Submitted:
03 July 2024
Posted:
04 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mintarjo, J.A.; Poerwanto, E.; Tedyanto, E.H. Current Non-Surgical Management of Knee Osteoarthritis. Cureus 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Uivaraseanu, B.; Vesa, C.M.; Tit, D.M.; Abid, A.; Maghiar, O.; Maghiar, T.A.; Hozan, C.; Nechifor, A.C.; Behl, T.; Patrascu, J.M.; et al. Therapeutic Approaches in the Management of Knee Osteoarthritis (Review). Exp Ther Med 2022, 23, 328. [Google Scholar] [CrossRef] [PubMed]
- Hamood, R.; Tirosh, M.; Fallach, N.; Chodick, G.; Eisenberg, E.; Lubovsky, O. Prevalence and Incidence of Osteoarthritis: A Population-Based Retrospective Cohort Study. J Clin Med 2021, 10, 4282. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, R.; Zhu, Y.; Zeng, Z.; Ye, Z.; Wang, Z.; Liu, W.; Xu, X. Self-Management for Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Res Manag 2022, 2022, 2681240. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Li, J.; Yu, C.; Zhang, C.; Chen, F.; Chen, J.; Ni, H.; Wang, J.; Kang, K.; Wei, Z.; et al. Knee Osteoarthritis: Current Status and Research Progress in Treatment (Review). Exp Ther Med 2023, 26, 481. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.W.-P.; Schlüter-Brust, K.U.; Eysel, P. The Epidemiology, Etiology, Diagnosis, and Treatment of Osteoarthritis of the Knee. Dtsch Arztebl Int 2010, 107, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Thomson, E.; Stefanyk, M.; Brennenstuhl, S. The Robust Association between Childhood Physical Abuse and Osteoarthritis in Adulthood: Findings from a Representative Community Sample. Arthritis Rheum 2009, 61, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, M.; Leclerc, A.; Allaert, F.; Rozenberg, S.; Valat, J.; Avouac, B.; Coste, P.; Litvak, E.; Hilliquin, P. Primary Osteoarthritis of Hip, Knee, and Hand in Relation to Occupational Exposure. Occup Environ Med 2005, 62, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, P.; Yin, K.-J.; Huang, J.-X.; Tian, T.; Cen, H.; Sui, C.; Xu, Z.; Pan, H.-F. Does Smoking Protect against Developing Osteoarthritis? Evidence from a Genetically Informed Perspective. Semin Arthritis Rheum 2022, 55, 152013. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ivorra, I.; Romera-Baures, M.; Roman-Viñas, B.; Serra-Majem, L. Osteoarthritis and the Mediterranean Diet: A Systematic Review. Nutrients 2018, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Siwiec, R.M. Knee Osteoarthritis. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Sutipornpalangkul, W.; Morales, N.P.; Harnroongroj, T. Free Radicals in Primary Knee Osteoarthritis. J Med Assoc Thai 2009, 92 Suppl 6, S268-274.
- Overview | Osteoarthritis in over 16s: Diagnosis and Management | Guidance | NICE. Available online: https://www.nice.org.uk/guidance/ng226 (accessed on 19 September 2023).
