1. Introduction
Sick sinus syndrome (SSS) is a sinoatrial node(SAN) and surrounding tissue impairment, causing sinus rhythm disorders and arrhythmias like sinus bradycardia and arrest [
1,
2,
3]. Epidemiological studies show SSS is prevalent among middle-aged and elderly individuals [
4], increasing with age [
5,
6]. Better diagnosis and treatment, require relevant research, which can be facilitated by constructing an animal SSS model. Current modeling methods include drugs and non-drugs. Drug modeling methods include sodium hydroxide(NaOH) chemical injury [
7,
8,
9], formaldehyde chemical injury [
10,
11], and amiodarone-induced [
12], etc. Non-drug modeling methods include ischemia-reperfusion injury [
7], genetic engineering animal-induced [
13,
14], natural aging-induced [
1], etc. Due to limited experimental conditions, the researcher initially chose the approach of 10% NaOH injection via the jugular vein(JV) for modeling but found it caused severe vascular damage in SD rats and was prone to induce malignant arrhythmia and even death when reducing heart rate(HR). Reducing concentrations to 8% and 5% did not improve the situation. Some improvement occurred using 1% but with instability HR and prolonged modeling time. Adjusting to 3% total modeling drug amount in the literature and intravenous push at 0.01 ml/s [
7], improved the condition. This study explored the feasibility of this modified method in establishing the SSS model and its impact on animals during the experimental process by observing the structural and functional changes of the sinus node after jugular vein injection of 3% NaOH.
2. Materials and Methods
2.1. Reagents
The 10%NaOH solution was purchased from Saint-Bio (product number: R21862-500); the SteadyPure rapid RNA extraction kit, SYBR premixed qPCR kit, reverse transcription premixed kit, and primers were from Accurate Biotechnology (Hunan, China, product number: AG21023、AG11701、AG11728); the pre-stained protein molecular weight standard was purchased from PageRuler (China, product number: 26616), HCN4 and α-tubulin polyclonal antibodies were acquired from Affinity Biosciences (China, product number: DF9771、AF4351), horseradish peroxidase (HRP) goat anti-rabbit IgG from FeiyuBio (product number: FY16309); Hematoxylin-eosin staining kit, calcium content chromogenic detection kit from Beyotime (product number: C0105S、S1063S), etc.
2.2. Animals and Grouping
All animal experiments adhered to relevant regulations. Healthy male Sprague-Dawley (SD) rats, specific pathogen-free (SPF), aged 6-8 weeks and weighed 272.02 ± 9.11 g, totaling 74, were purchased from SLAC Laboratory Animal (China), with the certification No. SYXK(Zhejiang)2021-0012. Five rats were housed per cage, with sufficient water and food in a room with suitable humidity, temperature, and light-dark cycle. Using a randomization tool, the rats were assigned to a sham group (n=12), a 3% NaOH group(n=31), and a 10% NaOH group(n=31). All rats received adaptive feeding for 3 days before modeling.
