Submitted:
09 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Resin Cements and Bonding Agents
2.2. Preparation of Lithium Disilicate (LDS; IPS e.max CAD) Surfaces
2.3. Shear Bond Strength Measurement
2.4. High-Performance Liquid Chromatography Analysis
2.5. Statistical analysis
3. Results
3.1. With and Without HP Pretreatment
3.1.1. Statistical comparison among three time periods for each resin cement (Table 3 and Table 4)
3.1.2. Statistical comparison within each time period among nine resin cements (Table 5)
| RelyX Universal Resin Cement / Scotchbond Universal Plus Adhesive. | PANAVIA SA Cement Universal / None | PANAVIA V5 / Clearfil Ceramic Primer Plus | |||
| Pretreating by HF(+) | Pretreating by HF(-) | Pretreating by HF(+) | Pretreating by HF(-) | Pretreating by HF(+) | Pretreating by HF(-) |
| Base a | Base c | Base a | Base b | Base a | Base b |
| TC 5k a | TC 5k d | TC 20k a | TC 5k c | TC 20k a | TC 5k c |
| TC 20k b | TC 20k e | TC 5k a | TC 20k c | TC 5k a | TC 20k d |
| G-Cem ONE / G-Cem ONE Adhesive Enhancing Primer | ESTECEM II / Tokuyama Universal Bond II (A+B) | Variolink Esthetic DC / Monobond Plus | |||
| Pretreating by HF(+) | Pretreating by HF(-) | Pretreating by HF(+) | Pretreating by HF(-) | Pretreating by HF(+) | Pretreating by HF(-) |
| TC 5k a | Base b | TC 5k a | Base a | TC 5k a | Base b |
| Base a | TC 5k c | Base a | TC 5k b | Base a | TC 5k c |
| TC 20k a | TC 20k c | TC 20k a | TC 20k c | TC 20k a | TC 20k d |
| ResiCem EX / BeautiBond Xtreme | Nexus Universal Chroma / OptiBond eXTRa Universal | Super-Bond Universal / M&C Primer A & B | |||
