Submitted:
06 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. OCT System
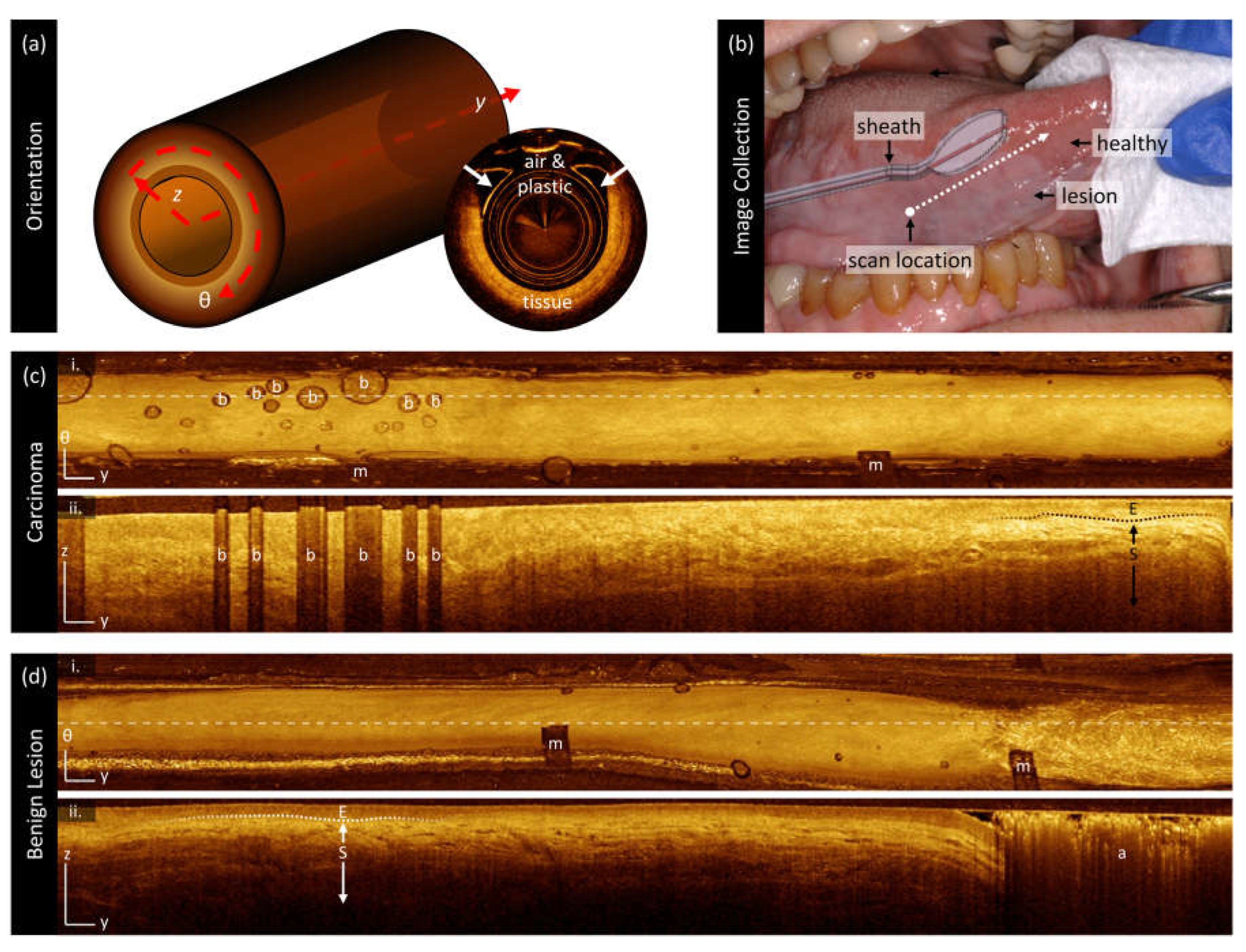
2.3. Image Collection
2.4. Deep Learning Segmentation
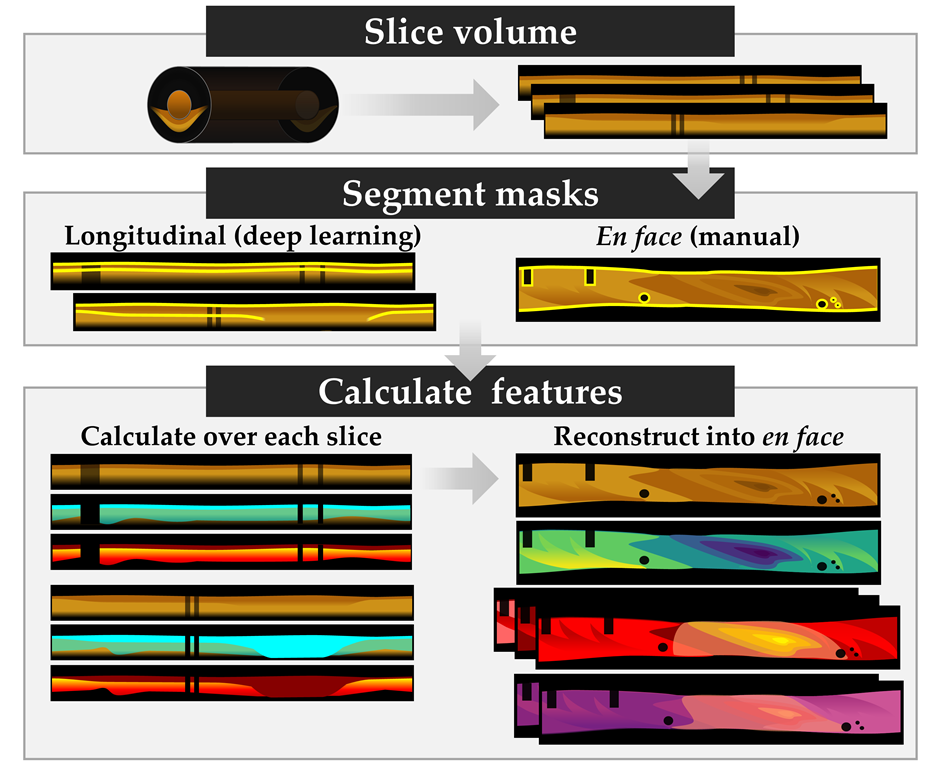
2.5. Image Processing
2.6. Biomarker Measurement
2.7. Quantitative and Statistical Analysis
3. Results
3.1. Datasets and Demographics
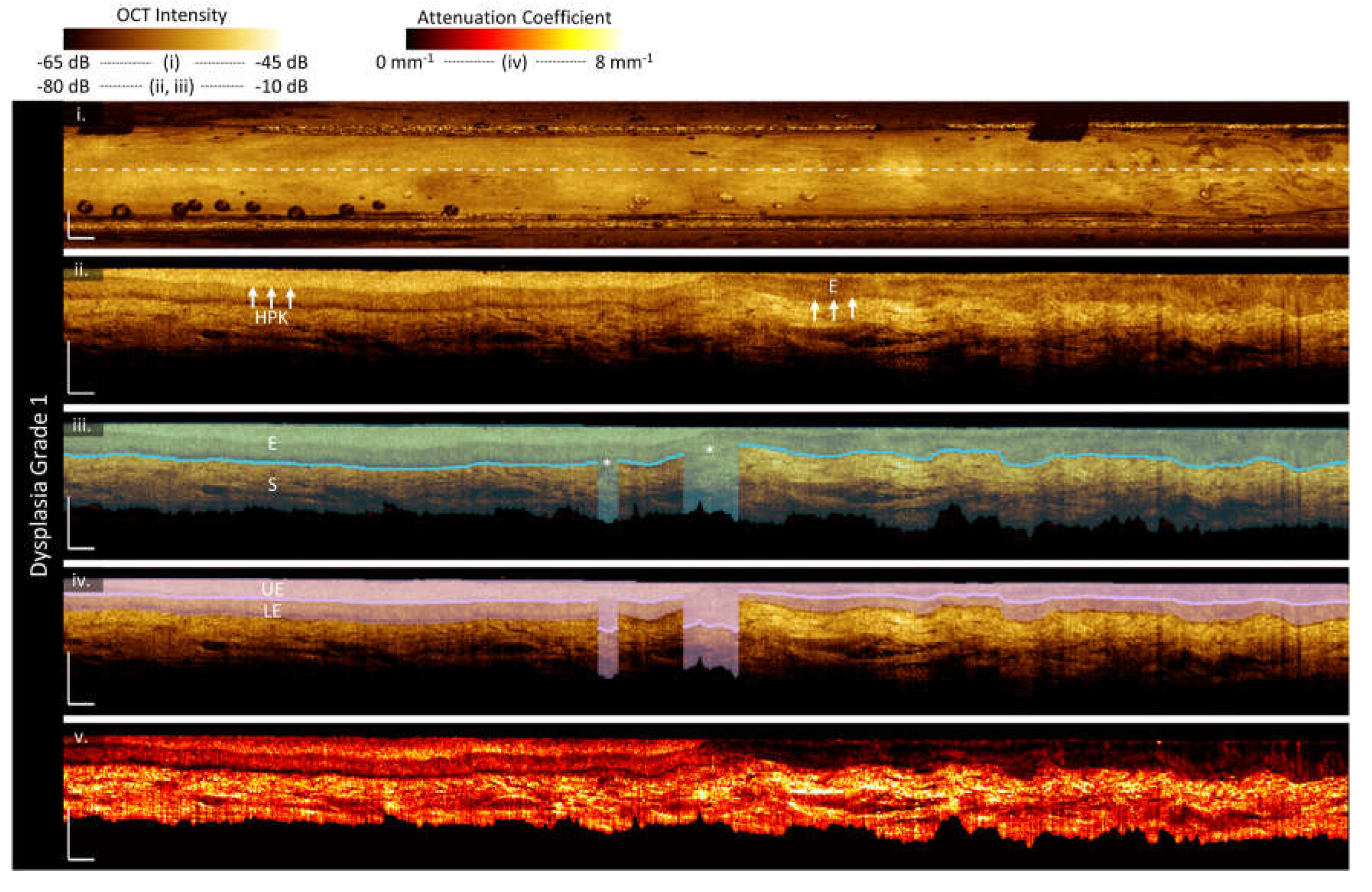
3.2. Sample Imaging
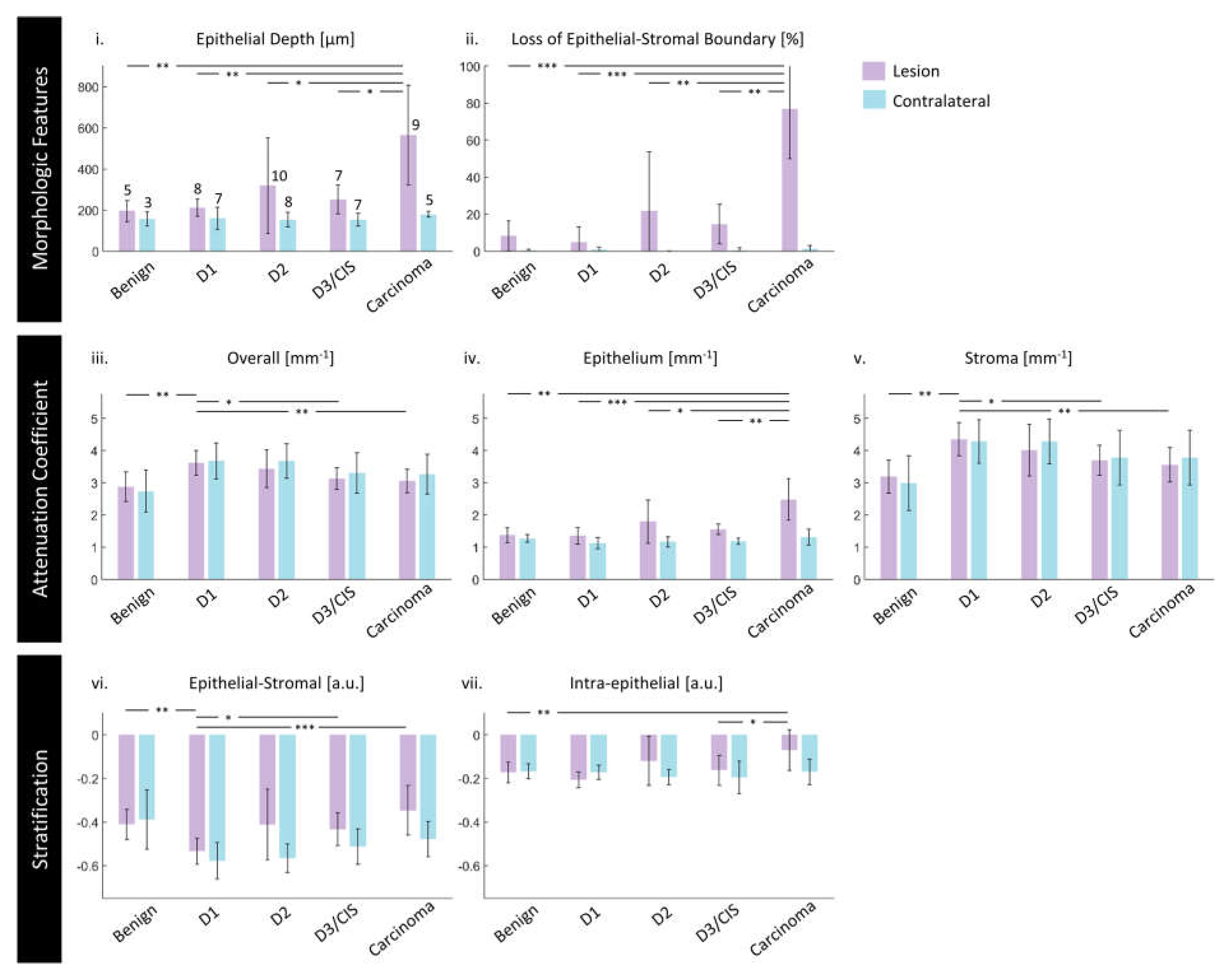
3.3. Quantitative Assessment of Disease Status and Contralaterals
3.4. Demographic and Other Pathologic Associations
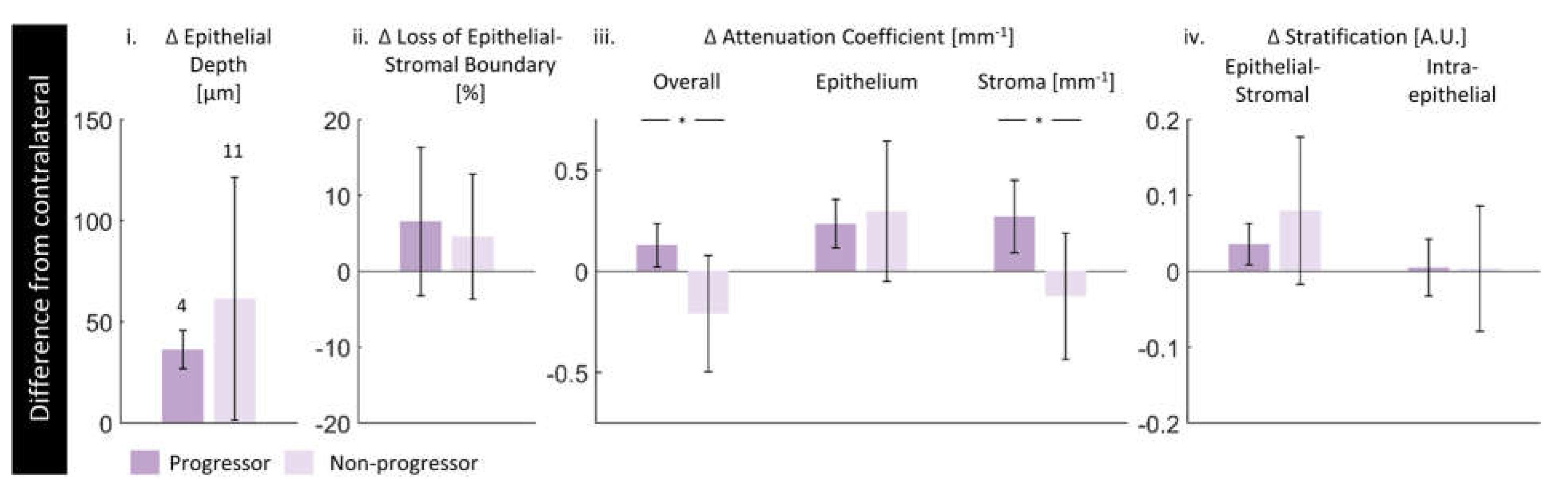
3.5. Future Progression
3.6. Reproducibility & Repeatability
4. Discussion
4.1. Dataset Limitations
4.2. Morphologic Measurements
4.3. Attenuation Coefficient Measurements
4.4. Stratification Measurements
4.4. Future Progression
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021, 0, 1–41. [Google Scholar] [CrossRef]
- Neville, B.W.; Day, T.A. Oral Cancer and Precancerous Lesions. CA: A Cancer Journal for Clinicians 2002, 52, 195–215. [Google Scholar] [CrossRef]