- Duong, V.; Oo, W.M.; Ding, C.; Culvenor, A.G.; Hunter, D.J. Evaluation and Treatment of Knee Pain: A Review. JAMA 2023, 330, 1568–1580. [Google Scholar] [CrossRef] [PubMed]
- Pesare, E.; Vicenti, G.; Kon, E.; Berruto, M.; Caporali, R.; Moretti, B.; Randelli, P.S. Italian Orthopaedic and Traumatology Society (SIOT) Position Statement on the Non-Surgical Management of Knee Osteoarthritis. J Orthop Traumatol 2023, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.-Y.; Zhang, Z.-R.; Tang, Z.-M.; Hua, F.-Z. Benefits and Mechanisms of Exercise Training for Knee Osteoarthritis. Front Physiol 2021, 12, 794062. [Google Scholar] [CrossRef] [PubMed]
- Wellsandt, E.; Golightly, Y. Exercise in the Management of Knee and Hip Osteoarthritis. Curr Opin Rheumatol 2018, 30, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, Y.; Chen, S.; Zhang, Y.; Zhang, Z.; Liu, C.; Lu, M.; Liu, F.; Li, S.; He, Z.; et al. The Effects of Resistance Exercise in Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Clin Rehabil 2016, 30, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, D.; Theysmans, T.; Mottola, R.; Verbrugghe, J. High-Intensity Training for Knee Osteoarthritis: A Narrative Review. Sports (Basel) 2023, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- van Doormaal, M.C.M.; Meerhoff, G.A.; Vliet Vlieland, T.P.M.; Peter, W.F. A Clinical Practice Guideline for Physical Therapy in Patients with Hip or Knee Osteoarthritis. Musculoskeletal Care 2020, 18, 575–595. [Google Scholar] [CrossRef] [PubMed]
- Peter, W.F.; Jansen, M.J.; Hurkmans, E.J.; Bloo, H.; Dekker, J.; Dilling, R.G.; Hilberdink, W.; Kersten-Smit, C.; de Rooij, M.; Veenhof, C.; et al. Physiotherapy in Hip and Knee Osteoarthritis: Development of a Practice Guideline Concerning Initial Assessment, Treatment and Evaluation. Acta Reumatol Port 2011, 36, 268–281. [Google Scholar] [PubMed]
- Berteau, J.-P. Knee Pain from Osteoarthritis: Pathogenesis, Risk Factors, and Recent Evidence on Physical Therapy Interventions. Journal of Clinical Medicine 2022, 11, 3252. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.; Luc-Harkey, B.A.; Harkey, M.S.; Davis-Wilson, H.C.; Pfeiffer, S.J.; Schwartz, T.A.; Nissman, D.; Padua, D.A.; Blackburn, J.T.; Spang, J.T. Using TENS to Enhance Therapeutic Exercise in Individuals with Knee Osteoarthritis. Medicine & Science in Sports & Exercise 2020, 52, 2086. [Google Scholar] [CrossRef]
