Submitted:
04 July 2024
Posted:
04 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
2.1. Description of the System
2.2. Case of Study
2.3. Modelling
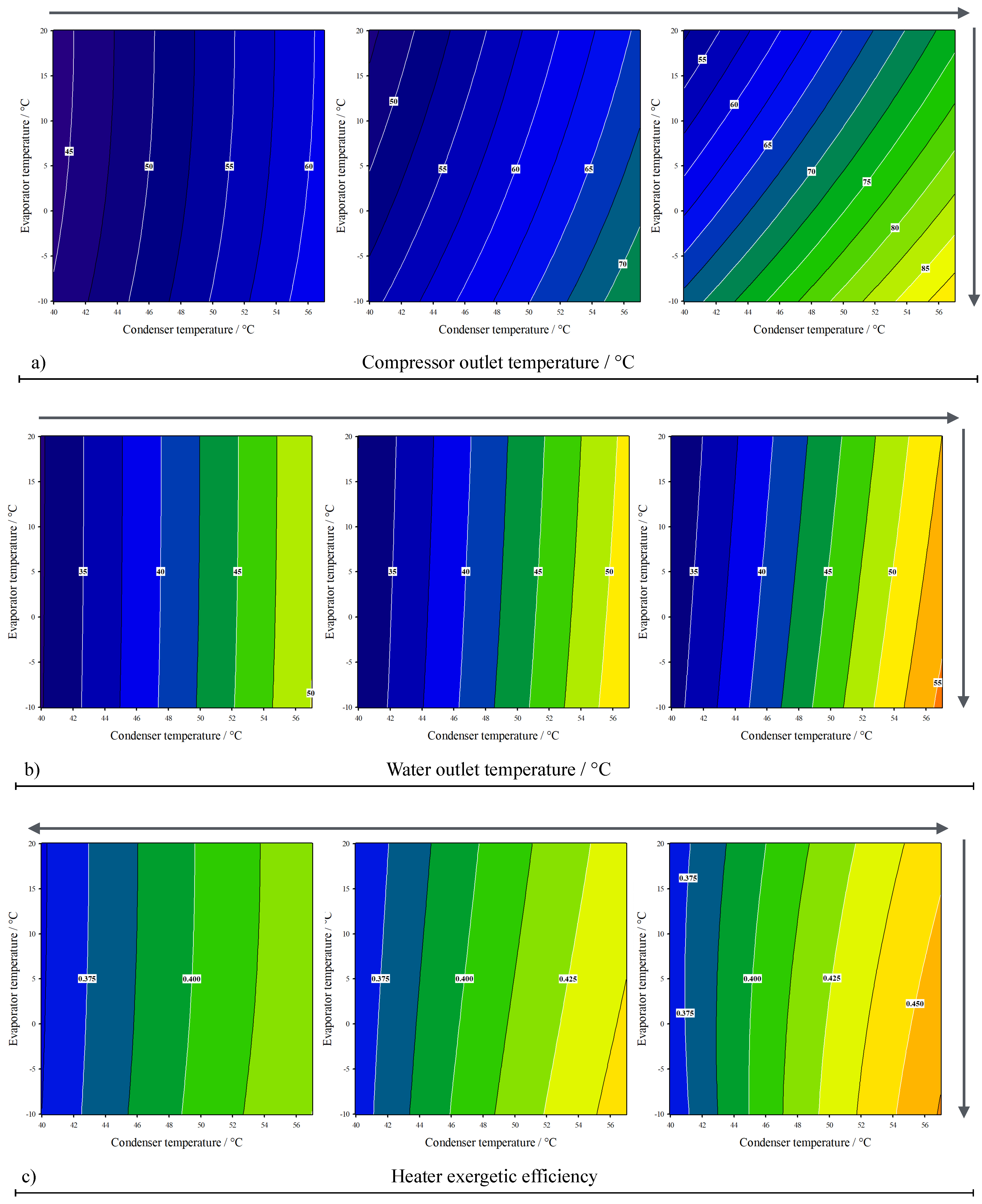
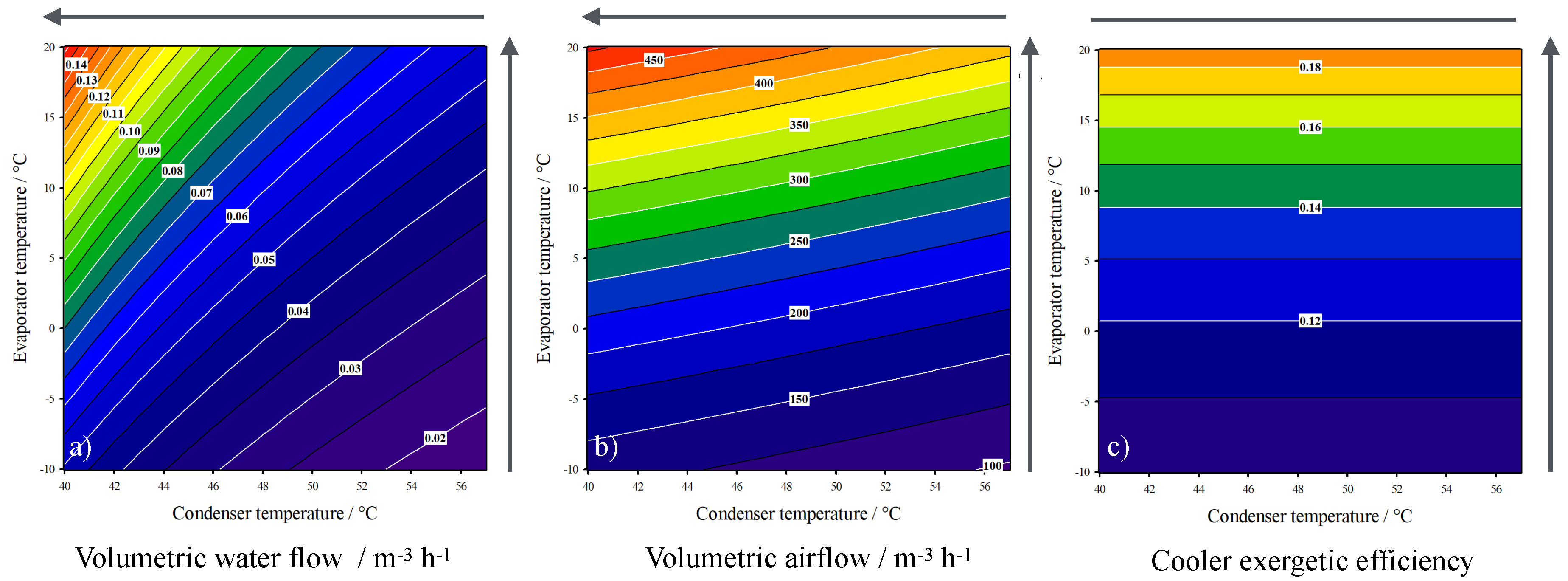
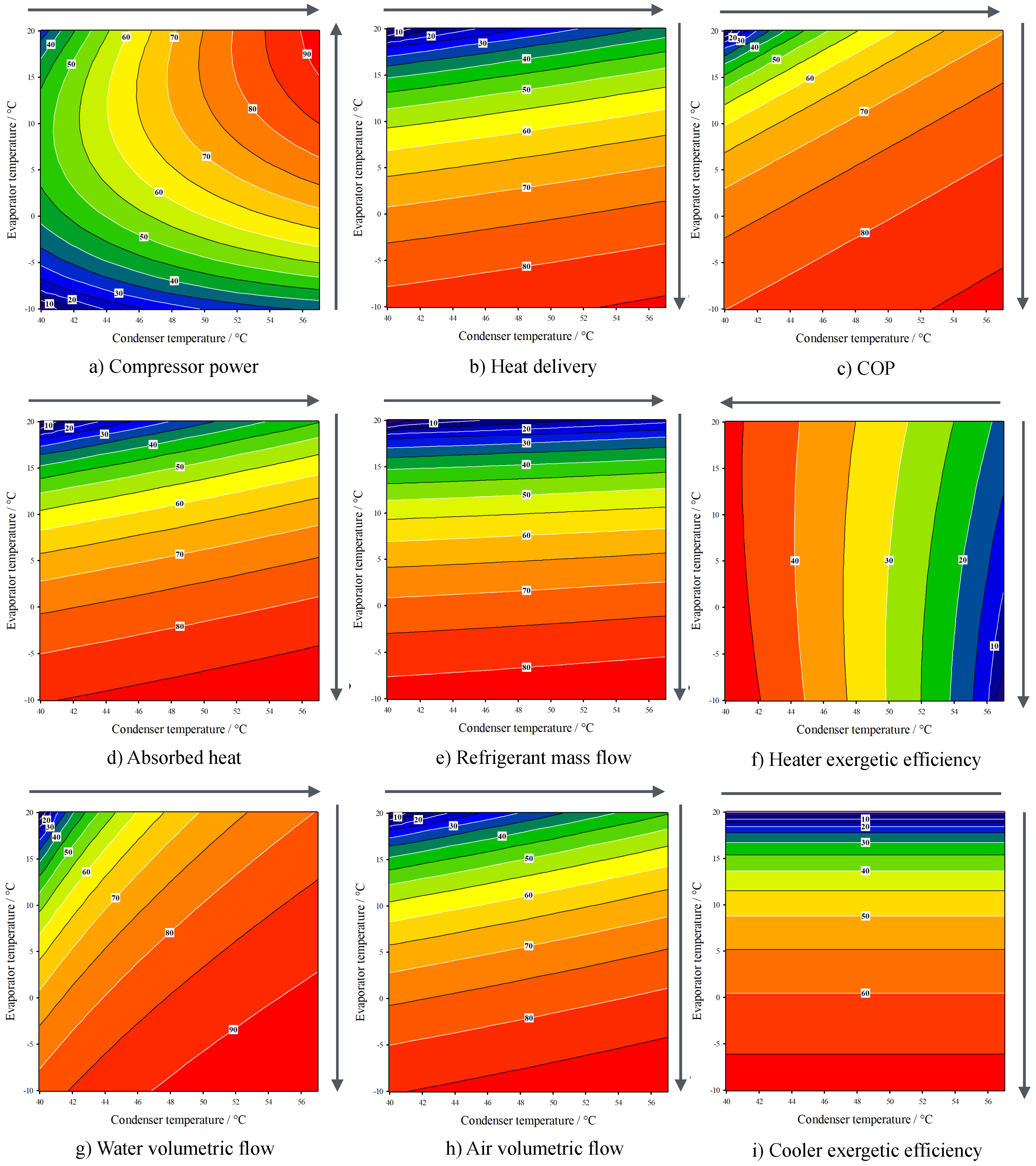
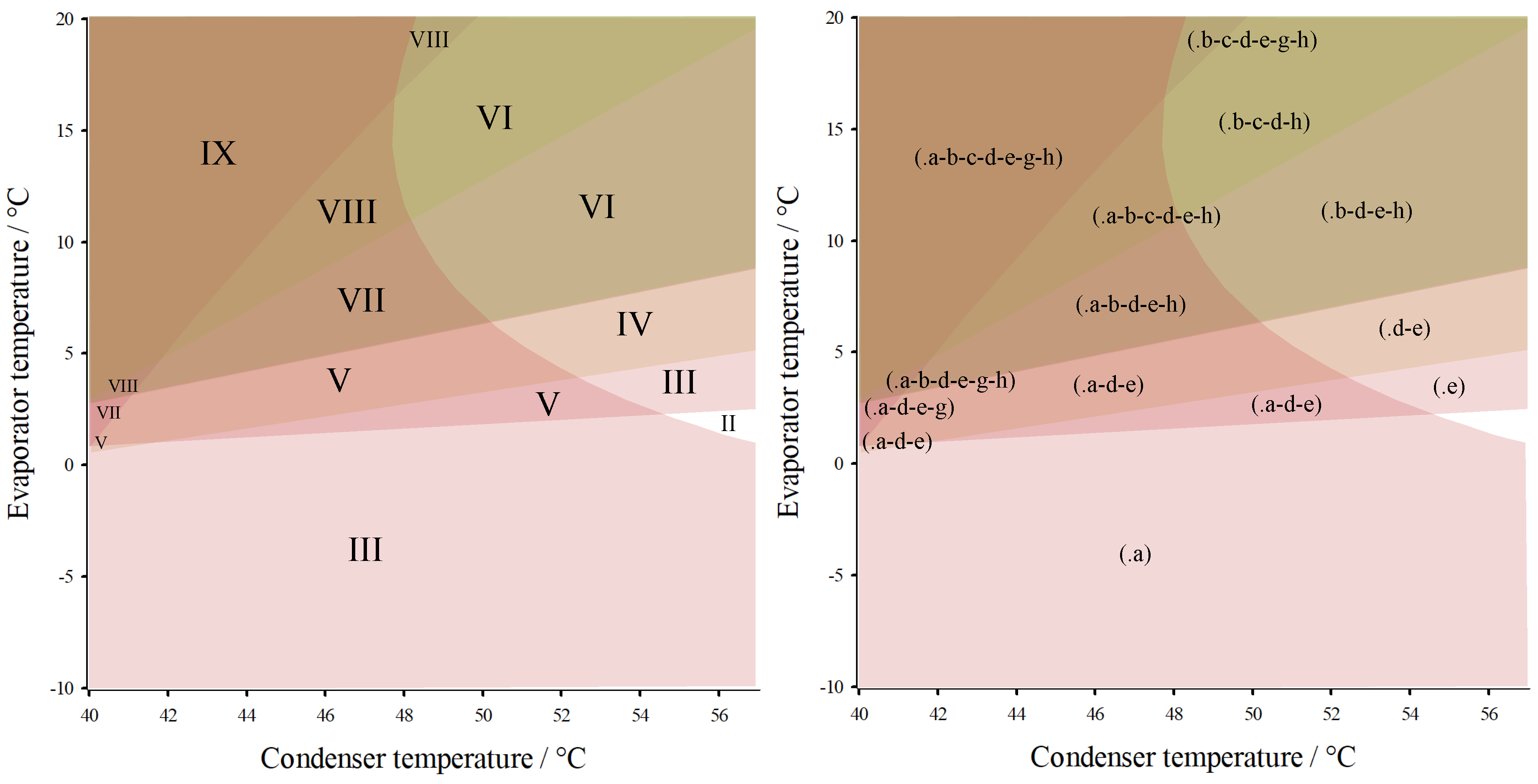
3. Results
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| COP | Coefficient of performance |
| EOS | Equation of state |
| HP | Heat Pump |
| LTTE | Low-temperature thermal energy |
| PC-SAFT | Pertubed-Chain Statistical Associating Fluid Theory |
References
- Al-Mansour, F. Energy efficiency trends and policy in Slovenia. Energy 2011, 36, 1868–1877. [CrossRef]
- de la Rue du Can, S.; Letschert, V.; Agarwal, S.; Park, W.Y.; Kaggwa, U. Energy efficiency improves energy access affordability. Energy for Sustainable Development 2022, 70, 560–568. [CrossRef]
- Chen, T.; Zhang, Y.; Jiang, C.; Li, H. How does energy efficiency affect employment? Evidence from Chinese cities. Energy 2023, 280, 128071. [CrossRef]
- González, J.; Llovell, F.; Garrido, J.M.; Quinteros-Lama, H. A rigorous approach for characterising the limiting optimal efficiency of working fluids in organic Rankine cycles. Energy 2022, 124191, 254. [CrossRef]
- Yekoladio, P.J.; Bello-Ochende, T.; Meyer, J.P. Thermodynamic analysis and performance optimization of organic rankine cycles for the conversion of low-to-moderate grade geothermal heat. International Journal of Energy Research 2015, 39, 1256–1271. [CrossRef]
