Submitted:
06 July 2024
Posted:
08 July 2024
You are already at the latest version
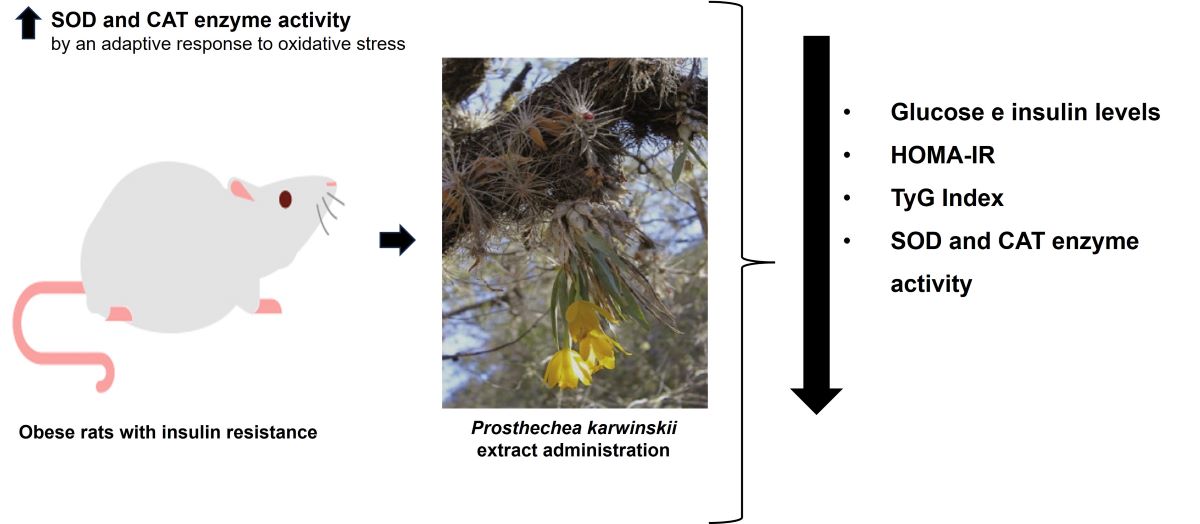
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Kits
2.2. Plant Material
2.3. Obtaining the Extract
2.4. Compound Identification with UHPLC-ESI-qTOF-MS/MS
2.5. Induction of Obesity
2.6. Experimental Design
2.7. Parameters Evaluated
2.8. Statistical Analysis
3. Results
3.1. Compounds Identified with UHPLC-ESI-qTOF-MS/MS
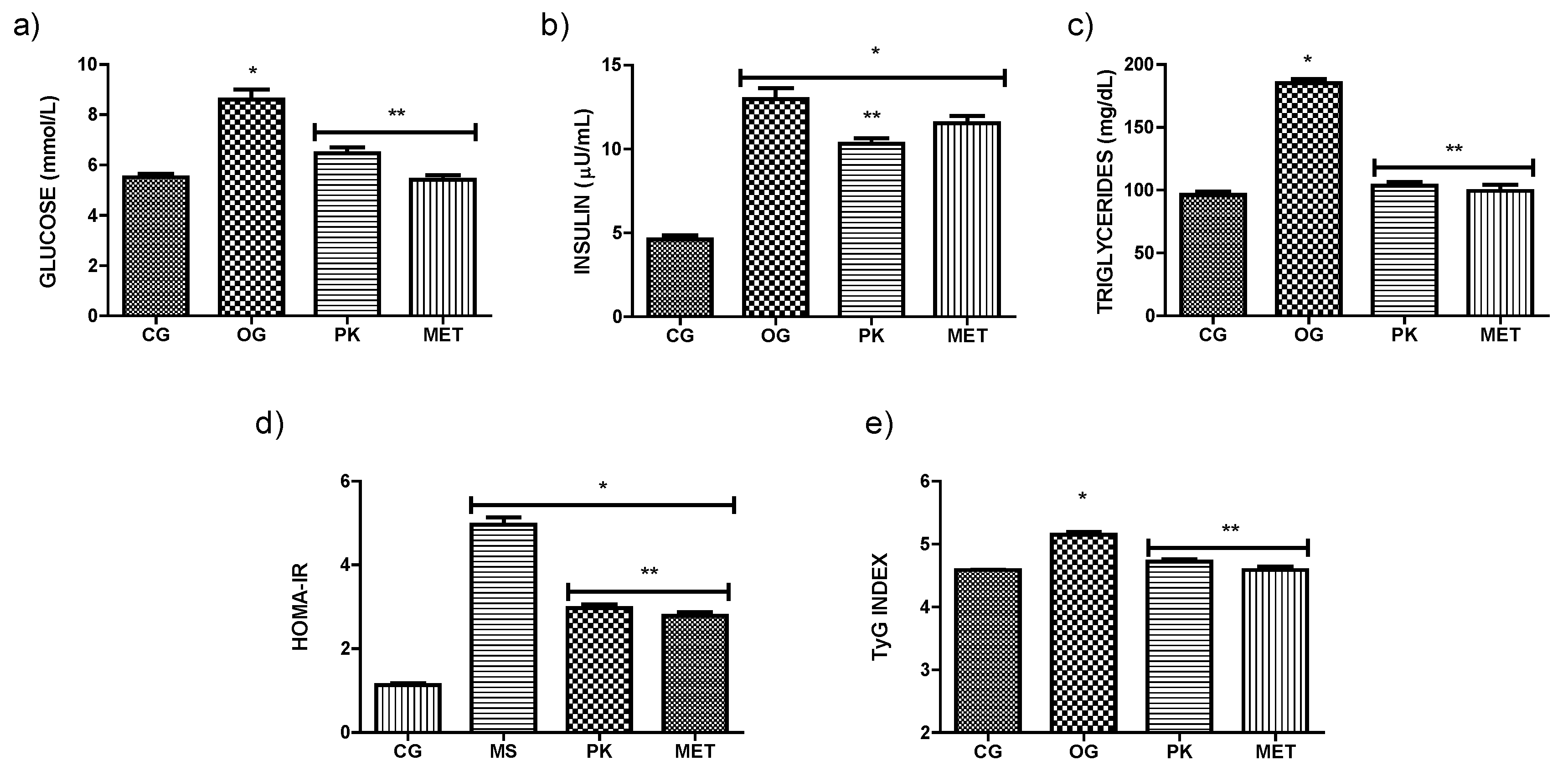
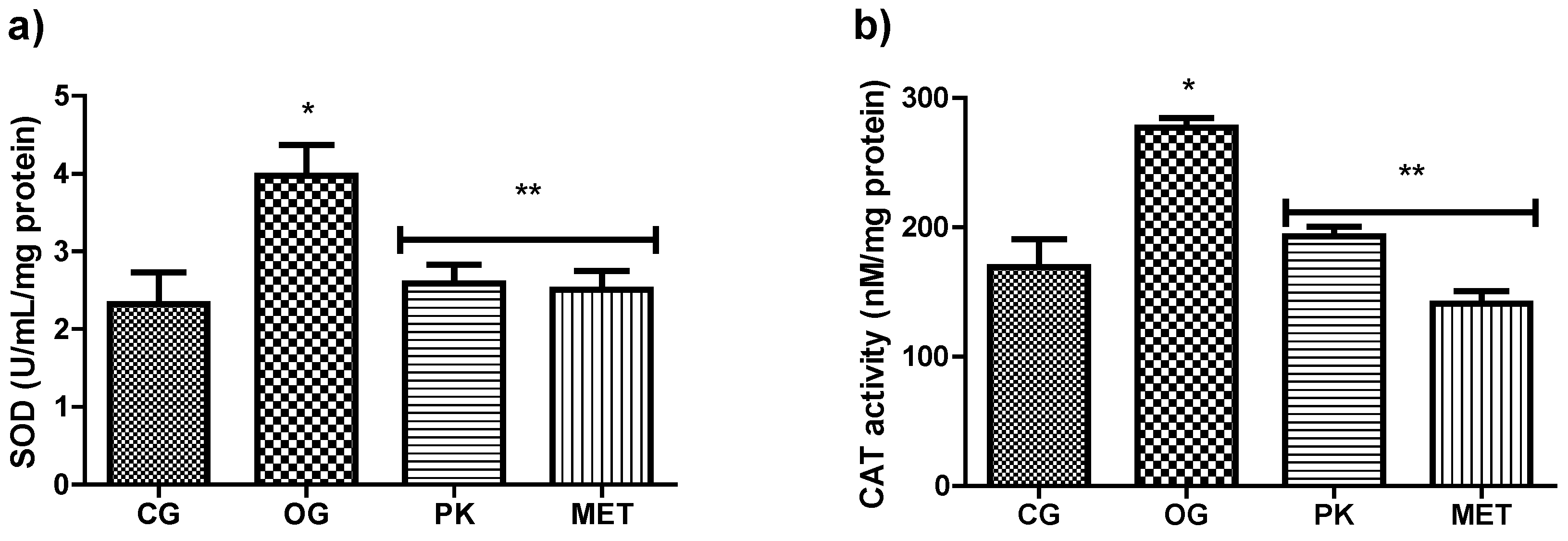
3.2. Effect of P. karwinskii Extract on Obesity, Insulin Resistance and Oxidative Stress Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The Molecular Link between Oxidative Stress, Insulin Resistance, and Type 2 Diabetes: A Target for New Therapies against Cardiovascular Diseases. Curr. Opin. Pharmacol. 2022, 62, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Hsouna, A. Ben; Dhibi, S.; Dhifi, W.; Saad, R. Ben; Brini, F.; Hfaidh, N.; Mnif, W. Essential Oil from Halophyte: Lobularia Maritima: Protective Effects against CCl4-Induced Hepatic Oxidative Damage in Rats and Inhibition of the Production of Proinflammatory Gene Expression by Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. RSC Adv. 2019, 9, 36758–36770. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Garcia, G.; Solano-Gomez, R.; Lagunez-Rivera, L. Documentation of the Medicinal Knowledge of Prosthechea Karwinskii in a Mixtec Community in Mexico. Rev. Bras. Farmacogn. 2014, 24, 153–158. [Google Scholar] [CrossRef]