- Nelson, A.E.; Allen, K.D.; Golightly, Y.M.; Goode, A.P.; Jordan, J.M. A Systematic Review of Recommendations and Guidelines for the Management of Osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum 2014, 43, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Sawitzke, A.D.; Shi, H.; Finco, M.F.; Dunlop, D.D.; Harris, C.L.; Singer, N.G.; Bradley, J.D.; Silver, D.; Jackson, C.G.; Lane, N.E.; et al. Clinical Efficacy and Safety of Glucosamine, Chondroitin Sulphate, Their Combination, Celecoxib or Placebo Taken to Treat Osteoarthritis of the Knee: 2-Year Results from GAIT. Ann Rheum Dis 2010, 69, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, M.; Jain, A.; Hurkat, P.; Jain, S.K. Chondroitin Sulphate: A Focus on Osteoarthritis. Glycoconj J 2016, 33, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, D.; Sirico, F.; Corrado, B.; Ruosi, C. Intra-Articular Hyaluronic Acid Injections and Oral Collagen Supplementation for Knee OA: A Case Report of an Elite Female Soccer Player. Journal of Human Sport and Exercise: JHSE 2021, 16, 1384–1394. [Google Scholar]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Zabrzyński, J.; Gagat, M.; Mobasheri, A.; Jeka, S. The Effect of Platelet-Rich Plasma on the Intra-Articular Microenvironment in Knee Osteoarthritis. International Journal of Molecular Sciences 2021, 22, 5492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-F.; Wang, C.-G.; Li, H.; Huang, Y.-T.; Li, Z.-J. Intra-Articular Platelet-Rich Plasma versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Meta-Analysis. Drug Des Devel Ther 2018, 12, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Duymus, T.M.; Mutlu, S.; Dernek, B.; Komur, B.; Aydogmus, S.; Kesiktas, F.N. Choice of Intra-Articular Injection in Treatment of Knee Osteoarthritis: Platelet-Rich Plasma, Hyaluronic Acid or Ozone Options. Knee Surg Sports Traumatol Arthrosc 2017, 25, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, D.; Mottola, R.; Palermi, S.; Sirico, F.; Corrado, B.; Gnasso, R. Intra-Articular Collagen Injections for Osteoarthritis: A Narrative Review. Int J Environ Res Public Health 2023, 20, 4390. