Submitted:
04 July 2024
Posted:
05 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
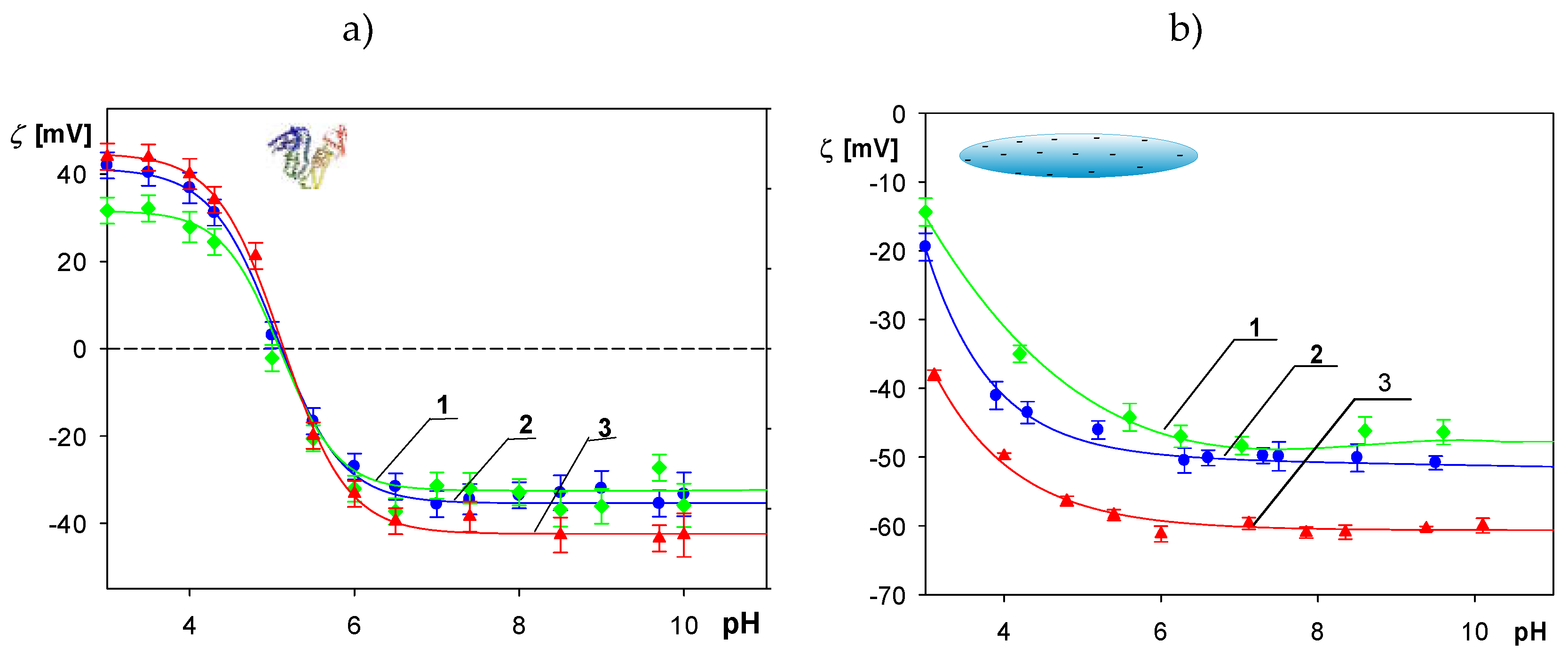
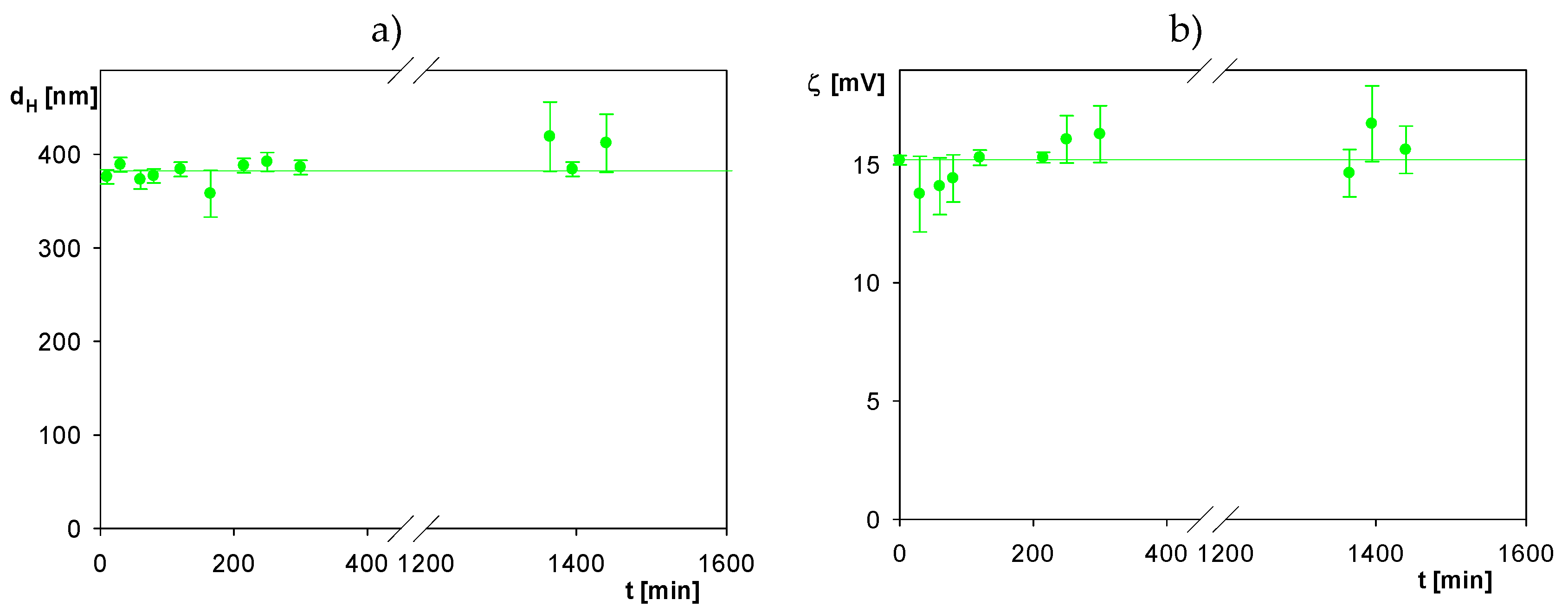
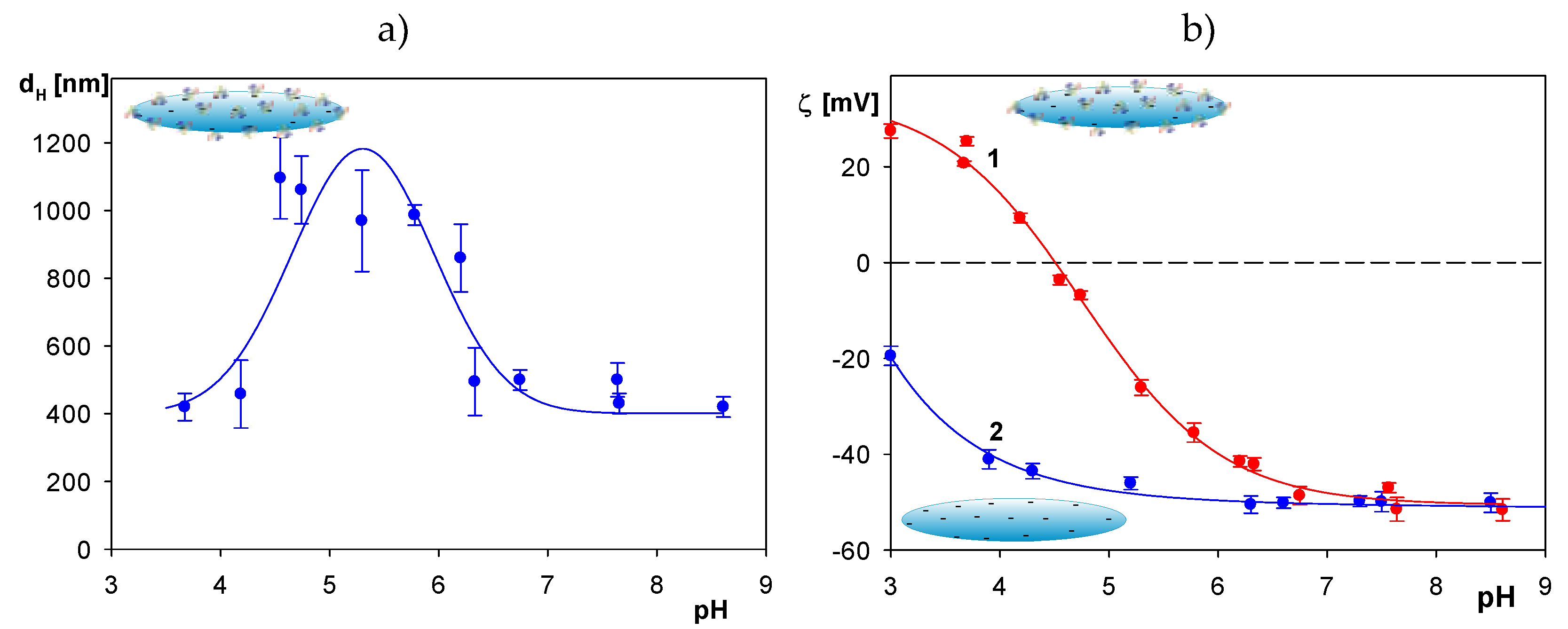
2.1. Physicochemical Characteristics of Albumin and Polymer Particles
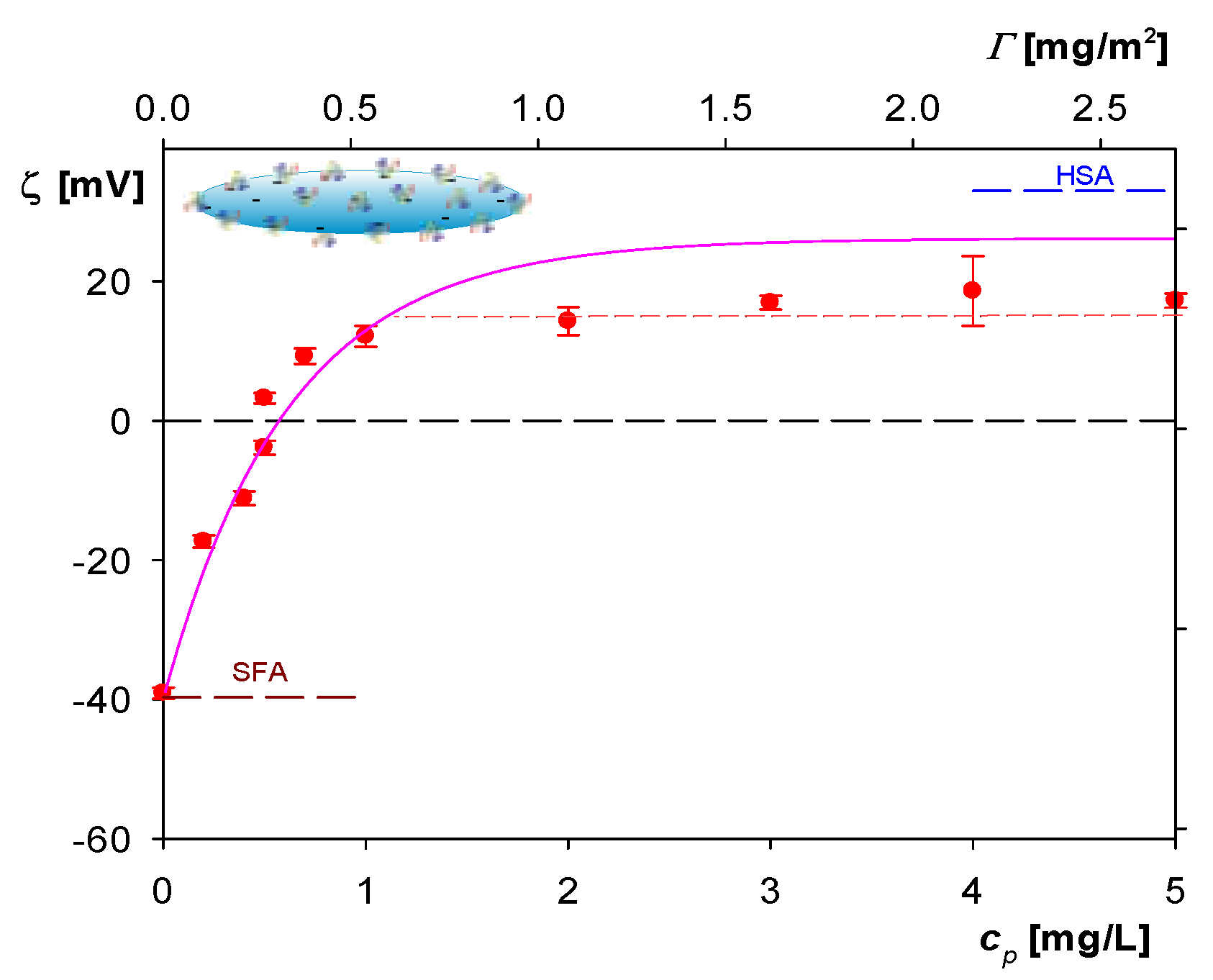
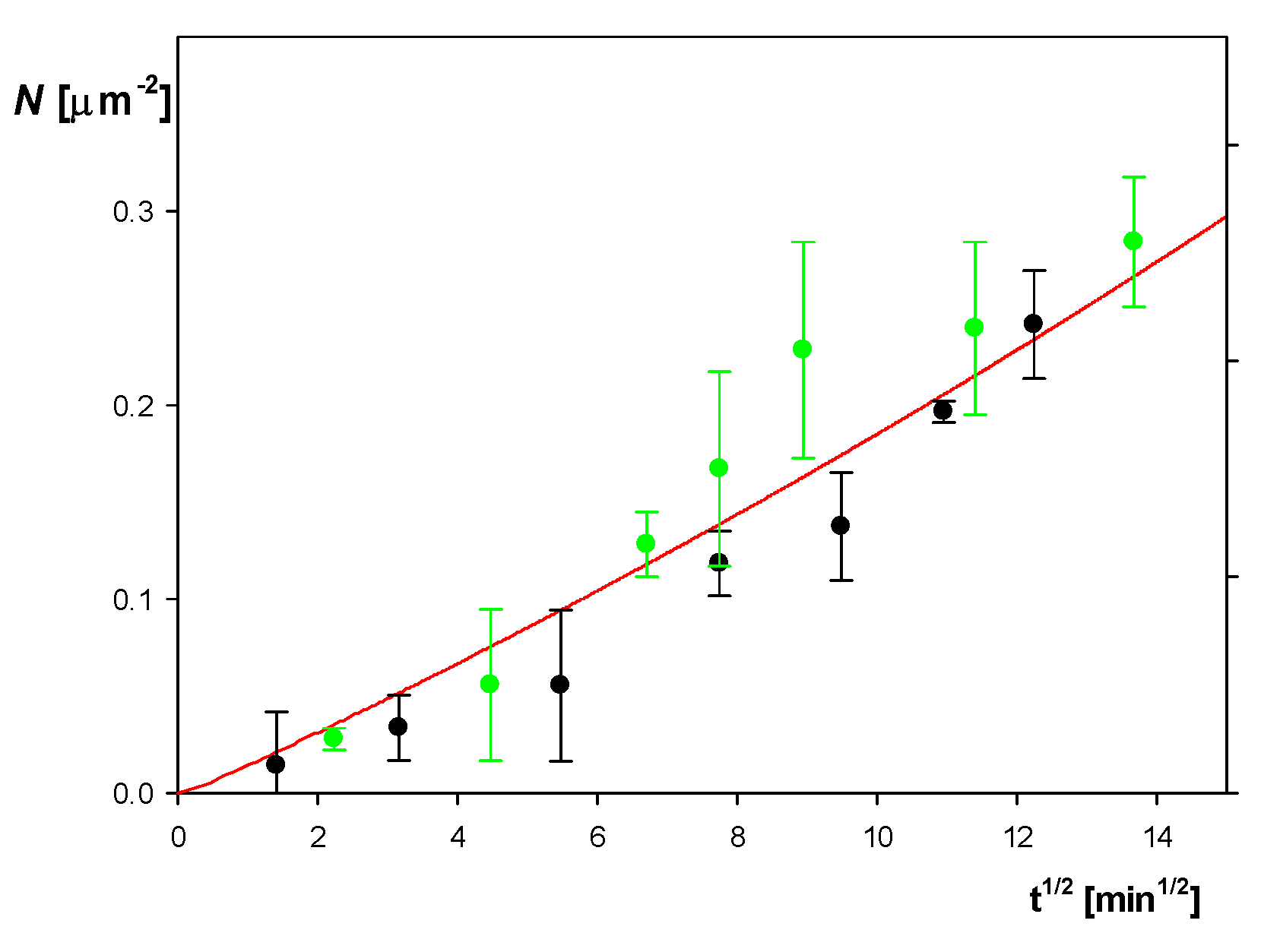
2.2. Formation of Albumin Corona at Polymer Particles
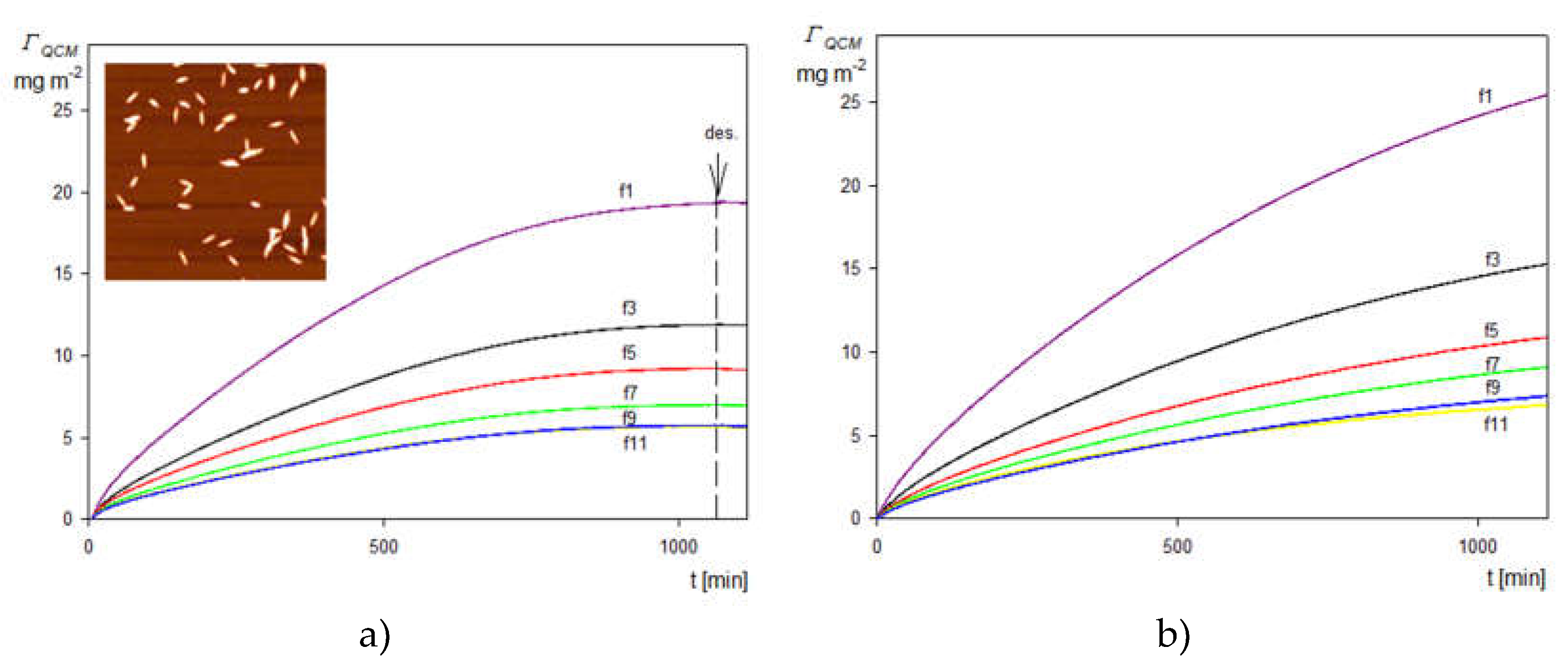
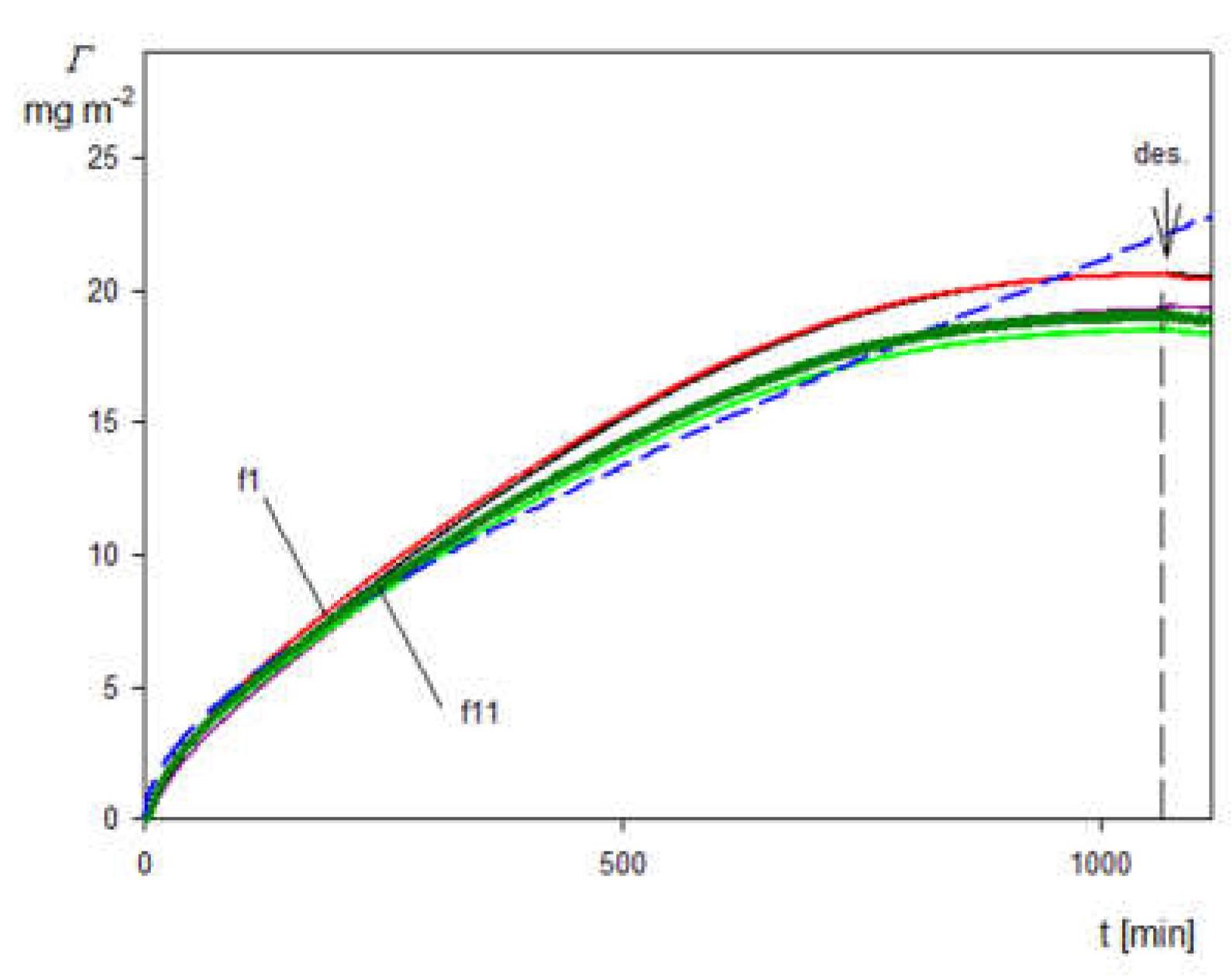
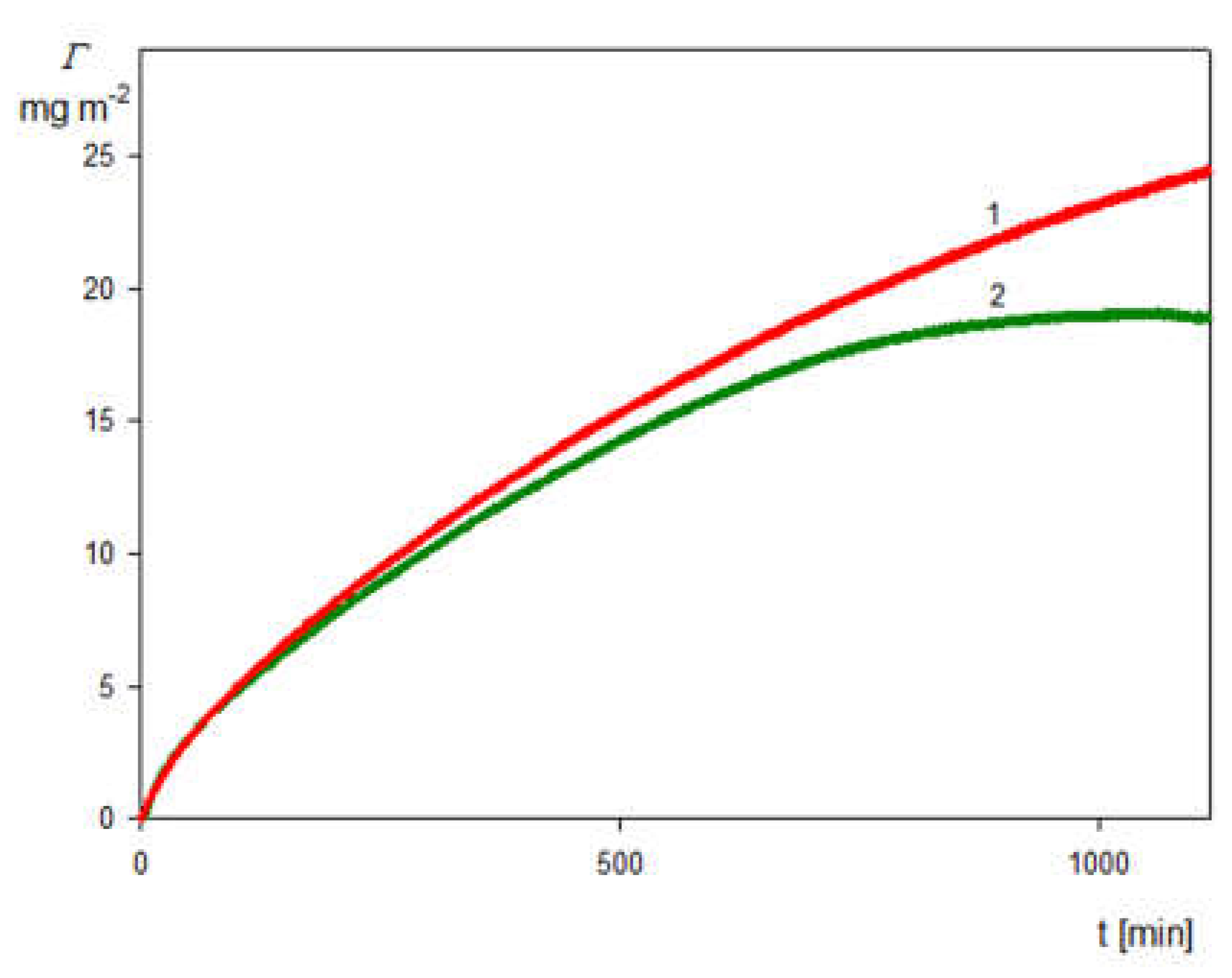
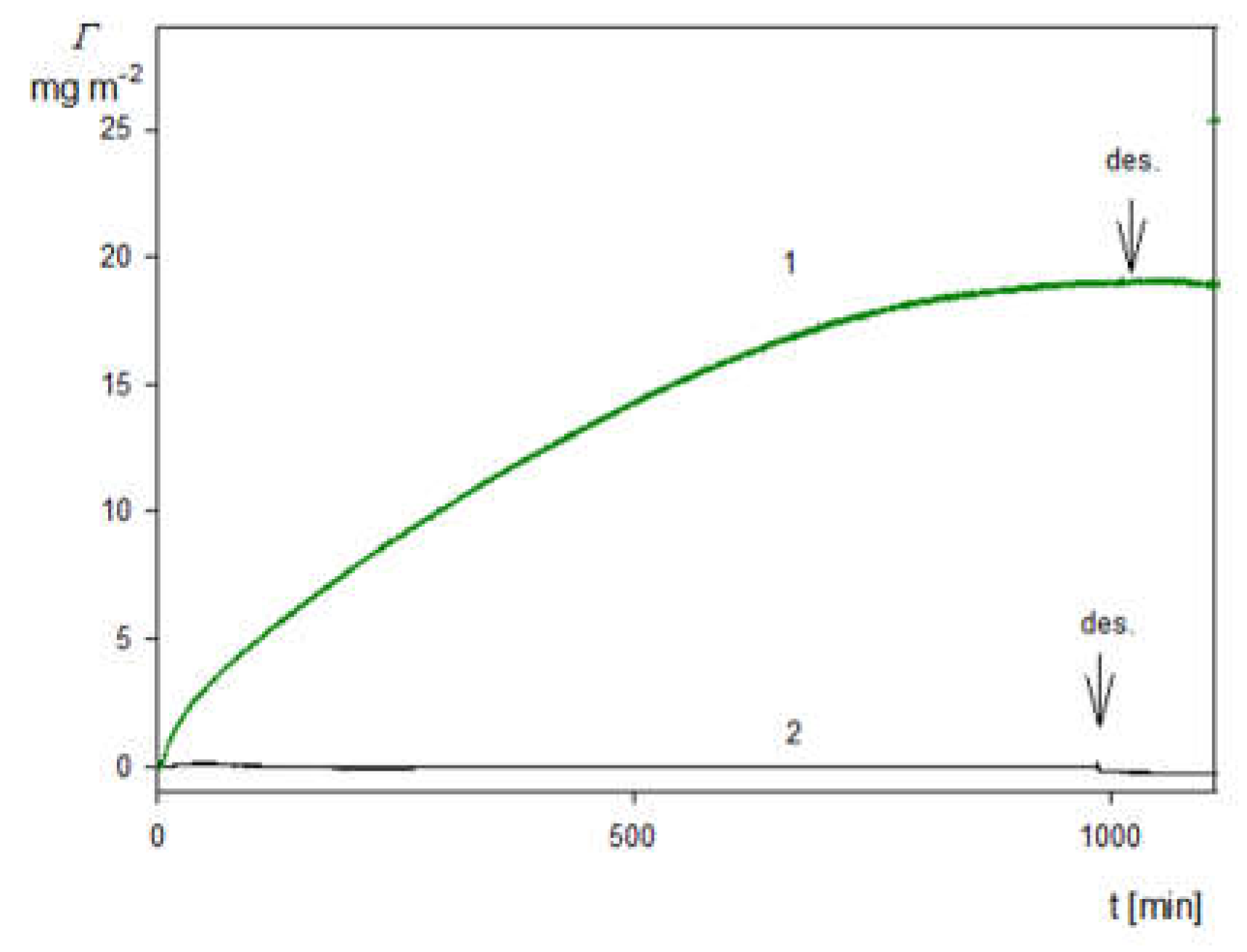
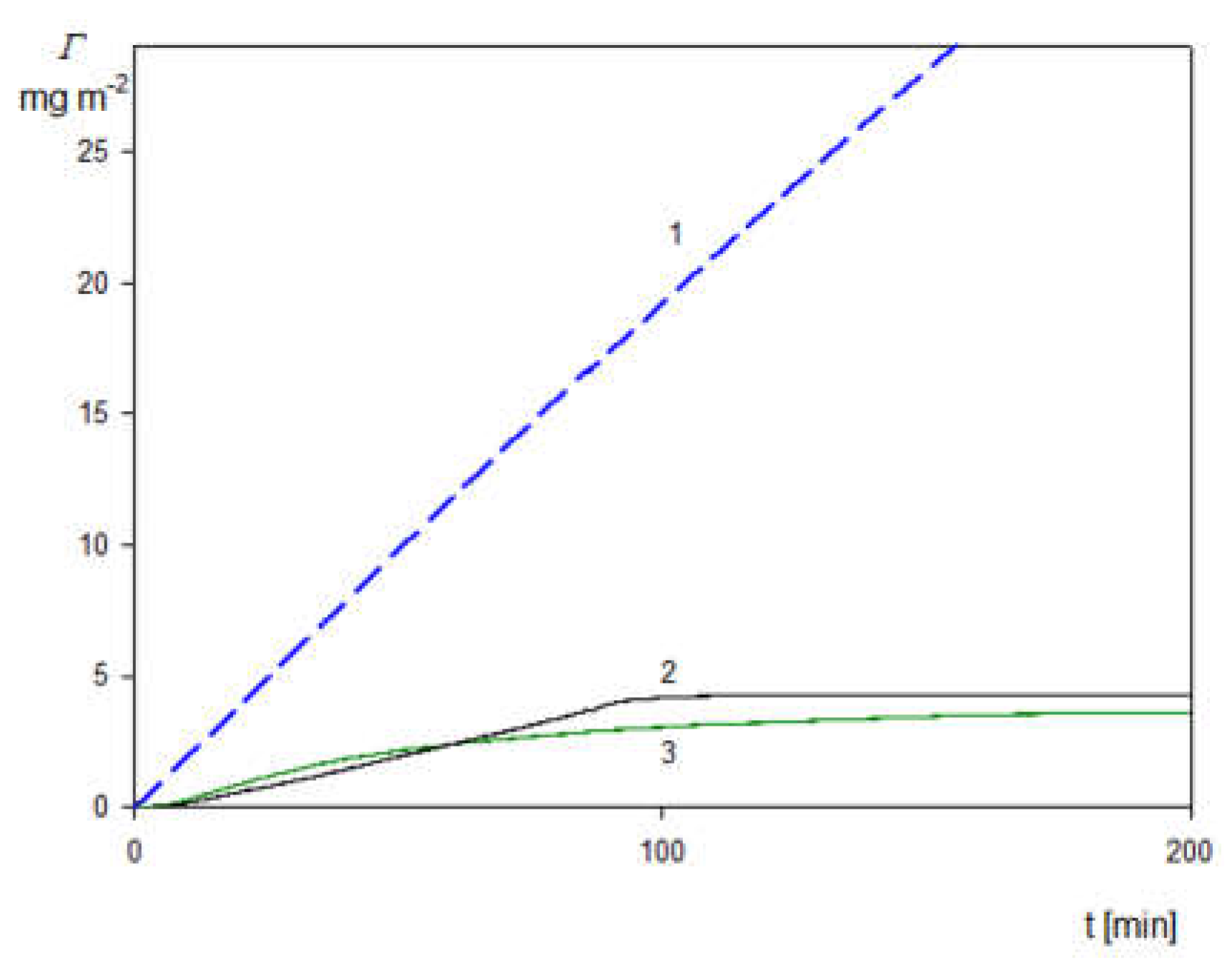
2.3. Deposition of the SHSA Particles on Abiotic Surfaces
3. Materials and Methods
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dávalos-Pantoja, L.; Ortega-Vinuesa, J.L.; Bastos-González, D.; Hidalgo- Álvarez, R. Colloidal stability of IgG- and IgY-coated latex microspheres. Colloids Surf. B: Biointerfaces. 2001, 20, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rodríguez, A.; Ortega-Vinuesa, J.L.; Hidalgo-Álvarez, R. Interfacial electrokinetics and electrophoresis. In Surfactant Science, 1st ed.; Á.V. Delgado (Ed.); Publisher: Marcel Dekker, Inc., New York, Basel, 2002, pp. 641–670. [CrossRef]
- Kawaguchi, H., Latex Diagnosis, Encyclopedia of Biocolloid and Biointerface Science 2V Set, 1st ed.; Hiroyuki Ohshima (Ed.); John Wiley& Sons INC, 2016, Volume 2, Chapter 50. ISBN: 978-1-118-54276-7.
- Chen, P.; Ding, F.; Cai, R.; Javed, I.; Yang, W.; Zhang, Z.; Li, Y.; Davis, Th.P.; Ke, P.Ch.; Chen, Ch. Amyloidosis inhibition, a new frontier of the protein corona. Nano Today. 2020, 35, 100937. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggard, T.; Thulin, E.; Nilsson, H.; Dawson, K.D.; Linse, S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. PNAS. 