2.3. Establishment of Animal Models
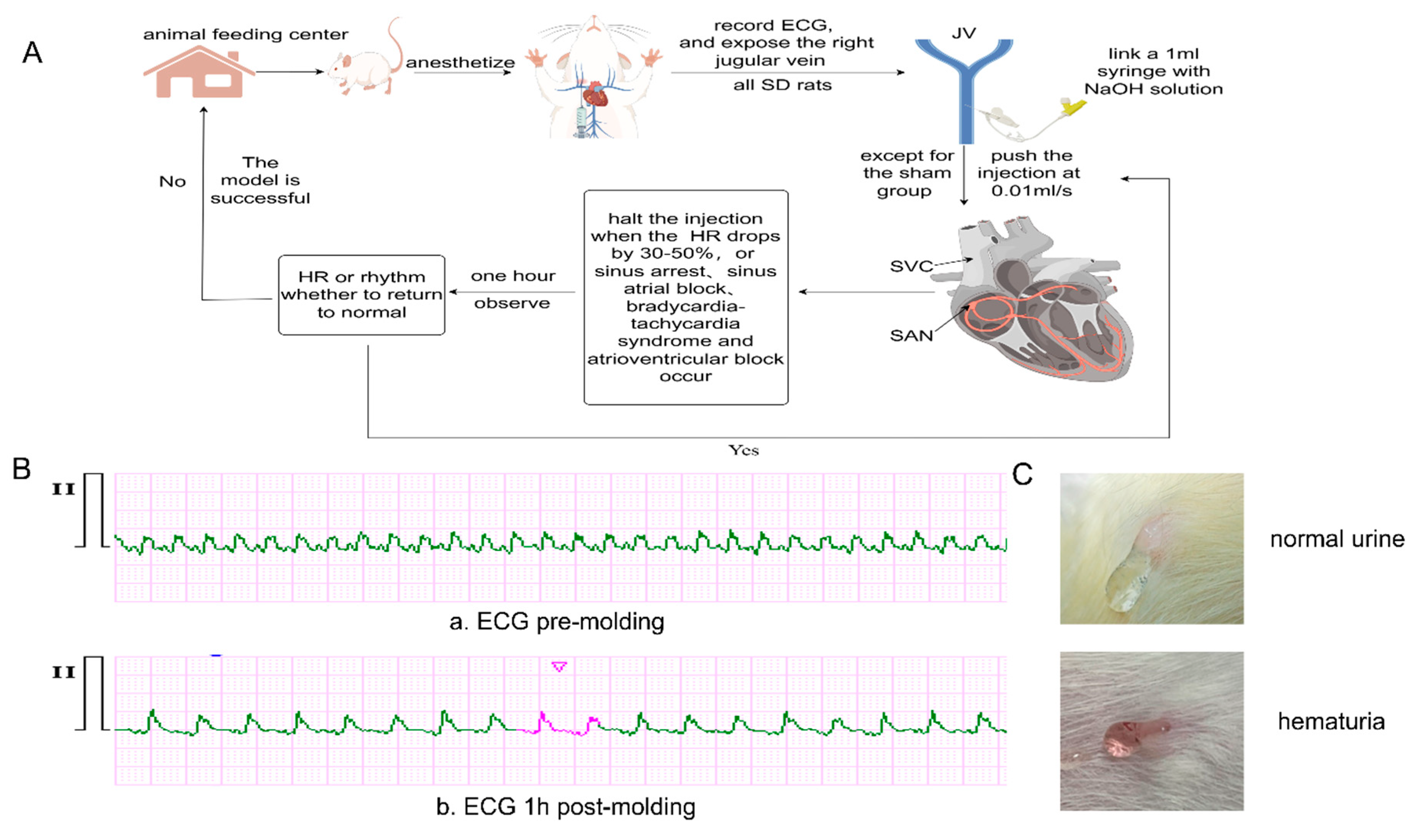
All surgeries were performed by the same individual in a quiet lab environment. Atropine sulfate (0.1 mg/kg) was injected subcutaneously to reduce salivary secretion, followed by Zoletil 50 (telazol 125 mg/zolazepam 125 mg, 0.2 mg/kg) injected intraperitoneally to anesthetize the rats. After connecting the electrodes and turning on the three-channel electrocardiogram(ECG) monitor (PC-80D, Shenzhen Lepu, China), the basic ECG was recorded for 3 minutes. The right JV was exposed, and an indwelling needle was inserted and connected to a 1 ml syringe. Except for the sham group, the other rats were injected with the corresponding NaOH concentration into the right JV at 0.01 ml/s. The injection was halted when HR decreased by 30-50%, or arrhythmias such as sinoatrial block, sinus arrest, slow-fast syndrome, escape rhythm, and atrioventricular block occurred [
7,
8,
9,
11,
15]. Observation was carried out for one hour. If the HR or rhythm did not return to normal, indicating the model was successful. Otherwise, continue to push the injection. Successful modeling rats were returned to the breeding center for continued feeding after debridement and suture (
Figure 1A,B).
