temperature also affect water chemistry and dissolved oxygen levels, impacting overall ecosystem dynamics.
- Total Dissolved Solids (TDS): Significant differences were found at Site 1 and Site 2, but not at Site 3. The lack of significant differences in TDS levels implies relatively uniform concentrations of dissolved solids across the sampling sites. While TDS levels indicate overall water quality, their non-significance at Site 3 suggests that factors influencing TDS, such as mineral content or salinity, may not have varied significantly among the sites during the sampling periods.
- Electrical Conductivity (EC): Significant differences were found only at Site 1. Like TDS, non-significant differences in EC levels at the other sites suggest consistent conductivity across the sampling sites. EC is often used as a proxy for TDS and provides insights into water quality and ion concentrations. The uniformity in EC levels indicates potential stability in ion concentrations and overall water chemistry among the sites.
- Turbidity: Significant differences were found only at Site 3. This site is near construction and ship repair activities on the riverside of Navotas River. Human activities that promote dynamic turbidity over time contribute to the murkiness of water.
- Dissolved Oxygen (DO): Significant differences were found across all sites. Variations in DO concentrations among sampling months could be attributed to changing weather conditions, salinity, total coliform, etc. DO is critical for the survival of aquatic organisms, and deviations from optimal levels indicate pollution, organic matter decomposition, or eutrophication. Monitoring DO levels is crucial for assessing water quality and ecosystem health.
- Nitrates and Phosphates: Both parameters showed significant differences in concentrations across sampling months at each site. These significant differences underscore variations in nutrient concentrations among the sampling sites. Excessive nutrient inputs, often from agricultural runoff or wastewater discharge, can lead to eutrophication, algal blooms, and oxygen depletion. Managing nutrient pollution is essential for maintaining water quality and preventing ecological degradation.
- Fecal Coliform: Significant differences were found across sampling sites, indicating varying levels of bacterial contamination. Elevated fecal coliform levels pose risks to human health and indicate sewage contamination or inadequate sanitation practices. Significant variations were observed in February, March, and April (p-value = 0.027), while January (p-value = 0.925) showed no significant difference. This indicates changes in water chemistry likely influenced by pollution sources or natural processes, with stability in January possibly due to balanced buffering capacity or reduced pollutant input.
4.5. Water Quality Index of Navotas River during the Sampling Periods
4.6. Ichthyofaunal Diversity of Navotas River
- Scatophagus argus (Linnaeus 1766), belonging to the family Scatophagidae, was the most abundant species. Known as spotted scat, it inhabits brackish estuaries and lower reaches of freshwater streams, frequently occurring among mangroves (Froese & Pauly, 2017; Randall, 2019). Scatophagus argus exhibits a wide salinity tolerance range and can survive in conditions ranging from freshwater to highly saline environments. It can tolerate elevated temperatures and low dissolved oxygen concentrations (Gupta, 2016).
- Pelates quadrilineatus (Bloch 1790), locally known as babanse, was the second most abundant species (18.22%). Found in brackish waters, this species often inhabits estuaries, seagrass beds, and mangrove bays, feeding on small fishes and invertebrates (Ching, 2023).
- Other species with lower abundances included Sarotherodon melanotheron (gloria tilapia) (17.10%), Terapon jarbua (bagaong) (15.61%), Lutjanus argentimaculatus (alakaak) (12.64%), Lutjanus argentimaculatus (kabang) (9.67%), and Nematalosa nasus (kabase) (8.18%). These species are commonly found in brackish waters, but their lower abundance may be due to seasonal variations, spawning environment differences, and tolerance for water quality changes.
- Sarotherodon melanotheron, an introduced invasive species, had the third-highest total occurrence. Its abundance can negatively impact native species by preying on their eggs and juveniles, contributing to the decline of native fish populations (Oluwale & Ugwumba, 2022).