- Rojas-Olivos, A.; Solano-Gómez, R.; Alexander-Aguilera, A.; Jiménez-Estrada, M.; Zilli-Hernández, S.; Lagunez-Rivera, L. Effect of Prosthechea Karwinskii (Orchidaceae) on Obesity and Dyslipidemia in Wistar Rats. Alex. J. Med. 2017. [CrossRef]
- Barragán-Zarate, G.S.; Alexander-Aguilera, A.; Lagunez-Rivera, L.; Solano, R.; Soto-Rodríguez, I. Bioactive Compounds from Prosthechea Karwinskii Decrease Obesity, Insulin Resistance, pro-Inflammatory Status, and Cardiovascular Risk in Wistar Rats with Metabolic Syndrome. J. Ethnopharmacol. 2021, 279. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Zarate, G.S.; Lagunez-Rivera, L.; Solano, R.; Pineda-Peña, E.A.; Landa-Juárez, A.Y.; Chávez-Piña, A.E.; Carranza-Álvarez, C.; Hernández-Benavides, D.M. Prosthechea Karwinskii, an Orchid Used as Traditional Medicine, Exerts Anti-Inflammatory Activity and Inhibits ROS. J. Ethnopharmacol. 2020, 253. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, A. M., Cribb, P. J., Chase, M. W., Rasmussen, F. N. Genera Orchidacearum Volume 4: Epidendroideae (Part One). Oxford University Press, New York 2005.
- Soto, M. A., Hágsater, E., Jiménez, R., Salazar, G. A., Solano, R., Flores, R., Ruiz, I. Orchids of Mexico. Digital Catalogue. Herbario AMO, México City 2007.