- Peyrovedin, H.; Haghbakhsh, R.; Duarte, A.R.C.; Shariati, A. Deep Eutectic Solvents as Phase Change Materials in Solar Thermal Power Plants: Energy and Exergy Analyses. Molecules 2022, 27. [CrossRef]
- Daniarta, S.; Nemś, M.; Kolasiński, P.; Pomorski, M. Sizing the Thermal Energy Storage Device Utilizing Phase Change Material (PCM) for Low-Temperature Organic Rankine Cycle Systems Employing Selected Hydrocarbons. Energies 2022, 15. [CrossRef]
- Bellocchi, S.; Guizzi, G.L.; Manno, M.; Pentimalli, M.; Salvatori, M.; Zaccagnini, A. Adsorbent materials for low-grade waste heat recovery: Application to industrial pasta drying processes. Energy 2017, 140, 729–745. [CrossRef]
- Urbano, D.G.; Aquino, A.; Scrucca, F. Energy Performance, Environmental Impacts and Costs of a Drying System: Life Cycle Analysis of Conventional and Heat Recovery Scenarios. Energies 2023, 16, 1523. [CrossRef]
- Hervás-Blasco, E.; Navarro-Peris, E.; Barceló-Ruescas, F.; Corberán, J.M. Improved water to water heat pump design for low-temperature waste heat recovery based on subcooling control. International Journal of Refrigeration 2019, 106, 374–383. [CrossRef]
- Ammar, Y.; Joyce, S.; Norman, R.; Wang, Y.; Roskilly, A.P. Low grade thermal energy sources and uses from the process industry in the UK. Applied Energy 2012, 89, 3–20. [CrossRef]