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Lippi, L.; Mezian, K.; Calafiore, D.; Pellegrino, R.; Mascaro, G.; Cisari, C.; Invernizzi, M. Ultrasound-Guided Platelet-Rich-Plasma Injections for Reducing Sacroiliac Joint Pain: A Paradigmatic Case Report and Literature Review. J Back Musculoskelet Rehabil 2022, 35, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-H.; Yang, R.-C.; Lee, C.-L.; Chen, T.-W.; Wang, M.-C. Preliminary Results of Integrated Therapy for Patients with Knee Osteoarthritis. Arthritis Rheum 2005, 53, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.; Manjoo, A.; Shaw, P.; Niazi, F.; Rosen, J. Rheological Properties of Commercially Available Hyaluronic Acid Products in the United States for the Treatment of Osteoarthritis Knee Pain. Clin Med Insights Arthritis Musculoskelet Disord 2018, 11, 1179544117751622. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The Mechanism of Action for Hyaluronic Acid Treatment in the Osteoarthritic Knee: A Systematic Review. BMC Musculoskelet Disord 2015, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Short, W.D.; Wang, X.; Keswani, S.G. Hyaluronidases in Human Diseases. Int J Mol Sci 2021, 22, 3204. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their Genomics, Structures, and Mechanisms of Action. Chem Rev 2006, 106, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Belcher, C.; Yaqub, R.; Fawthrop, F.; Bayliss, M.; Doherty, M. Synovial Fluid Chondroitin and Keratan Sulphate Epitopes, Glycosaminoglycans, and Hyaluronan in Arthritic and Normal Knees. Annals of the Rheumatic Diseases 1997, 56, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Ferkel, E.; Manjoo, A.; Martins, D.; Bhandari, M.; Sethi, P.; Nicholls, M. Intra-Articular Hyaluronic Acid Treatments for Knee Osteoarthritis: A Systematic Review of Product Properties. Cartilage 2023, 19476035231154530. [Google Scholar] [CrossRef] [PubMed]
- Miltner, O.; Schneider, U.; Siebert, C.H.; Niedhart, C.; Niethard, F.U. Efficacy of Intraarticular Hyaluronic Acid in Patients with Osteoarthritis--a Prospective Clinical Trial. Osteoarthritis Cartilage 2002, 10, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Al-Johani, A.H.; Kachanathu, S.J.; Ramadan Hafez, A.; Al-Ahaideb, A.; Algarni, A.D.; Meshari Alroumi, A.; Alenazi, A.M. Comparative Study of Hamstring and Quadriceps Strengthening Treatments in the Management of Knee Osteoarthritis. J Phys Ther Sci 2014, 26, 817–820. [Google Scholar] [CrossRef]