2.4. Observation Indicators
2.4.1. Changes in HR and Heart Rhythm
Collected and analyzed electrocardiographic data from each rat using computer software. Calculated HR by counting consecutive PP intervals over 10 seconds, then multiplying by 6 to obtain beats per minute (bpm) for each group. For arrhythmia episodes, calculated HR by averaging 10 consecutive PP intervals. Recorded changes in HR and heart rhythm pre-molding, 1 hour post-molding, 1 week post-molding, and 2 weeks post-molding. Combined with the literature, the collected ECGs were classified into types A, B, and C according to the classification criteria of SSS arrhythmia [
2,
3,
16] (
Table 1).
2.4.2. Record Dosage (g), the Number of Injections, and Hematuria Status
Registered the amount of NaOH during modeling in each rat and the number of injections. One injection was recorded from the start to reaching the standard suspension. Some rats exhibited hematuria during molding, which was also recorded (
Figure 1C).
2.4.3. Hematoxylin and Eosin(HE) Staining
At the scheduled time for pathology, each group of rats (n=3) were sacrificed by inhalation of CO2 after recording the ECGs 1 week after molding. The hearts were exposed by thoracotomy, and the right atrium(RA) and proximal segment of the superior vena cava(SVC) were cut along the lower edge of the right auricle, then immediately fixed with 4% paraformaldehyde. After 24 hours, dehydration, wax immersion, embedding, sectioning, and staining were carried out.
2.4.4. Detection of mRNA for Sodium Voltage-Gated Channel Type Vα (SCN5A), Hyperpolarization-Activated Cyclic Nucleotide-Gated Cation Channel 4 (HCN4), Potassium Voltage-Gated Channel Eag-Related Subfamily H2 (KCNH2), Potassium Voltage-Gated Channel KQT-like Subfamily 1 (KCNQ1), and Large-Conductance Calcium-Activated Potassium Channel Ma1 (KCNMA1)
In the qRT-PCR experiment, GAPDH was used as an internal reference gene. The primer sequences for GAPDH were: upstream 5′ GGCACAGTCAAGGCTGAGAATG 3′ and downstream 5′ ATGGTGGTGAAGACGCCAGTA 3′, with a fragment length of 45 bps. The primer sequences for SCN5A were: upstream 5′ GAGGACAGTGCTGCCAGAAT 3′ and downstream 5′ CCTCCCTCGTACCCCTAACA 3′ , with a fragment length of 40 bps. The primer sequences for HCN4 were: upstream 5′ CACTAAGGGCAACAAGGAGACCAAG 3′ and downstream 5′ TGAGTAGAGGCGGCAGTAAGTATCC 3′ , with a fragment length of 50 bps. The primer sequences for KCNH2 were: upstream 5′ GCCCCGACATGCTAGTACA 3′ and downstream 5′ AAGTTGAGGGTGATTTGGGGTAT 3′ , with a fragment length of 42 bps. The primer sequences for KCNMA1 were: upstream 5′ TACAGCACTCCGCAGACACTA 3′ and downstream 5′ ACAGATCACCATAACAACCACCAT 3′, with a fragment length of 45 bps. The primer sequences for KCNQ1 were: upstream 5′ GTTTGAACAGGGTGGAAGACAAG 3′ and downstream 5′ CCTTCATCACTGGCTACGACTT 3′, with a fragment length of 45 bps. The SAN tissue was removed and ground in liquid nitrogen (n = 3), and the ground powder was placed in the lysis solution to extract total mRNA, and reversed cDNA. The prepared cDNA products were subjected to PCR amplification in a fluorescence quantitative PCR instrument (CFX96 Touch). Exported the CT values to be analyzed.
2.4.5. Western Blotting
From each group, SAN tissues (n = 3) were harvested and lysed in buffer (lysis buffer: tissue = 6.5ul:1mg). Proteins were extracted on ice throughout the lysis process. Quantification was done by Bicinchoninic Acid Assay. Samples were leveled and heat-denatured at 37°C for 30 minutes. Treated samples were added to electrophoresis gel for electrophoresis, and then transferred to the membrane. After being blocked with 5% skim milk, the whole membrane was incubated with rabbit polyclonal antibody at 4°C for 15 hours. After TBST washing, goat anti-rabbit IgG-HRP was incubated for 1 hour. After TBST cleaning, super-sensitive ECL was added and immediately placed in a dual near-infrared laser imaging system (Odyssey Fc). Exposed for 30 seconds to 2 minutes in the 700 nm and chemical channels. Then, gray values were calculated using built-in Image J software and exported for statistical analysis.