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Milani, S.; Bombelli, F.B.; Pitek, A.S.; Dawson, K.A.; Radler, J. Reversible Versus Irreversible Binding of Transferrin to Polystyrene Nanoparticles: Soft and Hard Coronas. ACS Nano. 2012, 6, 2432–2541. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Aberg, Ch.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Treuel, L.; Brandholt, S.; Maffre, P.; Wiegele, S.; Shang, L.; Nienhaus, G.U. Impact of Protein Modificationon the Protein Corona on Nanoparticles and Nanoparticle-Cell Interactions. ACS Nano 2014, 8, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Choi, E.J.; Webster, T.J.; Kim, S.H.; Khang, D. Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int. J. Nanomed. 2015, 10, 97–113. [Google Scholar] [CrossRef]
- Winzen, S.; Schoettler, S.; Baier, G.; Rosenauer, C.; Mailaender, V.; Landfestera, K.; Mohr, K. Complementary Analysis of the Hard and Soft Protein Corona: Sample Preparation Critically Effects Corona Composition. Nanoscale 2015, 7, 2992–3001. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, F.; Garry, D.; Monopoli, M.P.; Salvati, A.; Dawson, K.A. The Intracellular Destiny of the Protein Corona: A Study on its Cellular Internalization and Evolution. ACS Nano. 2016, 10, 10471–10479. [Google Scholar] [CrossRef]
- Schöttler, S.; Landfester, K.; Mailänder, V. Controlling the Stealth Effect of Nanocarriers through Understanding the Protein Corona. Angew. Chem. Int. Ed. 2016, 55, 8806–8815. [Google Scholar] [CrossRef] [PubMed]
- Kokkinopoulou, M.; Simon, J.; Landfester, K.; Mailänder, V.; Lieberwirth, I. Visualization of the protein corona: towards a biomolecular understanding of nanoparticle-cell-interactions. Nanoscale. 2017, 9, 8858–8870. [Google Scholar] [CrossRef] [PubMed]
- Baimanov, D.; Cai, R.; Chen, Ch. Understanding the Chemical Nature of Nanoparticle-Protein Interactions. Bioconjugate Chem. 2019, 30, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.C.G.; Krüger, K.; Besford, Q.A.; Schlenk, M.; Kempe, K.; Förster, S.; Caruso, F. In Situ Characterization of Protein Corona Formation on Silica Microparticles Using Confocal Laser Scanning Microscopy Combined with Microfluidics. ACS Appl. Mater. Interfaces. 2019, 11, 2459–2469. [Google Scholar] [CrossRef]