4.6.1. Species Importance Value
4.6.2. Species Diversity of Ichthyofauna in Navotas River
4.6.3. Species Similarity and Species Evenness in Navotas River
4.7. Relationship of Water Quality Parameters in Four Sampling Periods among Three Sampling Sites in Navotas River
- pH and Nitrate Concentration: A significant and positive high correlation (r = 0.753) was found between pH and nitrate concentration, indicating a strong positive relationship. As pH levels increased, nitrate concentration also tended to increase, a finding statistically significant at the 0.01 level.
- pH and Water Temperature: A weak negative correlation (r = -0.382) was observed between pH and water temperature, suggesting a slight increase in water temperature as pH decreased.
- pH and Dissolved Oxygen: A weak negative correlation (r = -0.416) indicated that lower pH levels might lead to a slight decrease in dissolved oxygen concentration.
- Water Temperature and Electrical Conductivity: A moderate positive correlation (r = 0.528) suggested that as water temperature increased, electrical conductivity also tended to increase.
- EC and Total Dissolved Solids: A weak positive correlation (r = 0.066) indicated a slight increase in TDS as EC increased.
- Phosphate-Phosphorus: Showed moderate positive correlations with EC (r = 0.394) and nitrate-nitrogen (r = 0.459).
- Fecal Coliform Count and Electrical Conductivity: A strong negative correlation (r = -0.506) implied that as EC increased, the concentration of fecal coliform count decreased significantly.
4.8. Relationship between Water Quality Parameters and Fish Diversity in Navotas River
- pH: Exhibited significant positive moderate correlations with Lutjanus argentimaculatus (r = 0.527, p = 0.039) and Sarotherodon melanotheron (r = 0.602, p = 0.019).
- Nitrates: Showed positive moderate correlations with L. argentimaculatus (r = 0.651, p = 0.011) and S. melanotheron (r = 0.555, p = 0.031).
- Scatophagus argus: Displayed a negative moderate correlation with water temperature (r = -0.529, p = 0.039) and electrical conductivity (r = -0.536, p = 0.036).
5. Conclusions
Acknowledgments
References
- Arellano Law Foundation. (2004). Republic Act No. 9275. Lawphil. Retrieved October 2, 2023, from https://lawphil.net/statutes/repacts/ra2004/ra_9275_2004.html.
- Bhateria, R., & Jain, D. (2016). Water quality assessment of lake water: a review. Sustainable Water Resources Management, 2(2), 161–173. [CrossRef]
- Bobbitt, Z. (2021). Shannon Diversity Index: Definition & Example. Statology. Retrieved September 27, 2023, from https://www.statology.org/shannon-diversity-index/.
- Brett, J. (2014). Water Temperature - Environmental Measurement Systems. Fondriest Environmental. Retrieved October 4, 2023, from https://www.fondriest.com/environmental-measurements/parameters/water-quality/water-temperature/.
- Caabay, J. M. S. (2020). Determination of Some Physico-Chemical Parameters and Water Quality Index (WQI) of Laguna de Bay, Philippines. International Journal of Science and Management Studies, 3(1), 1-6. https://www.ijsmsjournal.org/2020/volume-3%20issue-1/ijsms-v3i1p101.pdf.
- Ching, T. H. (2023). Terapons - talk about fish. Retrieved May 23, 2024, from https://www.talkaboutfish.com/red-fishes-basses-congers-etc/terapon/.
- City Government of Navotas. (2015). COMPREHENSIVE LAND USE PLAN 2016 - 2025 PART 4: EXISTING LAND USE PROFILE. Navotas City. Retrieved September 30, 2023, from https://www.navotas.gov.ph/Content/clup/Part%204%20%20Existing%20Land%20Use%20Profile%20Final.pdf.
- Coliform Bacteria in Drinking Water. (2023). Washington State Department of Health. Retrieved October 14, 2023, from https://doh.wa.gov/community-and-environment/drinking-water/contaminants/coliform.
- Dacumos, J. (2012). Navotas, The Fish Capital. Vigattin Tourism. Retrieved September 20, 2023, from https://www.vigattintourism.com/tourism/articles/Navotas-The-Fish-Capital.
