Submitted:
09 July 2024
Posted:
10 July 2024
You are already at the latest version
Abstract
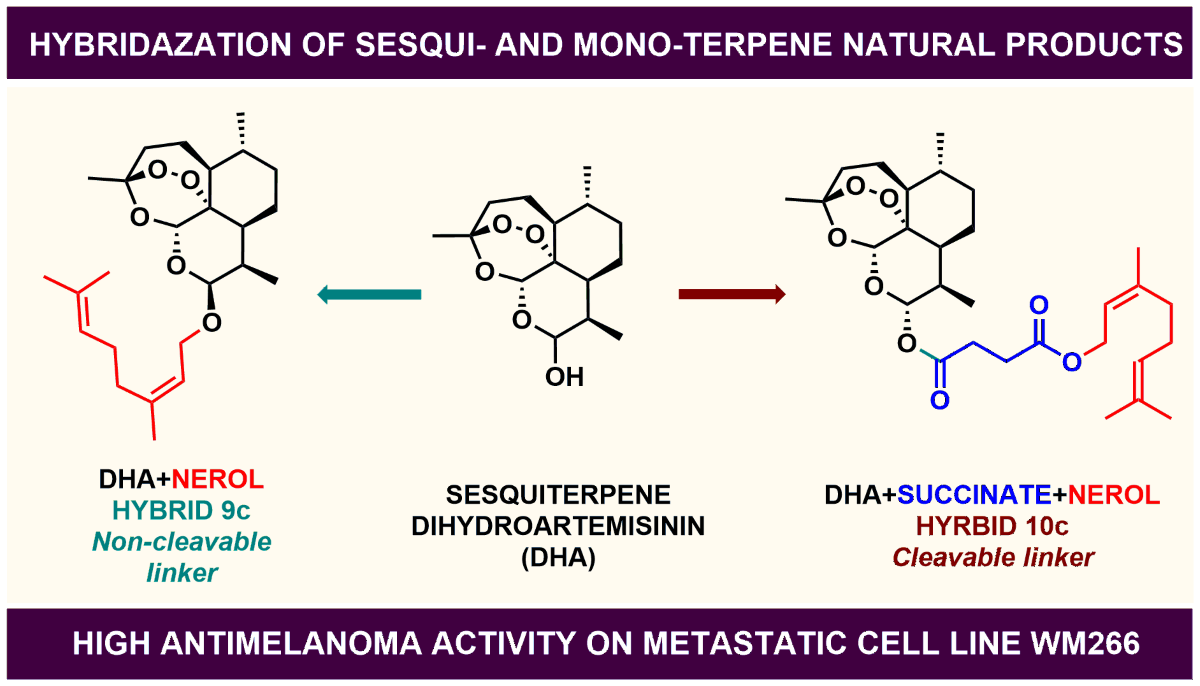
Keywords:
1. Introduction
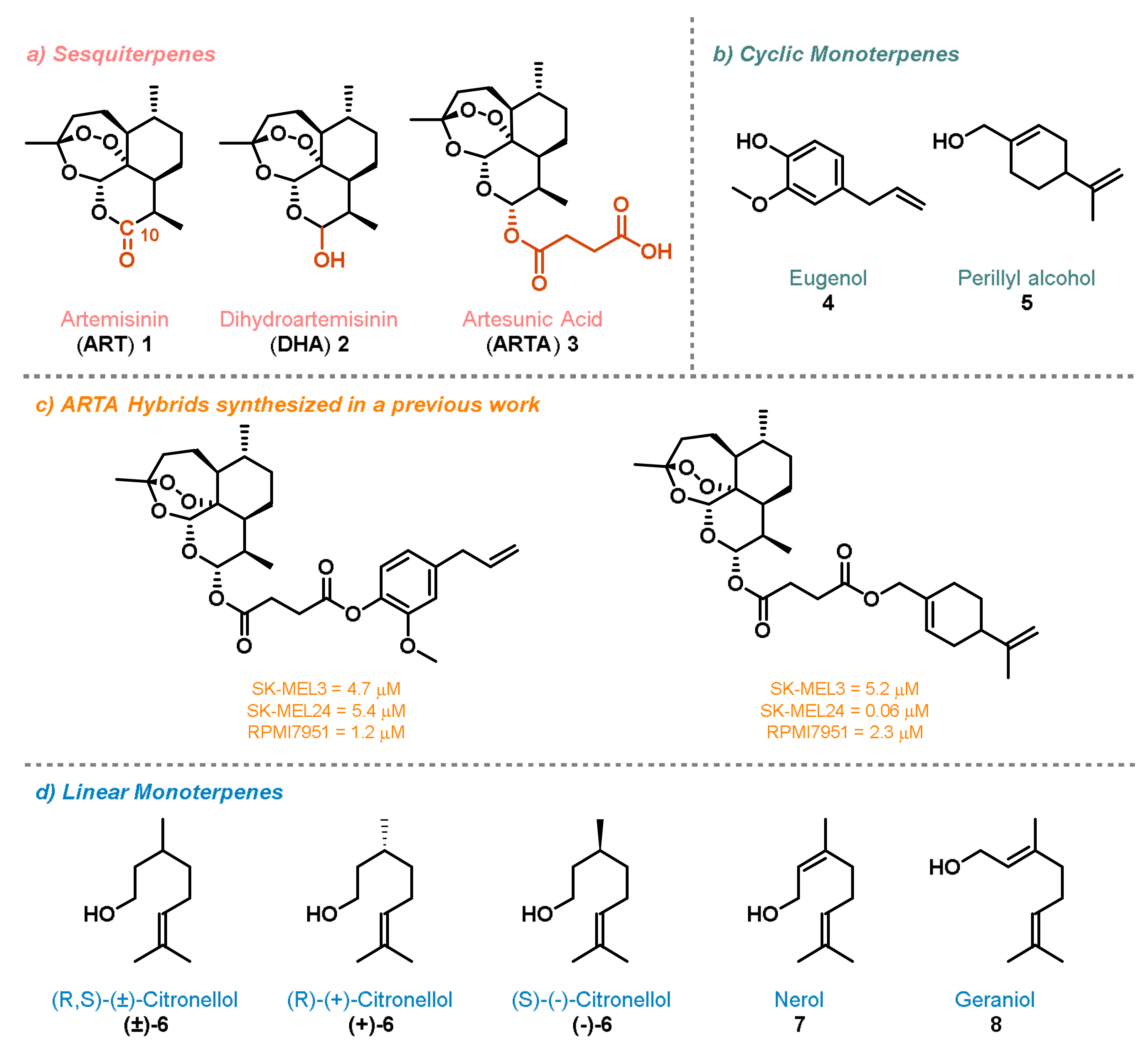
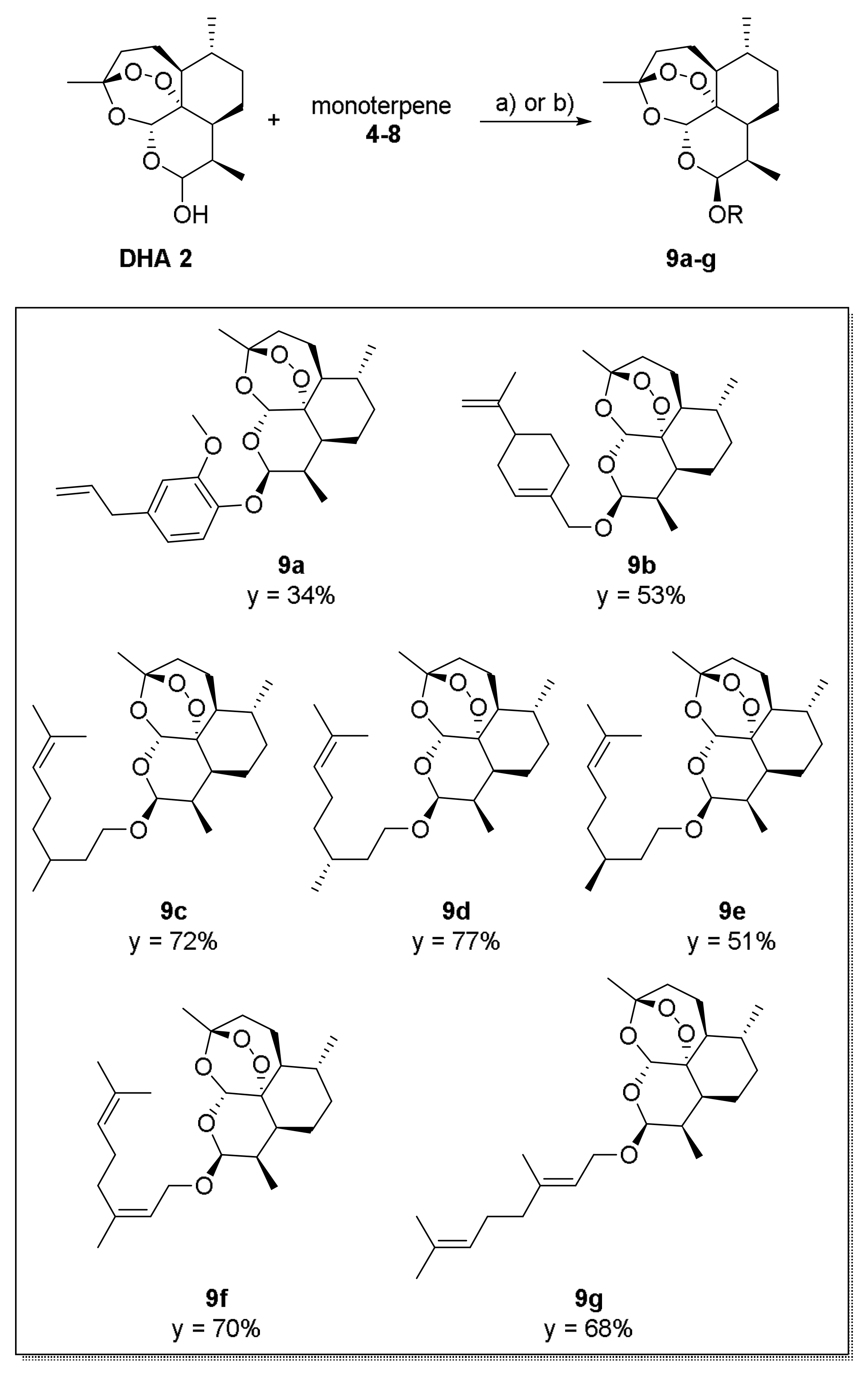
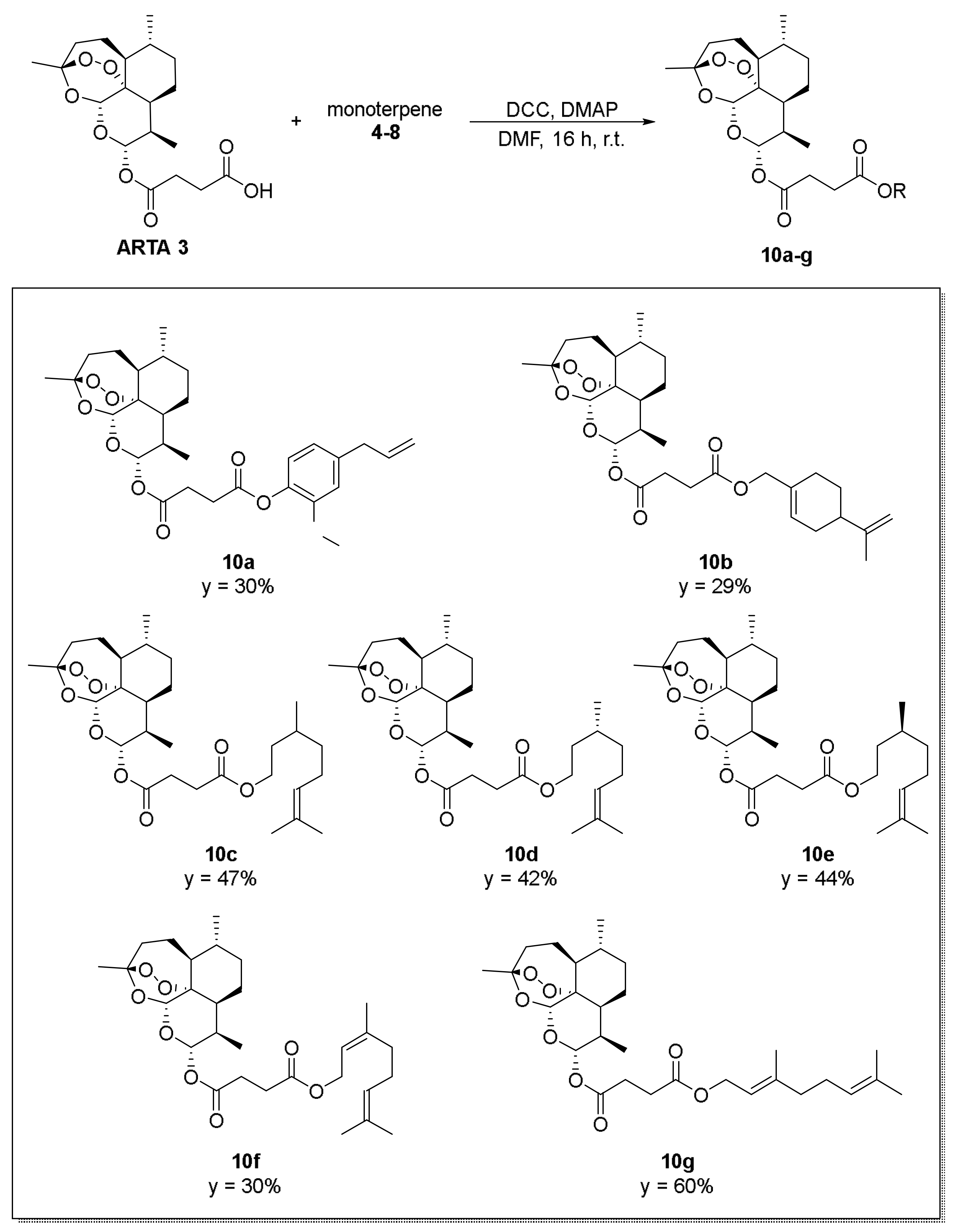
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry-General Part
3.2. Chemistry—Experimental Procedures and Compound Characterization
3.3. Biology
3.3.1. Cell Culture Conditions
3.3.2. General Treatment Protocol and Cell Viability Assay
3.3.3. Co-Administration Analyses
3.3.4. Treatment Protocol for DFO Assay
3.3.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anibogwu, R.; Jesus, K.D.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review. Molecules 2021, 26, 6995. [Google Scholar] [CrossRef]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Appalasamy, S.; Lo, K.Y.; Ch’ng, S.J.; Nornadia, K.; Othman, A.S.; Chan, L.K. Antimicrobial activity of artemisinin and precursor derived from in vitro plantlets of Artemisia annua L. Biomed Res. Int. 2014, 2014, 215872. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.R.; Efferth, T.; Serrano, M.A.; Castaño, B.; Macias, R.I.R.; Briz, O.; Marin, J.J.G. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiviral Res. 2005, 68, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Lai, H.C. Artemisinin induces apoptosis in human cancer cells. Anticancer Res. 2004, 24, 2277–2280. [Google Scholar] [PubMed]
- O’Neill, P.M.; Barton, V.E.; Ward, S.A. The molecular mechanism of action of artemisinin the debate continues. Molecules 2010, 15, 1705–1721. [Google Scholar] [CrossRef] [PubMed]
- Woodley, C.M.; Amado, P.S.M.; Cristiano, M.L.S.; O'Neill, P.M. Artemisinin inspired synthetic endoperoxide drug candidates: Design, synthesis, and mechanism of action studies. Med Res Rev. 2021, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Augustin, Y.; Staines, H.M.; Krishna, S. Artemisinins as a novel anti-cancer therapy: Targeting a global cancer pandemic through drug repurposing. Pharmacol Ther. 2020, 216, 107706. [Google Scholar] [CrossRef]
- Zeng, Z.W.; Chen, D.; Chen, L.; He, B.; Li, Y. A comprehensive overview of Artemisinin and its derivatives as anticancer agents. Eur J Med Chem. 2023, 247, 115000. [Google Scholar] [CrossRef] [PubMed]
- van Agtmael, M.A.; Eggelte, T.A.; van Boxtel, C.J. Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends Pharmacol Sci. 1999, 20, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cina Co-operative Research Group on Qinghaosu and its Derivatives as Antimalarials. J. Tradit. Chin. Med. 1982, 2, 9–16.
- China Co-operative Research Group on Qinghaosu and its Derivatives as Antimalarials. J. Tradit. Chin. Med. 1982, 2, 25–30.
- Berköz, M.; Özkan-Yılmaz, F.; Özlüer-Hunt, A.; Krośniak, M.; Türkmen, Ö.; Korkmaz, D.; Keskin, S. Artesunate inhibits melanoma progression in vitro via suppressing STAT3 signaling pathway. Pharmacol Rep. 2021, 73, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Curti, B.D.; Faries, M.B. Recent Advances in the Treatment of Melanoma. N. Engl. J. Med. 2021, 384, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers (Basel) 2022, 14, 4652. [Google Scholar] [CrossRef]
- Huangh, A.C.; Zappasodi, R.A. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat. Immunol. 2022, 23, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Shaveta; Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur J Med Chem. 2016, 124, 500–536. [CrossRef] [PubMed]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Decker, M. Hybrid molecules incorporating natural products: applications in cancer therapy, neurodegenerative disorders and beyond. Curr. Med. Chem. 2011, 18, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Filippi, S.; Bizzarri, B.M.; Zippilli, C.; Meschini, R.; Pogni, R.; Baratto, M.C.; Villanova, L.; Saladino, R. Synthesis and Evaluation of Artemisinin-Based Hybrid and Dimer Derivatives as Antimelanoma Agents. ACS Omega 2020, 5(1), 243−251.