- Marichal, L.; Giraudon-Colas, G.; Cousin, F.; Thill, A.; Labarre, J.; Boulard, Y.; Aude, J.Ch.; Pin, S.; Renault, J.P. Protein-Nanoparticle Interactions: What Are the Protein-Corona Thickness and Organization? Langmuir 2019, 35, 10831–10837. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Li.; Gao, J.; Liu, Y.; Gao, J.; Yao, L.; Yang, X.; Liu, X.; He, B.; Hu, L.; Shi, J.; Song, M.; Qu, G.; Jiang, G. Role of protein corona in the biological effect of nanomaterials: Investigating methods. Trends Anal. Chem. 2019, 118, 303–314. [Google Scholar] [CrossRef]
- Wang, H.; Ma, R.; Nienhaus, K.; Nienhaus, G.U. Formation of a Monolayer Protein Corona around Polystyrene Nanoparticles and Implications for Nanoparticle Agglomeration. Small. 2019, 15, 1900974. [Google Scholar] [CrossRef]
- Malaspina, D.C.; Pérez-Fuentes, L.; Drummond, C.; Bastos-González, D.; Faraudo, J. Protein-surface interactions at the nanoscale: Atomistic simulations with implicit solvent models, Curr. Opin. Colloid Interface Sci. 2019, 41, 40–49. [Google Scholar] [CrossRef]
- Lee, J.G.; Lannigan, K.; Shelton, W.A.; Meissner, J.; Bharti, B. Adsorption of Myoglobin and Corona Formation on Silica Nanoparticles. Langmuir. 2020, 36, 14157–14165. [Google Scholar] [CrossRef]
- Nienhaus, K.; Wang, H.; Nienhaus, G.U. Nanoparticles for biomedical applications: exploring and exploiting molecular interactions at the nano-bio interface. Mater. Today Adv. 2020, 5, 100036. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Tang, Q.; Yin, D.; Tang, Ch.; He, E.; Zou, L.; Peng, Q. The protein corona and its effects on nanoparticle-based drug delivery systems. Acta Biomater. 2021, 129, 57–72. [Google Scholar] [CrossRef]
