1. Introduction
The introduction and widespread adoption of genetically modified corn,
Zea maize L. (Poales: Poaceae) and Upland cotton,
Gossypium hirsutum L. (Malvales: Malvaceae), producing
Bacillus thuriengiensis (
Bt) (Bacillales: Bacillaceae) proteins has resulted in effective
Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) control while causing marginal to no harm to non-target organisms [
1,
2]. However, with the occurrence of resistance in
H. zea to one or more
Bt proteins, remedial insecticide sprays are often required to prevent unacceptable injury in
Bt cotton [
3,
4,
5,
6,
7,
8,
9,
10]. In the U.S., because of widespread issues with pyrethroid resistance, insecticides containing chlorantraniliprole are the primary means for managing
H. zea in cotton [
7,
11,
12]. Currently, there are numerous reports of field-evolved resistance of lepidopteran pests to chlorantraniliprole [
13]. However, to date, no chlorantraniliprole resistance has been reported for
H. zea, but, because of the heavy reliance on this insecticide for
H. zea management in cotton, grain sorghum, soybean, and other crops, there is concern that resistance may develop [
14,
15,
16,
17]. Thus, it is best to be proactive and develop additional management tactics targeting
H. zea in cotton.
Implementation of intercropping (also known as polyculture) systems has demonstrated utility for insect pest management. Intercropping involves the simultaneous cultivation of two or more companion crop species in one field [
18]. The companion crops may serve as repellents, trap crops, and/or natural enemy recruiters [
19,
20,
21,
22]. This ecosystem service provided by the intercropping system may promote insect pest suppression in the main crop, thus reducing/delaying the need for insecticide applications [
23,
24,
25,
26].
An intercropping system aimed at trap cropping involves cultivating a crop of interest simultaneously with another crop that is more preferred by the pests of concern; this favors the diversion of the pest from the main crop. The adoption of this system has resulted in the successful management of multiple key pests in several economic crops, including
H. zea in cotton [
24,
27,
28,
29].
Reports from several studies conducted in various regions in the world have demonstrated that grain sorghum,
Sorghum bicolor L. Moench (Poales: Poaceae), may serve as an effective diversionary trap crop for
H. zea and
Helicoverpa armigera (
Hübner) (Lepidoptera: Noctuidae) from cotton and as a source of
H. zea natural enemies [24,30]. Thus, the implementation of an intercropping system of cotton with grain sorghum may divert
H. zea from cotton to grain sorghum, while providing a valuable source of beneficial arthropods that may disperse from grain sorghum into cotton [
21].
Grain sorghum may also serve as an effective source for
Helicoverpa armigera nucleopolyhedrovirus (
HearNPV) dissemination to cotton [
24,
30,
31,
32].
HearNPV is a viral pesticide that is specific to Heliothines, including
H. zea [
33]. In the U.S.,
HearNPV has demonstrated high efficacy for
H. zea management in soybean,
Glycine max (L.) Merr. [
34]. In soybean,
HearNPV has been found to be very persistent in the canopy [
35], but, in cotton,
HearNPV persistence has not been sustained. This lack of persistence is thought to be primarily due to the high pH of dew on cotton leaves, resulting in virus deactivation as the dew dries [
36,
37,
38]. Although initial
HearNPV infection of
H. zea larvae in cotton is possible, it is unlikely an epizootic event will persist. Thus, the challenge of effectively integrating
HearNPV into cotton IPM is to devise a system where an epizootic nursery reservoir of
HearNPV can be initiated for persistent horizontal biotic and/or abiotic transmission into cotton.
This current study has two objectives. The first objective is to investigate the potential for utilizing grain sorghum as a trap crop for H. zea and a nursery crop for H. zea natural enemies. The second objective is to investigate the potential for utilizing grain sorghum as a nursery crop for HearNPV dissemination into the cotton canopy to manage H. zea.
2. Materials and Methods
2.1. Locations, Experimental Design, and Treatments
These experiments were conducted at three distinct geographical and environmental locations that are representative of the southern U.S. Cotton Belt. The sites include College Station, TX; Stoneville, MS; and Blackville, SC. Experiments were conducted over two years, with the first year serving as a proof-of-concept experiment and the second year serving as a validation experiment. The cotton used in these experiments was a non-Bt variety, DP 1822 XF (Bayer CropScience LP, St. Louis, MO). The grain sorghum used consisted of equal blends of seed from six hybrids with different levels of maturity (
Table 1, S&W Seed Company, Longmont, CO). The seed were blended to extend the bloom period of the planted area to approximately 21 days to extend the attractiveness of the grain sorghum to ovipositing
H. zea.
2.2. Proof-of-Concept Experiment
This experiment was conducted in 2020 and consisted of three treatments at each location. Each of the three fields were separated from one another by at least 0.5 kilometers to avoid unintended spread of HearNPV from one field to another. Two fields consisted of replicated (four each) alternating 8 rows wide strips of grain sorghum or cotton (with a row spacing of 0.97-1.02 m) and 61 m long. The third field consisted of a solid cotton block of 64 rows wide, with the same row spacing and length used in the interplanted fields. Each geographic location served as a field replicate. Grain sorghum was planted 7-10 days after planting cotton to closely time the expected first week of bloom of the cotton with the bloom of the earliest maturing grain sorghum hybrid.
All three fields and crops were grown using standard production practices but were not treated with insecticides that would harm H. zea. In one of the interplanted fields, the blooming grain sorghum was treated with HearNPV (Heligen®, AgBiTech, Fort Worth, TX) at 0.1 L/ha targeting 1st and 2nd instar H. zea larvae. The treatment was applied by ground using a high-clearance sprayer calibrated to deliver a spray volume of 93.54 L/ha. The interplanted nontreated field served as a non-HearNPV comparison. The cotton-only field served as a non-sorghum comparative treatment allowing evaluation of the effectiveness of grain sorghum as a H. zea trap crop and natural enemy nursery. Pre-treatment data and samples were collected from all fields before the HearNPV application and at 7, 14, and 21 days post-application.
Beneficial arthropods and
H. zea larvae were sampled from grain sorghum using the beat-bucket method [
39]. Four locations within each replicate were sampled. At each location, 25 heads were sampled (100 heads total per replicate) by bending the sorghum panicle into a 2.5-gallon bucket and vigorously shaking it against the bucket walls to dislodge
H. zea larvae and beneficial arthropods. Samples were collected into 1-gallon plastic bags and returned to the laboratory for counting. The number of
H. zea larvae were recorded and sized as small (1
st and 2
nd instar) or large (3
rd, 4
th, and 5
th instar). Beneficial arthropods were identified into families and counted. Samples of
H. zea and beneficial arthropods (pooled by family) were stored at -80
oC until they were evaluated for
HearNPV infection utilizing polymerase chain reaction (PCR). An additional beat bucket sample of
H.
zea larvae from 100 sorghum heads was collected from each sorghum replicate. When available, ≥3
rd instar
H. zea larvae from this sample were collected into 29 mL Solo condiment cups (Dart Container Corporation, Mason, MI, USA) containing laboratory-based meridic diet (WARD’S Stonefly Heliothis diet, Rochester, NY). Collected larvae were transported to the laboratory and held for parasitoid emergence and identification.