- Stevens, J.E.; Mizner, R.L.; Snyder-Mackler, L. Quadriceps Strength and Volitional Activation before and after Total Knee Arthroplasty for Osteoarthritis. J Orthop Res 2003, 21, 775–779. [Google Scholar] [CrossRef]
- Rice, D.A.; McNair, P.J. Quadriceps Arthrogenic Muscle Inhibition: Neural Mechanisms and Treatment Perspectives. Semin Arthritis Rheum 2010, 40, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.A.; McNair, P.J.; Lewis, G.N.; Dalbeth, N. Quadriceps Arthrogenic Muscle Inhibition: The Effects of Experimental Knee Joint Effusion on Motor Cortex Excitability. Arthritis Res Ther 2014, 16, 502. [Google Scholar] [CrossRef] [PubMed]
- Demeco, A.; de Sire, A.; Marotta, N.; Palumbo, A.; Fragomeni, G.; Gramigna, V.; Pellegrino, R.; Moggio, L.; Petraroli, A.; Iona, T.; et al. Effectiveness of Rehabilitation through Kinematic Analysis of Upper Limb Functioning in Wheelchair Basketball Athletes: A Pilot Study. Applied Sciences 2022, 12, 2929. [Google Scholar] [CrossRef]
- Brindisino, F.; Garzonio, F.; DI Giacomo, G.; Pellegrino, R.; Olds, M.; Ristori, D. Depression, Fear of Re-Injury and Kinesiophobia Resulted in Worse Pain, Quality of Life, Function and Level of Return to Sport in Patients with Shoulder Instability: A Systematic Review. J Sports Med Phys Fitness 2023, 63, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.J. Isokinetic Testing of Muscle Strength in Older Adults with Knee Osteoarthritis: An Integrative Review. Isokinetics and Exercise Science 2020, 28, 269–290. [Google Scholar] [CrossRef]
- Peat, G.; Thomas, E.; Duncan, R.; Wood, L.; Hay, E.; Croft, P. Clinical Classification Criteria for Knee Osteoarthritis: Performance in the General Population and Primary Care. Ann Rheum Dis 2006, 65, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann Rheum Dis 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Kim, S.S.; Lee, S.-Y.; Park, N.-H.; Park, J.-Y.; Choi, Y.-J.; Jeon, H.