- Villaseñor, J.L. Checklist of the native vascular plants of Mexico. Rev. Mex. De Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Solano, R., Salazar-Chávez, G., Jiménez-Machorro, R., Hagsater, E., Cruz-García, G. Actualización del catálogo de autoridades taxonómicas de Orchidaceae de México. Instituto Politécnico Nacional. Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional Unidad Oaxaca. Data base SNIB-CONABIO, Project KT005 (Mexico City) 2020.
- SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación), NORMA Oficial Mexicana NOM-062-ZOO-1999. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio 2001. Diario Oficial de la Federación. (accessed 13 March 2023).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Guerrero-Romero, F. The Correct Formula for the Triglycerides and Glucose Index. Eur. J. Pediatr. 2020, 179, 1171. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.P.; Bataglion, G.A.; da Silva, F.M.A.; de Almeida, R.A.; Paz, W.H.P.; Nobre, T.A.; Marinho, J.V.N.; Salvador, M.J.; Fidelis, C.H.V.; Acho, L.D.R.; et al. Phenolic and Aroma Compositions of Pitomba Fruit (Talisia Esculenta Radlk.) Assessed by LC-MS/MS and HS-SPME/GC-MS. Food Res. Int. 2016, 83, 87–94. [Google Scholar] [CrossRef]
- Ma, T.; Lin, J.; Gan, A.; Sun, Y.; Sun, Y.; Wang, M.; Wan, M.; Yan, T.; Jia, Y. Qualitative and Quantitative Analysis of the Components in Flowers of Hemerocallis Citrina Baroni by UHPLC–Q-TOF-MS/MS and UHPLC–QQQ-MS/MS and Evaluation of Their Antioxidant Activities. J. Food Compos. Anal. 2023, 120, 105329. [Google Scholar] [CrossRef]
- Dahibhate, N.L.; Dwivedi, P.; Kumar, K. GC–MS and UHPLC-HRMS Based Metabolite Profiling of Bruguiera Gymnorhiza Reveals Key Bioactive Compounds. South Afr. J. Bot. 2022, 149, 1044–1048. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic Acid Improves High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Durg, S.; Veerapur, V.P.; Neelima, S.; Dhadde, S.B. Antidiabetic Activity of Embelia Ribes, Embelin and Its Derivatives: A Systematic Review and Meta-Analysis. Biomed. Pharmacother. 2017, 86, 195–204. [Google Scholar] [CrossRef]