Cotton within the cotton-sorghum interplanting was sampled using three methods: visual sampling, beat-bucket sampling, and drop-cloth sampling. The visual sampling method was primarily aimed at detecting eggs and damaged fruiting forms, and the drop-cloth method was used to collect
H. zea larvae used to determine
HearNPV infection and parasitism rates of
H. zea. For the visual sampling method, each replicated strip was sampled by inspecting 25 individual plants using the method described by Calvin et al. [
15]. For each plant, the terminal was inspected for evidence of
H. zea feeding and the presence of
H. zea larvae. Four (2 small from the upper [top 5 nodes] canopy and 2 larger and lower) squares were sampled from each plant for evidence of injury and the presence of
H. zea larvae. Four (2 small [approximately 1 cm in diameter] with bloom tags [dried/attached blossoms] and 2 larger [approximately 2.0-2.5 cm in diameter] without bloom tags) bolls were sampled on each plant for injury and larvae. Injury to squares and bolls was only recorded as positive when the outer tissue was penetrated, when the fruit-feeding injury would result in square abortion, or when the carpel wall of the boll was penetrated. The size of each
H. zea larvae for all sampling was recorded as small (1
st and 2
nd instars) or large (3
rd, 4
th, and 5
th instars). Additionally, when inspecting the various plant structures, the number of Heliothine eggs were recorded for each plant.
Predators within the cotton plots were sampled using a beat bucket as described by Knutson et al. [
40]. A 5-gallon bucket was held at a 45
o angle to the ground and the sample plants were grasped near the base and quickly bent into the bucket. Ten beat-bucket samples per replicated strip of cotton were taken, with 3 plants sampled per beat bucket. The plants were rapidly beaten against the inside of the bucket 12-16 times for 3-4 seconds then were removed from the bucket. The leaves and fruiting forms that remained in the bucket and the dislodged predators were collected in 1-gallon plastic bags and transported to the laboratory for identification and counting. Leaves and fruiting forms dislodged were examined for predators. Additionally, four drop-cloth samples were collected per replicated strip of cotton. Black drop-cloths of 0.97 m long by 0.76 m wide were utilized. Approximately 1.5 m of cotton was vigorously shaken causing
H. zea to dislodge and drop onto the drop cloth. Dislodged fruits and leaves were examined for the presence of
H. zea larvae. The ≥3
rd instar
H. zea larvae from one-half of the larvae collected from each replicated strip were collected into 29 mL Solo condiment cups containing laboratory-based meridic diet; these larvae were transported to the laboratory and allowed to develop to estimate parasitism. The other half of each sample and the collected predators were pooled and stored at -80
oC. These samples were then analyzed to estimate
HearNPV presence using polymerase chain reaction (PCR). When the number of
H. zea larvae collected in a sample was low, all larvae collected were submitted for PCR analysis. Throughout the sampling period, precautions were taken to minimize anthropogenic dispersal of
HearNPV. Samples were taken in the untreated field first then in the
HearNPV treated field starting from the furthest to the closest transect to the sorghum block at each date.
2.3. Validation Experiment
The validation experiment was conducted similarly to the proof-of-concept experiment but instead of the grain sorghum being interplanted with cotton, it was planted on the edge of the field to simulate a practical means of implementation for growers. At each location, three approximately 2.0 ha blocks of cotton were utilized, with each block being separated from one another by at least 0.5 Km. Two of the fields were bordered on the predominantly upwind side with 8-12 rows of grain sorghum blended with 6 varied maturity hybrids (
Table 1). Sorghum was planted 7-10 days after planting cotton to synchronize bloom of the earliest maturing sorghum with the first week of bloom of the cotton. Planting the sorghum upwind from the cotton minimized the potential for herbicide drift from the cotton into the sorghum and maximized the potential for arthropods and
HearNPV dispersal from the sorghum into the cotton. Each geographic location served as a field replicate. Both crops were grown using standard production practices but were not treated with insecticides that would harm
H. zea. The blooming sorghum in one of the cotton-sorghum fields was treated with
HearNPV (Heligen
®, AgBiTech, Fort Worth, TX) at a rate of 0.1 L/ha targeting 1
st and 2
nd instar larvae. The treatment was applied using a high-clearance sprayer calibrated to deliver a spray volume of 93.54 L/ha. The untreated field bordered with sorghum served as a non-
HearNPV comparison. The cotton-only field served as a non-sorghum treatment to evaluate the effectiveness of grain sorghum as a
H. zea trap crop and natural enemy nursery. Pre-treatment data and samples were collected from all fields before the
HearNPV application and at 7, 14, and 21 days post-application.
Sorghum was sampled as described in the proof-of-concept experiment. Four locations, with 25 sorghum heads per location, were sampled within the sorghum. As previously described,
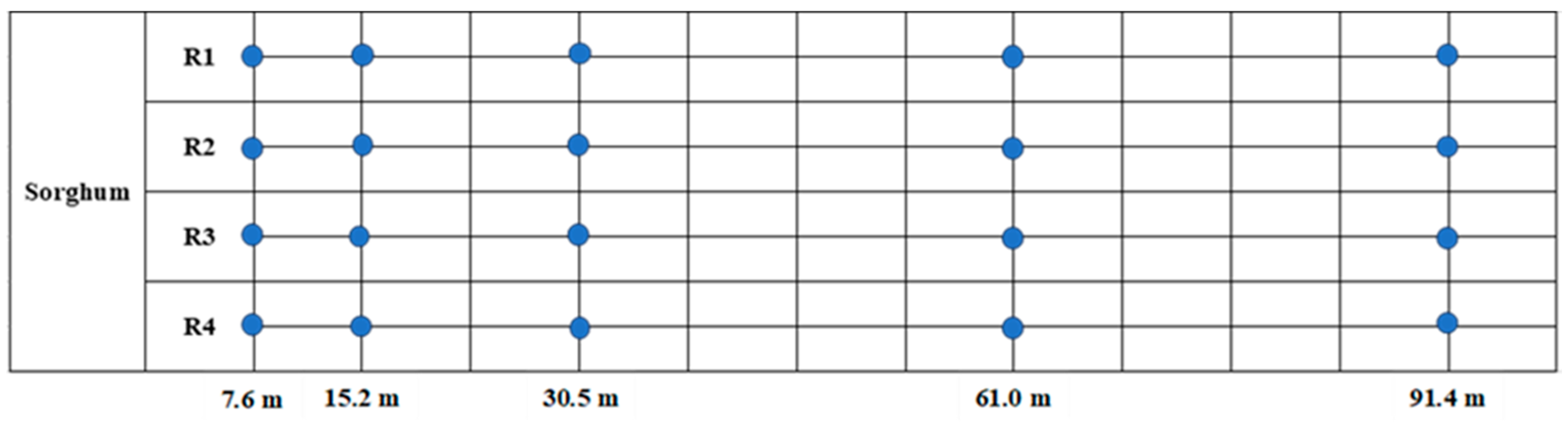
H. zea larvae and beneficial arthropod density were determined for each sample date. In both the cotton-only and cotton bordered by sorghum fields, the cotton was sampled based on replicated transects originating from the sorghum planting or the edge of the predominant upwind edge for the cotton-only planting. Each field was divided into equally spaced grids and the transects were divided into 4 equally spaced transects along those grids (
Figure 1). Data were collected along each transect at 7.6, 15.2, 30.5, 61.0, and 91.4 m. At each transect location, 10 plants were visually sampled, and 5 beat-bucket and 2 drop-cloth samples were taken as previously described. As in the proof-of-concept experiment, percentage of damaged fruiting forms, eggs,
H. zea larvae, predators, percent parasitism of larvae, and
HearNPV infection were determined for each sample transect distance by replicate and sample date. Data were collected, and samples were processed as previously described in the proof-of-concept experiment. Precautions, as described previously, were taken to minimize anthropogenic dispersal of
HearNPV.