- Martínez-Negro, M.; González-Rubio, G.; Aicart, E.; Landfester, K.; Guerrero-Martínez, A.; Junquera, E. Insights into colloidal nanoparticle-protein corona interactions for nanomedicine applications. Adv. Colloid Interface Sci. 2021, 289, 102366. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Ahmad, A.; Vyawahare, A.; Alam, P.; Khan, T.H.; Khana, R. Biological effects of formation of protein corona onto nanoparticles, Int. J. Biol. Macromol. 2021, 175, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Niaz, T. , Sarkar, A.; Mackie, A.; Imran, M. Impact of albumin corona onmucoadhesion and antimicrobial activity of carvacrol loaded chitosan nano-delivery systems under simulated gastro-intestinal conditions. Int. J. Biol. Macromol. 2021, 169, 171–182. [Google Scholar] [CrossRef]
- Farshbaf, M.; Valizadeh, H.; Panahi, Y.; Fatahi, Y.; Chen, M.; Zarebkohan, A.; Gao, H. The impact of protein corona on the biological behavior of targeting nanomedicines. Int. J. Pharm. 2022, 614, 121458. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhong, Z.; Huang, Z.; Fu, F.; Wang, W.; Wu, L.; Huang, Y.; Wu, Ch.; Pan, X. Two different protein corona formation modes on Soluplus® nanomicelles. Colloids Surf. B: Biointerfaces. 2022, 218, 112744. [Google Scholar] [CrossRef] [PubMed]
- Żeliszewska, P.; Wasilewska, M.; Cieśla, M.; Adamczyk, Z. Deposition of Polymer Particles with Fibrinogen Corona at Abiotic Surfaces under Flow Condition. , Molecules 2021, 26, 6299. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Nattich-Rak, M.; Dąbkowska, M.; Kujda-Kruk, M. Albumin Adsorption at Solid Substrates; A Quest for a Unified Approach. J. Colloid Interface Sci. 2018, 514, 769–790. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Sadlej, K.; Wajnryb, E.; Nattich, M.; Ekiel-Jeżewska, M.; Bławzdziewicz, J. Streaming Potential Studiem of Colloid, Polyelectrolytes and Protein Deposition. Adv. Colloid Interface Sci. 2010, 153, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Dogic, Z.; Fraden, S. Ordered phases of filamentous viruses. Curr. Opin. Colloid Interface Sci. 2006, 11, 47–55. [Google Scholar] [CrossRef]