2.4.6. Quantitative Experiment of Calcium Ions
From each group, SAN tissues were taken for quantitative detection of calcium ions (n = 3 rats). After ice cracking of the sample, centrifuged at 12,000 rpm for 4 minutes, extracted the superior liquid. Added calcium standard samples in a 96-well plate according to the concentration gradient, and added the sample after diluting the sample stock solution 4 times, mixed in the reaction solution, and placed it at room temperature in the dark for 8 minutes. Measured the absorbance in a multi-functional enzyme marker (Biotek, Synergy H1), drew a standard curve, calculated the calcium ion concentration, and performed the statistical analysis.
2.5. Statistical Analysis
Statistical analysis was performed using SPSS 25.0 software. Among the quantitative data, those that conformed to the normal distribution or approximately normal distribution were expressed as mean ± SD, and those that conformed to the severely skewed distribution were expressed as M (P25, P75). Qualitative data were expressed as n (%). For quantitative data among multiple groups that conformed to the normal distribution, one-way analysis of variance was used, and for data that conformed to the severely skewed distribution, the rank sum test was used; for qualitative data between two groups, the chi-square test was used. P < 0.05 indicated a statistically significant difference. The results were presented in tables or column charts drawn using GraphPad Prism.
4. Discussion
SSS is a complex and intractable cardiac electrophysiological disease with diverse etiologies including degenerative, ischemic, heritable heart diseases and ion channel remodeling [
2,
3,
4,
6,
17]. Current research connected with SSS is inadequate, with difficulty in animal modeling being one of the key reasons.
There are several modeling methods, with formaldehyde chemical injury being the most common [
10]. It causes chemical damage to the SAN and surrounding tissue. However, it poses harm to both subjects and practitioners, requiring high ventilation. Ischemia-reperfusion injury-induced modeling is also used [
7,
18], mainly simulating clinical coronary ischemia with SSS. However, it has difficult operation and high mortality risk. Recently, genetic engineering-induced and natural aging-induced methods emerged, simulating SSS pathogenesis from genetics or aging with less harm and closer reality [
1,
14]. However, they are costly and time-consuming. Each method has advantages and disadvantages, complicating experiments. This experiment chose NaOH chemical injury for low harm and high model formation rate. Its principle is similar to formaldehyde modeling, causing chemical damage [
7,
8]. Previous studies confirmed SAN in rats exists at the junction area between SVC and RA and the proximal wall above [
19,
20]. It was injected with 3% NaOH through JV into the area causing irreversible damage. Over time, the lesion can become fibrotic, similar to degenerative changes in the elderly.
A comparative experiment was conducted with the SSS model by JV injection of 3% NaOH and 10% NaOH to verify the success of modeling. The results showed decreased HRs at post-molding multiple points in both groups, with no difference in HR between them. Existing literature notes that decreased HR post-molding is a sign of SSS model success [
7,
8,
10], indicating successful model construction in our experiment. The modeling success rate reached 87%, far exceeding the 57% of the 10% NaOH group and significantly higher than the existing success rate of 44% in previous studies [
9]. The successful construction and high success rate of the 3% NaOH model prove the feasibility of this modeling method. Although similar to the 83% success rate of the 10% NaOH model by micro-pump [
7], statistical analysis shows no substantial correlation between injection times and modeling success. This is conducive to reducing instrument usage costs and high mortality rates within 5 minutes in animal experiments. Some researchers still construct the SSS model by pressing and permeating with a 10% NaOH needle tip, although this method has a relatively precise injury location, it may damage lung tissue or deviate SAN during locating. Therefore, it is not the first choice for researchers.
The study found HR was lower than sham after molding than before, but increased at 1 week post-molding, then decreased 2 weeks post-molding HR and was significantly lower than 1 week post-molding. This is likely due to rat autonomic nerve regulation compensating for damage [
21,
22]. As time passes, irreversible damage occurs and decompensation manifests, as decreased HR. Though unable to directly detect SAN function in rats by professional electrophysiological instruments, the literature suggests SSS was classified as type A, B, or C in clinical practice based on different injury sites and ECG manifestations [
2,
3,
16]. Results showed postoperative ECG of the 3% NaOH group was type A arrhythmia, especially at 1 week post-molding, indicating main SAN tissue injury with minimal extranodal damage. Based on this, we can initially conclude the successful limited SAN tissue SSS animal model construction via JV 3% NaOH injection, with mild extranodal damage.