| Pretreating by HF(+) | Pretreating by HF(-) | Pretreating by HF(+) | Pretreating by HF(-) | Pretreating by HF(+) | Pretreating by HF(-) |
| TC 5k a | Base b | Base a | Base b | Base a | Base b |
| Base a | TC 5k c | TC 20k a | TC 5k c | TC 5k a | TC 5k c |
| TC 20k a | TC 20k c | TC 5k a | TC 20k c | TC 20k a | TC 20k d |
| Base | TC 5k | TC 20k | |||
|---|---|---|---|---|---|
| Pretreating by HF(+) | Pretreating by HF(-) | Pretreating by HF(+) | Pretreating by HF(-) | Pretreating by HF(+) | Pretreating by HF(-) |
| PANAVIA V5 a | PANAVIA SA Cement Universal d | PANAVIA V5 a | PANAVIA SA Cement Universal d | G-Cem ONE a b | PANAVIA SA Cement Universal c |
| ResiCem EX a | Nexus Universal Chroma d | Nexus Universal Chroma a | Nexus Universal Chroma d e | RelyX Universal Resin Cement a b | Nexus Universal Chroma c |
| G-Cem ONE a | ResiCem EX d | Super-Bond Universal a | ResiCem EX d e | ResiCem EX a b | ResiCem EX c |
| Super-Bond Universal a b | PANAVIA V5 d e | ResiCem EX a b | PANAVIA V5 d e | PANAVIA V5 a b | Variolink Esthetic DC c |
| ESTECEM II a b | RelyX Universal Resin Cement e f | PANAVIA SA Cement Universal a b | G-Cem ONE e | Super-Bond Universal a b | PANAVIA V5 c |
| Nexus Universal Chroma a b c | G-Cem ONE e f | G-Cem ONE a b | RelyX Universal Resin Cement f | ESTECEM II a b | RelyX Universal Resin Cement c d |
| PANAVIA SA Cement Universal b c | Super-Bond Universal f | ESTECEM II b c | Variolink Esthetic DC f | Nexus Universal Chroma a b |
G-Cem ONE d |