- Almangush, A.; Mäkitie, A.A.; Triantafyllou, A.; De Bree, R.; Strojan, P.; Rinaldo, A.; Hernandez-Prera, J.C.; Suárez, C.; Kowalski, L.P.; Ferlito, A.; et al. Staging and Grading of Oral Squamous Cell Carcinoma: An Update. Oral Oncology 2020, 107, 104799. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, K.; Kavitha, L. Oral Epithelial Dysplasia: Classifications and Clinical Relevance in Risk Assessment of Oral Potentially Malignant Disorders. Journal of Oral and Maxillofacial Pathology: JOMFP 2019, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.F. Biopsy and Histopathologic Diagnosis of Oral Premalignant and Malignant Lesions. 2008, 74.
- Yang, G.; Wei, L.; Thong, B.K.S.; Fu, Y.; Cheong, I.H.; Kozlakidis, Z.; Li, X.; Wang, H.; Li, X. A Systematic Review of Oral Biopsies, Sample Types, and Detection Techniques Applied in Relation to Oral Cancer Detection. BioTech 2022, 11, 5. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: A Consensus Report from an International Seminar on Nomenclature and Classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral Diseases 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, I.; Glaun, M.D.E.; Prabhash, K.; Busheri, A.; Lai, S.Y.; Noronha, V.; Hosni, A. Current Treatment Strategies and Risk Stratification for Oral Carcinoma. American Society of Clinical Oncology Educational Book 2023, e389810. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; Drexler, W. Introduction to OCT; 2015; ISBN 978-3-319-06419-2.
- Drexler, W.; Chen, Y.; Aguirre, A.D.; Považay, B.; Unterhuber, A.; Fujimoto, J.G. Ultrahigh Resolution Optical Coherence Tomography. In Optical Coherence Tomography; Drexler, W., Fujimoto, J.G., Eds.; Springer International Publishing: Cham, 2015; pp. 277–318. ISBN 978-3-319-06418-5. [Google Scholar]
- Gora, M.J.; Suter, M.J.; Tearney, G.J.; Li, X. Endoscopic Optical Coherence Tomography: Technologies and Clinical Applications. Biomedical Optics Express 2017, 8, 2405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Fujimoto, J.G.; Tsai, T.-H.; Mashimo, H. Endoscopic OCT. In Optical Coherence Tomography: Technology and Applications, Second Edition; 2015; pp. 2077–2108 ISBN 978-3-319-06419-2.