-J. A Practical MRI Grading System for Osteoarthritis of the Knee: Association with Kellgren–Lawrence Radiographic Scores. European Journal of Radiology 2013, 82, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Åström, M.; Thet Lwin, Z.M.; Teni, F.S.; Burström, K.; Berg, J. Use of the Visual Analogue Scale for Health State Valuation: A Scoping Review. Qual Life Res 2023, 32, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Monticone, M.; Ferrante, S.; Salvaderi, S.; Rocca, B.; Totti, V.; Foti, C.; Roi, G.S. Development of the Italian Version of the Knee Injury and Osteoarthritis Outcome Score for Patients with Knee Injuries: Cross-Cultural Adaptation, Dimensionality, Reliability, and Validity. Osteoarthritis and Cartilage 2012, 20, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-H.; Lu, P.W.-A.; Lin, C.-W.; Lu, E.W.-H.; Yang, S.-F. Different Molecular Weights of Hyaluronan Research in Knee Osteoarthritis: A State-of-the-Art Review. Matrix Biol 2023, 117, 46–71. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Franceschi, F. Viscosupplementation with High Molecular Weight Native Hyaluronan. Focus on a 1500-2000 KDa Fraction (Hyalubrix®). Eur Rev Med Pharmacol Sci 2014, 18, 3326–3338. [Google Scholar] [PubMed]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of Student’s t-Test, Analysis of Variance, and Covariance. Ann Card Anaesth 2019, 22, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Foti, C.; Cisari, C.; Carda, S.; Giordan, N.; Rocco, A.; Frizziero, A.; Della Bella, G. A Prospective Observational Study of the Clinical Efficacy and Safety of Intra-Articular Sodium Hyaluronate in Synovial Joints with Osteoarthritis. Eur J Phys Rehabil Med 2011, 47, 407–415. [Google Scholar] [PubMed]
- Filardo, G.; Kon, E.; Di Martino, A.; Di Matteo, B.; Merli, M.L.; Cenacchi, A.; Fornasari, P.M.; Marcacci, M. Platelet-Rich Plasma vs Hyaluronic Acid to Treat Knee Degenerative Pathology: Study Design and Preliminary Results of a Randomized Controlled Trial. BMC Musculoskelet Disord 2012, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Di Matteo, B.; Di Martino, A.; Merli, M.L.; Cenacchi, A.; Fornasari, P.; Marcacci, M.; Kon, E. Platelet-Rich Plasma Intra-Articular Knee Injections Show No Superiority Versus Viscosupplementation: A Randomized Controlled Trial. Am J Sports Med 2015, 43, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Galluccio, F.; Gazar, Y.A.; Negm, A.A.; Fajardo Perez, M.; Yamak Altinpulluk, E.; Ergönenç, T.; Chang, K.