- Ghorbani, A. Biomedicine & Pharmacotherapy Mechanisms of Antidiabetic efects of Flavonoid Rutin. Biomed. Pharmacother. J. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Free. Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.O.; Tanus-santos, J.E.; Cavalli, R.D.C.; Bengtsson, T.; Barbosa, P.O.; Tanus-santos, J.E.; Cavalli, R.D.C.; Bengtsson, T. The Nitrate-Nitrite-Nitric Oxide Pathway: Potential Role in Mitigating Oxidative Stress in Hypertensive Disorders of Pregnancy The Nitrate-Nitrite-Nitric Oxide Pathway: Potential Role in Mitigating Oxidative Stress in Hypertensive Disorders of Pregnancy. 2024. [CrossRef]
- Zeng, L.; Wang, Y.H.; Ai, C.X.; Zhang, B.; Zhang, H.; Liu, Z.M.; Yu, M.H.; Hu, B. Differential Effects of Oxytetracycline on Detoxification and Antioxidant Defense in the Hepatopancreas and Intestine of Chinese Mitten Crab under Cadmium Stress. Sci. Total Environ. 2024, 930, 172633. [Google Scholar] [CrossRef] [PubMed]
- Aouacheri, O.; Saka, S.; Krim, M.; Messaadia, A.; Maidi, I. The Investigation of the Oxidative Stress-Related Parameters in Type2 Diabetes Mellitus. Can. J. Diabetes 2015, 39, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Leghi, G.E.; Domenici, F.A.; Vannucchi, H. Influence of Oxidative Stress and Obesity in Patients with Nonalcoholic Steatohepatitis. Arq. De Gastroenterol. 2015, 52, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.O.; Souza, M.O.; Silva, M.P.S.; Santos, G.T.; Silva, M.E.; Bermano, G.; Freitas, R.N. Açaí (Euterpe Oleracea Martius) Supplementation Improves Oxidative Stress Biomarkers in Liver Tissue of Dams Fed a High-Fat Diet and Increases Antioxidant Enzymes’ Gene Expression in Offspring. Biomed. Pharmacother. 