| Variolink Esthetic DC b c | Variolink Esthetic DC f | RelyX Universal Resin Cement c | ESTECEM II g | PANAVIA SA Cement Universal a b | ESTECEM II e |
| RelyX Universal Resin Cement c | ESTECEM II f | Variolink Esthetic DC c |
Super-Bond Universal g | Variolink Esthetic DC b | Super-Bond Universal e |
3.1.3. Statistical comparison between HF(+) and HF(-) specimens of each resin cement for each time period (Table 3 and Table 6)
3.2. Pretreatment with Tokuyama Universal Bond II Only versus Manufacturers′ Recommended Pretreating Agents Without HF Pretreatment
3.2.1. Statistical Comparison between Base and TC 20k (Table 8)
3.2.2. Statistical comparison within each time period among nine resin cements (Table 9(a) and Table 9(b))
| Base (after 1-day) | TC 20k | ||
|---|---|---|---|
| Pretreating by Tokuyama Universal Bond II (A+B) | Pretreating by recommended bonding agent | Pretreating by Tokuyama Universal Bond II (A+B) | Pretreating by recommended bonding agent |
| PANAVIA SA Cement Universal a | PANAVIA SA Cement Universal c | ESTECEM II f | PANAVIA SA Cement Universal i |
| Nexus Universal Chroma a | Nexus Universal Chroma c | Nexus Universal Chroma f g | Nexus Universal Chroma i |
| PANAVIA V5 a b | ResiCem EX c | ResiCem EX f g | ResiCem EX i |
| ESTECEM II a b | PANAVIA V5 c | Super-Bond Universal f g | Variolink Esthetic DC i |
| RelyX Universal Resin Cement a b | RelyX Universal Resin Cement d | G-Cem ONE EM f g h | PANAVIA V5 i |
| G-Cem ONE EM a b | G-Cem ONE EM d e | RelyX Universal Resin Cement f g h | RelyX Universal Resin Cement i |