- Bianchi, M.; De Pascale, A. Bottoming cycles for electric energy generation: Parametric investigation of available and innovative solutions for the exploitation of low and medium temperature heat sources. Applied Energy 2011, 88, 1500–1509. [CrossRef]
- Brogioli, D.; La Mantia, F. Heat recovery in energy production from low temperature heat sources. AIChE Journal 2019, 65, 980–991. [CrossRef]
- Lee, J.Y.; Chen, P.Y. Optimization of Heat Recovery Networks for Energy Savings in Industrial Processes. Processes 2023, 11. [CrossRef]
- Pandey, G.K.; Sikha, S.S.; Thakur, A.; Yarlagadda, S.S.; Thatikonda, S.S.; Baiju suja, B.; Mystkowski, A.; Dragašius, E.; Gundabattini, E. Thermal Mapping and Heat Transfer Analysis of an Induction Motor of an Electric Vehicle Using Nanofluids as a Cooling Medium. Sustainability (Switzerland) 2023, 15. [CrossRef]
- Borgeson, S.; Brager, G. Comfort standards and variations in exceedance for mixed-mode buildings. Building Research and Information 2011, 39, 118–133. [CrossRef]
- Singh, S.; Hanna, E.G.; Kjellstrom, T. Working in Australia’s heat: Health promotion concerns for health and productivity. Health Promotion International 2015, 30, 239–250. [CrossRef]
- Zhao, Z.; Zhang, Y.; Mi, H.; Zhou, Y.; Zhang, Y. Experimental research of a water-source heat pump water heater system. Energies 2018, 11. [CrossRef]
- Janghorban Esfahani, I.; Ifaei, P. Optimal Design of a Renewable-Energy-Driven Integrated Cooling–Freshwater Cogeneration System. Processes 2024, 12, 1164. [CrossRef]