- Buitenhuis, J. Electrophoresis of fd-virus particles: experiments and an analysis of the effect of finite rod lengths. Langmuir. 2012, 28, 13354–13363. [Google Scholar] [CrossRef]
- Armanious, A.; Aeppli, M.; Jacak, R.; Refardt, D.; Sigstam, T.; Kohn, T.; Sander, M. Viruses at Solid–Water Interfaces: A Systematic Assessment of Interactions Driving Adsorption. Environ. Sci. Technol. 2016, 50, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.T.T.; Tarabara, V.V. Virus deposition onto polyelectrolyte-coated surfaces: A study with bacteriophage MS2. J. Colloid Interface Sci. 2019, 540, 155–166. [Google Scholar] [CrossRef] [PubMed]
- de Martín Garrido, N.; Crone, M.A.; Ramlaul, K.; Simpson, P.A.; Freemont, P.S.; Aylett, CH.H.S. Bacteriophage MS2 displays unreported capsid variability assembling T = 4 and mixed capsids. Mol. Microbiol. 2020, 113, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Turoňová, B.; Sikora, M.; Schürmann, C.; Hagen, W.J.H.; Welsch, S.; Blanc, F.E.C.; von Bülow, S.; Gecht, M.; Bagola, K.; Hörner, C.; van Zandbergen, G.; Landry, J.; de Azevedo, N.T.D.; Mosalaganti, S.; Schwarz, A.; Covino, R.; Mühlebach, M.D.; Hummer, G.; Locker, J.K.; Beck, M. In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges. Science. 2020, 370, 203–208. [Google Scholar] [CrossRef]
- Ken, Z.; Oton, J.; Qu, K.; Cortese, M.; Zila, V.; McKeane, L.; Nakane, T.; Zivanov, J.; Neufeldt, C.J.; Cerikan, B.; Lu, J.M.; Peukes, J.; Xiong, X.; Kräusslich, H.-G.; Scheres, S.H.W.; Bartenschlager, R.; Briggs, J.A.G. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020, 588, 498–502. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y.; et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278. [Google Scholar] [CrossRef]