- van Manen, L.; Dijkstra, J.; Boccara, C.; Benoit, E.; Vahrmeijer, A.L.; Gora, M.J.; Mieog, J.S.D. The Clinical Usefulness of Optical Coherence Tomography during Cancer Interventions. Journal of Cancer Research and Clinical Oncology 2018, 144, 1967–1990. [Google Scholar] [CrossRef]
- Pahlevaninezhad, H.; Lee, A.M.D.; Rosin, M.; Sun, I.; Zhang, L.; Hakimi, M.; MacAulay, C.; Lane, P.M. Optical Coherence Tomography and Autofluorescence Imaging of Human Tonsil. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Vermeer, K.A.; Mo, J.; Weda, J.J.A.; Lemij, H.G.; Boer, J.F. de Depth-Resolved Model-Based Reconstruction of Attenuation Coefficients in Optical Coherence Tomography. Biomedical Optics Express 2013, 5, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Efficacy of Optical Coherence Tomography in the Diagnosing of Oral Cancerous Lesion: Systematic Review and Meta-analysis. Head & Neck 2023, 45, 473–481. [Google Scholar] [CrossRef]
- Yang, C.C.; Tsai, M.-T.; Lee, H.-C.; Lee, C.-K.; Yu, C.-H.; Chen, H.-M.; Chiang, C.-P.; Chang, C.-C.; Wang, Y.-M.; Yang, C.C. Effective Indicators for Diagnosis of Oral Cancer Using Optical Coherence Tomography. Opt. Express 2008, 16, 15847. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-K.; Chi, T.-T.; Wu, C.-T.; Tsai, M.-T.; Chiang, C.-P.; Yang, C.-C. (C. C.) Diagnosis of Oral Precancer with Optical Coherence Tomography. Biomed. Opt. Express 2012, 3, 1632. [Google Scholar] [CrossRef] [PubMed]
- Hamdoon, Z.; Jerjes, W.; Upile, T.; McKenzie, G.; Jay, A.; Hopper, C. Optical Coherence Tomography in the Assessment of Suspicious Oral Lesions: An Immediate Ex Vivo Study. Photodiagnosis and Photodynamic Therapy 2013, 10, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Jerjes, W.; Hamdoon, Z.; Yousif, A.A.; Al-Rawi, N.H.; Hopper, C. Epithelial Tissue Thickness Improves Optical Coherence Tomography’s Ability in Detecting Oral Cancer. Photodiagnosis and Photodynamic Therapy 2019, 28, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Obade, A.Y.; Pandarathodiyil, A.K.; Oo, A.L.; Warnakulasuriya, S.; Ramanathan, A. Application of Optical Coherence Tomography to Study the Structural Features of Oral Mucosa in Biopsy Tissues of Oral Dysplasia and Carcinomas. Clin Oral Invest 2021, 25, 5411–5419. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, J.; Liu, C.; Zhang, J.; Liang, Y. Identification of Oral Precancerous and Cancerous Tissue by Swept Source Optical Coherence Tomography. Lasers Surg Med 2022, 54, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Cheng, L.; Yang, J.; Yin, B.; Fan, X.; Yang, J.; Li, S.; Zhong, J.; Huang, X. Noninvasive Oral Cancer Screening Based on Local Residual Adaptation Network Using Optical Coherence Tomography. Med Biol Eng Comput 2022, 60, 1363–1375. [Google Scholar] [CrossRef]
- James, B.L.; Sunny, S.P.; Heidari, A.E.; Ramanjinappa, R.D.; Lam, T.; Tran, A.V.; Kankanala, S.; Sil, S.; Tiwari, V.; Patrick, S.; et al. Validation of a Point-of-Care Optical Coherence Tomography Device with Machine Learning Algorithm for Detection of Oral Potentially Malignant and Malignant Lesions. Cancers 2021, 13, 3583. [Google Scholar] [CrossRef]
- Heidari, A.E.; Suresh, A.; Kuriakose, M.A.; Chen, Z.; Wilder-Smith, P.; Sunny, S.P.; James, B.L.; Lam, T.M.; Tran, A.V.; Yu, J.; et al. Optical Coherence Tomography as an Oral Cancer Screening Adjunct in a Low Resource Settings. IEEE J. Select. Topics Quantum Electron. 2019, 25, 1–8. [Google Scholar] [CrossRef]
- Wilder-Smith, P.; Jung, W.; Brenner, M.; Osann, K.; Beydoun, H.; Messadi, D.; Chen, Z. In Vivo Optical Coherence Tomography for the Diagnosis of Oral Malignancy. Lasers Surg Med 2004, 35, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Sunny, S.P.; Agarwal, S.; James, B.L.; Heidari, E.; Muralidharan, A.; Yadav, V.; Pillai, V.; Shetty, V.; Chen, Z.; Hedne, N.; et al. Intra-Operative Point-of-Procedure Delineation of Oral Cancer Margins Using Optical Coherence Tomography. Oral Oncology 2019, 92, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Panzarella, V.; Buttacavoli, F.; Gambino, A.; Capocasale, G.; Di Fede, O.; Mauceri, R.; Rodolico, V.; Campisi, G. Site-Coded Oral Squamous Cell Carcinoma Evaluation by Optical Coherence Tomography (OCT): A Descriptive Pilot Study. Cancers 2022, 14, 5916. [Google Scholar] [CrossRef] [PubMed]
- Stasio; Lauritano; Iquebal; Romano; Gentile; Lucchese Measurement of Oral Epithelial Thickness by Optical Coherence Tomography. Diagnostics 2019, 9, 90. [CrossRef]
- Lee, A.M.D.; Cahill, L.; Liu, K.; MacAulay, C.; Poh, C.; Lane, P. Wide-Field in Vivo Oral OCT Imaging. Biomedical Optics Express 2015, 6, 2664. [Google Scholar] [CrossRef] [PubMed]
- Gambino, A.; Martina, E.; Panzarella, V.; Ruggiero, T.; Haddad, G.E.; Broccoletti, R.; Arduino, P.G. Potential Use of Optical Coherence Tomography in Oral Potentially Malignant Disorders: In-Vivo Case Series Study. BMC Oral Health 2023, 23, 540. [Google Scholar] [CrossRef]
- Trebing, C.T.; Sen, S.; Rues, S.; Herpel, C.; Schöllhorn, M.; Lux, C.J.; Rammelsberg, P.; Schwindling, F.S. Non-Invasive Three-Dimensional Thickness Analysis of Oral Epithelium Based on Optical Coherence Tomography—Development and Diagnostic Performance. Heliyon 2021, 7, e06645. [Google Scholar] [CrossRef]
- Yang, Z.; Shang, J.; Liu, C.; Zhang, J.; Liang, Y. Identification of Oral Cancer in OCT Images Based on an Optical Attenuation Model. Lasers Med Sci 2020, 35, 1999–2007. [Google Scholar] [CrossRef]
- Hill, C.; Malone, J.; Liu, K.; Ng, S.P.-Y.; MacAulay, C.; Poh, C.; Lane, P. 3-Dimension Epithelial Segmentation in Optical Coherence Tomography of the Oral Cavity Using Deep Learning 2024.