- DENR-Environmental Management Bureau. (2018). NCR, List of Waterbodies. Water Quality Management Section. Retrieved September 30, 2023, from https://water.emb.gov.ph/?page_id=809.
- Fletcher, J. (2023). Is distilled water safe to drink? Medical News Today. https://www.medicalnewstoday.com/articles/317698.
- Floyd, R. F. (2021). Dissolved oxygen for fish production. Fisheries and Aquatic Sciences. https://edis.ifas.ufl.edu/publication/FA002#FOOTNOTE_2.
- Fondriest Environmental, Inc. (2013). Dissolved Oxygen. Fundamentals of Environmental Measurements. https://www.fondriest.com/environmental-measurements/parameters/water-quality/dissolved-oxygen/.
- Fondriest Environmental, Inc. (2014). Conductivity, Salinity & Total Dissolved Solids - Environmental Measurement Systems. Fundamentals of Environmental Measurements. Retrieved October 1, 2023, from https://www.fondriest.com/environmental-measurements/parameters/water-quality/conductivity-salinity-tds/.
- Fondriest Environmental, Inc. (2014). Turbidity, Total Suspended Solids & Water Clarity - Environmental Measurement Systems. Fundamentals of Environmental Measurements. Retrieved October 1, 2023, from https://www.fondriest.com/environmental-measurements/parameters/water-quality/turbidity-total-suspended-solids-water-clarity/.
- Gan, M. (2017). Development for Whom? How Navotas fisherfolk resist the displacement of their people and livelihood - IBON INTERNATIONAL. ibon international. Retrieved September 20, 2023, from https://iboninternational.org/2017/08/11/development-for-whom-how-navotas-fisherfolk-resist-the-displacement-of-their-people-and-livelihood/.
- Gupta, S. (2016). An Overview on Morphology, Biology, and Culture of Spotted Scat Scatophagus argus (Linnaeus 1766). Reviews in Fisheries Science & Aquaculture, 24(2), 203–212. [CrossRef]
- Hamid, A., Bhat, S. U., & Jehangir, A. (2019). Local determinants influencing stream water quality. Applied Water Science, 10. https://link.springer.com/article/10.1007/s13201-019-1043-4.
- Hsu, E., & Du Pasquier, L. (Eds.). (2015). Pathogen-Host Interactions: Antigenic Variation V. Somatic Adaptations. Springer International Publishing.
- Ismail, M. H., Zaki, P. H., Fuad, M. F. A., & Jemali, N. (2017). Analysis of importance value index of unlogged and logged peat swamp forest in Nenasi Forest Reserve, Peninsular Malaysia. BONOROWO WETLANDS, 7(2), 74–78. [CrossRef]
- Iticescu, C., Georgescu, L. P., Murariu, G., Topa, C., Timofti, M., Pintilie, V., & Arseni, M. (2019). Lower Danube Water Quality Quantified through WQI and Multivariate Analysis. Water, 11(6), 1305. [CrossRef]
- JoVE Science Education Database. (2023). Turbidity and Total Solids in Surface Water. Environmental Science. JoVE, Cambridge, MA. https://app.jove.com/v/10015/turbidity-and-total-solids-in-surface-water.
- Kumar, P. (2022). Calculating forest species diversity with information-theory based indices using sentinel-2A sensor's of Mahavir Swami Wildlife Sanctuary (M. Dobriyal, A. Kale, A. K. Pandey, R. S. Tomar, & E. Thounaojam, Eds.). PLoS One, 17(5), e0268018. [CrossRef]
- Libretexts. (2024). 22.2: Diversity indices. Biology LibreTexts. https://bio.libretexts.org/Courses/Gettysburg_College/01%3A_Ecology_for_All/22%3A_Biodiversity/22.02%3A_Diversity_Indices#:~:text=A%20diversity%20index%20is%20a,%2C%20evenness%2C%20and%20dominance).
- Malabon, Navotas spared due to flood mitigation measures: Ang. (2020). Philippine News Agency. https://www.pna.gov.ph/articles/1122789.