-V.; Pan, J.L.; Allam, A.E.-S. The Booster Effect of a Single Quarterly Dose of Hyaluronic Acid in Knee Osteoarthritis: Five-Year Results of a Registry-Based Study. Cureus 2022, 14, e31592. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, L.S.; Marelli, B.M.; Crapanzano, C.; De Martinis, S.E.; Gala, L.; Ferraro, M.; Marelli, N.; Albisetti, W. A Randomized Double-Blind Clinical Trial on the Treatment of Knee Osteoarthritis: The Efficacy of Polynucleotides Compared to Standard Hyaluronian Viscosupplementation. Knee 2014, 21, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Cudoni, S.; Zedde, P. Trattamento conservativo della gonartrosi: Platelet Rich Plasma vs acido ialuronico. LO SCALPELLO 2019, 33, 226–229. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front Vet Sci 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Moreland, L.W. Intra-Articular Hyaluronan (Hyaluronic Acid) and Hylans for the Treatment of Osteoarthritis: Mechanisms of Action. Arthritis Res Ther 2003, 5, 54–67. [Google Scholar] [CrossRef]
- Migliore, A.; Procopio, S. Effectiveness and Utility of Hyaluronic Acid in Osteoarthritis. Clin Cases Miner Bone Metab 2015, 12, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Diracoglu, D.; Vural, M.; Baskent, A.; Dikici, F.; Aksoy, C. The Effect of Viscosupplementation on Neuromuscular Control of the Knee in Patients with Osteoarthritis. J Back Musculoskelet Rehabil 2009, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maia, P.A.V.; Cossich, V.R.A.; Salles-Neto, J.I.; Aguiar, D.P.; de Sousa, E.B. Viscosupplementation Improves Pain, Function and Muscle Strength, but Not Proprioception, in Patients with Knee Osteoarthritis: A Prospective Randomized Trial. Clinics (Sao Paulo) 2019, 74, e1207. [Google Scholar] [CrossRef] [PubMed]
- Bayramoğlu, M.; Karataş, M.; Cetin, N.; Akman, N.; Sözay, S.; Dilek, A. Comparison of Two Different Viscosupplements in Knee Osteoarthritis -- a Pilot Study. Clin Rheumatol 2003, 22, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.F.T.; Chen, C.P.C.; Chen, M.J.L.; Hong, W.-H.; Yu, T.-Y.; Tsai, W.-C. Improvement of Muscle Strength in Osteoarthritic Knee Patients after Intraarticular Knee Injection of Hyaluronan. Am J Phys Med Rehabil 2005, 84, 274–277. [Google Scholar] [CrossRef] [PubMed]