2.4. HearNPV Infection Analysis
HearNPV infection of H. zea larvae was determined using methods described by Black et al. (2019). For each sample, HearNPV occlusion bodies were purified and extracted, and the DNA was subsequently separated and extracted utilizing a DNA extraction kit (DNeasy Blood and Tissue Kit: Qiagen, Germantown, MD). Extracted DNA was amplified with HearNPV polyhedrin-specific primers HzSpolh-2F (5′-CCCTACTTTGGGCAAAACC-3′) and HzSpolh-2R (5′-TCGGTTTGGTTGGTCGCATA-3′) (IDT, Coralville, IA) using a Veriti™ 96-Well Thermal Cycler (Applied Biosystem, Foster City, CA). A volume of 50 µl of PCR mixture was used and consisted of 1 μl extracted DNA sample, 2.5 mM MgCl2, 0.2 mM dNTPs, 0.5 μM each primer, 1× GoTaq Flexi Buffer, and 1.25 U of GoTaq DNA polymerase (Promega, Madison, WI). To confirm the effective amplification of the target gene, a positive control and a negative control consisting of HearNPV and deionized water, respectively, were included in each individual thermocycler run. Once amplified, samples were visualized using a 4200 TapeStation with D1000 ScreenTape Assay (Agilent Technologies, Inc, Waldbronn, Germany) for HearNPV confirmation. HearNPV presence was confirmed when a band was present at 400 base pairs (bp). For the HearNPV-positive samples, PCR products were sequenced (Eurofins, Louisville, KY) to confirm the HearNPV polyhedron sequence.
2.5. Statistical Analyses
For the proof-of-concept experiment, the percentage of damaged fruiting forms, beneficial arthropods, parasitized larvae, and
H. zea eggs and larvae were compared between treatments using a multiple Student’s t-test [
41]. For the validation experiment
, the percentage of damaged fruiting forms, beneficial arthropods, and
H. zea larvae were compared between treatments and between distances within treatment using a multiple Student’s t-test [
41]. To compare the virus detection frequency between treatments, the Kruskal–Wallis test [
41] was performed.
3. Results
3.1. Proof-of-Concept Experiment
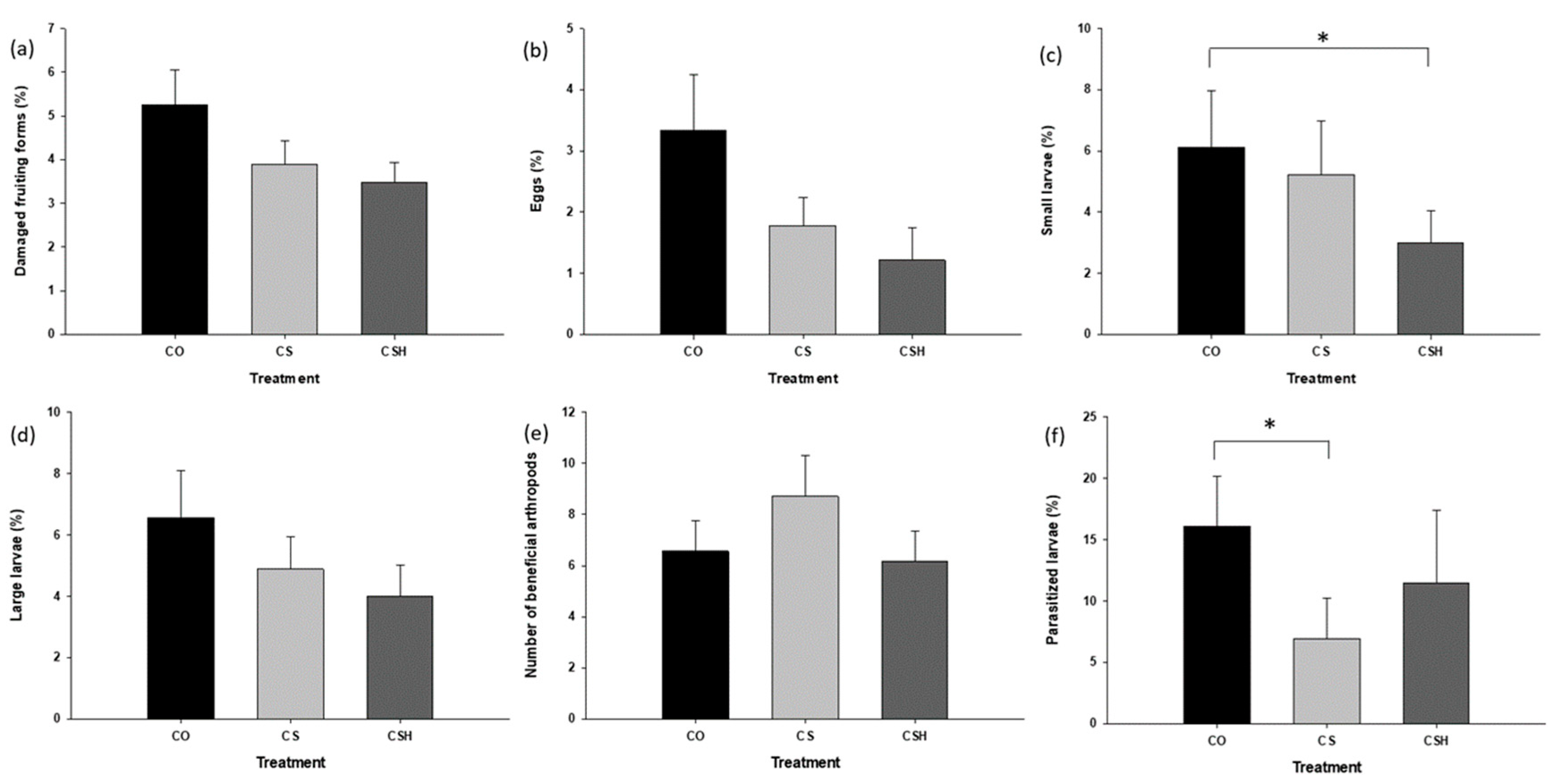
When the cotton-only treatment was compared with the non-treated cotton-sorghum for
H. zea parameters, no significant differences were detected for the percentage of damaged fruiting forms (
t = 1.42, df = 76.806,
P = 0.1591), percentage of eggs (
t = 1.48, df = 64.723,
P = 0.1435), percentage of small larvae (
t = 0.89, df = 86,
P = 0.3781), or percentage of large larvae (
t = 0.86, df = 75.8,
P = 0.3942). Additionally, there were no differences detected for the number of beneficial arthropods (
t = -1.07, df = 86,
P = 0.288). However, significant differences were detected in the percentage of parasitized
H. zea larvae with cotton-only exhibiting a greater incidence of parasitized larvae (
Figure 2f;
t = 2.03, df = 43,
P = 0.0484).