P cells generate spontaneous electrical activity in the SAN through multiple ion channels, including SCN5A, HCN4, KCNQ1, KCNH2, and KCNMA1, etc. The sodium current (I
Na+), pacemaker current (I
f), and related potassium current (I
k+) generated by these channels are involved in phase 4 automatic depolarization of P cells [
23,
24,
25]. Ca
2+ in the SAN also contributes to P cell automaticity [
26]. As the membrane potential, L-type calcium channels are activated, allowing Ca
2+ to flow inward slowly. The subsequent increase in intracellular Ca
2+ activates the sodium-calcium exchange current (I
NCX). The transient increase in Ca
2+ concentration causes cell contraction and induces action potentials [
27,
28,
29]. HE staining, detection of membrane ion channel transcription and expression, and quantitative experiments on calcium ion distribution were used to explore the structural and functional changes of SAN P cells after modeling. The results show that modeling disrupted SAN tissue was disordered, damaged P cell structure, reduced the membrane ion channels transcription and expression, and decreased Ca
2+ content. HCN4 damage was notable, possibly due to its abundant SAN distribution in rats [
30]. The results further confirm that JV injection of 3% NaOH damages P cell structure and function, which is the pathological basis for inducing sinus rhythm generation or conduction disorders of SSS.
In our experiment, a positive correlation was discovered between model construction and hematuria. Previous studies have observed that NaOH forms a stop interface when inducing tissue necrosis. It is speculated that the phenomenon probably results from excessive NaOH and ruptured red blood cells being excreted through urine, preventing drug accumulation causing malignant arrhythmia or death. This discovery could inform future SSS modeling.
In SSS experiments, animals like dogs, rabbits, rats, and zebrafish have been used [
10,
31,
32]. Numerous studies have shown SD rats share similarities in physiological structure and function of the sinoatrial node with humans. Given their easy breeding, strong reproduction, lower cost, and abundant prior SSS research [
7,
8,
9], they were selected. However, rats are prone to fright, bringing certain difficulties to HR collection. Therefore, ECG collection after anesthesia is a good method in vivo. A quiet laboratory environment can avoid HR monitoring errors from fright. The modified NaOH concentration caused less damage to blood vessels and lower mortality, indicating the improved method aligns with animal welfare needs.
The modified method represents a rapid modeling approach, suitable for mechanistic simulations and drug research involving multiple SAN-related ion channels or degenerative diseases. Advantages include low cost, minimal lab requirements, and reduced animal harm. It is ideal for studies with limited resources and time constraints. However, it is inapplicable to single ion channel lesion genetic research or cardiovascular ischemia-related SSS research. It may cause damage outside the SAN and is unsuitable for diseases with originally combined ventricular or atrial arrhythmias. In this SSS modeling study, failure to collect dynamic ECGs may miss transient arrhythmias. Due to limited conditions, specific SAN action potential changes could not be deeply explored. With improved experimental conditions, more in-depth research is needed.
Figure 1.
The process of model establishment, changes in the ECG after molding, and different urine during molding. A) illustrates the process of model establishment, by Figdraw. B) shows the ECG before and after model establishment, 20mm/Mv, 25mm/sec. a. HR is 341 bpm and b. HR is 219 bpm, escape rhythm. C) displays the changes in urine during the model establishment process, the upper picture depicts normal urine, clear and bright, while the lower image shows hematuria, slightly red and turbid.
Figure 1.
The process of model establishment, changes in the ECG after molding, and different urine during molding. A) illustrates the process of model establishment, by Figdraw. B) shows the ECG before and after model establishment, 20mm/Mv, 25mm/sec. a. HR is 341 bpm and b. HR is 219 bpm, escape rhythm. C) displays the changes in urine during the model establishment process, the upper picture depicts normal urine, clear and bright, while the lower image shows hematuria, slightly red and turbid.