- Ministry of Environment. (2020). Electrical conductivity (EC). Electrical conductivity (EC) - Environmental Water Quality Information. Retrieved October 1, 2023, from https://wq.moenv.gov.tw/EWQP/en/Encyclopedia/NounDefinition/Pedia_48.aspx.
- National Ocean Service. (2023). What is water quality? Florida Keys National Marine Sanctuary. Retrieved September 30, 2023, from https://floridakeys.noaa.gov/ocean/waterquality.html.
- Negi, R. K., & Mamgain, S. K. (2013). Species Diversity, Abundance and Distribution of Fish Community and Conservation Status of Tons River of Uttarakhand State, India. Journal of Fisheries and Aquatic Science, 8(5), 617-626. [CrossRef]
- Nguyen, Q. (2017). The Use of Simpson ’s Diversity Index to Develop a Diverse Community for the Enrichment of Experiential Learning. UReCA: The NCHC Journal of Undergraduate Research & Creative Activity, 42. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1038&context=ureca.
- Oluwale, F. V., & Ugwumba, A. A. A. (2022). Biological invasion: evidence from a tropical reservoir (Eleiyele, South West Nigeria). The Zoologist, 20(1), 1–10. [CrossRef]
- Omayio, D., & Mzungu, E. (2019). Modification of Shannon-Wiener Diversity Index towards Quantitative Estimation of Environmental Wellness and Biodiversity Levels under a Non-comparative Scenario. Journal of Environment and Earth Science, 9(9), 46-47. ISSN 2225-0948. [CrossRef]
- OpenStax. (2021). 15.6: Vertebrates. Biology LibreTexts. Retrieved October 2, 2023, from https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Concepts_in_Biology_(OpenStax)/15%3A_Diversity_of_Animals/15.06%3A_Vertebrates.
- Ozkan, K., Ozdemir, S., Senol, A., & Kucuksille, E. U. (2024). A New Species Richness Measure Improved from Margalef and Menhinick Indices. Journal of Science, 37(3), 1057–1062.
- Philippine Statistics Authority. (2020). Solid, Responsive, World Class. Philippine Statistics Authority National Capital Region. Retrieved September 19, 2023, from https://rssoncr.psa.gov.ph/ncr4.
- Rahman, I. U., Hart, R., Afzal, A., Iqbal, Z., Ijaz, F., Abdullah, E. F., Ali, N., Khan, S. M., Alqarawi, A. A., Alsubeie, M. S., & Bussmann, R. W. (2019). A new ethnobiological similarity index for the evaluation of novel use reports. Applied Ecology and Environmental Research, 17(2), 2765-2777. [CrossRef]
- Randall, J. E. (2019). Spotted Scat (Scatophagus argus) Ecological Risk Screening Summary. U.S. Fish And Wildlife Service. https://www.fws.gov/sites/default/files/documents/Ecological-Risk-Screening-Summary-Spotted-Scat.pdf.
- Regmi, R. K. (2018). Urbanization and Related Environmental Issues of Metro Manila. Journal of Advanced College of Engineering and Management, 3, 79-92. [CrossRef]
- Sharashy, O. (2023). Application of Shannon and Simpson Diversity Index to Study Plant biodiversity on Coastal Rocky Ridges Habitats with Reference to Census Data in the Ras El-Hekma and Omayed Area on the Western Coastal Region of Egypt. Journal of Pure & Applied Sciences, 21(1), 41-45. [CrossRef]
- Simpson's Diversity Index. (2023). Barcelona Field Studies Centre. Retrieved September 18, 2023, from https://geographyfieldwork.com/Simpson'sDiversityIndex.htm.
- United States EPA. (2023). The Effects: Dead Zones and Harmful Algal Blooms | US EPA. Environmental Protection Agency. Retrieved October 2, 2023, from https://www.epa.gov/nutrientpollution/effects-dead-zones-and-harmful-algal-blooms.