2021, 139, 111627. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, A.; Pourakbar, L.; Siavash Moghaddam, S. Effects of Malic Acid and EDTA on Oxidative Stress and Antioxidant Enzymes of Okra (Abelmoschus Esculentus L.) Exposed to Cadmium Stress. Ecotoxicol. Environ. Saf. 2022, 248, 114320. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Siddique, A.; Ashraf, M.A.; Rasheed, R.; Ibrahim, M.; Iqbal, M.; Akbar, S.; Imran, M. Does Exogenous Application of Ascorbic Acid Modulate Growth, Photosynthetic Pigments and Oxidative Defense in Okra (Abelmoschus Esculentus (L.) Moench) under Lead Stress? Acta Physiol. Plant. 2017, 39, 1–13. [Google Scholar] [CrossRef]
- Londero, É.P.; Bressan, C.A.; Pês, T.S.; Saccol, E.M.H.; Baldisserotto, B.; Finamor, I.A.; Pavanato, M.A. Rutin-Added Diet Protects Silver Catfish Liver against Oxytetracycline-Induced Oxidative Stress and Apoptosis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 239, 108848. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, C.; Zhang, H. Hepatoprotective Effects of Kaempferol 3-O-Rutinoside and Kaempferol 3-O-Glucoside from Carthamus Tinctorius L. on CCl4-Induced Oxidative Liver Injury in Mice. J. Food Drug Anal. 2015, 23, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, A.K.; Sharma, M.C.; Gupta, R.S. Antioxidant Activity and Protection of Pancreatic b -Cells by Embelin in Streptozotocin-Induced Diabetes. J. Diabetes 2012, 4, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Nonose, Y.; Pieper, L.Z.; da Silva, J.S.; Longoni, A.; Apel, R. V.; Meira-Martins, L.A.; Grings, M.; Leipnitz, G.; Souza, D.O.; de Assis, A.M. Guanosine Enhances Glutamate Uptake and Oxidation, Preventing Oxidative Stress in Mouse Hippocampal Slices Submitted to High Glutamate Levels. Brain Res. 2020, 1748, 147080. [Google Scholar] [CrossRef]
| Peak | RT (min) | m/z [M-H]- |
Error (ppm) | MS/MS fragments | Compound (Chemical formula) |