- Masip, X.; Navarro-Peris, E.; Corberán, J.M. Influence of the thermal energy storage strategy on the performance of a booster heat pump for domestic hot water production system based on the use of low temperature heat source. Energies 2020, 13. [CrossRef]
- Dávila, P.; Bourouis, M.; Francisco Nicolalde, J.; Martínez-Gómez, J. Modelling and analysis of a compression/resorption heat pump system with a zeotropic mixture of acetone/CO2. Applied Thermal Engineering 2023, 227. [CrossRef]
- Min, M.; Kim, Y.; Lee, S.; Ko, C. Performance evaluation of a water-to-water heat pump with a floor panel heating system. KSME International Journal 1999, 13, 642–649. [CrossRef]
- Ritchie, M.J.; Engelbrecht, J.A.; Booysen, M.J. Which strategy saves the most energy for stratified water heaters? Energies 2021, 14. [CrossRef]
- Oh, S.; Cho, Y.; Yun, R. Raw-water source heat pump for a vertical water treatment building. Energy and Buildings 2014, 68, 321–328. [CrossRef]
- Pitarch, M.; Hervas-Blasco, E.; Navarro-Peris, E.; Corberán, J.M. Exergy analysis on a heat pump working between a heat sink and a heat source of finite heat capacity rate. International Journal of Refrigeration 2019, 99, 337–350. [CrossRef]
- Stene, J. Residential CO2 heat pump system for combined space heating and hot water heating. International Journal of Refrigeration 2005, 28, 1259–1265. [CrossRef]
- SECOP. Technical Data Model SC10GHH. Technical Data 2023, 1, 1–4. https://www.secop.com/fileadmin/user_upload/SEPS/datasheets/en/sc1212g_104g8280_r134a_220v_50hz_03-2024_ds.pdf.