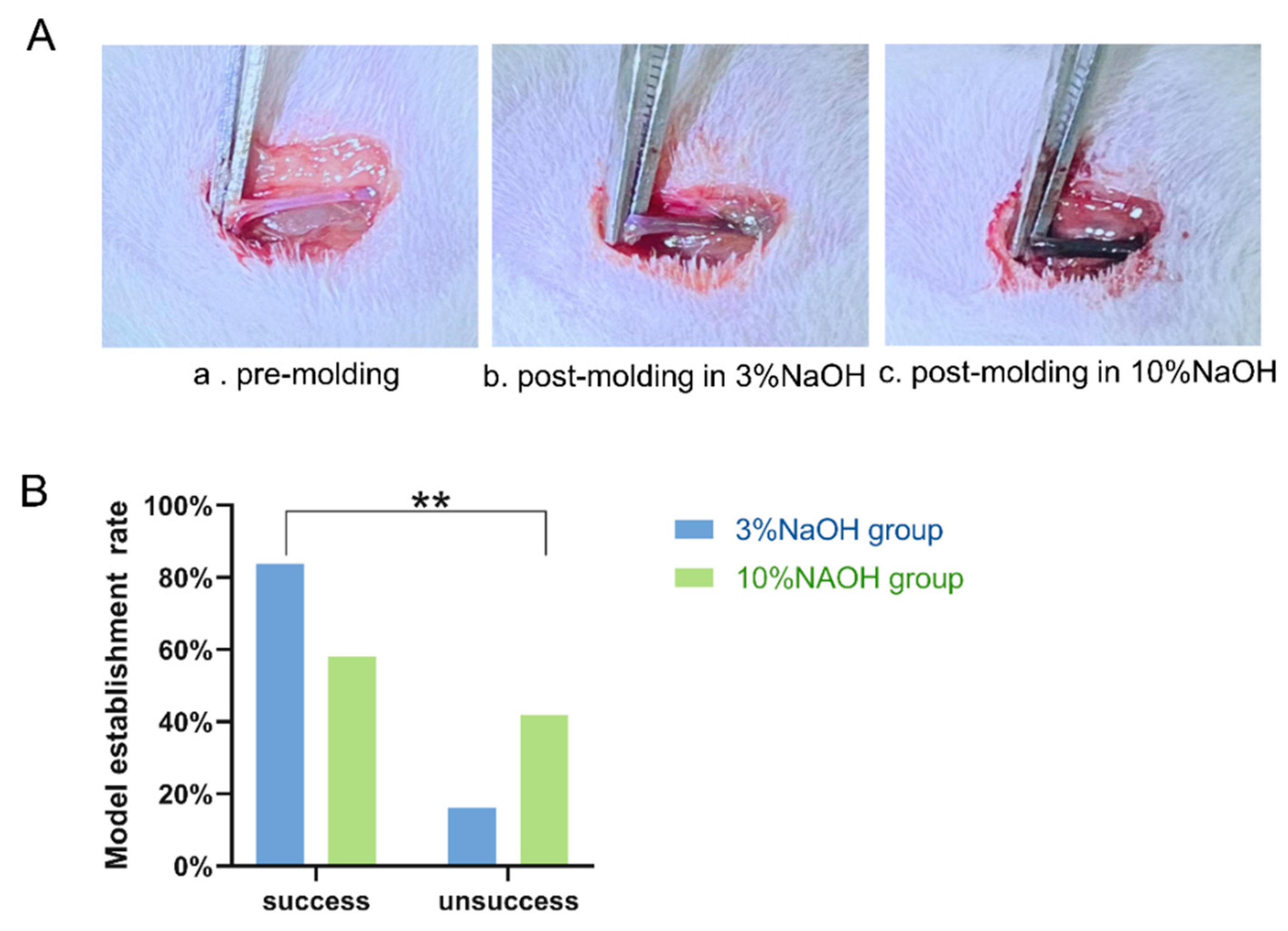
Figure 2.