- United States EPA. (2023). Indicators: Phosphorus | US EPA. Environmental Protection Agency. Retrieved October 4, 2023, from https://www.epa.gov/national-aquatic-resource-surveys/indicators-phosphorus.
- Vergara, D. C. D. M., & Blanco, A. C. (2023). SURFACE URBAN HEAT ISLANDS AND RELATED HEALTH RISK IN THE PHILIPPINES: a GEOSPATIAL ASSESSMENT USING MODIS DATA. the International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences/International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, XLVIII-4/W6-2022, 451–456. [CrossRef]
- Wilson, J. W., & Primack, R. B. (2023). 22.2: Diversity Indices. Biology LibreTexts. Retrieved September 17, 2023, from https://bio.libretexts.org/Courses/Gettysburg_College/01%3A_Ecology_for_All/22%3A_Biodiversity/22.02%3A_Diversity_Indices.
| Anthropogenic Activities | Mean | Remarks |
| Fishing | 2.86 | Often |
| Boating | 2.71 | Often |
| Washing of clothes | 2.29 | Seldom |
| Discharging water from the laundry | 2.29 | Seldom |
| Disposing of untreated wastewater from household and transport system | 2.00 | Seldom |
| Excreting domestic wastes | 1.14 | Never |
| Throwing garbage in the river | 1.07 | Never |
| Irrigating plants with water from the river | 1.00 | Never |
| Practicing aquaculture | 1.00 | Never |
| Water Quality Parameter | Sampling Site |
Sampling Period | DENR Standard | |||
| Jan | Feb | Mar | Apr | |||
| pH | 1 | 7.55 | 7.3 | 7.1 | 7.68 | 6.5-9.0* |
| 2 | 7.48 | 6.83 | 6.87 | 6.56 | ||
| 3 | 7.52 | 7.05 | 6.70 | 6.88 | ||
| Temperature (°C) | 1 | 23.20 | 29.13 | 28.90 | 34.5 | 25-31* |
| 2 | 23.67 | 28.73 | 28.77 | 35.67 | ||
| 3 | 28.83 | 27.47 | 28.60 | 37.50 | ||
| TDS (mg/L) | 1 | 9144.33 | 8733.33 | 9866.67 | 10700 | - |
| 2 | 9200 | 1173.33 | 9700 | 10433.33 | ||
| 3 | 9533.33 | 1153.33 | 9666.67 | 9350 | ||
| Conductivity (µS/cm) | 1 | 18243.33 | 17653.33 | 19733.33 | 21333.33 | - |
| 2 | 18700 | 19610 | 19400 | 20800 | ||
| 3 | 19000 | 19466.67 | 19266.67 | 18800 | ||
| Turbidity (NTU) | 1 | 5 | 5 | 6 | 5 | - |
| 2 | 5 | 5 | 5 | 5 | ||
| 3 | 5 | 5 | 6 | 5 | ||
| Dissolved Oxygen (mg/L) | 1 | 6.35 | 7.09 | 7.63 | 6.92 | (minimum) 5* |
| 2 | 6.22 | 6.99 | 7.84 | 6.80 | ||
| 3 | 6.99 | 6.81 | 7.66 | 6.62 | ||
| Nitrates (mg/L) | 1 | 0.35 | 0.05 | 0.16 | 0.53 | 7* |
| 2 | 0.11 | 0.02 | 0.14 | 0.01 | ||
| 3 | 0.17 | 0.08 | <0.01 | 0.03 | ||
| Phosphates (mg/L) | 1 | 2.61 | 2.32 | 3.71 | 3.25 | 0.025** |
| 2 | 1.23 | 2.05 | 3.72 | 2.61 | ||
| 3 | 1.20 | 1.86 | 1.12 | 0.55 | ||
| Fecal Coliform (MPN/100mL) | 1 | 92000 | 160000 | 14000 | 350 | 200** |