- Portal, E.E. Electrical characteristic of the motor, 2024.
- Moshinsky, M. Chapter 3. In Manual de refrigeración; Reverté, 2012.
- SECOP. SECOP LBP-MBP-HBP COMPRESSORS: Evaporation Pressures, 2023.
- Wagner, W.; Pruß, A. The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use. Journal of Physical and Chemical Reference Data 2002, 31, 387–535. [CrossRef]
- Keenan, J.; Chao, J.; Kaye, J. Gas Tables: Thermodynamic Properties of Air Products of Combustion and Component Gases, Compressible Flow Functions; Wiley, 1980.
- Siddiqui, M.U.; Owes, A.; Al-Amri, F.G.; Saeed, F. Recent developments in the search for alternative low-global-warming-potential refrigerants: A review. International Journal of Air-Conditioning and Refrigeration 2020, 28. [CrossRef]
- Shaik, S.V.; Babu, T.P. Theoretical Performance Investigation of Vapour Compression Refrigeration System Using HFC and HC Refrigerant Mixtures as Alternatives to Replace R22. Energy Procedia 2017, 109, 235–242. [CrossRef]
- Gross, J.; Sadowski, G. Perturbed-Chain SAFT: An equation of state based on a perturbation theory for chain molecules. Industrial & Engineering Chemistry Research 2001, 40, 1244 – 1260. [CrossRef]
- Gross, J.; Sadowski, G. Application of the Perturbed-Chain SAFT equation of state to associating systems. Industrial & Engineering Chemistry Research 2002, 41, 5510 – 5515. [CrossRef]
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. SAFT: equation-of-state solution model for associating fluids. Fluid Phase Equilibria 1989, 52, 31 – 38. [CrossRef]
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. New reference equation of state for associating liquids. Industrial & Engineering Chemistry Research 1990, 29, 1709 – 1721. [CrossRef]
- González, J.; Llovell, F.; Garrido, J.M.; Quinteros-Lama, H. Accurate and Model-Free Control Function for a Single Stage Transcritical Refrigerator Cycle. ACS Omega 2020, 5, 19217–19226. [CrossRef]
- Parvaneh, K.; Rasoolzadeh, A.; Shariati, A. Modeling the phase behavior of refrigerants with ionic liquids using the QC-PC-SAFT equation of state. Journal of Molecular Liquids 2019, 274, 497–504. [CrossRef]