| Super-Bond Universal a b | Super-Bond Universal d e | PANAVIA SA Cement Universalf g h | G-Cem ONE EM j |
| ResiCem EX a b | Variolink Esthetic DC e | Variolink Esthetic DC g h | ESTECEM II k |
| Variolink Esthetic DC b | ESTECEM II e | PANAVIA V5 h | Super-Bond Universal k |
| Pretreating by Tokuyama Universal Bond II (A+B) | Pretreating by recommended bonding agent | |||
|
Base (after 1-day) |
TC 20k |
Base (after 1-day) |
TC 20k | |
| PANAVIA SA Cement Universal | a | f | c | i |
| Nexus Universal Chroma | a | f g | c | i |
| PANAVIA V5 | a b | f g | c | i |
| ESTECEM II | a b | f g | c | i |
| RelyX Universal Resin Cement | a b | f g h | d | i |
| G-Cem ONE EM | a b | f g h | d e | i |
| Super-Bond Universal | a b | f g h | d e | j |
| ResiCem EX | a b | g h | e | k |
| Variolink Esthetic DC | b | h | e | k |
3.2.3. Statistical comparison between two pretreating agents at each time period (Table 10)
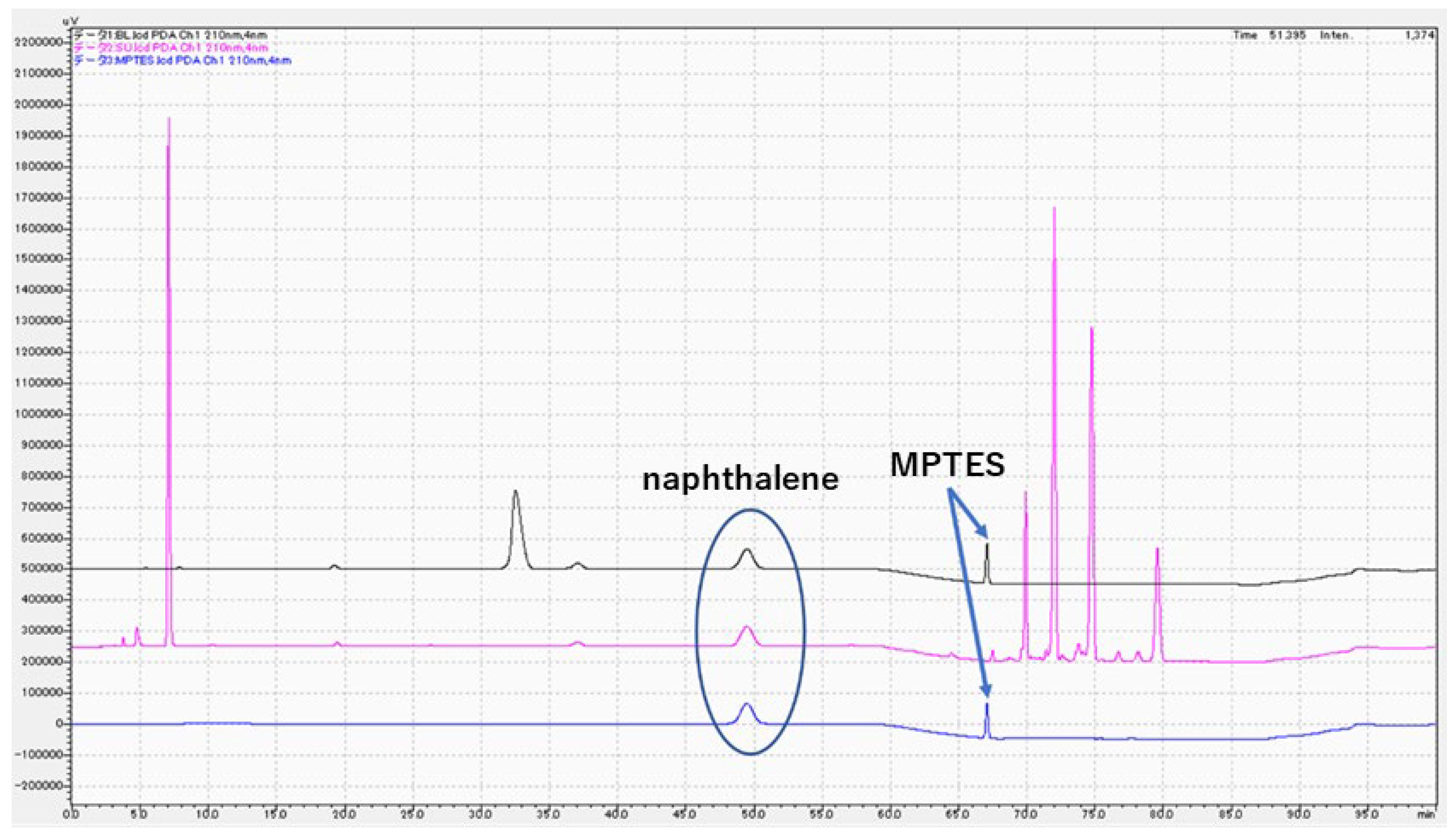
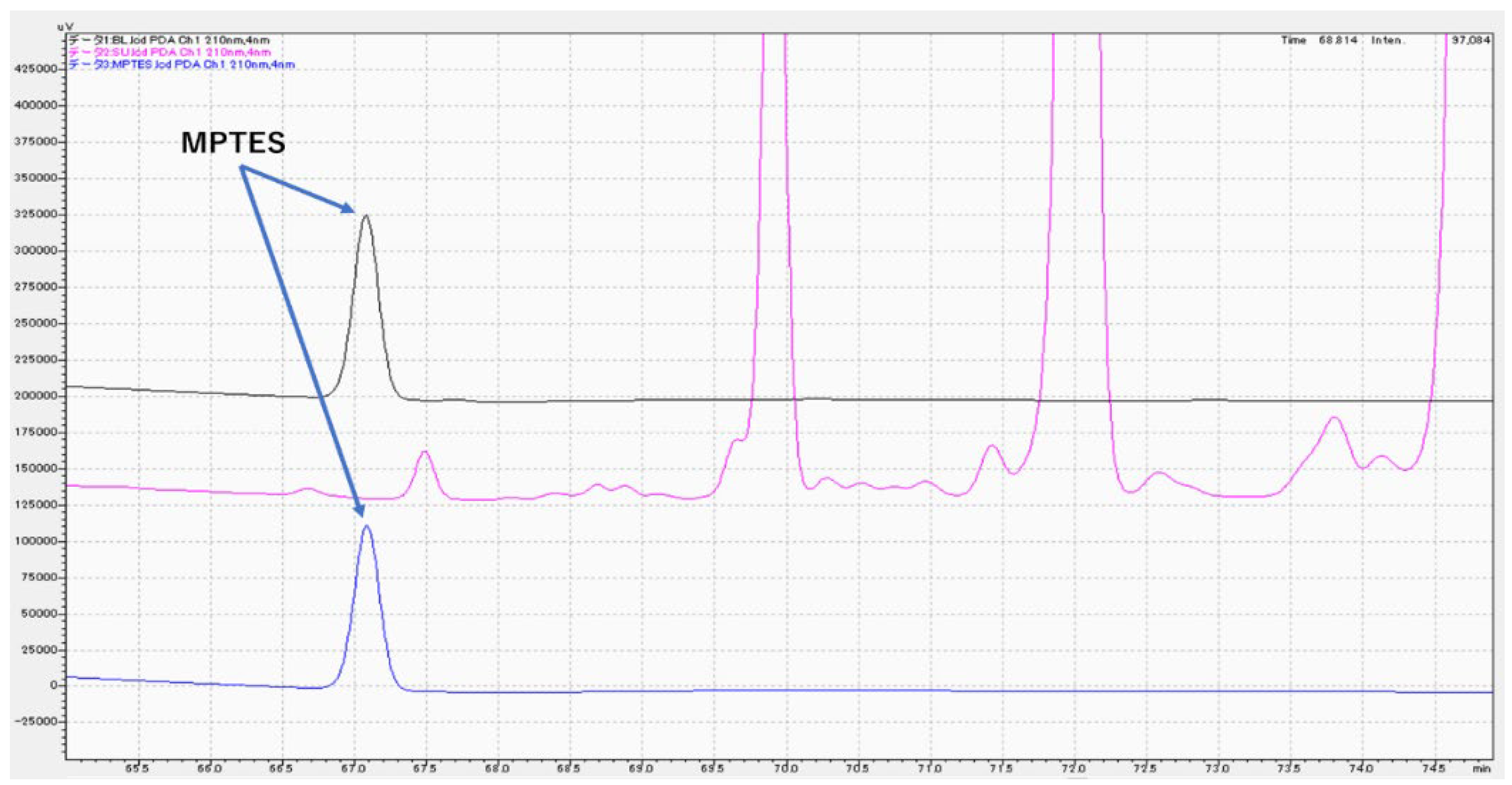
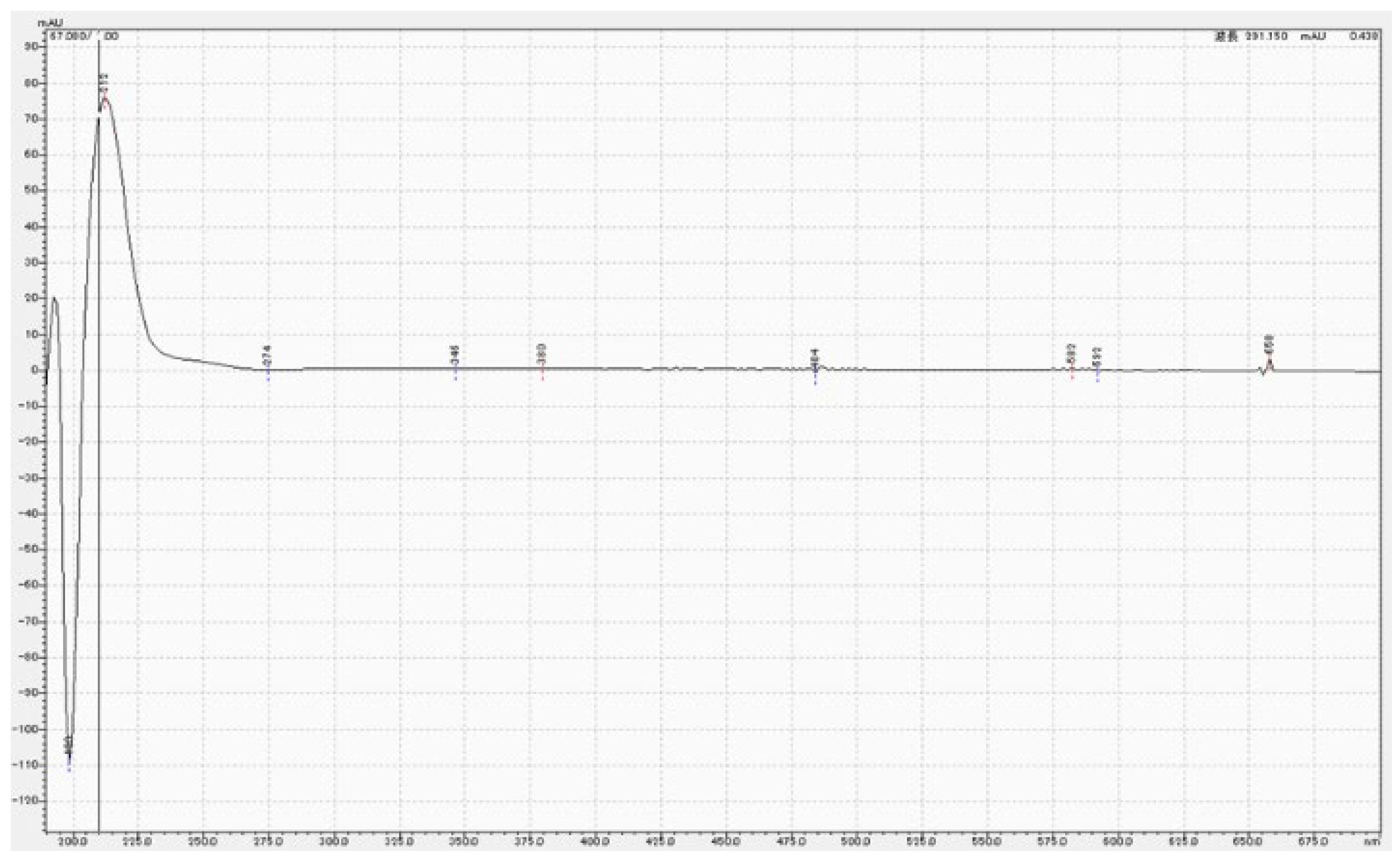
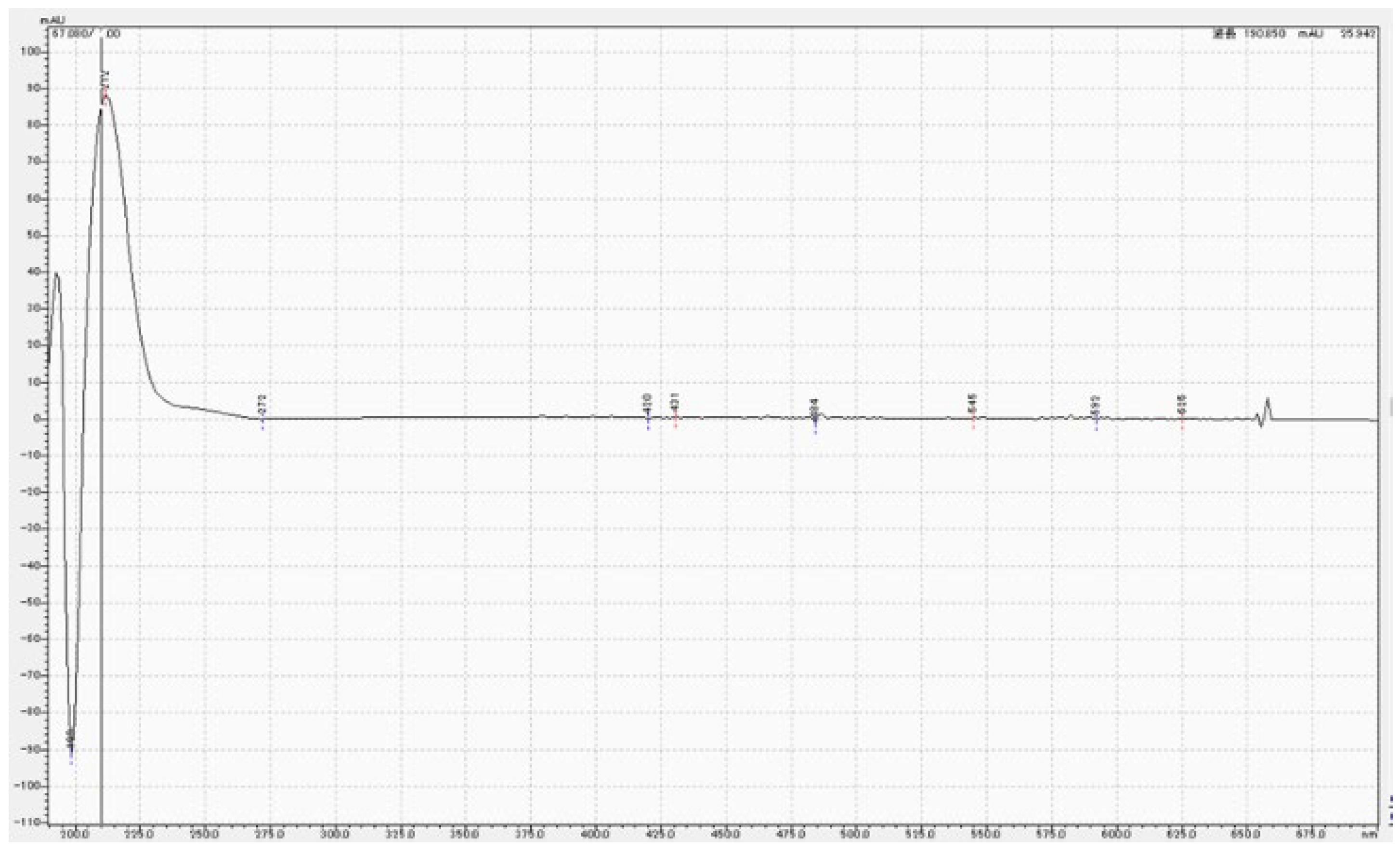
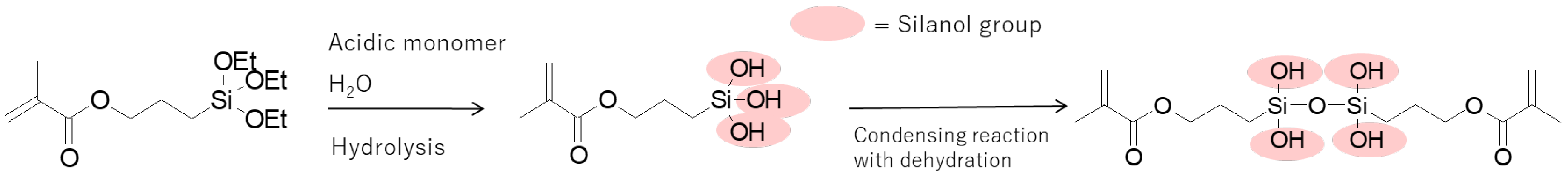
3.3. Chemical analysis of MPTES by HPLC
4. Discussion
4.1. HF(+) versus HF(-)
4.2. One-Bottle versus Two-Bottle
4.3. Analysis of MPTES by HPLC
4.4. Applicability of Tokuyama Universal Bond II as a Pretreating Agent
4.5. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powers, J.M.; Wataha, J.C. (Eds.). Dental Materials: Properties and manipulation, 9th ed.; Mosby Elsevier: St. Louis, MI, USA, 2008; pp. 152-159.