When the cotton-only treatment was compared with the
HearNPV-treated cotton-sorghum for
H. zea parameters, no significant differences were detected in the percentage of damaged fruiting forms (
t = 1.88, df = 69.031,
P = 0.0642), percentage of eggs (
t = 1.45, df = 78.588,
P = 0.1521), or the percentage of large larvae (
t = 1.39, df = 73.512,
P = 0.1676). There were also no differences detected in the number of beneficial arthropods (
t = 0.23, df = 86,
P = 0.8165) or percentage of parasitized
H. zea larvae (
t = 0.82, df = 37,
P = 0.4179). Significant differences were detected for the percentage of small
H. zea larvae (
t = 2.18, df = 63.927,
P = 0.0328), with cotton-only exhibiting greater incidence (
Figure 2c).
The non-treated cotton-sorghum did not differ from HearNPV-treated cotton-sorghum in either the percentage of damaged fruiting forms (t = 0.49, df = 86, P = 0.6278), percentage of eggs (t = 0.13 , df = 77.868, P = 0.8944), percentage of small larvae (t = 1.27, df = 69.662, P = 0.2072), percentage of large larvae (t = 0.66, df = 86, P = 0.509), number of beneficial arthropods (t = 1.28, df = 78.158, P = 0.2057), or percentage of parasitized larvae (t = -0.77, df = 40, P = 0.4449).
3.2. Validation Experiment
3.2.1. Comparison of Treatment
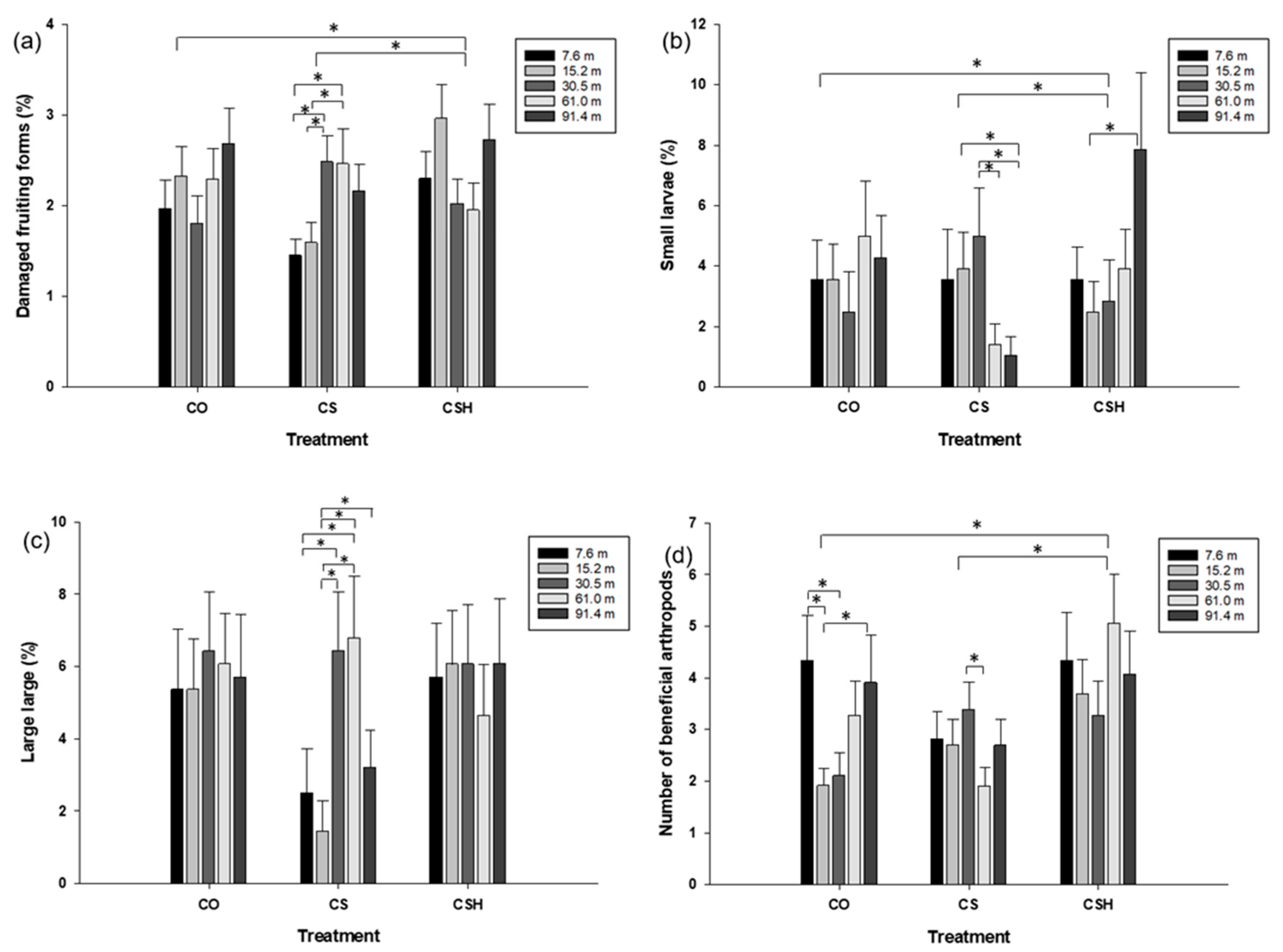
For this experiment, the percentage of eggs and parasitized larvae were not evaluated due to the incompleteness of the data for these variables. Significant differences between cotton-only and non-treated cotton-sorghum were not observed for either the percentage of fruiting forms damaged by H. zea (t = -0.56, df = 390.42, P = 0.5792), percentage of small larvae (t = -0.92, df =398, P =0.3585), percentage of large larvae (t = 1.53, df = 398, P = 0.1261), or the number of beneficial arthropods (t = 1.08, df = 332.67, P = 0.2826).
The cotton-only plots had significantly fewer damaged fruiting forms (
Figure 3a;
t = -2.76, df = 398,
P = 0.006) and small larvae (
Figure 3b:
t = -3.01, df = 361.55,
P = 0.0028) than cotton from the
HearNPV-treated cotton-sorghum plots. However,
HearNPV-treated cotton-sorghum plots resulted in a greater number of beneficial arthropods in the cotton (
Figure 3d;
t = -2.04, df = 396.6,
P = 0.0416). There was no significant difference between the two treatments for the percentage of large larvae (
t = -0.25, df = 398,
P = 0.8024).
HearNPV-treated cotton-sorghum resulted in a significantly greater number of injured fruiting forms (
Figure 3a;
t = -2.39, df = 398,
P = 0.0174), small larvae (
Figure 3b;
t = -2.23, df = 365.84,
P = 0.0265), as well as a greater number of beneficial arthropods (
Figure 3d;
t = -3.27, df = 357.57,
P = 0.0012) than the non-treated cotton-sorghum, but the two treatments did not differ in large larvae incidence (
Figure 3c;
t = -1.78, df = 398,
P = 0.0766).