| 2 | 35000 | 92000 | 160000 | 92000 | ||
| 3 | 160000 | 160000 | 92000 | 160000 | ||
| Parameters | January | February | March | April | ||||
|---|---|---|---|---|---|---|---|---|
| p-value | Remarks | p-value | Remarks | p-value | Remarks | p-value | Remarks | |
| pH | 0.925 | NS | 0.027 | S | 0.027 | S | 0.027 | S |
| Temperature | 0.058 | NS | 0.026 | S | 0.020 | S | 0.021 | S |
| TDS | 0.575 | NS | 0.065 | NS | 0.517 | NS | 0.027 | S |
| EC | 0.924 | NS | 0.190 | NS | 0.295 | NS | 0.035 | S |
| Turbidity | 0.066 | NS | 0.026 | S | 0.066 | NS | 0.148 | NS |
| DO | 0.027 | S | 0.027 | S | 0.295 | NS | 0.027 | S |
| Nitrates | 0.018 | S | 0.018 | S | 0.018 | S | 0.018 | S |
| Phosphates | 0.066 | NS | 0.050 | NS | 0.061 | NS | 0.027 | S |
| Fecal Coliform | 0.018 | S | 0.018 | S | 0.018 | S | 0.018 | S |
| Parameters | Site 1 | Site 2 | Site 3 | |||
|---|---|---|---|---|---|---|
| p-value | Remarks | p-value | Remarks | p-value | Remarks | |
| pH | 0.029 | S | 0.042 | S | 0.029 | S |
| Temperature | 0.029 | S | 0.039 | S | 0.032 | S |
| TDS | 0.042 | S | 0.029 | S | 0.117 | NS |
| EC | 0.042 | S | 0.334 | NS | 0.958 | NS |
| Turbidity | 0.241 | NS | 0.051 | NS | 0.042 | S |
| Dissolved Oxygen | 0.029 | S | 0.029 | S | 0.029 | S |
| Nitrates | 0.029 | S | 0.029 | S | 0.029 | S |
| Phosphates | 0.029 | S | 0.029 | S | 0.042 | S |
| Fecal Coliform | 0.029 | S | 0.029 | S | 0.029 | S |
| WnQn | ||||||
|---|---|---|---|---|---|---|
| Parameter | January | February | March | April | ||
| pH | Site 1 | 3.48 | 3.38 | 3.27 | 3.54 | |
| Site 2 | 3.44 | 3.15 | 3.16 | 3.02 | ||
| Site 3 | 3.46 | 3.25 | 3.09 | 3.17 | ||
| Temperature | Site 1 | 30.88 | 38.77 | 38.46 | 45.92 | |
| Site 2 | 31.50 | 38.24 | 38.29 | 47.47 | ||
| Site 3 | 38.37 | 36.56 | 38.06 | 49.91 | ||
| Total Dissolved Solids | Site 1 | 1.22 | 1.16 | 1.31 | 1.42 | |
| Site 2 | 1.22 | 0.16 | 1.29 | 1.39 | ||
| Site 3 | 1.27 | 0.15 | 1.29 | 1.24 | ||
| Electrical Conductivity | Site 1 | 200.66 | 194.17 | 217.05 | 234.65 | |
| Site 2 | 205.68 | 215.69 | 213.38 | 228.78 | ||
| Site 3 | 208.98 | 214.12 | 211.92 | 206.78 | ||
| Turbidity | Site 1 | 6.65 | 6.65 | 7.99 | 6.65 | |
| Site 2 | 6.65 | 6.65 | 6.65 | 6.65 | ||
| Site 3 | 6.65 | 6.65 | 7.99 | 6.65 | ||
| Dissolved Oxygen | Site 1 | 5.87 | 6.55 | 7.05 | 6.40 | |
| Site 2 | 5.75 | 6.46 | 7.25 | 6.28 | ||
| Site 3 | 6.46 | 6.29 | 7.08 | 6.12 | ||
| Nitrates | Site 1 | 0.12 | 0.02 | 0.05 | 0.18 | |
| Site 2 | 0.04 | 0.01 | 0.05 | 0.00 | ||
| Site 3 | 0.06 | 0.03 | 0.00 | 0.01 | ||