- Mishra, P.M.; Uversky, V.N.; Nandi, C.K. Serum albumin-mediated strategy for the effective targeting of SARS-CoV-2. Medical Hypotheses 2020, 140, 109790. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Batys, P.; Barbasz, J. SARS-CoV-2 virion physicochemical characteristics pertinent to abiotic substrate attachment, Curr. Opin. Colloid Interface Sci. 2021, 55, 101466. [Google Scholar] [CrossRef]
- Wang, Y.; Hammes, F.; Duggelin, M.; Egli, Th. Influence of Size, Shape, and Flexibility on Bacterial Passage through Micropore Membrane Filters. Environ. Sci. Technol. 2008, 42, 6749–6754. [Google Scholar] [CrossRef]
- Ngo, V.K.Th.; Nguyen, D.G.; Nguyen, H.P.U.; Tran, V.M.; Nguyen, Th.Kh.M.; Huynh, T.P.; Lam, Q.V.; Huynh, Th.D.; Truong, Th.N.L. Quartz crystal microbalance (QCM) as biosensor for the detecting of Escherichia coli O157:H7. Adv. Nat. Sci.: Nanosci. Nanotechnol. 2014, 5, 045004. [Google Scholar] [CrossRef]
- Strauss, J.; Kadilak, A.; Cronin, Ch.; Mello, Ch.M.; Camesano, T.A. Binding, inactivation, and adhesion forces between antimicrobial peptide cecropin P1 and pathogenic E. coli. Colloids Surf. B: Biointerfaces. 2010, 75, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.L.J.; Wargenau, A.; Tufenkji, N. Optimizing Bacteriophage Surface Densities for Bacterial Capture and Sensing in Quartz Crystal Microbalance with Dissipation Monitoring. ACS Appl. Mater. Interfaces. 2016, 8, 13698–13706. [Google Scholar] [CrossRef] [PubMed]
- Taneja, P.; Manjuladevi, V.; Gupta, R. K.; Kumar, S.; Gupta, K. K. Facile ultrathin film of silver nanoparticles for bacteria sensing. Colloids Surf. B: Biointerfaces. 2020, 196, 111335. [Google Scholar] [CrossRef] [PubMed]
- Peters Jr, T. All About Albumin: Biochemistry. Genetics and Medical Applications. Academic Press. Elsevier, Amsterdam, Netherlands 1996. ISBN 0080527043, 9780080527048.