3.2.2. Comparison of Distance
Within the cotton-only field, there was no difference between any of the distances for damaged fruiting forms, small larvae, or large larvae (
P > 0.05;
Figure 3a, b, c). However, beneficial arthropod incidence was statistically greater at 7.6 m from the grain sorghum than at 15.2 and 30.5 m and significantly greater at 91.4 m than at 15.2 m (
P < 0.05;
Figure 3d).
Within the non-treated cotton-sorghum, a lower incidence of injured fruiting forms was observed in cotton at 7.6 and 15.2 m than at 30.5 m; fewer damaged fruiting forms were found at7.6 and 15.2 m than at 61.0 m (
P < 0.05;
Figure 3a). Fewer small
H. zea larvae were detected at 61.0 m than at 15.2 or 30.5 m, and the 30.5 m distance exhibited a greater incidence of small larvae (
P < 0.05;
Figure 3b). Fewer large larvae were observed at 7.6 m than at 30.5 and 61.0 m, and significantly fewer large larvae were found at 15.2 m than at 30.5, 61.0, and 91.4 m from the grain sorghum (
P < 0.05;
Figure 3c). Significantly more beneficial arthropods were detected at 30.5 m than at 61.0 m (
P < 0.05;
Figure 3d).
Within the
HearNPV-treated cotton-sorghum, none of the distances differed in the number of damaged
H. zea fruiting forms, large larvae, or beneficial arthropods (
P > 0.05;
Figure 3a, b, c). However, significantly fewer small larvae were observed at 15.2 m than at 91.m from the grain sorghum (
P < 0.05;
Figure 3b).
3.3. Beneficial Arthropods Observed
A variety of predators and parasitoids of
H. zea were observed in cotton during both years of the study (
Table 2). Minute pirate bug (Hemiptera: Anthocoridae), fire ants (Hymenoptera: Formicidae), lady beetles (Coleoptera: Coccinellidae), lacewings (Neuroptera: Chrysopidae, Hemerobiidae), cotton fleahopper (Hemiptera: Miridae), big-eyed bug (Hemiptera: Geocoridae), and spiders (Araneae: Thomisidae, Salticidae, Araneidae, and Oxyopidae) were the most common predators. Tachinid flies (Diptera: Tachinidae) and braconid wasps (Hymenoptera: Braconidae) were the most abundant parasitoids.
3.4. PCR Analysis
3.4.1. Helicoverpa zea Samples
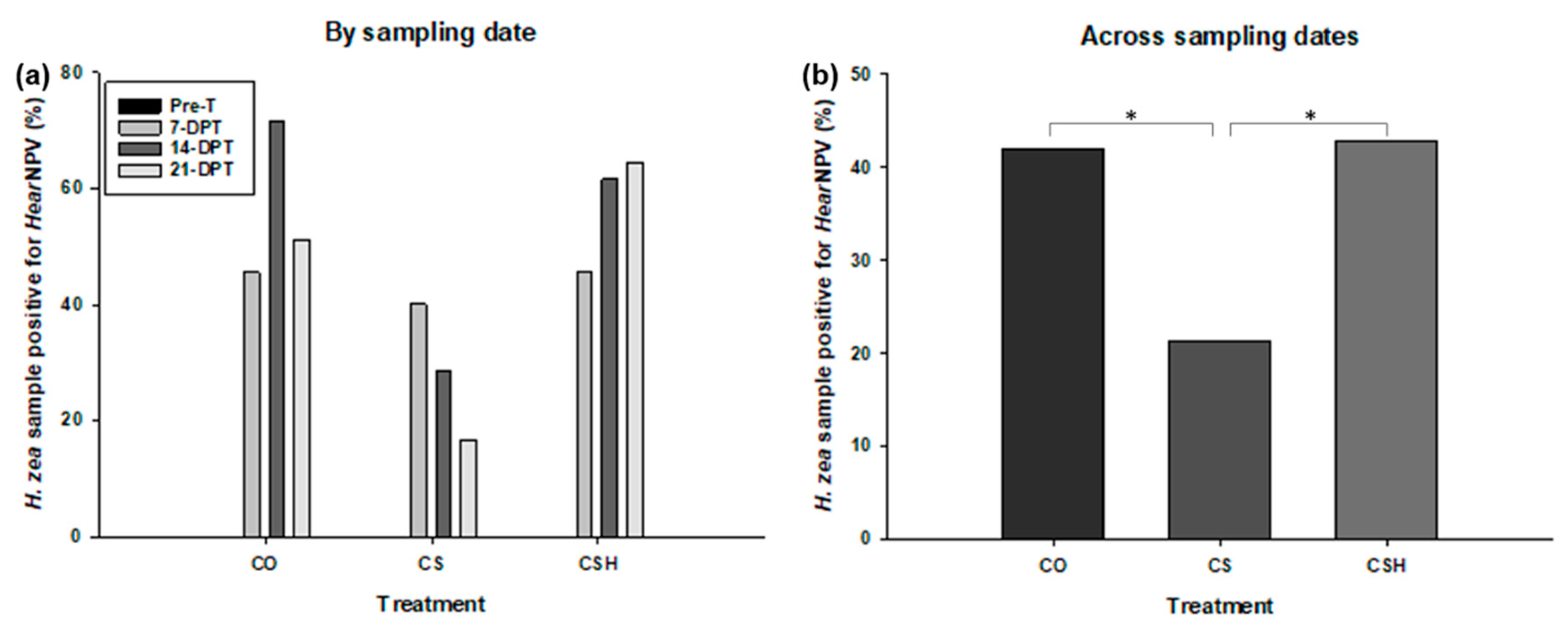
In 2020,
HearNPV was not detected in
H. zea samples collected from pre-treated cotton of any treatment. However, the virus was detected in
H. zea samples collected throughout the subsequent sampling dates for all treatments (
Figure 4a). Based on the Kruskal-Wallis test results, there was a difference in the
HearNPV prevalence between the cotton-only and non-treated cotton-sorghum (χ
2 = 3.8571, df = 1,
P = 0.0495) with cotton-only having greater prevalence of
HearNPV (
Figure 4b). Additionally, there was a significant difference between non-treated cotton-sorghum and
HearNPV-treated cotton-sorghum (χ
2 = 3.8571, df = 1,
P = 0.0495) with
HearNPV-treated cotton-sorghum exhibiting greater incidence of
HearNPV (
Figure 4b). There was no statistical difference in virus detection in
H.
zea between the cotton-only and
HearNPV-treated cotton-sorghum (χ
2 = 0, df = 1,
P = 1).