- Tzenkova, R.; Kamenarska, Z.; Draganov, A.; Atanassov, A. Composition of Artemisia Annua Essential Oil Obtained from Species Growing Wild in Bulgaria. Biotechnol. Biotechnol. Equip. 2010, 24, 1833–1835. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int J Mol Sci. 2020, 21, 4986. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.K.; Chan, H.-W.; Cheung, M.-K.; Lam, W.-L.; Soo, M.-K.; Tsang, H.-W.; Voerste, A.; Williams, I.D. C-10 Ester and Ether Derivatives of Dihydroartemisinin 2 10-α Artesunate, Preparation of Authentic 10-β Artesunate, and of Other Ester and Ether Derivatives Bearing Potential Aromatic Intercalating Groups at C-10. Eur. J. Org. Chem. 2002, 113–132. [Google Scholar] [CrossRef]
- Herrmann, L.; Hahn, F.; Grau, B.W.; Wild, M.; Niesar, A.; Wangen, C.; Kataev, E.; Marschall, M.; Tsogoeva, S.B. Autofluorescent Artemisinin-Benzimidazole Hybrids via Organo-Click Reaction: Study of Antiviral Properties and Mode of Action in Living Cells. Chemistry. 2023, 29, e202301194. [Google Scholar] [CrossRef] [PubMed]
| Entry | Compound | S.C.b | IC50 (µM±SD)c | TSId | |||
|---|---|---|---|---|---|---|---|
| C3PV | WM115 | WM266 | WM115 | WM266 | |||
| 1 | DHA 2 | - | 0.7 ± 0.19 | 1.6 ± 0.4 | 1.6 ± 0.03 | 0.4 | 0.4 |
| 2 | ARTA 3 | - | 1.7 ± 0.44 | 1.5 ± 0.01 | 1.3 ± 0.2 | 1.1 | 1.3 |
| 3 | Eugenol 4 | - | 1.0±0.1 | 3.0±0.02 | 0.9±0.05 | 0.3 | 1.1 |
| 4 | Perillyl alcohol 5 | - | 52.5±9.5 | 1.2±0.02 | 0.6±0.04 | 43.8 | 87.5 |
| 5 | (±)-citronellol (±)-6 | - | 3.0±1.2 | 2.6±0.05 | 0.3±0.23 | 1.2 | 10.0 |
| 6 | (+)-citronellol (+)-6 | - | 1.9 ± 0.8 | 1.5 ± 0.07 | 0.6± 0.02 | 1.3 | 3.2 |
| 7 | (-)-citronellol (-)-6 | - | 1.0 ± 0.9 | 0.9 ± 0.03 | 0.5 ± 0.3 | 1.1 | 2.0 |
| 8 | Nerol 7 | - | 3.8±1.5 | 1.4±0.03 | 0.5±0.01 | 2.7 | 7.6 |