- Chen, S.-W.; Zhang, Q.; Guo, Z.-M.; Chen, W.-K.; Liu, W.-W.; Chen, Y.-F.; Li, Q.-L.; Liu, X.-K.; Li, H.; Ou-Yang, D.; et al. Trends in Clinical Features and Survival of Oral Cavity Cancer: Fifty Years of Experience with 3,362 Consecutive Cases from a Single Institution. CMAR 2018, Volume 10, 4523–4535. [Google Scholar] [CrossRef]
- Lin, N.-C.; Hsien, S.-I.; Hsu, J.-T.; Chen, M.Y.C. Impact on Patients with Oral Squamous Cell Carcinoma in Different Anatomical Subsites: A Single-Center Study in Taiwan. Sci Rep 2021, 11, 15446. [Google Scholar] [CrossRef] [PubMed]
- Pahlevaninezhad, H.; Lee, A.; Cahill, L.; Lam, S.; MacAulay, C.; Lane, P. Fiber-Based Polarization Diversity Detection for Polarization-Sensitive Optical Coherence Tomography. Optics Letters 2014, 1, 283–295. [Google Scholar] [CrossRef]
- Liu, J.; Ding, N.; Yu, Y.; Yuan, X.; Luo, S.; Luan, J.; Zhao, Y.; Wang, Y.; Ma, Z. Optimized Depth-Resolved Estimation to Measure Optical Attenuation Coefficients from Optical Coherence Tomography and Its Application in Cerebral Damage Determination. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Zimmerman, D.W. A Note on Preliminary Tests of Equality of Variances. Brit J Math & Statis 2004, 57, 173–181. [Google Scholar] [CrossRef]
- Welch, B.L. THE GENERALIZATION OF ‘STUDENT’S’ PROBLEM WHEN SEVERAL DIFFERENT POPULATION VARLANCES ARE INVOLVED. Biometrika 1947, 34, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biometrics Bulletin 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Student The Probable Error of a Mean. Biometrika 1908, 6, 1. [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Statist. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for Equality of Variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Stanford University Press: Palo Alto, Calif, 1960; pp. 278–292. [Google Scholar]
- Grubbs, F.E. Procedures for Detecting Outlying Observations in Samples. Technometrics 1969, 11, 1–21. [Google Scholar] [CrossRef]
- Spearman, C. The Proof and Measurement of Association between Two Things. The American Journal of Psychology 1904, 15, 72. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, T.; Biswal, N.C.; Wang, X.; Sanders, M.; Brewer, M.; Zhu, Q. Optical Scattering Coefficient Estimated by Optical Coherence Tomography Correlates with Collagen Content in Ovarian Tissue. Journal of Biomedical Optics 2011, 16, 090504. [Google Scholar] [CrossRef] [PubMed]
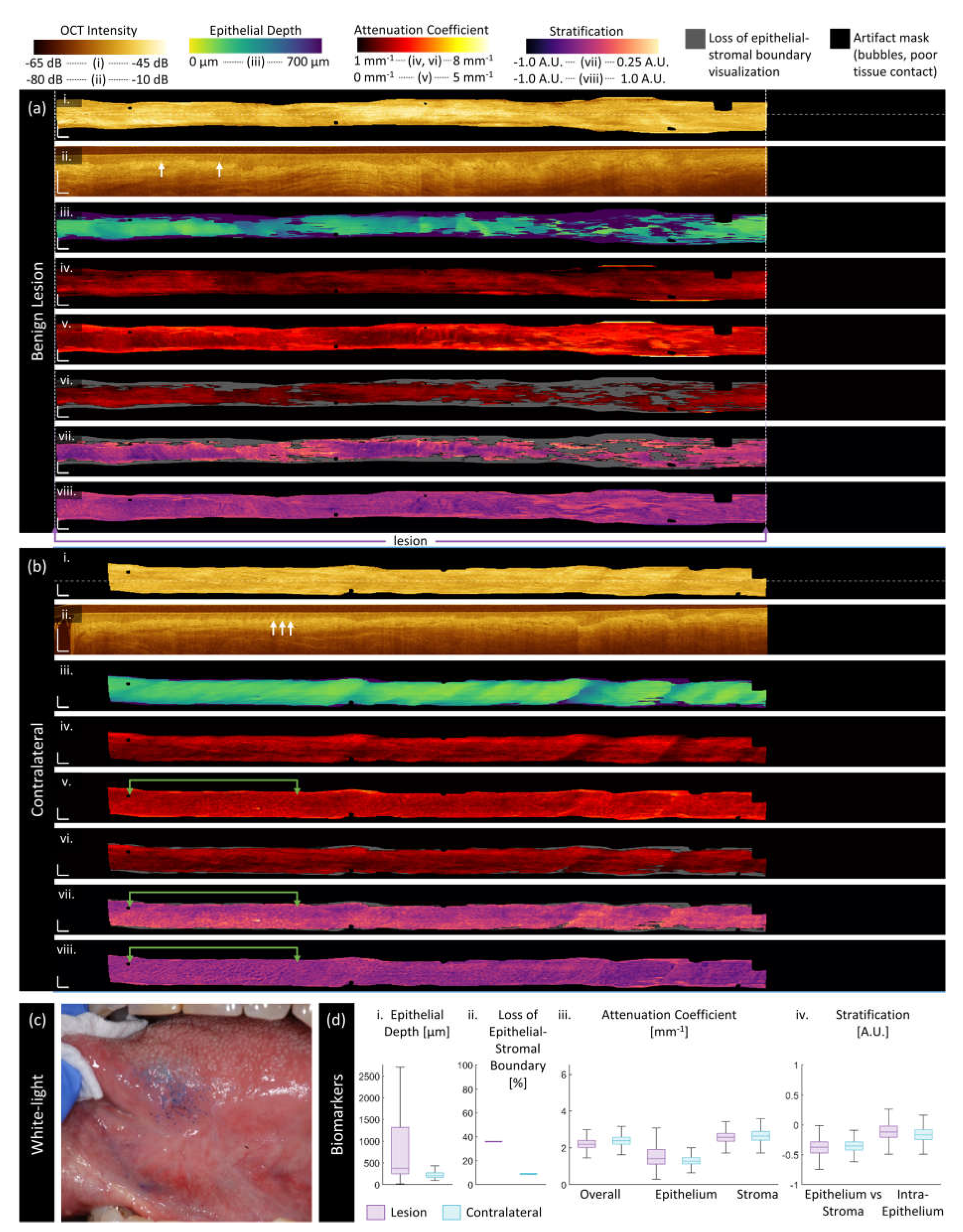
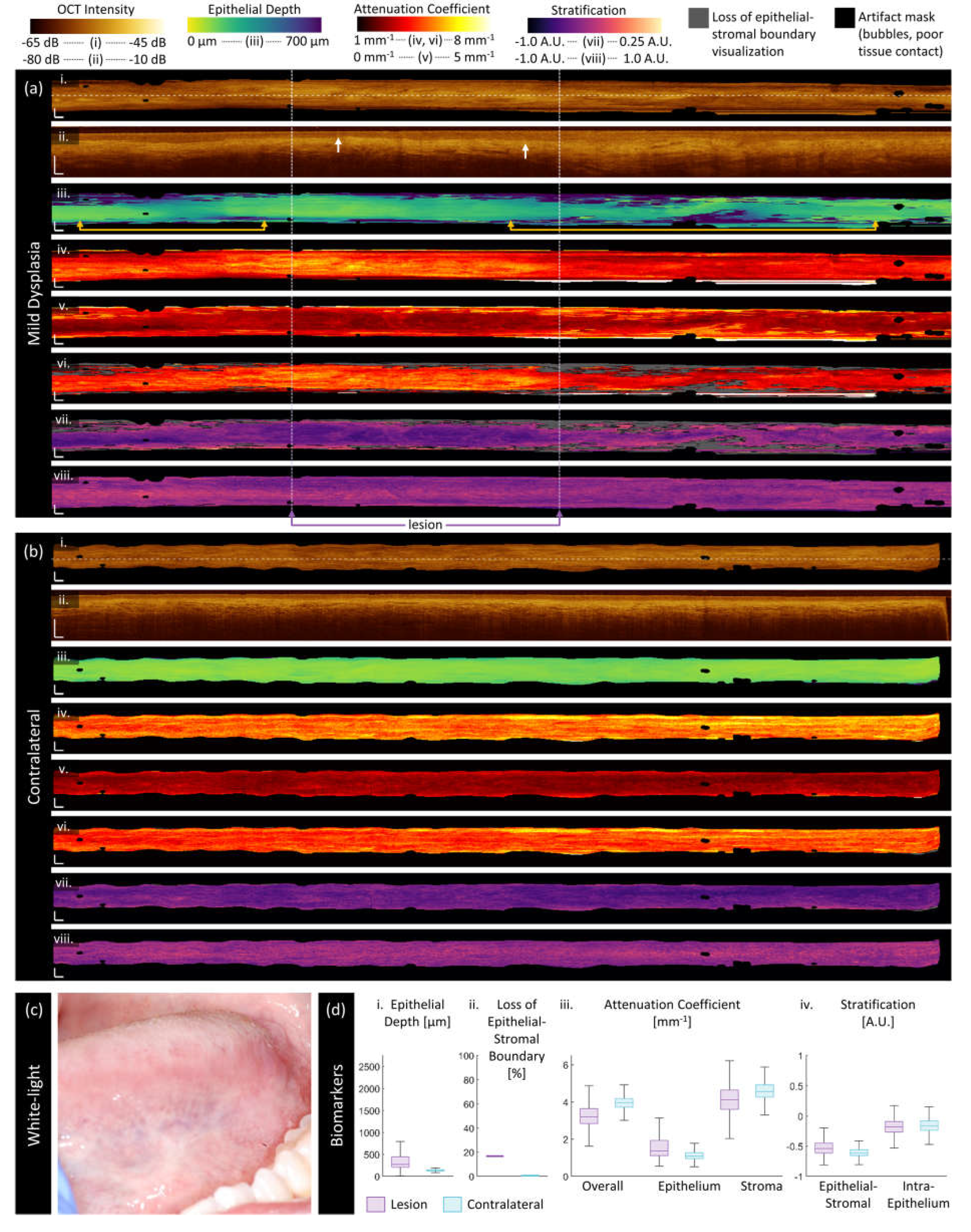
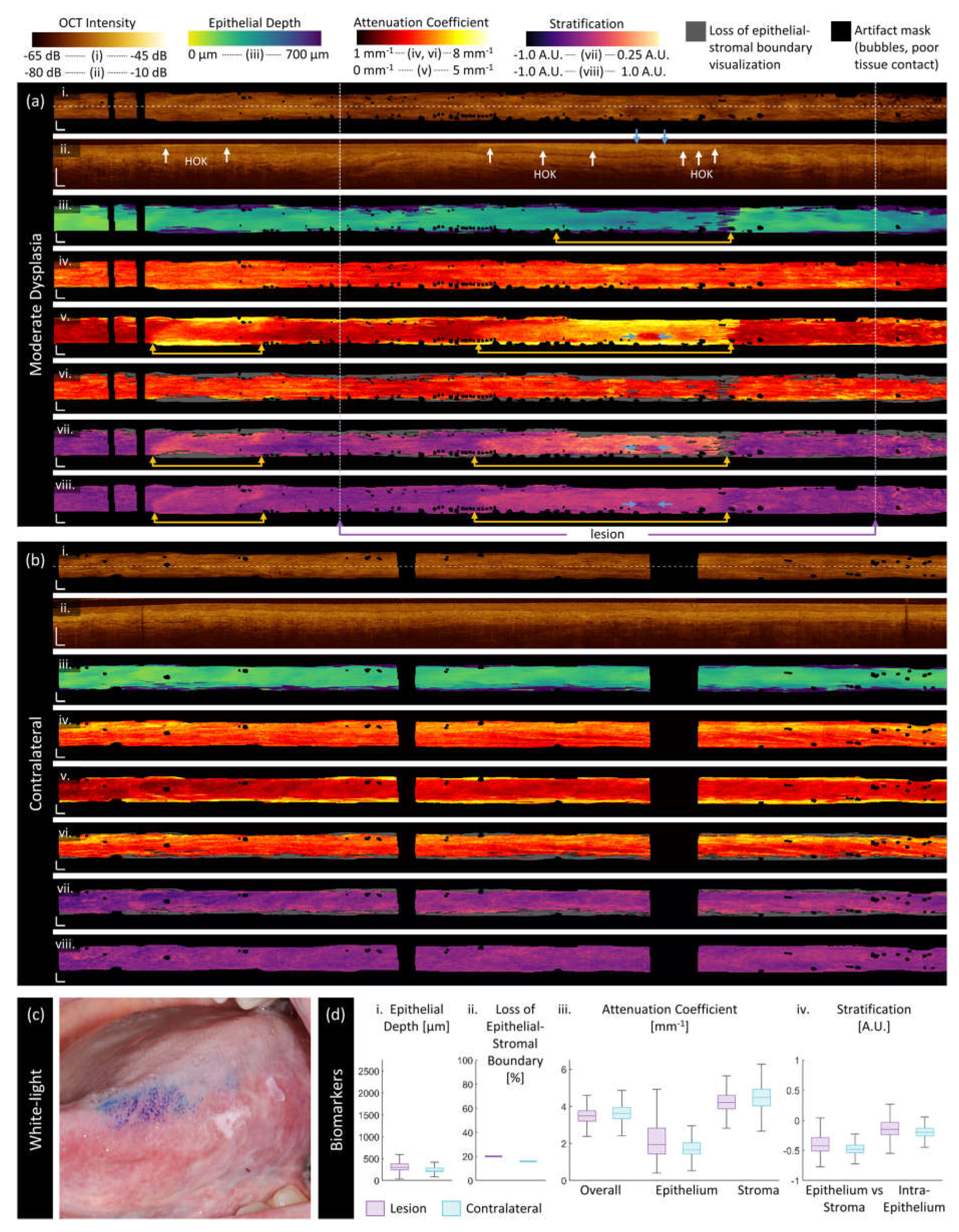
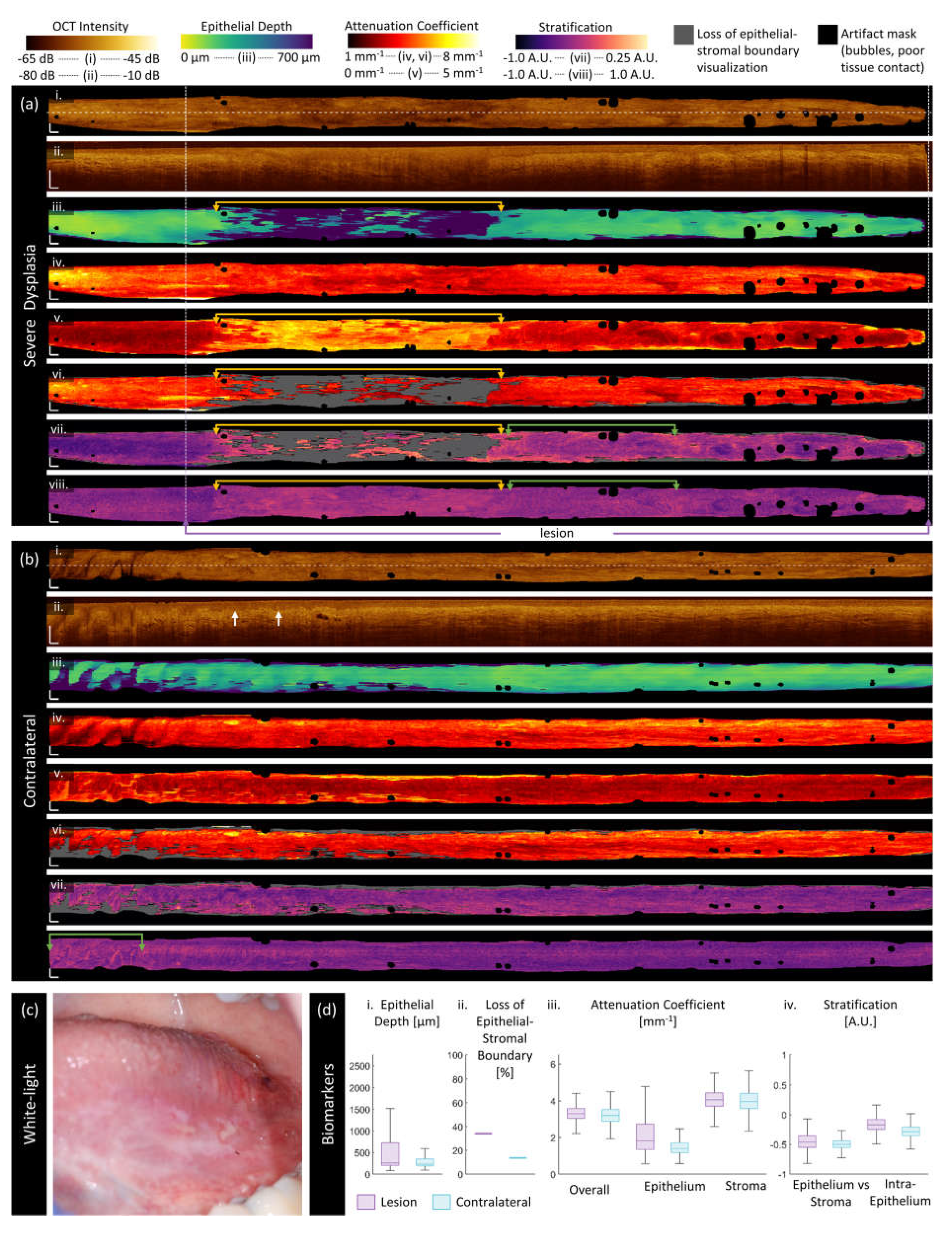
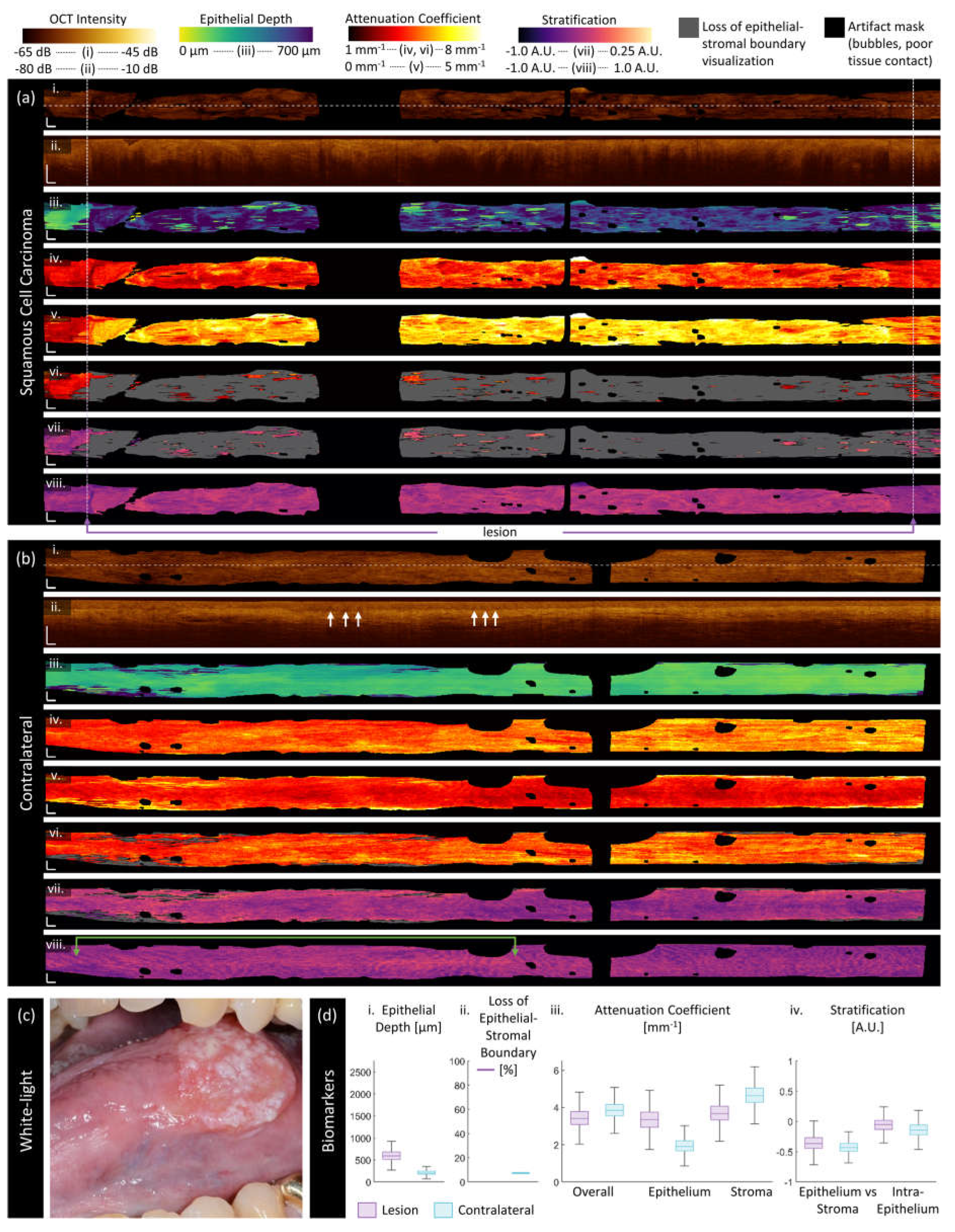
| Category | Biomarker | Description | Dimensionality |
|---|---|---|---|
| ‘Morphologic’ | Epithelium depth [μm] |
Height of segmented epithelium region | 2D en face image Range: 0 to ~ 2 mm |
| Loss of epithelial-stromal boundary [%] |
Percentage of loss over volume, excluding artifacts | single value per volume Range: 0 to 100% |
|
| ‘Attenuation’ | Overall attenuation coefficient [mm-1] |