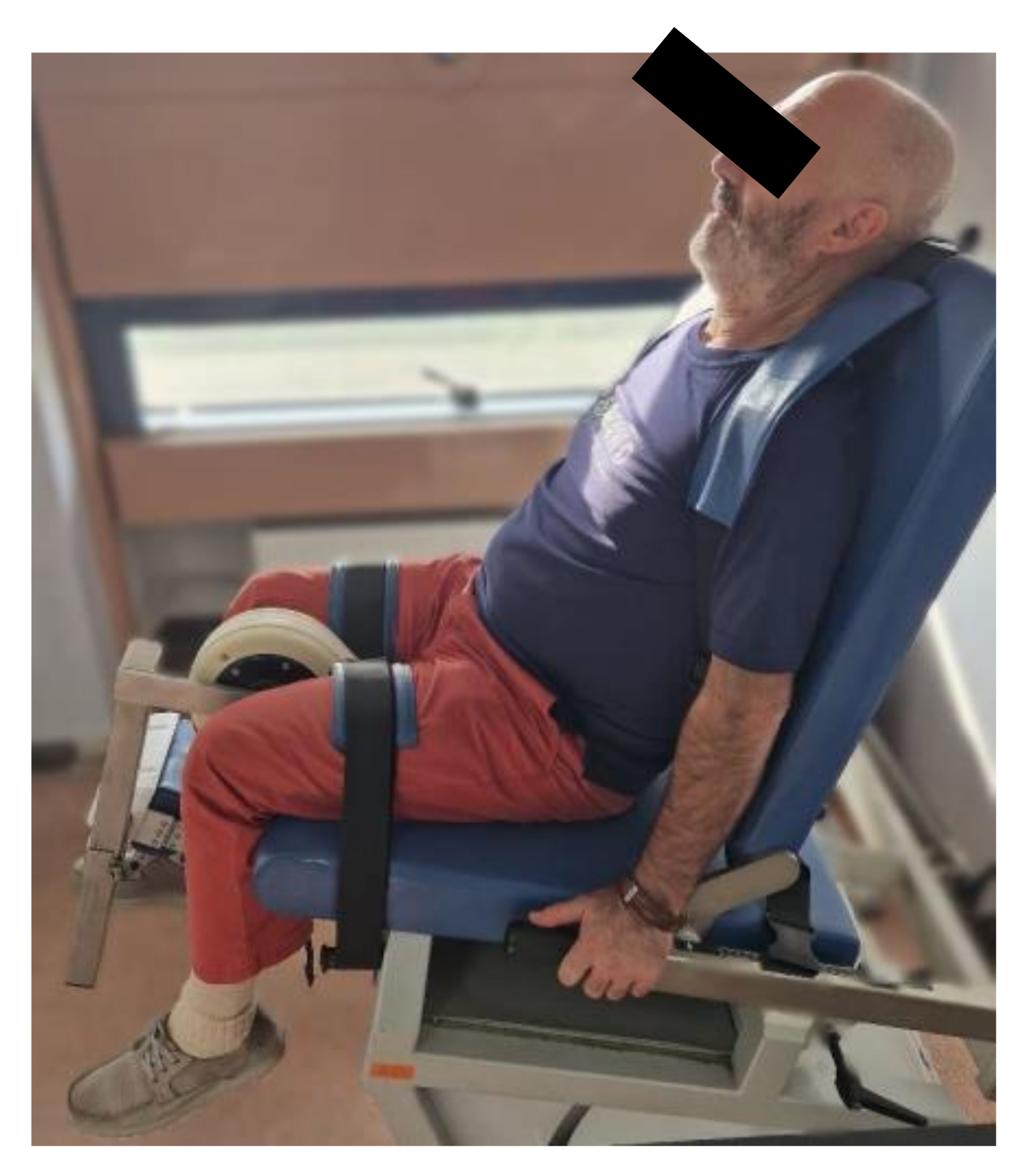
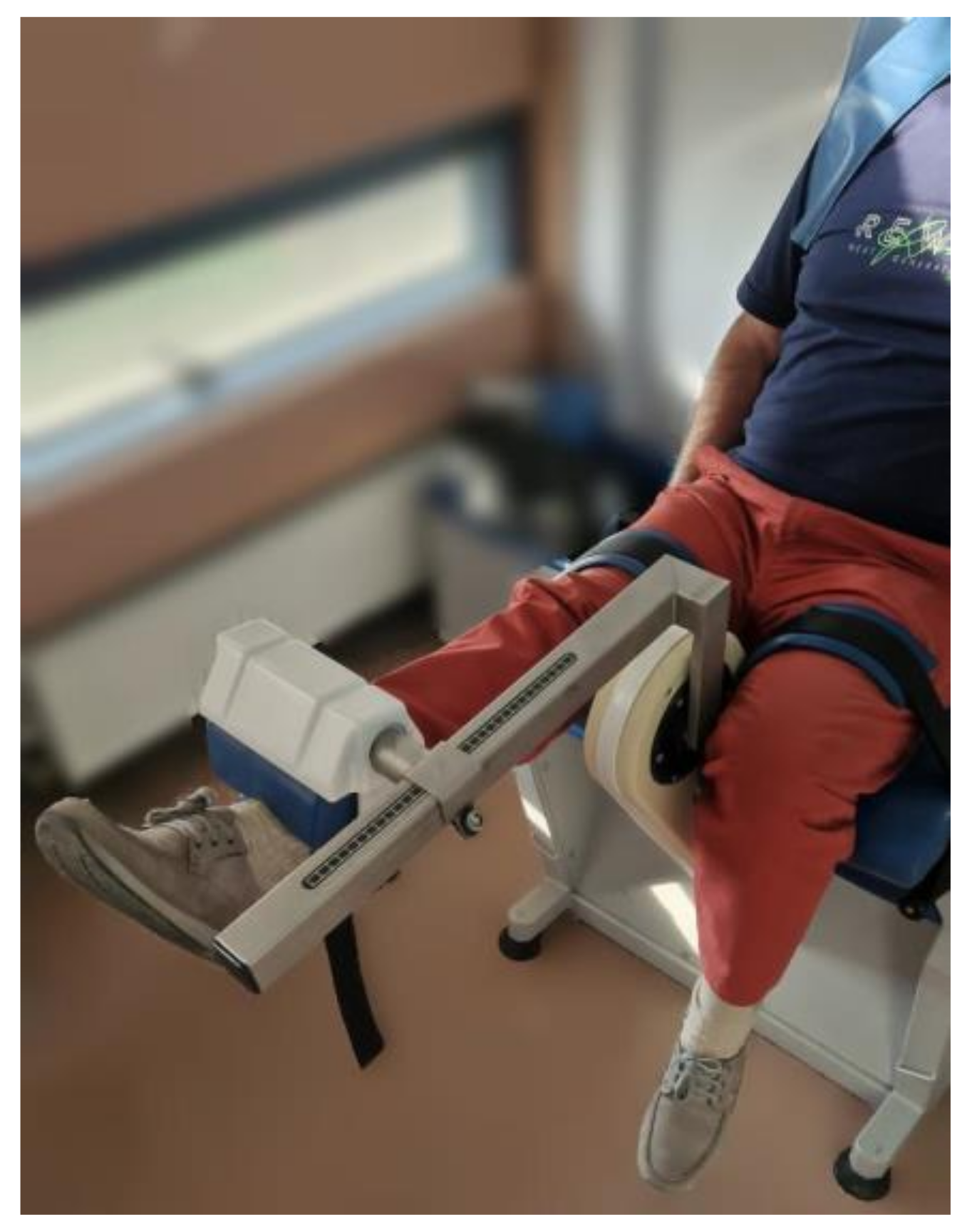
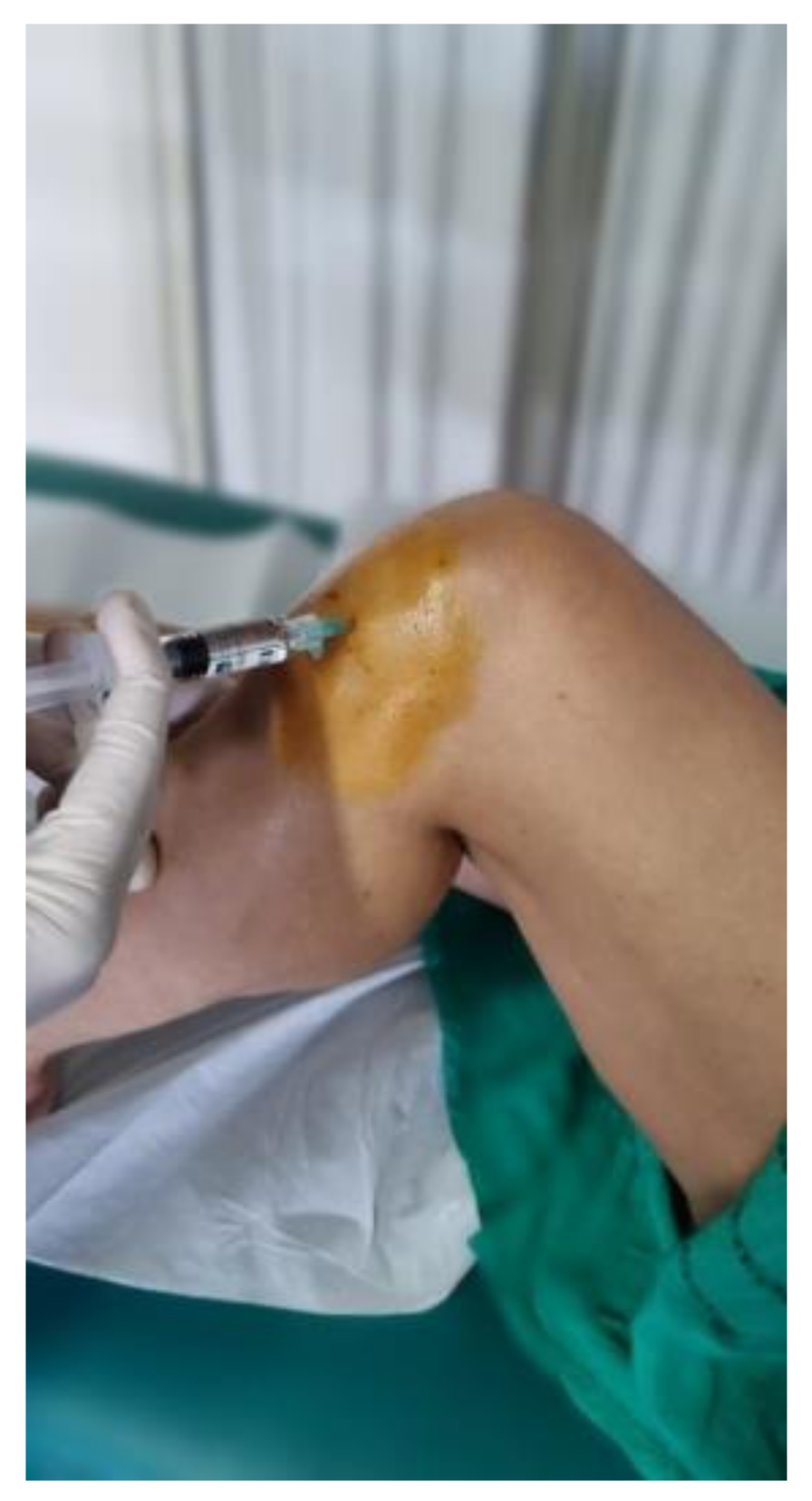
| Total sample (n=30) | Males (n=14) | Females (n=16) | |
|---|---|---|---|
| Age | 58.35 (8.82) | 52.57 (8.6) | 62.63 (5) |
| BMI (kg/m2) | 26.42 (2.71) | 25.34 (2.11) | 27.37 (2.95) |
| Knees (DX:SX) | 26:20 | 12:8 | 14:12 |
| Active workers | 22/30 | 14/14 | 8/16 |
| Physical exercise | 14/30 | 6/14 | 8/16 |
| K-L I | K-L II | K-L III | |
|---|---|---|---|
| Knees (DX:SX) | 0:2 | 16:18 | 10:0 |
| Age | 40 | 58.29 (8.64) | 62.2 (5.12) |
| Sex (M:F) | 2:0 | 12:16 | 4:6 |
| BMI (kg/m2) | 26.47 | 26.65 (2.61) | 27.38 (3.14) |
| Active workers | 2/2 | 20/28 | 8/10 |
| Physical exercise | 0/2 | 14/28 | 4/10 |
| T0 | T1 | p-value | |
|---|---|---|---|
| VAS (all K-L groups) | 4.7 (2.1) | 1.6 (1.34) * | <0,001 |
| VAS (K-L I) | 2 | 0 | / |
| VAS (K-L II) | 4.6 (1.9) | 1.6 (1.37) * | <0,001 |
| VAS (K-L III) | 5.8 (2) | 1.8 (1.3) * | 0,006 |
| T0 | T1 | p-value | |
|---|---|---|---|
| KOOS (all K-L groups) | 75% (0,1) | 91% (0,1) * | <0,001 |
| KOOS (K-L I) | 96% | 100% | / |
| KOOS (K-L II) | 76% (0,1) | 91% (0,1) * | <0,001 |
| KOOS (K-L III) | 70% (0,1) | 91% (0,1) * | 0,001 |
| T0 | T1 | p-values | |
|---|---|---|---|
| EXT:FLX (N/m) (all K-L groups) | 88.7(22.8):50.1 (18.5) | 92.3 (31.7):51 (22.5) | 0.66:0.87 |
| EXT:FLX (N/m) (K-L I) | 142:101 | 171:119 | / |
| EXT:FLX (N/m) (K-L II) | 88.8 (21.7):48.7 (15.7) | 89.6 (27.6):49.2 (18.7) | 0.93:0.93 |
| EXT:FLX (N/m) (K-L III) | 77.4 (10.8):44.6 (14.2) | 85.8 (29):43.2 (11.3) | 0.58:0.87 |
| T0 | T1 | p-values | |
|---|---|---|---|
| EXT:FLX (N/m) (all K-L groups) | 68.3 (17.7):42.5 (16) | 69.7(18.7):41.7 (12.7) | 0.87:0.8 |
| EXT:FLX (N/m) (K-L I) | 77:37 | 90:44 | / |
| EXT:FLX (N/m) (K-L II) | 69 (18.2):43.5 (15.8) | 68.4(17.5):42.5 (13.3) | 0.93:0.85 |
| EXT:FLX (N/m) (K-L III) | 64.2 (17.2):40 (18) | 70.2 (24.3):38.4(12.7) | 0.67:0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