| Phosphates | Site 1 | 347.36 | 308.77 | 493.76 | 432.54 | |
| Site 2 | 163.70 | 272.83 | 495.09 | 347.36 | ||
| Site 3 | 159.71 | 247.55 | 149.06 | 73.20 | ||
| Fecal Coliform | Site 1 | 3.06 | 5.32 | 3.06 | 0.01 | |
| Site 2 | 1.16 | 3.06 | 5.32 | 3.06 | ||
| Site 3 | 5.32 | 5.32 | 3.06 | 5.32 | ||
| Water Quality Index | 517 | 582 | 701 | 617 | ||
| Interpretation | Unfit | Unfit | Unfit | Unfit | ||
| Family | Fish Species | Site 1 | Site 2 | Site 3 | Total Occurrence (%) |
|---|---|---|---|---|---|
| Scatophagidae | Scatophagus argus (kitang) | 31 | 8 | 11 | 50 (18.59%) |
| Terapontidae | Pelates quadrilineatus (babanse) | 26 | 8 | 15 | 49 (18.22%) |
| Cichlidae | Sarotherodon melanotheron (gloria tilapia) | 26 | 7 | 13 | 46 (17.10%) |
| Terapontidae | Terapon jarbua (bagaong) | 27 | 9 | 6 | 42 (15.61%) |
| Lutjanidae | Lutjanus argentimaculatus (alakaak) | 17 | 3 | 14 | 34 (12.64%) |
| Lutjanidae | Lutjanus argentimaculatus (kabang) | 21 | 3 | 2 | 26 (9.67%) |
| Dorosomatidae | Nematalosa nasus (kabase) | 13 | 8 | 1 | 22 (8.18%) |
| Total | 161 | 46 | 62 | 269 (100%) |
| Species | RF | RD | RA | SIV | Rank |
|---|---|---|---|---|---|
| Scatophagus argus (kitang) | 16.67 | 18.59 | 18.58 | 53.84 | 1 |
| Pelates quadrilineatus (babanse) | 14.58 | 18.21 | 18.23 | 51.02 | 2 |
| Sarotherodon melanotheron (gloria tilapia) | 16.67 | 17.10 | 17.10 | 50.87 | 3 |
| Terapon jarbua (bagaong) | 14.58 | 15.61 | 15.62 | 45.81 | 4 |
| Lutjanus argentimaculatus (alakaak) | 12.50 | 12.64 | 12.65 | 37.79 | 5 |
| Lutjanus argentimaculatus (kabang) | 14.58 | 9.67 | 9.66 | 33.91 | 6 |
| Nematalosa nasus (kabase) | 10.42 | 8.18 | 8.17 | 26.76 | 7 |
| Family | Species | Common Name | Biodiversity Status | Conservation Status |
|---|---|---|---|---|
| Scatophagidae | Scatophagus argus | Kitang | Native | Least Concern |
| Dorosomatidae | Nematalosa nasus | Kabase | Native | Least Concern |
| Terapontidae | Terapon jarbua | Bagaong | Native | Least Concern |
| Pelates quadrilineatus | Babanse | Native | Not Evaluated | |
| Lutjanidae | Lutjanus argentimaculatus | Alakaak | Native | Least Concern |
| Lutjanus argentimaculatus | Kabang | Native | Least Concern | |
| Cichlidae | Sarotherodon melanotheron | Gloria Tilapia | Introduced | Least Concern |
| Diversity Index | Sampling Sites | ||
|---|---|---|---|
| Site 1 | Site 2 | Site 3 | |
| Shannon-Wiener (H’) | 1.91 | 1.87 | 1.71 |
| Simpson’s Diversity (D) | 0.15 | 0.14 | 0.18 |
| Simpson’s Index of Diversity (1-D) | 0.85 | 0.86 | 0.82 |
| Reciprocal (1/D) | 6.81 | 7.04 | 5.48 |