- Cea-Klapp, E.; Polishuk, I.; Canales, R.I.; Quinteros-Lama, H.; Garrido, J.M. Estimation of Thermodynamic Properties and Phase Equilibria in Systems of Deep Eutectic Solvents by PC-SAFT EoS. Industrial & Engineering Chemistry Research 2020, 59, 22292–22300. [CrossRef]
- González, J.; Llovell, F.; Garrido, J.M.; Quinteros-Lama, H. Selection of a suitable working fluid for a combined organic Rankine cycle coupled with compression refrigeration using molecular approaches. Fluid Phase Equilibria 2023, 572, 113847. [CrossRef]
- Yang, L.; Zhao, L.X.; Zhang, C.L.; Gu, B. Loss-efficiency model of single and variable-speed compressors using neural networks. International Journal of Refrigeration 2009, 32, 1423–1432. [CrossRef]
- Willem, H.; Lin, Y.; Lekov, A. Review of energy efficiency and system performance of residential heat pump water heaters. Energy and Buildings 2017, 143, 191–201. [CrossRef]
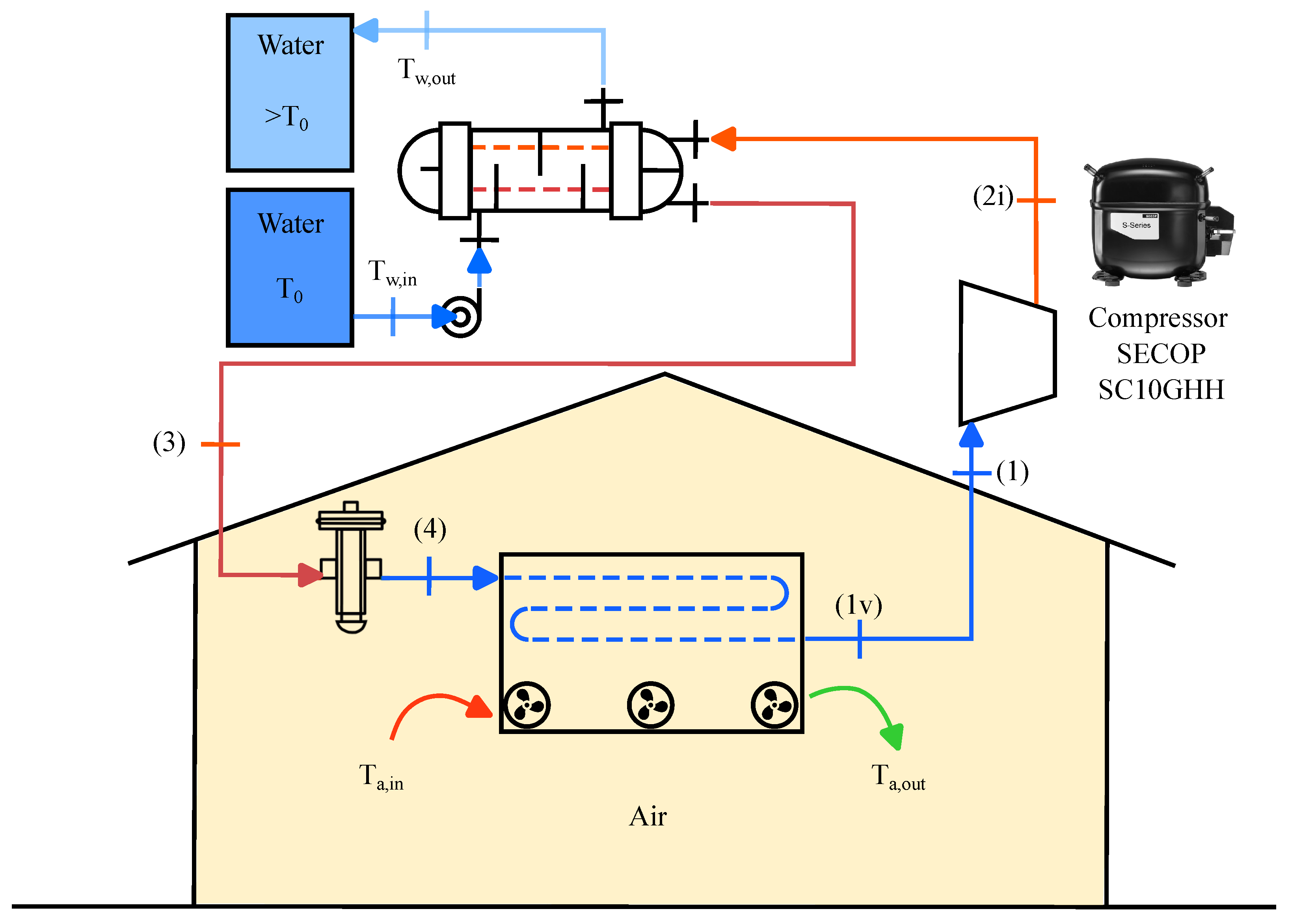
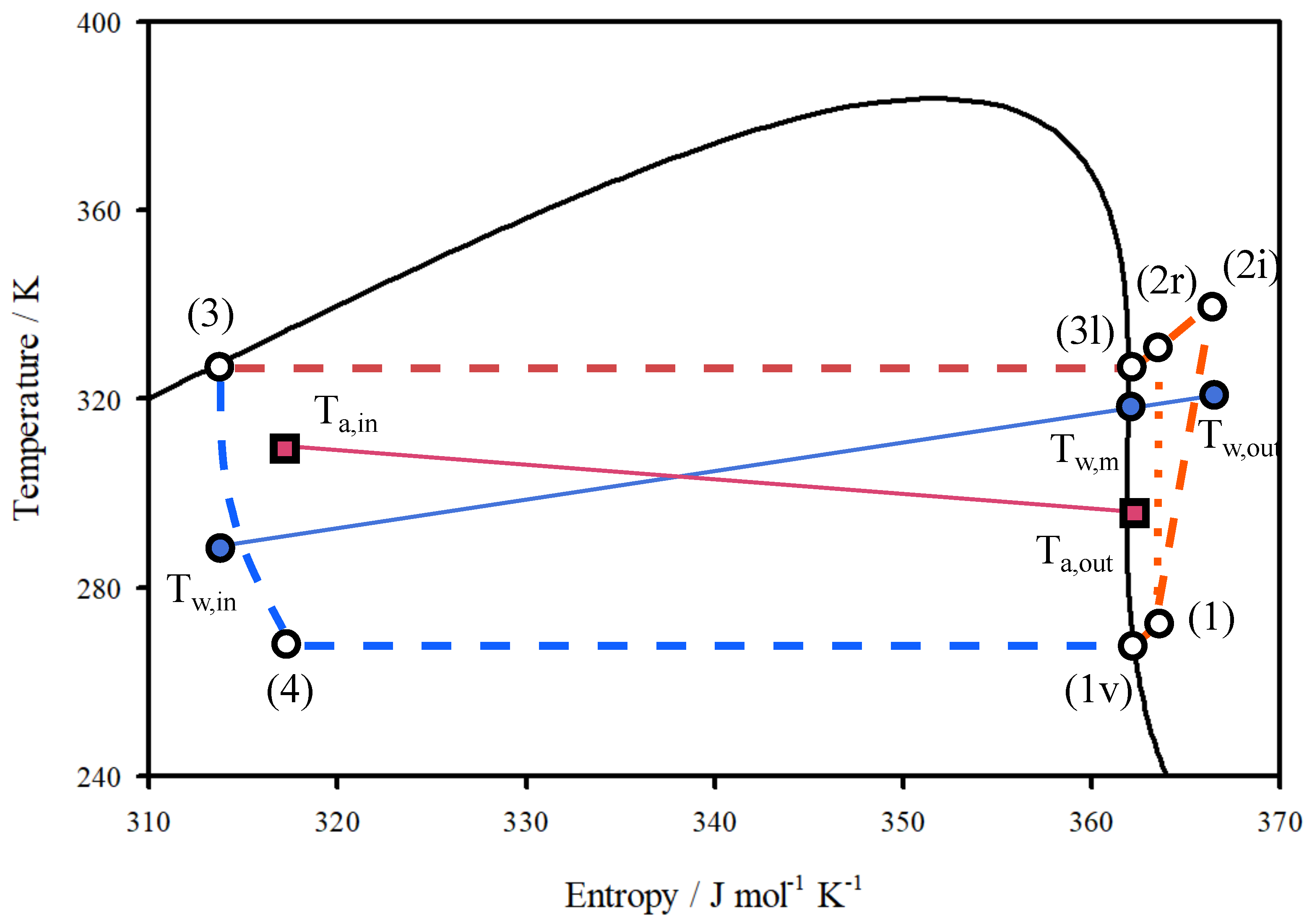
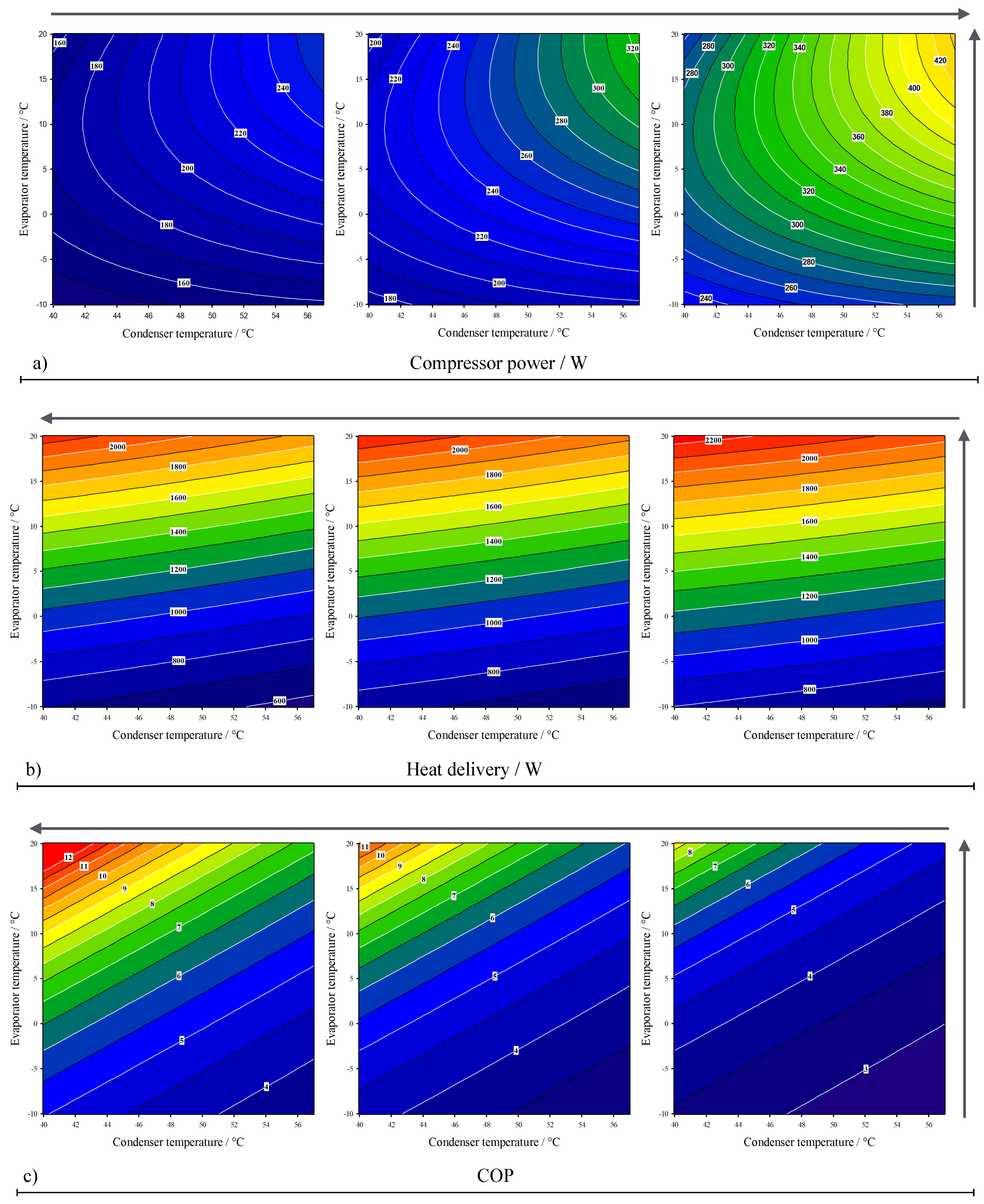