- Tian, T.; Tsoi, J.K.-H.; Matinlinna, J.P.; Burrow, M.F. Aspects of bonding between resin luting cements and glass ceramic materials. Dent. Mater. 2014, 30, e147-e162. [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Maruo, Y.; Nishigawa, G.; Irie, M.; Yoshida, Y.; Van Meerbeek, B. Sandblasting may damage the surface of composite CAD/CAM blocks. Dent. Mater. 2017, 33, e124-135. [CrossRef]
- Maruo, Y.; Nishigawa, G.; Irie, M.; Yoshihara, K.; Matumoto, T.; Minagi, S. Does acid etching morphologically and chemically affect lithium disilicate glass ceramic surfaces? J. Appl. Biomater. Funct. Mater. 2017, 15, e93-e100. [CrossRef]
- Maruo, Y.; Nishigawa, G.; Yoshihara, K.; Minagi, S.; Matsumoto, T.; Irie, M. Does 8-methacryloxyoctyl trimethoxy silane (8-MOTS) improve initial bond strength on lithium disilicate glass ceramics? Dent. Mater. 2017, 33, e95-e100. [CrossRef]
- Lee, H.Y.; Han, G.J.; Chang, J.; Son, H.H. Bonding of the silane containing multi-mode universal adhesive for lithium disilicate ceramics. Restor. Dent. Endod. 2017, 42, 95-104.
- Blatz, M.B.; Vonderheide, M.; Conejo, J. The effect of resin bonding on long-term success of high-strength ceramics. J. Dent. Res. 2018, 97, 132-139. [CrossRef]
- Guimaraes, H.A.B.; Cardoso, P.C.; Decurcio, R.A.; Monteiro, L.J.E.; de Almeida, L.N.; Martins, W.F. Simplified surface treatments for ceramic cementation: Use of universal adhesive and self-etching ceramic primer. Int. J. Biomater. 2018, Dec 31:2018:2598073. eCollection 2018. [CrossRef]
- Murillo-Gomez, F.; Wanderley, R.B.; de Goes, M.F. Impact of silane-containing universal adhesive on the biaxial flexural strength of a resin cement/glass-ceramic system. Oper. Dent. 2019, 44, 200-209. [CrossRef]
- Inokoshi, M.; Nozaki, K.; Takagaki, T.; Okazaki, Y.; Yoshihara, K.; Minakuchi, S.; Van Meerbeek, B. Initial curing characteristics of composite cements under ceramic restorations. J. Prosthodont. Res. 2021, 65, 39-45. [CrossRef]
- Ueda, N.; Takagaki, T.; Nikaido, T.; Takahashi, R.; Ikeda, M.; Tagami, J. The effect of different ceramic surface treatments on the repair bond stregnth of resin composite to lithium disilicate ceramic. Dent. Mater. J. 2021, 40, 1073-1079. [CrossRef]
- Shen, C.; Rawls, H.R.; Esquivel-Upshaw, J.F. (Eds.). Phillips’ Science of Dental Materials, 13th ed.; Mosby Elsevier: St. Louis, MI, USA, 2022; pp. 202-232.
- Jurado, C.; Pinedo, F.; Trevino, D.A.C.; Williams, Q.; Marquez-Conde, A.; Irie, M.; Tsujimot, A. CAD/CAM lithium disilicate ceramic crowns: Effect of occlusal thickness on fracture resistance and fractographic analysis. Dent. Mater. J. 2022, 41, 705-709. [CrossRef]
- Irie, M.; Okada, M.; Maruo, Y.; Nishigawa, G.; Matsumoto T. Shear bond strength of resin luting materials to lithium disilicate ceramic: Correlation between flexural strength and modulus of elasticity. Polymers 2023, 15, 1128. [CrossRef]
- Bertolini, J.C. Hydrofluoric acid: A review of toxicity. J. Emerg. Med. 1992, 10, 163-168.
- https://www.ivoclar.com/en_us/products/cementation/monobond-etch-prime (accessed on 2 May 2024).
- Blum, I.R.; Nikolinakos, N.; Lynch, C.D.; Wilson, N.H.F.; Millar, B.J.; Jagger, D.C. An in vitro comparison of four intra-oral ceramic repair systems. J. Dent. 2012, 40, 906-912.