Mean en face projection of attenuation coefficient over the entire depth of visualized tissue | 3D data Range: 0 to ~10 mm-1 |
| Epithelium attenuation coefficient [mm-1] | Mean en face projection of attenuation coefficient over the segmented epithelium region | 3D data Range: 0 to ~10 mm-1 |
|
| Stroma attenuation coefficient [mm-1] |
Mean en face projection of attenuation coefficient over the visualized stroma region | 3D data Range: 0 to ~10 mm-1 |
|
| ‘Stratification’ | Epithelial-Stromal Stratification [a.u.] | 2D en face image Range: -1 to 1 a.u. |
|
| Intraepithelial Stratification [a.u.] |
2D en face image Range: -1 to 1 a.u. |
| Diagnosis | Lesion | Contralateral | Total | Males | Females |
|---|---|---|---|---|---|
| Only contralateral imaged | 0 | 1 | 1 | 1 | 0 |
| Benign | 5 | 3 | 8 | 3 | 2 |
| Dysplasia grade 1 | 8 | 7 | 15 | 3 | 5 |
| Dysplasia grade 2 | 10 | 8 | 18 | 4 | 6 |
| Dysplasia grade 3 & carcinoma in situ | 7 | 7 | 14 | 3 | 4 |
| Carcinoma (squamous cell, verrucous) | 9 | 5 | 14 | 6 | 3 |
| Total | 39 sites | 31 sites |
40 patients (70 sites) |
20 patients (50%) | 20 patients (50%) |
| Diagnosis | Lesion | Contralateral | Total | Males | Females |
|---|---|---|---|---|---|
| Progressors | 4 | 4 | 8 | 1 | 3 |
| Non-progressors | 11 | 11 | 22 | 5 | 6 |
| Total | 15 sites | 15 sites |
15 patients (33 sites) |
6 patients (40%) | 9 patients (60%) |
| Patient number | Previous biopsy (time difference) [months] |
Timepoint 1 | Time difference [months] |