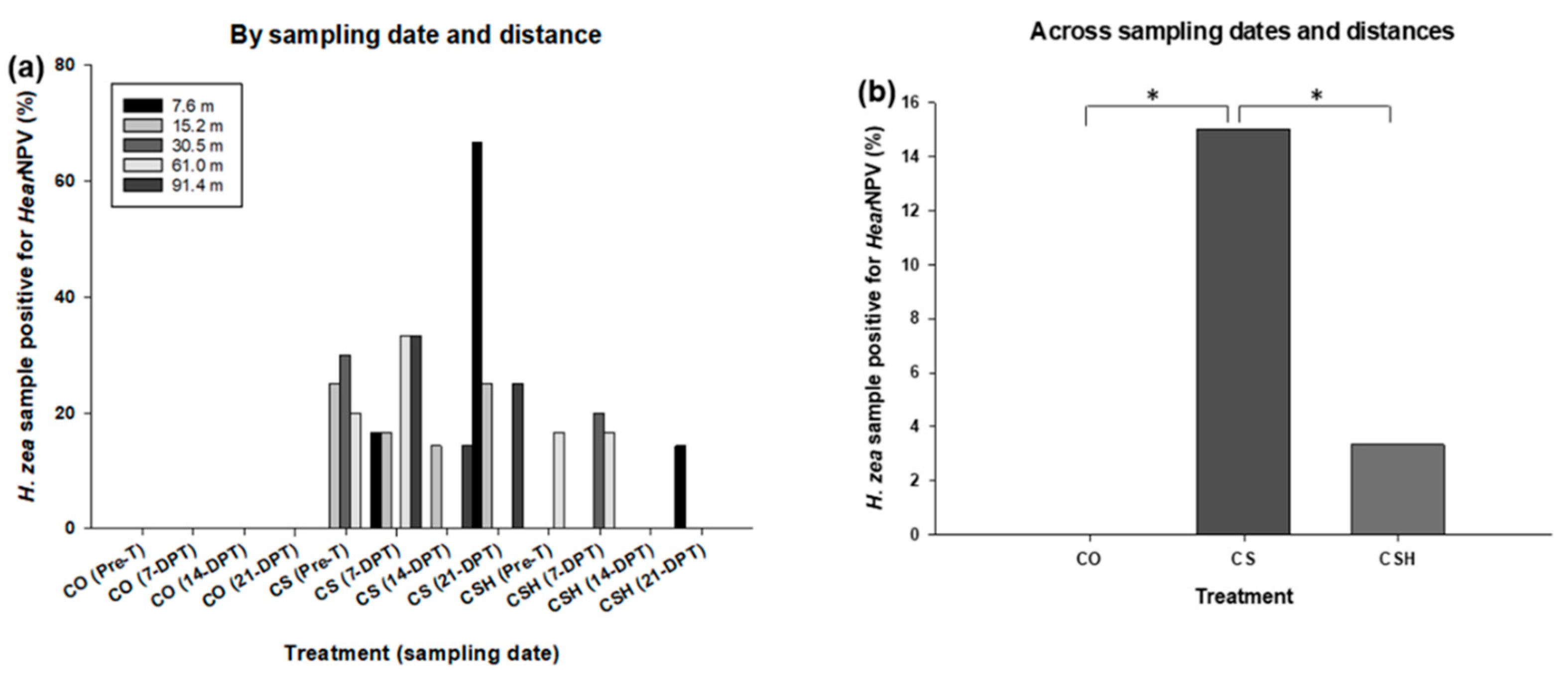
In 2021,
HearNPV was detected in
H. zea samples collected from cotton at all sampling dates for both treated cotton-sorghum and non-treated cotton-sorghum. Additionally, throughout the subsequent sampling dates, the virus was detected in
H. zea samples collected from both fields and across most distance locations except at 91.4 m from the
HearNPV
-treated grain sorghum. However,
HearNPV was not detected in any
H. zea samples collected from the cotton-only field (
Figure 5a). We observed a statistical difference in
HearNPV frequency between the cotton-only and non-treated cotton-sorghum (χ
2 = 7.8125, df = 1,
P = 0.0052), with non-treated cotton-sorghum exhibiting greater
HearNPV incidence (
Figure 5b). Additionally, there was a significant difference between non-treated cotton-sorghum and
HearNPV-treated cotton-sorghum (χ
2 = 6.9018, df = 1,
P = 0.0086), with non-treated cotton-sorghum exhibiting greater
HearNPV incidence (
Figure 5b). No statistical difference between the cotton-only and
HearNPV-treated cotton-sorghum were observed (χ
2 = 3.7156, df = 1,
P = 0.0539).
3.4.2. Beneficial Arthropod Samples
In 2020, none of the beneficial arthropod samples collected from the cotton-only and non-treated cotton-sorghum fields were positive for
HearNPV, while the virus was detected in 7 samples collected from the treated cotton-sorghum treatment. Arthropods in the families Chrysopidae, Coccinellidae, Pentatomidae, and Reduviidae were the only arthropod groups that appeared to be carriers for
HearNPV (
Table 3). In 2021, the virus was detected in beneficial arthropod samples collected from both treated and non-treated cotton-sorghum fields. The arthropod groups that carried the virus included spiders (Thomisidae, Salticidae, Araneidae, and Oxyopidae), Formicidae, Anthocoridae, Reduviidae, Coccinellidae, and Pentatomidae. Coccinellids, pentatomids, and reduviids were the only arthropod groups in which the virus was detected consistently in both years of the study (
Table 3).
4. Discussion
Several studies have reported the utility of intercropping for insect pest management. Growing crops in an intercropping setting may favor pest diversion and increase natural enemy populations [
21,
24,
30,
31,
32]. Based on the results of this current study, growing cotton in an intercropping system with grain sorghum did not result in consistent increase in
H. zea control and beneficial arthropods relative to the cotton-only treatment. Surprisingly, the cotton-sorghum treatment exhibited a significantly lower percentage of parasitized larvae relative to the cotton-only. Hence, the results of this study did not show evidence that sorghum could serve as a
H. zea trap crop and a source of
H. zea natural enemies. However, a previous study has found sorghum to be a desirable diversionary
H. zea trap crop and favored measurable
H. zea control, but, similarly to our study, sorghum did not serve as a source for
H. zea natural enemies [
24].
Additionally, the results of our current study did not provide sufficient evidence to support our hypothesis that grain sorghum interplanted with cotton will serve as a source of
HearNPV that would favor persistent dissemination of the virus into the cotton canopy. Surprisingly,
HearNPV was detected in samples collected from all treatments indicating that the virus is naturally occurring in the locations where this current study was conducted. In the first year of the study,
HearNPV was more prevalent in the treated cotton-sorghum field compared with the non-treated cotton-sorghum field, but the virus became more prevalent in non-treated cotton-sorghum fields in the second year of the study. However, we observed an interesting pattern. When
HearNPV was more prevalent in the treated field, reduced incidence of damaged fruiting forms and fewer larvae were detected, and when
HearNPV was more prevalent in the non-treated field, there was also a reduction in injury to fruiting forms and lower larval counts. This indicated that the presence of
HearNPV that originated from either natural sources or nearby
HearNPV-treated grain sorghum favored some level of
H. zea suppression in cotton. Previous studies have demonstrated that
HearNPV applied to nearby grain sorghum favored a greater level of
H. armigera control in cotton compared with direct applications to cotton and facilitated the persistence of the virus in the cotton canopy [
30,
31,
32].