- Reston, E.G.; Filho, S.C.; Arossi, G.; Cogo, R.B.; Rocha, C.S.; Closs, L.Q. Repairing ceramic restorations: Final solution or alternative procedure? Oper. Dent. 2008, 33, 461-466.
- Lung, C.Y.K.; Mathinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012, 28, 467-477. [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Sonoda, A.; Maruo, Y.; Makita, Y.; Okihara, T.; Irie, M.; Yoshida, Y.; Van Meerbeek, B. Effectiveness and stability of silane coupling agent incorporated in “universal” adhesives. Dent. Mater. 2016, 32, 1218-1225. [CrossRef]
- Stape, T.H.S.; Tulkki, O.; Salim, .IA.; Jamal, K.N.; Mutluay, M.M.; Tezvergil-Mutluay, A. Composite repair: On the fatigue strength of universal adhesives. Dent. Mater. 2022, 38, 231-241. [CrossRef]
- Hooshmand, T.; van Noort, R.; Keshvad, A. Storage effect of a pre-activated silane on the resin to ceramic bond. Dent. Mater. 2004, 20, 635-642. [CrossRef]
- Tian, T.; Tsoi, J.K.-H.; Matinlinna, J.P.; Burrow, M.F. Aspects of bonding between resin luting cements and glass ceramic materials. Dent. Mater. 2014, 30, e147-e162. [CrossRef]
- Irie, M.; Suzuki, K.; Watts, D.C. Marginal and flexural integrity of three classes of luting cement, with early finishing and water storage. Dent. Mater. 2004, 20, 3-11. [CrossRef]
- Irie, M.; Hatanaka, K.; Suzuki, K.; Watts, D.C. Immediate versus water-storage performance of class V flowable composite restoratives. Dent. Mater. 2006, 22, 875-883. [CrossRef]
- Irie, M.; Maruo, Y.; Nishigawa, G.; Suzuki, K.; Watts, D.C. Physical properties of dual-cured luting-agents correlated to early no interfacial-gap incidence with composite inlay restorations. Dent. Mater. 2010, 26, 608-615. [CrossRef]
- https://tokuyama-dental.eu/en/shop/bonding-agents-adhesives/15312-universal-bond-ii (accessed on dd mmm yyyy).
- Hayashi, K.; Ishii, R.; Takamizawa, T.; Suda, S.; Aoki, R.; Hayashi, K.; Kamimoto, A.; Miyazaki, M. Treatment of saliva contaminatin of resin core foundation before adhesive luting. Dent. Mater. J. 2024, 43, 36-43. [CrossRef]
| Product | Composition | Manufacturer | Batch No. |
|---|---|---|---|
| RelyX Universal Resin Cement | Surface treated glass powder filler, Phosphate ester monomer, TEGDMA, DiurethaneDimethacrylate, Silica filler, Initiator, Titanium Dioxide | 3M, Seefeld, Germany | 8697260 |
| PANAVIA SA Cement Universal (Automix) | Paste A: MDP, Bis-GMA, TEGDMA, HEMA, Silanated barium glass filler, Silanated colloidal silica, dl-Camphorquinone, Peroxide, Catalysts, Pigments Paste B: Hydrophobic aromatic dimethacrylate, Silane coupling agent, Silanated barium glass filler, Aluminum oxide filler, Surface treated sodium fluoride (Less than 1%), dl-Camphorquinone, Accelerators, Pigments, Filler content: 40 vol.%, 62 wt.% |
Kuraray Noritake Dental, Tainai, Niigata, Japan | RRO194 |
| PANAVIA V5 | Paste A: Bis-GMA, TEGDMA, Hydrophobic aromatic dimethacrylate, Hydrophilic aliphatic dimethacrylate, Initiators, Accelerators, Silanated barium glass filler, Silanated fluoroalminosilicate glass filler, Colloidal silica Paste B: Bis-GMA, Hydrophobic aromatic dimethacrylate, Hydrophilic aliphatic dimethacrylate, Silanated barium glass filler, Silanated alminium oxide filler, Accelerators, dl-Camphorquinone, Pigments, Filler content: 38 vol %, 61 wt.% |
Kuraray Noritake Dental, Tainai, Niigata, Japan |
4EO236 |
| G-Cem ONE EM | Fluoro-alumino-silicate-glass, Urethanedimethacrylate, Dimethacrylate, Phosphoric ester monomer, Silicone dioxide, Initiator, 54 ~ 67 wt% | GC, Hasunuma, Itabashi, Tokyo, Japan | 2302201 |
| ESTECEM II | Paste A: Bis-GMA, TEGDMA, Bis-MPEPP, Silica-Zirconia Filler Paste B: Bis-GMA, TEGDMA, Bis-MPEPP, Silica-Zirconia Filler, Camphorquinone, Peroxide, Filler content: 74 wt.% |
Tokuyama Dental, Tokyo, Japan | 078001 |