| 9 | Geraniol 8 | - | 0.5±0.02 | 0.5±0.01 | 0.5±0.09 | 1.0 | 1.0 |
| 10 | 9a | DHA | 0.7±0.04 | 0.1±0.01 | 1.0±0.09 | 7.0 | 0.7 |
| 11 | 9b | DHA | 0.3±0.1 | 0.2±0.01 | 0.2±0.01 | 1.5 | 1.5 |
| 12 | 9c | DHA | 364.2±7.9 | 2.1±0.3 | 1.4±0.56 | 173.4 | 260.1 |
| 13 | 9d | DHA | 51.0±0.3 | 2.9±0.6 | 2.7±0.2 | 17.6 | 18.9 |
| 14 | 9e | DHA | 50.0±0.03 | 2.4±0.1 | 2.2±0.6 | 20.8 | 22.7 |
| 15 | 9f | DHA | 87.3±2.5 | 3.0±0.4 | 1.9±0.5 | 29.1 | 45.9 |
| 16 | 9g | DHA | 6.2±0.7 | 14.5±1.1 | 13.4±1.5 | 0.4 | 0.5 |
| 17 | 10a | ARTA | 1.6±0.3 | 1.5±0.8 | 0.6±0.1 | 1.1 | 2.7 |
| 18 | 10b | ARTA | 20.3±5.5 | 0.03±0.01 | 0.02±0.01 | 676.7 | 1015.0 |
| 19 | 10c | ARTA | 4.4±2.9 | 1.3±0.9 | 0.6±0.02 | 3.4 | 7.3 |
| 20 | 10d | ARTA | 4.8±0.9 | 1.7±0.5 | 1.3±0.8 | 2.8 | 3.7 |
| 21 | 10e | ARTA | 5.1±1.7 | 1.9±0.6 | 1.6±0.9 | 2.7 | 3.2 |
| 22 | 10f | ARTA | 7.9±4.5 | 0.4±0.04 | 0.09±0.03 | 19.8 | 87.8 |
| 23 | 10g | ARTA | 2.4±0.9 | 1.5±0.7 | 1.0±0.1 | 1.6 | 2.4 |
| 24 | PTX | - | 78.9 ± 0.8 | 2.3±0.7 | 0.9±0.04 | 34.3 | 87.7 |
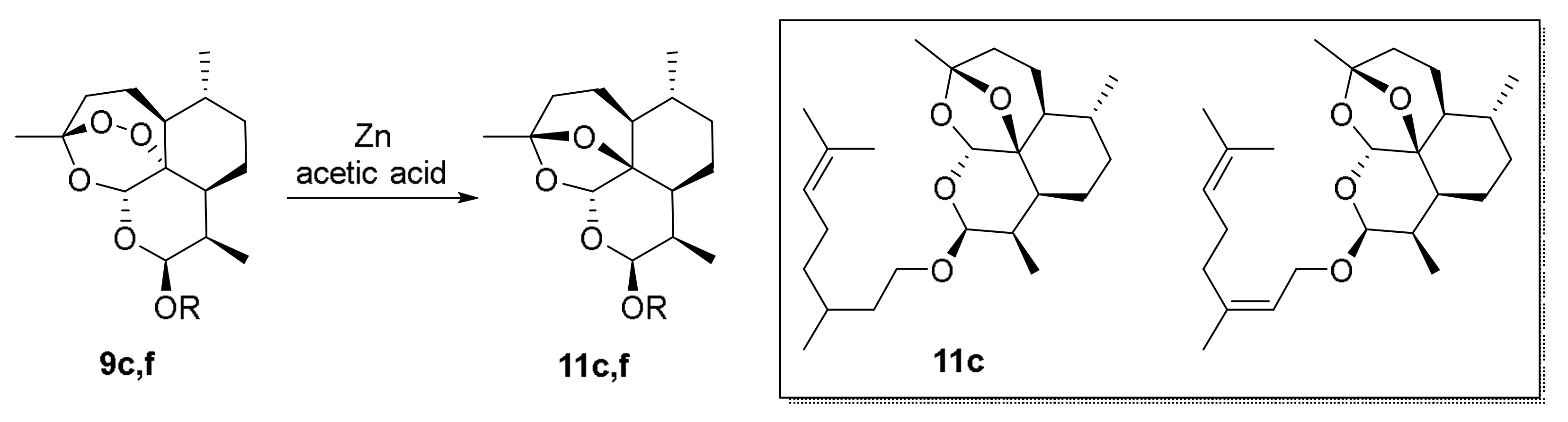
| 25 | 11c | 2-dDHAe | - | - | 35.1±2.5 | - | - |
| 26 | 11f | 2-dDHAe | - | - | 76.3±9.5 | - | - |
| Entry | Combination | R.H.b | IC50±SD WM266c | IC50±SD R.H.d |
|---|---|---|---|---|
| 1 | DHA+citronellol (±)-6 | 9c | 4.56±07 | 1.4±0.56 |
| 2 | DHA+nerol 7 | 9f | 5.49±0.6 | 1.9±0.5 |
| 3 | ARTA+perillyl acohol 5 | 10b | 1.8±0.3 | 0.02±0.01 |
| 4 | ARTA+nerol 7 | 10f | 2.9±0.9 | 0.09±0.03 |
| Entry | Compoundb | IC50±SD WM266b,c | |
|---|---|---|---|
| without DFO | with DFO | ||
| 1 | 9c | 1.4±0.56 | 12.7 ± 0.3 |
| 2 | 9f | 1.9±0.5 | 132.7±0.4 |
| 3 | 10b | 0.02±0.01 | 0.31±0.1 |
| 4 | 10f | 0.09±0.03 | 0.5±0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