Several factors could have impacted the results of this study. For instance, to maintain isolation, the fields (treatments) were planted distantly from each other. Thus, the field for each individual treatment could have been exposed to significantly different levels of
H. zea infestation and had considerably varied densities of beneficial arthropods. The natural occurrence of the virus could have also been inherently varied among field locations. In College Station, we observed higher
H. zea pressure in the
HearNPV-treated cotton-sorghum field than the non-treated cotton-sorghum and the cotton-only fields in 2021. This condition might have caused the data to be biased. Additionally, populations of
H. zea in these locations could have had varied levels of susceptibility to
HearNPV. Resistance to Cry Bt proteins in
H. zea is widespread [
3,
4], and laboratory bioassays showed that
H. zea strains resistant to Cry
Bt proteins are significantly less susceptible to
HearNPV relative to a
Bt susceptible strain [
42]. This situation has caused this study to be extremely challenging.
Our data suggest that the effectiveness of using grain sorghum as a trap crop and a nursery for natural enemies and HearNPV will not consistently result in beneficial outcomes. However, we suggest that further investigation on virus efficacy against H. zea in cotton using the sorghum-cotton system as well as the ability of grain sorghum to serve as a trap crop and source of natural enemies for H. zea be considered in future studies which may allow a better understanding of these systems.
Author Contributions
Conceptualization, D.K. and W.C.; methodology, D.K. and W.C.; validation, D.K., W.C., J.G., J.G., and L.P.; formal analysis, W.C. and D.K.; investigation, D.K., W.C., J.G., and J.G.; resources, D.K., J.G., J.G., and L.P.; data curation, W.C.; writing—original draft preparation, W.C..; writing—review and editing, D.K., W.C., J.G., J.G., and L.P.; visualization, D.K. and W.C.; supervision, D.K.; project administration, D.K.; funding acquisition, D.K., J.G., and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Crop Protection and Pest Management Competitive Grants Program [grant no. 2019-70006-30449/project accession no. 1021163] from the USDA National Institute of Food and Agriculture.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We thank all the graduate students, student workers, and laboratory staff under Drs. David Kerns, Jeff Gore, and Jeremy Greene at Texas A&M University, Mississippi State University, and Clemson University, respectively, for technical assistance. We are also thankful to Dr. Joseph Black for providing guidance during the PCR analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carpenter, J.E. Peer-reviewed surveys indicate positive impact of commercialized GM crops. Nat. Biotechnol. 2010, 28, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Li, Y.H.; Wu, K.M. Risk assessment and ecological effects of transgenic Bacillus thuringiensis crops on non-target organisms. J. Invert. Plant Biol., 2011, 53, 520–538. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y. Successes and failures of transgenic Bt crops: Global patterns of field-evolved resistance. In Bt resistance: Characterization and strategies for GM crops producing Bacillus thuringiensis toxins, Soberón, M., Gao, Y., Bravo, A., Eds.; CABI Press, 2015, pp. 1-14.
- Dively, G.P.; Venugopal, P.D.; Finkenbinder, C. Field-evolved resistance in corn earworm to Cry proteins expressed by transgenic sweet corn. PLoS ONE, 2016, 11, e0169115. [Google Scholar] [CrossRef] [PubMed]
- Kerns, D.L.; Yang, F.; Lorenz, G.M.; Gore, J.; Catchot, A.L.; Stewart, S.D.; Brown, S.A.; Cook, D.R.; Seiter, N. Value of Bt technology for bollworm management. Proceedings of the Beltwide Cotton Conference, Memphis, TN, 2018.
- Reisig, D.D.; Huseth, A.S.; Bachelor, J.S.; Aghaee, M.; Braswell, L.; Burrack, H.J.; Flanders, K.; Greene, J.K.; Herbert, D.A.; Jacobson, A.; Palua-Moraes, S.V.; Roberts, P.; Taylor, S.V. Long-term empirical and observational evidence of practical Helicoverpa zea resistance to cotton with pyramided Bt toxins. J. Econ. Entomol. 2018, 111, 1824–1833. [Google Scholar] [CrossRef]
- Reisig, D.; Kerns, D.; Gore, J.; Musser, F. Managing pyrethroid- and Bt-resistant bollworm in southern U.S. cotton. Crops & Soils 2019. [Google Scholar] [CrossRef]
- Little, N.S.; Elkins, B.H.; Mullen, R.M.; Perera, O.P.; Parys, K.A.; Allen, K.C.; Boykin, D.L. Differences between two populations of bollworm, Helicoverpa zea (Lepidoptera: Noctuidae), with variable measurements of laboratory susceptibilities to Bt toxins exposed to non-Bt and Bt cottons in large field cages. PLoS ONE 2019, 14, e0212567. [Google Scholar] [CrossRef]
- Yang, F.; González, J.C.S.; Williams, J.; Cook, D.C.; Gilreath, R.T.; Kerns, D.L. Occurrence and ear damage of Helicoverpa zea on transgenic Bacillus thuringiensis maize in the field in Texas, US and its susceptibility to Vip3A protein. Toxins, 2019, 11, 102. [Google Scholar] [CrossRef]
- Kaur, G.; Guoa, J.; Brown, S.; Head, G.P.; Priced, P.A.; Paula-Moraese, S.; Nif, X.; Dimase, M.; Huanga, F. Field-evolved resistance of Helicoverpa zea (Boddie) to transgenic maize expressing pyramided Cry1A. 105/Cry2Ab2 proteins in northeast Louisiana, the United States. J. Invertebr. Pathol. 2019, 163, 11–20. [Google Scholar]
- Musser, F.R.; Greene, J.K.; Kerns, D.; Stewart, S.D.; Parajulee, M.N; Lorenz, G.M.; Jones, M.; Herbert, D.A.; Taylor, S.; Phillips, P.M.; Reisig, D. Monitoring bollworms for pyrethroid resistance. Proceedings of the Beltwide Cotton Conference, Memphis, TN, 2017.
- Vyavhare, S.S.; Kerns, D.L. Managing cotton insects in Texas. Texas A&M AgriLife Extension Publication ENTO-PU-158, College Station, TX, USA: 2022. (https://lubbock.tamu.edu/files/2022/07/managing-cotton-insects-in-texas.pdf). (Accessed 1 December 2022).
- Roditakis, E.; Vasakis, E.; Grispou, M.; Stavrakaki, M.; Nauen, R.; Gravouil, M.; Bassi, A. First report of Tuta absoluta resistance to diamide insecticides. J. Pest Sci. 2015, 88, 9–16. [Google Scholar] [CrossRef]
- Adams, A.; Gore, J.; Catchot, A.; Musser, F.; Cook, D.; Krishnan, N.; Irby, T. Susceptibility of Helicoverpa zea (Lepidoptera: Noctuidae) neonates to diamide insecticides in the Midsouthern and Southeastern United States. J. Econ. Entomol. 2016, 109, 2205–2209. [Google Scholar] [CrossRef]
- Calvin, W.; Yang, F.; Brown, S.A.; Catchot, A.L.; Crow, W.D.; Cook, D.R.; Gore, J.; Kurtz, R.; Lorenz, G.M.; Seiter, N.J.; Stewart, S.D.; Towles, T.; Kerns, D.L. Development of economic thresholds toward bollworm (Lepidoptera: Noctuidae), management in Bt cotton, and assessment of the benefits from treating Bt cotton with insecticide. J. Econ. Entomol. 2021, 114, 2493–2504. [Google Scholar] [CrossRef]
- Musser, F.R.; Catchot, B.D. Insecticide resistance monitoring for bollworm and soybean looper. Proceedings of the Beltwide Cotton Conference, Memphis, TN, 2022.