| Variolink Esthetic DC | Monomer matrix: urethane dimethacrylate and further methacrylate monomers, Inorganic filler: mixed oxides, ytterbium trifluoride Additional contents: initiators, stabilizers, pigments (< 1 %) Total content of inorganic filler: approx. 65 wt.% |
Ivoclar Vivadent AG, Schaan, Liechtenstein | ZO47JD |
| ResiCem EX | Paste A: S-PRG filler, Bis-GMA, Silicate glass, Initiators, Others Paste B: S-PRG filler, Bis-GMA, Silicate glass, Initiators, Others Filler content: 65 wt.%, 45 vol.% |
Shofu, Kyoto, Japan | 062209 |
| Nexus Universal Chroma | TEGDMA, Urethane dimethacrylate, Bisphenol A diglycidylmethacrylate (Bis-GMA), GPDM, photoinitiator, redox initiator, bariumaluminosilicate glass filler, ytterbium fluoride, silica, Filler content: 43 vol.%, 68 wt.% | Kerr, Orange CA, USA |
8764312 |
| Super-Bond Universal | PMMA, 4-META, MMA, TBB, Self-cure type (Bulk-mix technique) | SUN MEDICAL, Moriyama, Japan | Universal Polymer: EW1, Catalyst V: FW12 |
| Adhesive | Batch No. | Composition | Manufacturer | Surface treatment of IPS e.max |
|---|---|---|---|---|
| Scotchbond Universal Plus Adhesive | 7836013 | Brominated dimethacrylate, HEMA, Silane Treated Silica, Vitrabond Copolymer, MDP, Initiators, MPTES,Ethanol, Water | 3M, Seefeld, Germany | Scotchbond Universal Plus Adhesive (20 s) – air (5 s) |
| CLEAFIL CERAMIC PRIMER PLUS | 3L0094 | 3-Methacryloxypropyl trimethoxysilane, MDP, Ethanol |
Kuraray Noritake Dental, Tainai, Niigata, Japan | CLEAFIL CERAMIC PRIMER PLUS (1-2 s) – air (5 s) |
| G-Cem One Multi Primer | 2104221 | Vinyl silane, Phosphate ester monomer, Thiophosphate ester monomer, Methacrylic ester, Ethanol |
GC, Hasunuma, Itabashi, Tokyo, Japan | G-Cem One Multi Primer (10 s) – air (5 s) |
| Tokuyama Universal Bond II (A+B) | Bond A: 019 Bond B:514 |
Liquid A: Phosphoric acid monomer (New 3D-SR monomer), MTU-6, HEMA, Bis-GMA, TEGDMA, Acetone, Others. Liquid B: γ-MPTES, Borate, Peroxide, Acetone, Ethanol, Water, Others |
Tokuyama Dental, Tokyo, Japan | Tokuyama Universal Bond II Mix (Liquid A + Liquid B, 1-2 s) – air (5 s) |
| Monobond Plus | ZO1LG8 | Phosphoric acid monomer, Silane methacylate, Ethanol | Ivoclar Vivadent AG, Schaan, Liechtenstein | Monobond Plus (60 s) – air |
| BeautiBond Xtreme | 042347 | Acetone, Water, Bis-GMA, TEGDMA, Phosphoric ester monomer, Silane coupling agent, Initiator, Others | Shofu, Kyoto, Japan | BeautiBond Xtreme (20 s) – air |
| OptiBond eXTRa Universal | Primer: 8199022 Adhesive: 8181793 |
HEMA, dimethacrylate monomers, tri-functional methacrylate monomer, Ethanol, Photo-initiator, Bariumaluminosilicate filler, Silica, Sodium hexafluorosilicate | Kerr, Orange CA, USA | OptiBond eXTRa Adhesive (15 s) – air (5 s) – LED light (5 s) |
| M&C Primer A & B | Liquid A: EW1, Liquid B: ES1 |
M&C Primer A: MDP, VTD, MMA, Acetone M&C Primer B: γ -MPTS, MMA | SUN MEDICAL, Moriyama, Japan | Mix (Liquid A + Liquid B,1-2 s) – air (5 s) |
| Resin cement / Pretreating agent | Pretreating by HF | Time | ||
|---|---|---|---|---|
| Base (1-day) | TC 5k | TC 20k | ||
| RelyX Universal Resin Cement / Scotchbond Universal Plus Adhesive |
(+) | 43.4 (5.1, 0) | 41.6 (6.2, 0) | 29.3 (3.3, 0) |
| (-) | 30.1 (8.2, 0) | 17.0 (4.7, 0) | 1.7 (0.9, 0) | |
| PANAVIA SA Cement Universal / None | (+) | 38.3 (3.7, 0) | 32.6 (5.4, 0) | 33.7 (6.6, 0) |
| (-) | 14.3 (2.6, 0) | 1.9 (0.8, 2) | 0.9 (0.3, 5) | |
| PANAVIA V5 / Clearfil Ceramic Primer Plus |
(+) | 30.9 (4.0, 0) | 28.3 (2.4, 0) | 30.8 (3.7, 0) |
| (-) | 22.1 (6.2, 0) | 5.4 (2.1, 0) | 1.5 (0.6, 0) | |
| G-Cem ONE EM /G-Multi Primer | (+) | 31.2 (4.6, 1) | 34.1 (7.7, 0) | 26.2 (3.4, 0) |
| (-) | 30.4 (7.1, 0) | 8.5 (2.4, 0) | 7.3 (2.1, 0) | |
| ESTECEM II / Tokuyama Universal Bond II (A+B) |
(+) | 33.3 (6.0, 0) | 37.9 (4.1, 0) | 31.1 (4.9, 0) |
| (-) | 38.3 (8.2, 0) | 27.5 (3.6, 0) | 20.5 (4.4, 0) | |
| Variolink Esthetic DC / Monobond Plus |
(+) | 41.6 (3.7, 0) | 41.7 (5.1, 0) | 35.8 (5.5, 0) |
| (-) | 38.1 (5.8, 0) | 21.0 (3.3, 0) | 1.3 (0.6, 0) | |