Type of compound | Relative yield (%) | Chemical structure |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.7 | 191.0557 | 1.9 | 85.0293, 87.0078, 111.0443, 127.6945 | Quinic Acidbd (C7H12O6) |
Cyclitol, cyclic polyol, and cyclohexanecarboxylic acid | 22.21 | 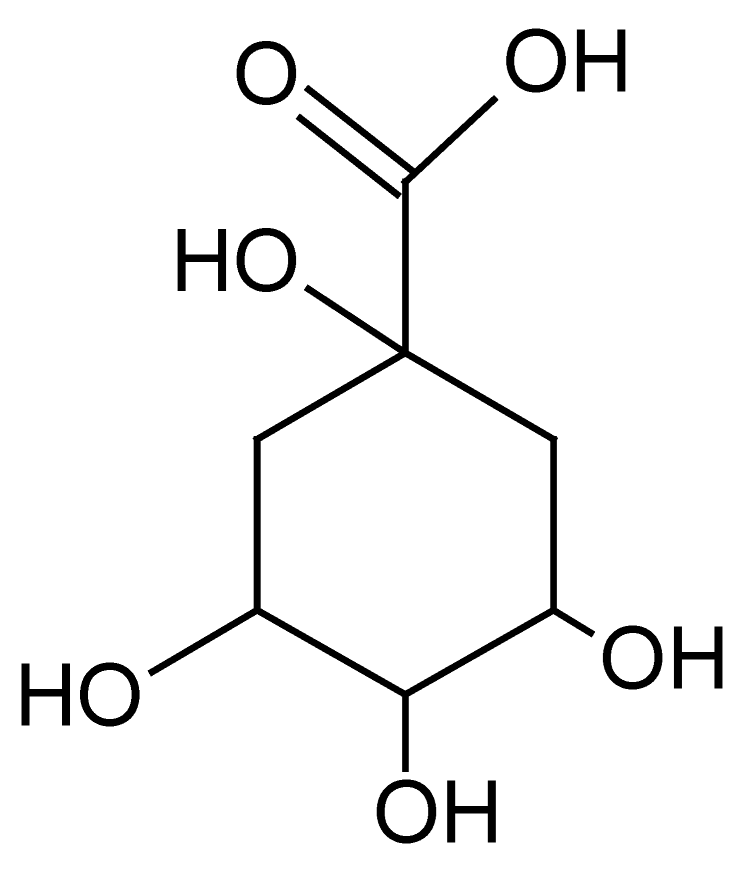 |
| 2 | 0.8 | 133.0140 | 1.8 | 115.0032 | Malic acidd (C4H6O5) |
Dicarboxylic organic acid | 5.71 | 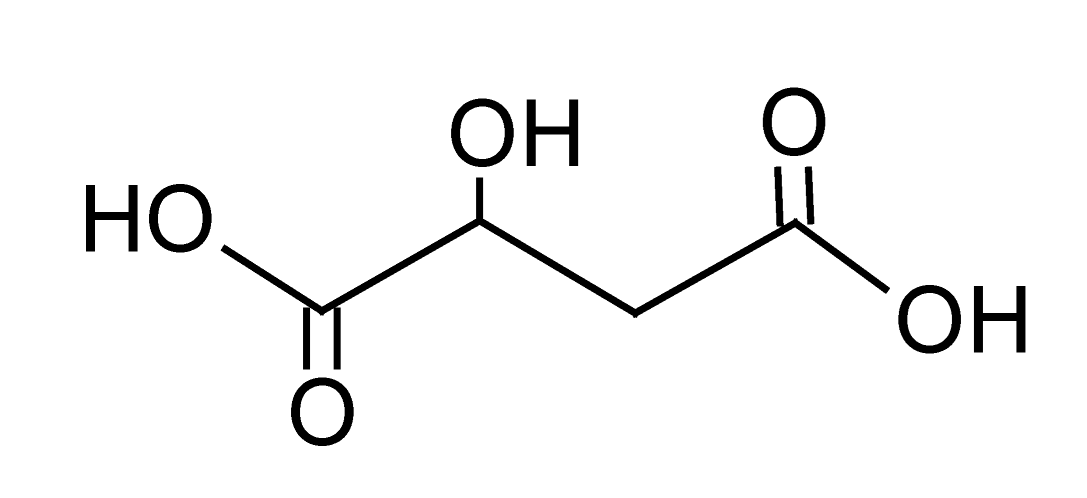 |
| 3 | 1.2 | 117.0191 | 2.3 | 73.0290, 99.0072 | Succinic acidd (C4H604) |
Dicarboxylic organic acid | 1.89 | 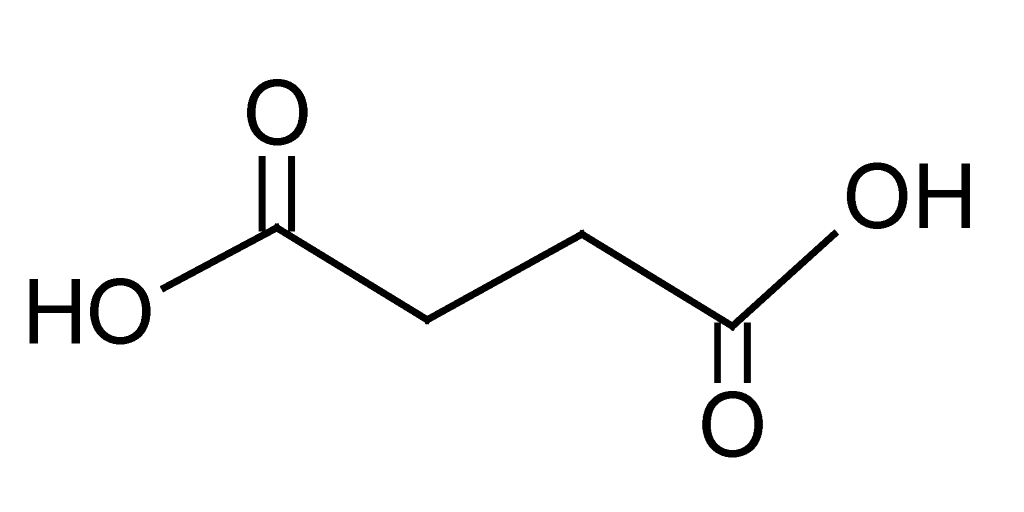 |
| 4 | 2.3 | 164.0712 | 3.2 | 72.0072, 103.0539, 147.0442 | L-(-)-Phenylalaninee (C9H11NO2) |
Amino acid | 0.53 | 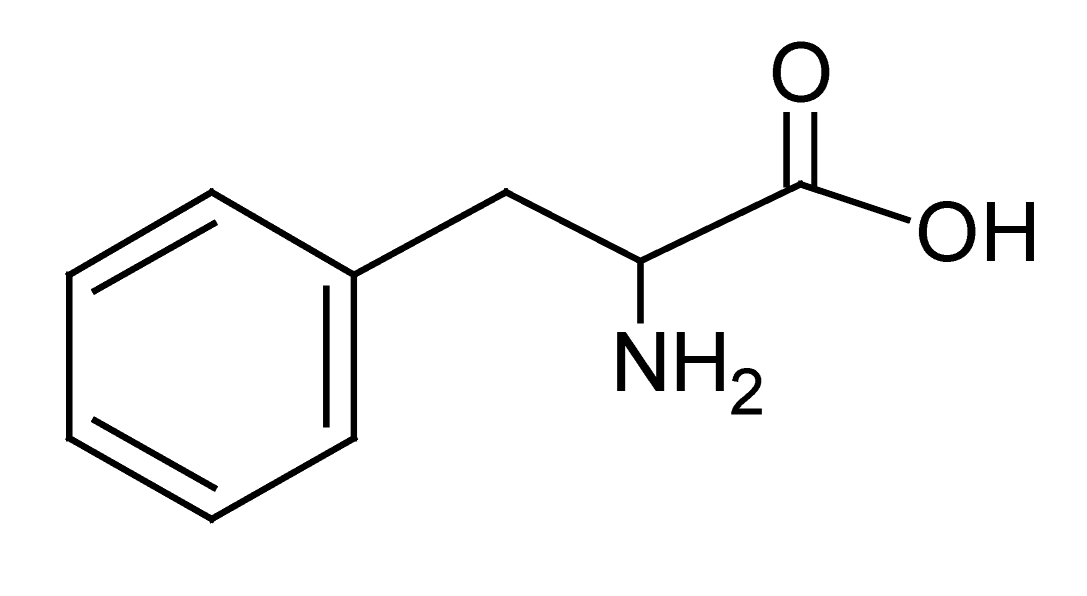 |
| 5 | 2.7 | 282.0833 | 3.9 | 108.5347, 133.0157, 150.0429 | Guanosinee (C10H13N5O5) |
Nucleoside | 0.46 | 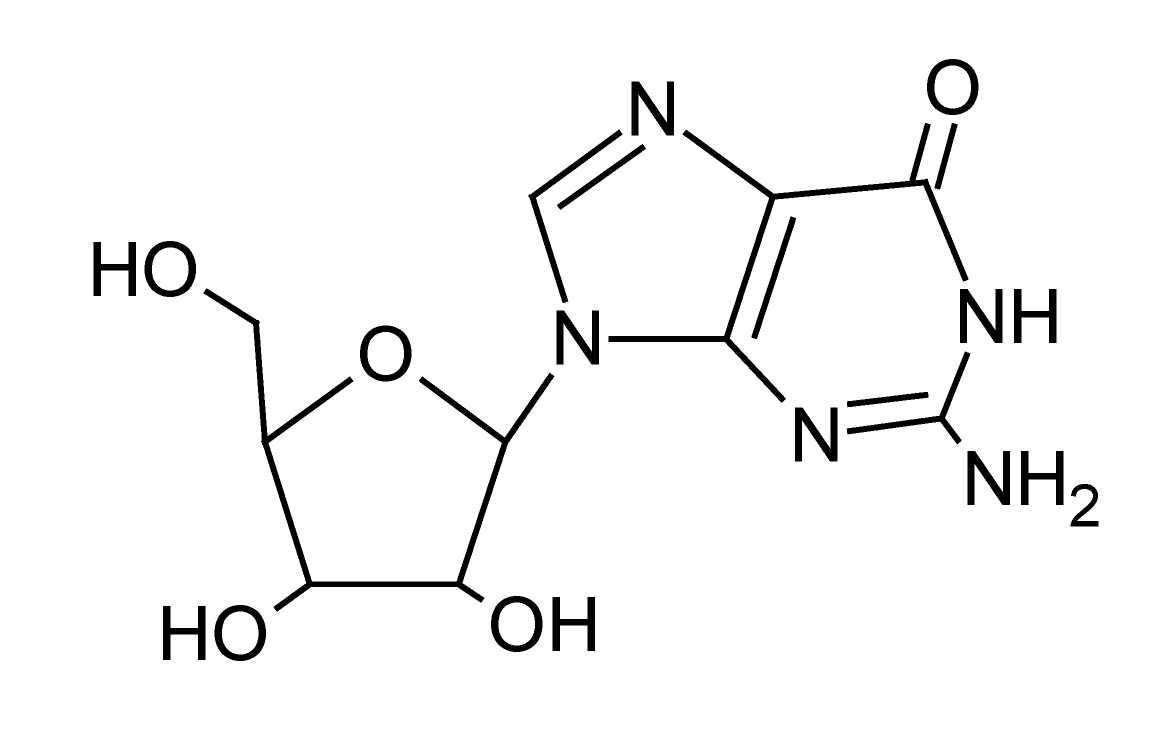 |
| 6 | 6.0 | 353.0867 | 3.1 | 179.0365, 191.0556 | Neochlorogenic acidb (C16H18O9) |
Caffeoylquinic acid, phenolic compound | 4.35 | 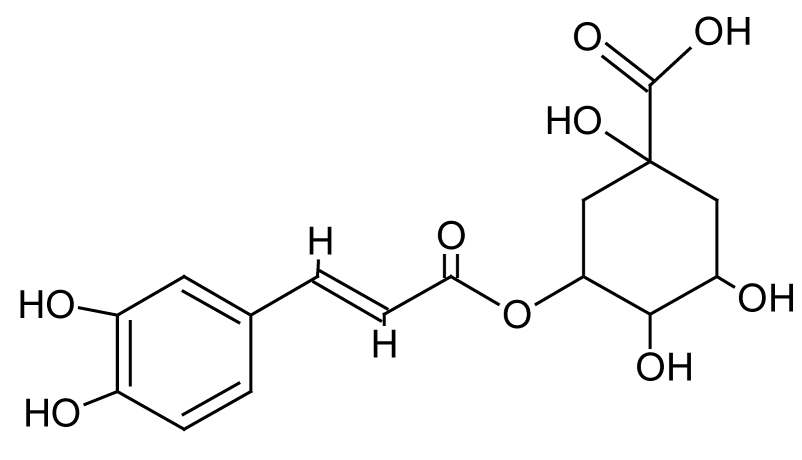 |
| 7 | 6.3 | 353.0866 | 3.4 | 173.0452, 179.0365, 191.0556 | Chlorogenic acida (C16H18O9) |
Caffeoylquinic acid, phenolic compound | 8.44 | 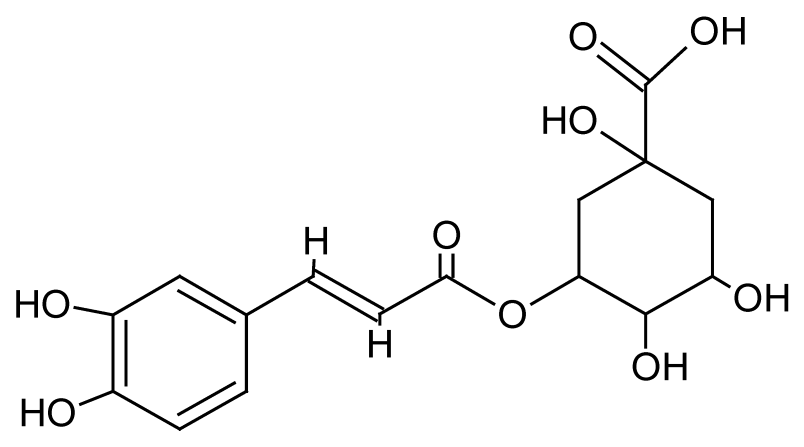 |
| 8 | 6.5 | 609.1438 | 3.8 | 300.0266, 301.0335 | Rutinabe (C27H30O16) |
Flavonoid glycoside | 15.52 | 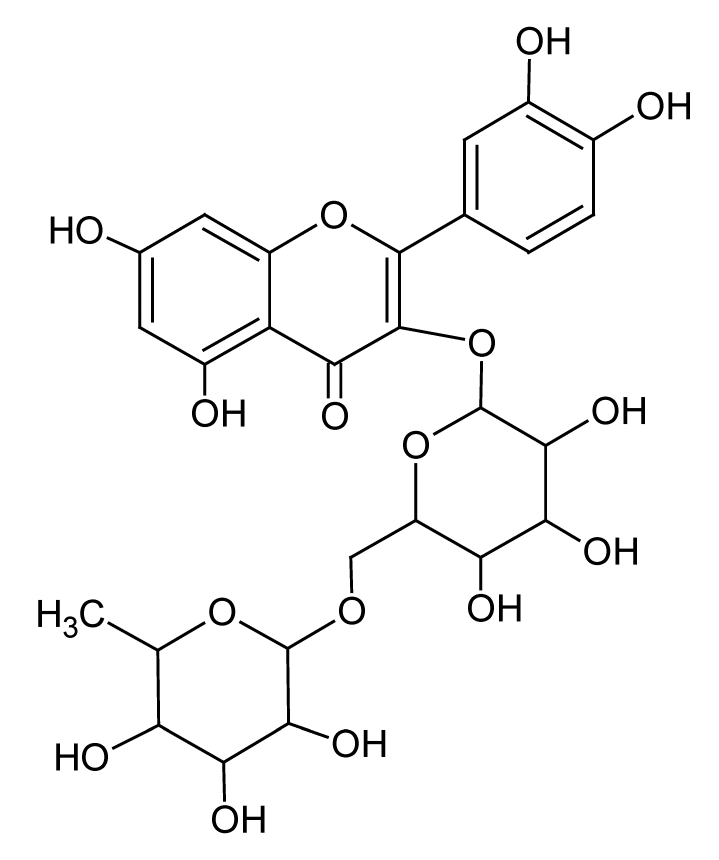 |
| 9 | 6.6 | 593.1489 | 3.9 | 284.0314, 285.0393 | Kaempferol-3-O-rutinosidee (C27H30O15) |
Flavonol glycoside | 25.30 | 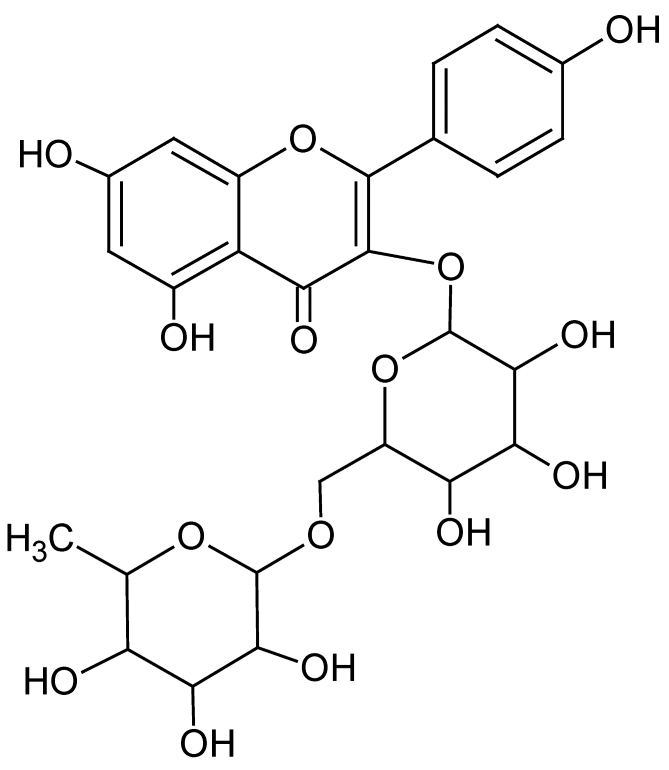 |
| 10 | 6.8 | 187.0970 | 3.1 | 97.0653, 125.0963, 169.0889 | Azelaic acidc (C9H1604) |
Dicarboxylic acid | 2.10 | 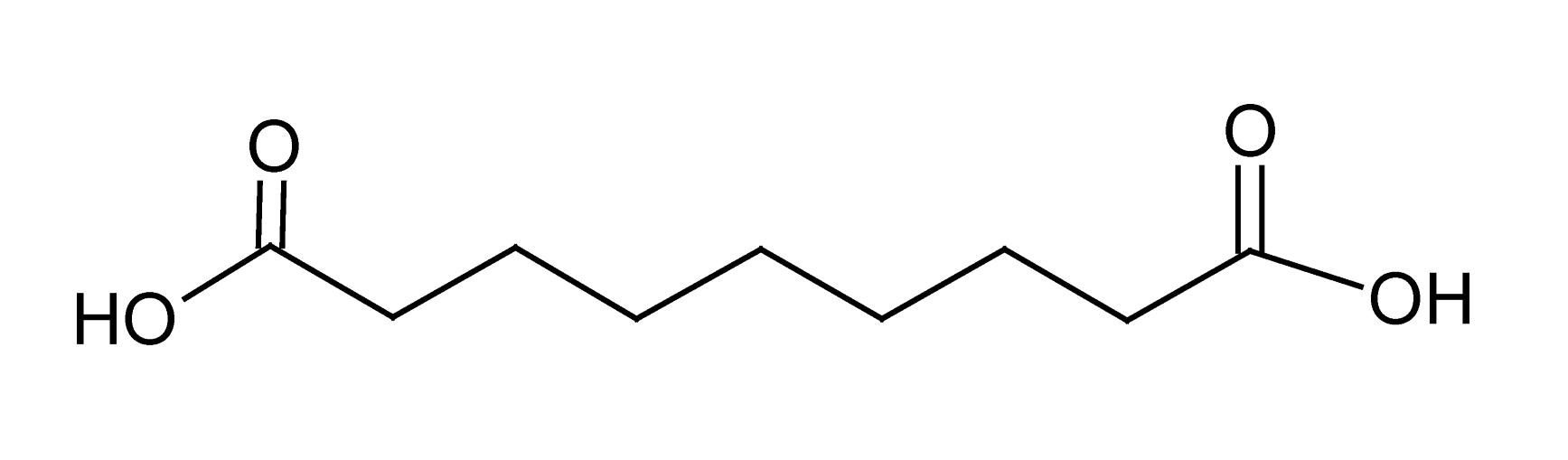 |
| 11 | 8.2 | 329.2321 | 3.9 | 171.1023, 229.1436 | Pinellic acidc (C18H34O5) |
Carboxylic acid | 1.09 | 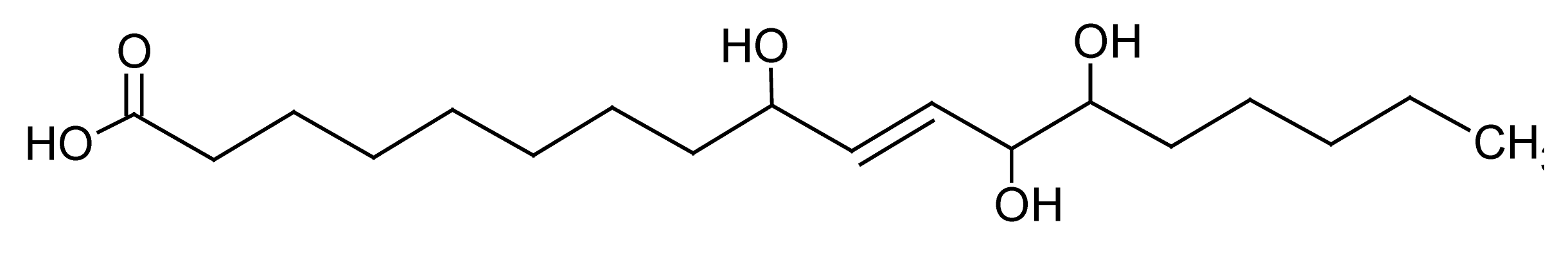 |