- Allen, K.C.; Little, N.S.; Perera, O.P. Susceptibilities of Helicoverpa zea (Lepidoptera: Noctuidae) populations from the Mississippi Delta to a Diamide insecticide. J. Econ. Entomol. 2023, 116, 160–176. [Google Scholar] [CrossRef]
- Stomph, T.; Dordas, C.; Baranger, A.; Rijk, J.; Dong, B.; Evers, J.; Gu, C.; Li, L.; Simon, J.; Jensen, E.S.; Wang, Q.; Wang, Y.; Wang, Z.; Xu, H.; Zhang, C.; Zhang, L.; Zhang, W-P.; Bedoussac, L.; van der Werf, W. Adv. Agron. 2020, 160, 1–50. [CrossRef]
- Smith, H.A.; McSorley, R. Intercropping and pest management: A review of major concepts. Am. Entomol., 2000, 46, 154–161. [Google Scholar] [CrossRef]
- VanTine, M.; Verlinden, S. Managing insects and disease damage under an organic system. Extension service, West Virginia University, Morgantown, WV: 2003. (https://organic.wvu.edu/files/d/f47962a9-d1e3-4bc8-a4d3-b7728548c30a/insects.pdf). (Accessed 3 April 2023).
- Jones, G.A.; Gillett, J.L. Intercropping with sunflowers to attract beneficial insects in organic agriculture. Fla. Entomol. 2005, 88, 91–96. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C. Can we make crops more attractive to the natural enemies of herbivores? Entomol. Ornithol. Herpetol. 2012, 1, e102. [Google Scholar] [CrossRef]
- King, E.G.; Coleman, R.J. Potential for biological control of Heliothis species. Ann. Rev. Entomol. 1989, 34, 53–75. [Google Scholar] [CrossRef]
- Tillman, P.G.; Mullinix, B.G. Grain sorghum as a trap crop for corn earworm (Lepidoptera: Noctuidae) in cotton. Environ. Entomol., 2004, 33, 1371–1380. [Google Scholar] [CrossRef]
- Knutson, A.; Ruberson, J. Field Guide to predators, parasites and pathogens attacking insect and mite pests of cotton. Texas A&M University extension publication E-357, College Station, TX, 2005. (https://www.researchgate.net/publication/26904773_Field_Guide_to_Predators_Parasites_and_Pathogens_Attacking_Insect_and_Mite_Pests_of_Cotton_Recognizing_the_Good_Bugs_in_Cotton). (Accessed 22 February 2021).
- Safarzoda, S.; Bahlai, C.A; Fox, A.F.; Landis, D.A. The role of natural enemy foraging guilds in controlling cereal aphids in Michigan wheat. PLoS ONE, 2014, 9, e114230. [Google Scholar] [CrossRef]
- Ratnadass, A.; Togola, M.; Cissé, B.; Vassal, J.M. Potential of sorghum and physic nut (Jatropha curcas) for management of plant bugs (Hemiptera: Miridae) and cotton bollworm (Helicoverpa armigera) on cotton in an assisted trap-cropping strategy. SAT eJournal, 2009, 7, 1–7. [Google Scholar]
- Piñero, J.C. , Manandhar, R. Effects of increased crop diversity using trap crops, flowering plants, and living mulches on vegetable insect pests. Trends Entomol. 2015, 11, 91–109. [Google Scholar]
- Sarkar, S.C.; Wang, E.; Wu, S.; Lei, Z. Application of trap cropping as companion plants for the management of agricultural pests: A review. Insects, 2018, 9, 128. [Google Scholar] [CrossRef]
- Duraimurugan, P.; Regupathy, A. Push-pull strategy with trap crops, neem and nuclear polyhedrosis virus for insecticide resistance management in Helicoverpa armigera (Hubner) in cotton. Amer. J. of Applied Sci. 2005, 2, 1042–1048. [Google Scholar]
- Roome, R.E. Field trials with a nuclear polyhedrosis virus and Bacillus thuringiensis against larvae of Heliothis armigera (Hb.) (Lepidoptera, Noctuidae) on sorghum and cotton in Botswana. Bull. Entomol. Res. 1975, 65, 507–514. [Google Scholar] [CrossRef]
- Roome, R.E.; Daoust, R.A. Survival of the nuclear polyhedrosis virus of Heliothis armigera on crops and in soil in Botswana. J. Invert. Path., 1975, 27, 7–12. [Google Scholar] [CrossRef]
- Gettig, R.R.; McCarthy, W.J. Genotypic variation among wild isolates of Heliothis spp nuclear polyhedrosis viruses from different geographical regions. Virology. 1982, 117, 245–252. [Google Scholar] [CrossRef]
- Black, J.L.; Lorenz, G.M.; Cato, A.J.; Bateman, N.R.; Seiter, N.J. Efficacy of Helicoverpa armigera Nucleopolyhedrovirus on Soybean for Control of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in Arkansas Agriculture. Insects. 2022, 13, 91. [Google Scholar] [CrossRef]
- Black, J.L.; Lorenz, G.M.; Cato, A.J.; Faske, T.R.; Popham, H.J.R.; Paddock, K.J.; Bateman, N.R.; Seiter, N.J. Field Studies on the horizontal transmission potential by voluntary and involuntary carriers of Helicoverpa armigera nucleopolyhedrovirus (Baculoviridae). J. Econ. Entomol. 2019, 112, 1098–1104. [Google Scholar] [CrossRef]
- Yearian, W.C.; Young, S.Y. Persistence of Heliothis nuclear polyhedrosis virus on cotton plant parts. Environ. Entomol., 1974, 3, 1035–1036. [Google Scholar] [CrossRef]
- Young, S.Y.; Yearian, W.C.; Kim, K. S. Effect of dew from cotton and soybean foliage on activity of Heliothis nuclear polyhedrosis virus. J. Invert. Path., 1977, 29, 105–111. [Google Scholar] [CrossRef]
- McLeod, P.J.; Yearian, W.C.; Young III, S.Y. Inactivation of Baculovirus heliothis by ultraviolet irradiation, dew, and temperature. J. Invertebr. Pathol. 1977, 30, 237–241. [Google Scholar] [CrossRef]
- Merchant, M.E.; Teetes, G.L. Evaluation of selected sampling methods for panicle infesting insect pests of sorghum. J. Econ. Entomol. 1992, 85, 2418–2424. [Google Scholar] [CrossRef]
- Knutson, A.E.; Muegge, M.A.; Wilson, L.T.; Naranjo, S.E. Evaluation of sampling methods and development of sample plans for estimating predator densities in cotton. J. Econom. Entomol. 2008, 101, 1501–1509. [Google Scholar] [CrossRef]
- SAS Institute. User’s manual. SAS Institute, Cary, NC, USA: 2013. Version 13.1.
- Calvin, W.; Yang, F.; Kennedy, H.; Marcon, P.G.; Kerns, D.L. Susceptibility of field and laboratory Bt-susceptible and resistant strains of Helicoverpa zea to HearNPV. Plants. 2024, 13, 529. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).