Timepoint 2 | ||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | Lesion | Contra- lateral |
Diagnosis | Lesion | Contralateral | |||
| 1 | D1 (unknown) |
D1 | 1 | 1 | 5 | D1 | 2 | 1 |
| 2 | D2 (10) |
D2 | 2 | 1 | 2 | Hyperplastic candidiasis | 1 | 1 |
| 3 | N/A |
D2 | 1 | 1 | 21 | D2 | 1 | 1 |
| 4 | D2 (16) |
D3 | 1 | 1 | 6 | D3 | 1 | 1 |
| 5 | D2 (13) |
Verrucous carcinoma |
1 | 1 | 6 | Verrucous carcinoma |
1 | 1 |
| Morphologic Features | Mean Attenuation Coefficient | Stratification | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient number | Epithelium depth | Loss of epithelial-stromal boundary visualization |
Overall | Epithelium | Stroma | Epithelial-Stromal | Intra- epithelial |
|||
| [µm] | [%] | [mm-1] | [mm-1] | [mm-1] | [a.u.] | [a.u.] | ||||
| Contralateral (between timepoints) |
1 | 240 50 |
2 3 |
3.05 0.24 |
1.22 0.18 |
3.71 0.55 |
-0.51 0.00 |
-0.21 0.08 |
||
| 2 | 150 20 |
0 0 |
3.59 0.21 |
1.15 0.00 |
4.21 0.25 |
-0.57 0.01 |
-0.12 0.07 |
|||
| 3 | 160 30 |
0 0 |
3.76 1.41 |
1.12 0.11 |
4.47 2.00 |
-0.59 0.11 |
-0.15 0.00 |
|||
| 4 | 120 10 |
0 0 |
4.25 0.90 |
0.95 0.20 |
4.95 1.33 |
-0.68 0.02 |
-0.14 0.06 |
|||
| 5 | 220 80 |
0 0 |
3.60 0.09 |
1.36 0.05 |
4.52 0.41 |
-0.54 0.02 |
-0.23 0.01 |
|||
| Lesion (single timepoint) |
1 | 440 40 |
15 2 |
2.79 0.04 |
1.58 0.21 |
3.62 0.09 |
-0.41 0.05 |
-0.22 0.04 |
||
| 2 | 220 50 |
20 29 |
3.69 0.15 |
1.50 0.39 |
4.59 0.00 |
-0.53 0.08 |
-0.21 0.01 |
|||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