| Margalef’s Richness (dmg) | 1.18 | 1.57 | 1.45 |
| Sites | % Similarity |
|---|---|
| Site1-Site2 | 60% |
| Site1-Site3 | 80% |
| Site2-Site3 | 60% |
| Sampling Month | EH | ||
|---|---|---|---|
| Site 1 | Site 2 | Site 3 | |
| January | 0.63 | 0.64 | 0.35 |
| February | 0.91 | 0.76 | 0.75 |
| March | 0.83 | 0.55 | 0.76 |
| April | 0.85 | 0.33 | 0 |
| Parameters | pH | WT | TDS | EC | Tn | DO | NO3-N | PO4-P | FCC |
| pH | 1 | ||||||||
| WT | -0.382 | 1 | |||||||
| TDS | 0.205 | 0.244 | 1 | ||||||
| EC | -0.206 | 0.528 | 0.066 | 1 | |||||
| Tn | -0.008 | 0.013 | -0.021 | 0.123 | 1 | ||||
| DO | -0.416 | 0.114 | 0.121 | 0.185 | 0.316 | 1 | |||
| NO3-N | 0.753 | -0.071 | 0.313 | 0.317 | 0.12 | -0.174 | 1 | ||
| PO4-P | 0.078 | -0.039 | 0.168 | 0.394 | 0.265 | 0.440 | 0.459 | 1 | |
| FCC | -0.276 | 0.069 | -0.267 | -0.506 | 0.437 | 0.081 | -0.489 | -0.36 | 1 |
| Parameters | Pearson R | Scatophagus argus | Nematalosa nasus | Terapon jarbua | Pelates quadrilineatus | Lutjanus argentimaculatus | Lutjanus argentimaculatus | Sarotherodon melanotheron |
| pH | Pearson Correlation Sig. (2-tailed) |
0.179 0.288 |
0.328 0.149 |
0.075 0.409 |
0.263 0.204 |
0.527 0.039 |
0.121 0.354 |
0.602 0.019 |
| Temperature (°C) | Pearson Correlation Sig. (2-tailed) |
-0.529 0.039 |
-0.091 0.389 |
0.143 0.328 |
-0.120 0.355 |
-0.191 0.277 |
-0.146 0.325 |
-0.460 0.066 |
| TDS (mg/L) | Pearson Correlation Sig. (2-tailed) |
-0.222 0.224 |
-0.079 0.403 |
-0.048 0.442 |
-0.086 0.396 |
-0.377 0.114 |
0.138 0.335 |
-0.120 0.355 |
| EC (µS/cm) | Pearson Correlation Sig. (2-tailed) |
-0.536 0.036 |
-0.272 0.196 |
0.071 0.413 |
0.135 0.337 |
0.244 0.223 |
-0.456 0.068 |
-0.301 0.171 |
| Turbidity (NTU) | Pearson Correlation Sig. (2-tailed) |
0.141 0.331 |
-0.218 0.248 |
0.282 0.188 |
0.332 0.146 |
-0.244 0.223 |
0.297 0.174 |
-0.385 0.108 |
| DO (mg/L) | Pearson Correlation Sig. (2-tailed) |
0.049 0.440 |
-0.118 0.358 |
0.338 0.142 |
0.264 0.203 |
-0.282 0.188 |
0.231 0.235 |
-0.259 0.208 |
| Nitrates (mg/L) | Pearson Correlation Sig. (2-tailed) |
0.094 0.386 |
0.013 0.484 |
0.059 0.428 |
0.231 0.235 |
0.651 0.011 |
-0.160 0.310 |
0.555 0.031 |
| Phosphates (mg/L) | Pearson Correlation Sig. (2-tailed) |
0.273 0.195 |
0.001 0.499 |
0.316 0.159 |
0.331 0.147 |
0.155 0.315 |
0.245 0.221 |
0.276 0.193 |
| Fecal Coliform (MPN/100 mL) | Pearson Correlation Sig. (2-tailed) |
0.113 0.364 |
-0.101 0.378 |
-0.289 0.181 |
-0.321 0.162 |
-0.111 0.365 |
-0.13 0.483 |
0.196 0.271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