- Kragh-Hansen, U., Minchiotti, L., Galliano, M., Peters Jr. T. Human serum albumin isoforms: genetic and molecular aspects and functional consequences. Biochim. Biophys. Acta. 2013, 1830, 5405–5417. [CrossRef] [PubMed]
- Adamczyk, Z.; Morga, M.; Nattich-Rak, M.; Sadowska, M. Nanoparticle and bioparticle deposition kinetics. Adv. Colloid Interface Sci. 2022, 302, 102630. [Google Scholar] [CrossRef] [PubMed]
- Nattich-Rak, M.; Sadowska, M.; Motyczyńska, M.; Adamczyk, Z. Mimicking Pseudo-Virion Interactions with Abiotic Surfaces: Deposition of Polymer Nanoparticles with Albumin Corona. Biomolecules MDPI. 2022, 12, 1658. [Google Scholar] [CrossRef]
- Wasilewska, M.; Adamczyk, Z.; Pomorska, A.; Nattich-Rak, M.; Sadowska, M. Human Serum Albumin Adsorption Kinetics on Silica: Influence of Protein Solution Stability. Langmuir. 2019, 35, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Nattich-Rak, M.; Kosior, D.; Morga, M.; Adamczyk, Z. Kinetics of Human Serum Albumin Adsorption on Polycation Functionalized Silica. Biomolecules. 2024, 14, 531. [Google Scholar] [CrossRef] [PubMed]
- Höök, F.; Vörös, J.; Rhodahl, M.; Kurrat, R.; Böni, P.; Ramsden, J.J.; Textor, M.; Spencer, N.D.; Tengvall, P.; Gold, J.; Kasemo, B. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloids Surf. B: Biointerfaces. 2002, 24, 155–170. [Google Scholar] [CrossRef]
- Makaraviciute, A.; Ruzgas, T.; Ramanavicius, A.; Ramanaviciene, A. Antibody fragment immobilization on planar gold and gold nanoparticle modified quartz crystal microbalance with dissipation sensor surfaces for immunosensor applications. Anal. Methods. 2014, 6, 2134. [Google Scholar] [CrossRef]
- Plikusiene, I.; Maciulis, V.; Juciute, S.; Ramanavicius, A.; Balevicius, Z.; Slibinskas, R.; Kucinskaite-Kodze, I.; Simanavicius, M.; Balevicius, S.; Ramanaviciene, A. Investigation of SARS-CoV-2 nucleocapsid protein interaction with a specific antibody by combined spectroscopic ellipsometry and quartz crystal microbalance with