| 12 | 11.5 | 293.2112 | 3.6 | 275.2013, 235.1680, 223.1685 | Embelinc (C17H26O4) |
Para-benzoquinone | 8.60 |  |
| CG | OG | PK | MET | |
|---|---|---|---|---|
| Weight at week 0 (g) | 74.3 ± 17.9 | 78.2 ± 15.5 | - | - |
| Weight at week 20 (g) | 368.5 ± 38.1 | 456.2 ± 42.6* | - | - |
| TyG index at week 20 | 7.73 ± 0.48 |
8.85 ± 0.53* |
- | - |
| HOMA-IR at week 20 | 1.73 ± 0.25 | 5.01 ± 0.47* | - | - |
| Weight at the end of the experiment (g) | 423.1 ± 30.2 | 527.0 ± 45.3* | 487.0 ± 42.8# | 489.0 ± 55.2# |
| Total adipose tissue at the end of the experiment (g) | 14.3 ± 3.1 | 37.92 ± 4.4* | 29.38 ± 3.5* ** | 33.35 ± 4.9* |
| Week 1 | CG | OG | PK | MET |
|---|---|---|---|---|
| Weight (g) | 389.00±28.70a | 479.00±35.97b | 472.00±32.49b | 494.00±67.77b |
| Liquid consumption (mL/day) | 30.60±4.59a | 17.42±2.61b | 13.85±2.07bc | 9.85±1.47c |
| Liquid consumption (mL /day/100 g) | 7.86±0.58a | 3.63±0.27b | 2.93±0.20b | 1.99±0.27c |
| Equivalent in kcal in drinkable water | 0a | 13.58±1.02b | 10.96±0.75b | 7.44±1.02c |
| Feed consumption (g/day) | 24.28±3.64a | 17.57±2.63ab | 17.71±2.65bc | 18.42±2.76bc |
| Feed consumption (g/day/100 g) | 6.24±0.46a | 3.66±0.27b | 3.75±0.25b | 3.73±0.51b |
| Equivalent in kcal in feed | 19.35±1.42a | 11.37±0.85b | 11.63±0.80b | 11.56±1.58b |
| Total Kcal/day/100 g body weight | 19.35±1.42ab | 24.95±1.87b | 22.59±1.55b | 19.01±2.60ab |
| Week 2 | CG | OG | PK | MET |
| Weight (g) | 398.00 ± 25.21a | 482.00 ± 33.55b | 459.00 ± 35.41b | 482.00 ± 55.16b |
| Liquid consumption (mL/day) | 32.57 ± 4.88a | 28.14 ± 4.22a | 33.60 ± 5.04a | 30.77 ± 4.61a |
| Liquid consumption (mL /day/100 g) | 8.18 ± 1.10a | 5.83 ± 0.95b | 7.32 ± 0.38b | 6.38 ± 0.06b |
| Equivalent in kcal in drinkable water | 0a | 65.39 ± 4.55b | 76.80 ± 5.92bc | 75.08 ± 10.15bc |
| Feed consumption (g/day) | 27.28 ± 4.09a | 16.00 ± 2.4b | 11.85 ± 1.77b | 12.71 ± 1.90b |