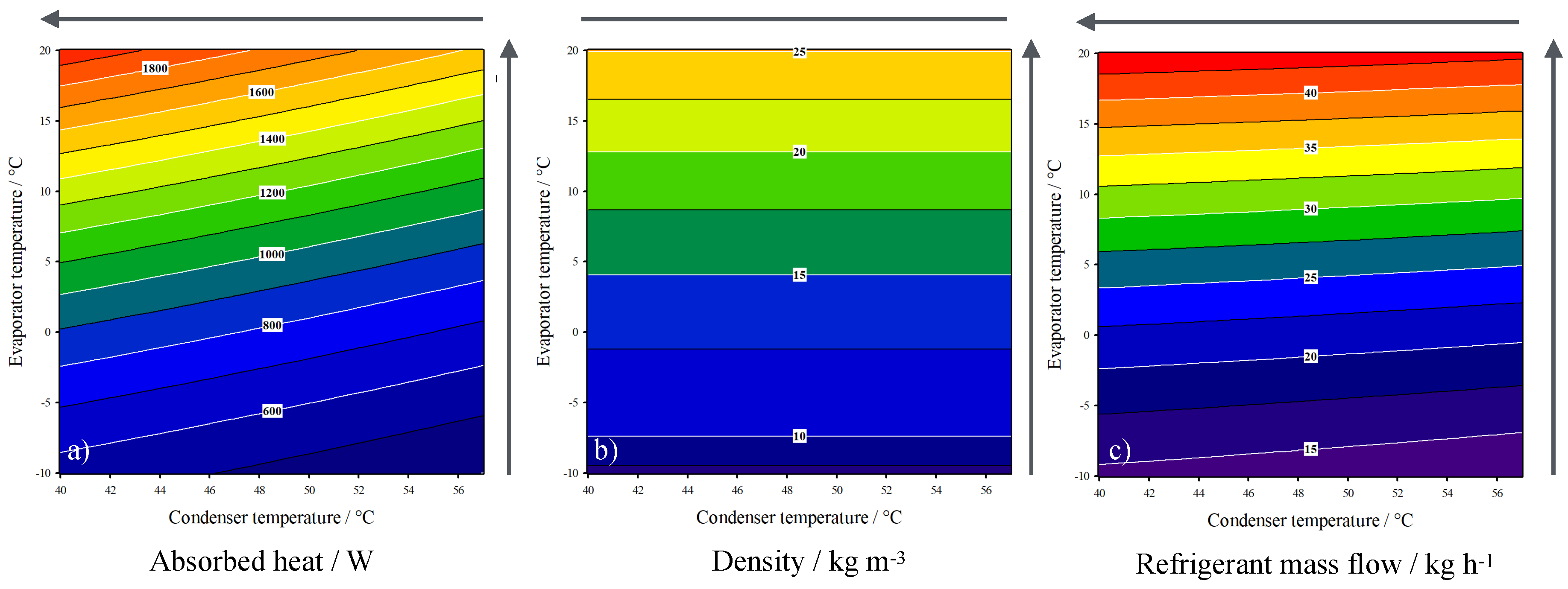
| Value | Range | |
|---|---|---|
| HP system compressor technical conditions | ||
| Displaced volume | 10.29 cm3 | – |
| Revolutions per minute | 2900.00 rpm | 2900.00 to 3500.00 rpm |
| Pressure operation maximum | 1600.00 kPa | – |
| Temperature operation maximum | 330.15 K | – |
| Isentropic performance | – | 60 to 100 % |
| General boundary and limit conditions | ||
| Condenser temperature | – | 313.15 to 330.15 K |
| Evaporator temperature | – | 263.15 to 293.15 K |
| Initial water temperature | 293.15 K | – |
| Initial industrial building temperature | 313.15 K | – |
| Comfort industrial building temperature | 297.15 K | – |
| i | |||
|---|---|---|---|
| 1 | J kg−1 | J kg−1 K−1 | J kg−1 K−2 |
| 5.8341 | 9.6110 | 6.6642 | |
| 2 | J kg−1 K−1 | J kg−1 K−2 | J kg−1 K−3 |
| 0.4713 | 4.9931 | –2.9897 |
| R134a | m | |||
|---|---|---|---|---|
| Residual | m−10 | K | ||
| 3.2483 | 3.0157 | 170.60 | ||
| Ideal gas | ||||
| 7.4912 | 0.01707 | –6.0249 | –115348.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