dissipation. J. Colloid Interface Sci. 2022, 626, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Sadowska, M.; Żeliszewska, P. Applicability of QCM-D for Quantitative Measurements of Nano- and Microparticle Deposition Kinetics: Theoretical Modeling and Experiments. Anal. Chem. 2020, 92, 15087–15095. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, M.; Nattich-Rak, M.; Morga, M.; Adamczyk, Z.; Basinska, T.; Mickiewicz, D.; Gadzinowski, M. Anisotropic Particle Deposition Kinetics from Quartz Crystal Microbalance Measurements: Beyond the Sphere Paradigm. Langmuir. 2024, 40, 7907−7919. [Google Scholar] [CrossRef] [PubMed]
- Tarnapolsky, A.; Freger, V. Modeling QCM-D response to deposition and attachment of microparticles and living cells. Anal. Chem. 2018, 90, 13960–13968. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, C.; Schmudde, M.; Noufele, C. N.; Graf, C.; Risse, T. Ordered structures of functionalized silica nanoparticles on gold surfaces: Correlation of quartz crystal microbalance with structural characterization. Anal. Chem. 2015, 87, 10642–10649. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Keller, A.; Odell, J.A.; Ottewill, R.H. Preparation of monodisperse ellipsoidal polystyrene particles. Colloid Polym. Sci. 1993, 271, 469–479. [Google Scholar] [CrossRef]
- Komar, P.; Gosecka, M.; Gadzinowski, M.; Gosecki, M.; Makowski, T.; Slomkowski, S.; Basinska, T. Core-shell spheroidal microparticles with polystyrene cores and rich in polyglycidol shells. Polymer. 2018, 146, 6–11. [Google Scholar] [CrossRef]
- Ohshima, H. Approximate Analytic Expression for the Electrophoretic Mobility of Moderately Charged Cylindrical Colloidal Particles. Langmuir. 2015, 31, 13633–13638. [Google Scholar] [CrossRef]
- Henry, D.C. The cataphoresis of suspended particles. Part I.—The equation of cataphoresis. Proc. R. Soc. A. London. 1931, 133. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