| Feed consumption (g/day/100 g) | 6.85 ± 0.44a | 3.31 ± 0.23b | 2.58 ± 0.19b | 2.63 ± 0.35b |
| Equivalent in kcal in feed | 21.25 ± 1.36a | 10.29 ± 0.71b | 8.008 ± 0.61c | 8.177 ± 1.10c |
| Total Kcal/day/100 g body weight | 21.25 ± 1.36a | 75.68 ± 5.26b | 84.80 ± 6.54bc | 83.26 ± 11.25bc |
| Week 3 | CG | OG | PK | MET |
| Weight (g) | 402.00 ± 30.75a | 492.00 ± 37.76b | 468.00 ± 29.42b | 486.00 ± 54.94b |
| Liquid consumption (mL/day) | 30.42 ± 4.56a | 44.85 ± 6.72b | 46.28 ± 6.94b | 31.00 ± 4.650a |
| Liquid consumption (mL /day/100 g) | 7.56 ± 0.57a | 9.11 ± 0.69b | 9.89 ± 0.62b | 6.37 ± 0.90a |
| Equivalent in kcal in drinkable water | 0a | 102.1 ± 7.83b | 110.7 ± 6.96b | 71.44 ± 10.13c |
| Feed consumption (g/day) | 23.14 ± 3.47a | 12.14 ± 1.82b | 9.571 ± 1.43b | 9.571 ± 1.43b |
| Feed consumption (g/day/100 g) | 17.84 ± 1.36a | 7.65 ± 0.58b | 6.34 ± 0.39b | 6.10 ± 0.86b |
| Equivalent in kcal in feed | 5.75 ± 0.44a | 2.46 ± 0.18b | 2.04 ± 0.12b | 1.96 ± 0.27b |
| Total Kcal/day/100 g body weight | 17.84 ± 1.36a | 109.7 ± 8.42b | 117.10 ± 7.36b | 77.54 ± 11.00c |
| Week 4 | CG | OG | PK | MET |
| Weight (g) | 423.1 ± 30.20a | 527.0 ± 45.30b | 487.0 ± 42.80ab | 489.0 ± 55.20ab |
| Liquid consumption (mL/day) | 33.11 ± 4.96a | 48.91 ± 7.33b | 44.57 ± 6.68ab | 52.97 ± 8.54b |
| Liquid consumption (mL /day/100 g) | 7.82 ± 0.63a | 9.28 ± 0.75b | 9.15 ± 0.61b | 10.83 ± 1.61b |
| Equivalent in kcal in drinkable water | 0a | 103.93 ± 8.40b | 102.55 ± 6.85b | 121.32 ± 18.09b |
| Feed consumption (g/day) | 24.42 ± 3.66a | 11.71 ± 1.75b | 10.71 ± 1.60b | 9.57 ± 1.43b |
| Feed consumption (g/day/100 g) | 5.77 ± 0.46a | 2.22 ± 0.17b | 2.19 ± 0.14b | 1.96 ± 0.27b |
| Equivalent in kcal in feed | 17.89 ± 1.44a | 6.88 ± 0.55b | 6.81 ± 0.45b | 6.09 ± 0.84b |
| Total Kcal/day/100 g body weight | 17.89 ± 1.44a | 110.81 ± 8.95b | 109.36 ± 10.34bc | 127.41 ± 12.94c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