| ResiCem EX / BeautiBond Extreme |
(+) | 31.2 (3.3, 0) | 31.7 (2.8, 0) | 30.5 (3.3, 0) |
| (-) | 19.4 (3.9, 0) | 3.8 (1.4, 2) | 1.1 (0.5, 0) | |
| Nexus Universal Chroma / OptiBond eXTRa Universal |
(+) | 37.1 (6.1, 0) | 29.9 (2.6, 0) | 31.8 (4.6, 0) |
| (-) | 18.0 (4.8, 0) | 2.5 (1.5, 0) | 0.9 (0.3, 6) | |
| Super-Bond Universal / M&C Primer A & B |
(+) | 33.2 (3.8, 0) | 31.2 (5.5, 0) | 30.9 (1.6, 0) |
| (-) | 31.7 (4.1, 0) | 28.2 (5.1, 0) | 22.5 (5.2, 0) |
|
RelyX Universal Resin Cement / Scotchbond Universal Plus Adhesive |
PANAVIA SA Cement Universal / None |
PANAVIA V5 / Clearfil Ceramic Primer Plus |
||||||
| Base | TC 5k | TC 20k | Base | TC 5k | TC 20k | Base | TC 5k | TC 20k |
| S | S | S | S | S | S | S | S | S |
| G-Cem ONE /G-Cem ONE Adhesive Enhancing Primer |
ESTECEM II / Tokuyama Universal Bond II (A+B) |
Variolink Esthetic DC / Monobond Plus |
||||||
| Base | TC 5k | TC 20k | Base | TC 5k | TC 20k | Base | TC 5k | TC 20k |
| NS | S | S | NS | NS | S | NS | S | S |
|
ResiCem EX / BeautiBond Extreme |
Nexus Universal Chroma/ OptiBond eXTRa Universal | Super-Bond Universal /M&C Primer A & B | ||||||
| Base | TC 5k | TC 20k | Base | TC 5k | TC 20k | Base | TC 5k | TC 20k |
| S | S | S | S | S | S | NS | NS | S |
| Resin cement | Pretreating agent | Time | |
|---|---|---|---|
| Base (1-day) | TC 20k | ||
| RelyX Universal Resin Cement | Tokuyama Universal Bond II (A+B) | 40.1 (5.5, 0) | 24.7 (2.1, 0) |
| Scotchbond universal Adhesive Plus | 30.1 (8.2, 0) | 1.7 (0.9, 0) | |
| PANAVIA SA Cement Universal / None | Tokuyama Universal Bond II (A+B) | 34.8 (4.9, 0) | 25.0 (2.8, 0) |
| None | 14.3 (2.6, 0) | 0.9 (0.3, 5) | |
| PANAVIA V5 |
Tokuyama Universal Bond II (A+B) | 37.2 (4.4, 0) | 29.0 (4.0, 0) |
| Clearfil Ceramic Primer Plus | 22.1 (6.2, 0) | 1.5 (0.6, 0) | |
| G-Cem ONE EM | Tokuyama Universal Bond II (A+B) | 40.5 (6.5, 1) | 24.6 (2.0, 0) |
| G-Multi Primer | 30.4 (7.1, 0) | 7.3 (2.1, 0) | |
| ESTECEM II / Tokuyama Universal Bond II | Tokuyama Universal Bond II (A+B) | 38.3 (8.2, 0) | 20.5 (4.4, 0) |
| Variolink Esthetic DC /Monobond Plus | Tokuyama Universal Bond II (A+B) | 43.1 (4.0, 0) | 25.1 (4.2, 0) |
| Monobond Plus | 38.1 (5.8, 0) | 1.3 (0.6, 0) | |
| ResiCem EX /BeautiBond Xtreme | Tokuyama Universal Bond II (A+B) | 42.6 (6.9, 0) | 23.7 (3.6, 0) |
| BeautiBond Extreme | 19.4 (3.9, 0) | 1.1 (0.5, 0) | |
| Nexus Universal Chroma |
Tokuyama Universal Bond II (A+B) | 34.9 (4.3, 0) | 23.4 (1.8, 0) |
| OptiBond eXTRa Universal | 18.0 (4.8, 0) | 0.9 (0.3, 6) | |
| Super-Bond Universal |
Tokuyama Universal Bond II (A+B) | 40.7 (4.3, 0) | 23.8 (2.6, 0) |
| M&C Primer A & B | 31.7 (4.1, 0) | 22.5 (5.2, 0) | |
| RelyX Universal Resin Cement | PANAVIA SA Cement Universal | PANAVIA V5 | |||
| Tokuyama Universal Bond II (A+B) | Scotchbond universal Adhesive Plus | Tokuyama Universal Bond II (A+B) | None | Tokuyama Universal Bond II (A+B) | Clearfil Ceramic Primer Plus |
| S | S | S | S | S | S |
| G-Cem ONE | ESTECEM II | Variolink Esthetic DC | |||
| Tokuyama Universal Bond II (A+B) | G-Cem ONE Adhesive Enhancing Primer | Tokuyama Universal Bond II (A+B) | Tokuyama Universal Bond II (A+B) | Monobond Plus | |
| S | S | S | S | S | |
| ResiCem EX | Nexus Universal Chroma | Super-Bond Universal | |||
| Tokuyama Universal Bond II (A+B) | BeautiBond Extreme | Tokuyama Universal Bond II (A+B) | OptiBond eXTRa Universal | Tokuyama Universal Bond II (A+B) |
M&C Primer A & B |
| S | S | S | S | S | S |
| RelyX Universal Resin Cement | PANAVIA SA Cement Universal | PANAVIA V5 | G-Cem ONE EM | ||||
| Base | TC 20k | Base | TC 20k | Base | TC 20k | Base | TC 20k |
| S | S | S | S | S | S | S | S |
| Variolink Esthetic DC | ResiCem EX | Nexus Universal Chroma | Super-Bond Universal | ||||
| Base | TC 20k | Base | TC 20k | Base | TC 20k | Base | TC 20k |
| NS | S | S | S | S | S | S | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