Compares JV changes and model establishment rate between rats injected with 3% and 10% NaOH. A) JV changes pre and post-molding establishment, a. Normal vascular; b.3% NaOH injection, causing vascular dilation and darkening; c.10% NaOH injection, causing significant dilation and blackening. B) Model establishment success rate between the two modeled groups, since the pathological staining appointment was scheduled 1 week post-molding, the numbers at 1 week post-molding were used for the statistics. The P value was obtained by Chi-square test. **: vs 10% NaOH group P<0.01, statistically significant.
Figure 2.
Compares JV changes and model establishment rate between rats injected with 3% and 10% NaOH. A) JV changes pre and post-molding establishment, a. Normal vascular; b.3% NaOH injection, causing vascular dilation and darkening; c.10% NaOH injection, causing significant dilation and blackening. B) Model establishment success rate between the two modeled groups, since the pathological staining appointment was scheduled 1 week post-molding, the numbers at 1 week post-molding were used for the statistics. The P value was obtained by Chi-square test. **: vs 10% NaOH group P<0.01, statistically significant.
Figure 3.
Changes in HR and rhythm after modeling. A) HR changes in each group of rats 2 weeks after molding, 10 mm/mV, 25 mm/sec. The HR of a is 366 bpm, b is 228 bpm, and c has a slow HR with second-degree atrioventricular block 2:1 conduction. The PP interval is about 261 bpm, and the RR interval is about 143 bpm. B) HR results in each group of SD rats before molding, 1 hour after molding, 1 week after molding, and 2 weeks after molding. Circles represent degree of dispersion, and error bars represent standard deviation. The corrected P value was obtained through one-way analysis of variance and post hoc Bonferroni. ***: vs sham P < 0.001, statistically significant difference; ns: vs 10% NaOH P > 0.05, no statistical significance. C) ECG examples of SSS arrhythmia types in SD rats. a. Type A was collected 2 weeks after molding, with intermittent sinus arrest, pause time reached 588 ms, and basic sinus HR was 269 bpm; b. Type B was collected 1 hour after molding, HR decreased significantly, to about 188 bpm, accompanied by paroxysmal supraventricular tachycardia, HR about 349 bpm, consistent with bradycardia-tachycardia syndrome; c. Type C was collected 1 hour after molding, slow HR and second-degree atrioventricular block 2:1 and 3:1 conduction. PP interval about 259 bpm. When 2:1 conduction, RR interval about 134 bpm, when 3:1 conduction, RR interval about 88 bpm, consistent with sick sinus node - atrioventricular node syndrome. D) Results of clinical classification in the two modeling groups. The top graph is for 1 week after molding, and the bottom is for 2 weeks after molding. The P value was obtained by Chi-square test. *: 3% NaOH vs 10% NaOH P < 0.05, statistically remarkable difference.
Figure 3.
Changes in HR and rhythm after modeling. A) HR changes in each group of rats 2 weeks after molding, 10 mm/mV, 25 mm/sec. The HR of a is 366 bpm, b is 228 bpm, and c has a slow HR with second-degree atrioventricular block 2:1 conduction. The PP interval is about 261 bpm, and the RR interval is about 143 bpm. B) HR results in each group of SD rats before molding, 1 hour after molding, 1 week after molding, and 2 weeks after molding. Circles represent degree of dispersion, and error bars represent standard deviation. The corrected P value was obtained through one-way analysis of variance and post hoc Bonferroni. ***: vs sham P < 0.001, statistically significant difference; ns: vs 10% NaOH P > 0.05, no statistical significance. C) ECG examples of SSS arrhythmia types in SD rats. a. Type A was collected 2 weeks after molding, with intermittent sinus arrest, pause time reached 588 ms, and basic sinus HR was 269 bpm; b. Type B was collected 1 hour after molding, HR decreased significantly, to about 188 bpm, accompanied by paroxysmal supraventricular tachycardia, HR about 349 bpm, consistent with bradycardia-tachycardia syndrome; c. Type C was collected 1 hour after molding, slow HR and second-degree atrioventricular block 2:1 and 3:1 conduction. PP interval about 259 bpm. When 2:1 conduction, RR interval about 134 bpm, when 3:1 conduction, RR interval about 88 bpm, consistent with sick sinus node - atrioventricular node syndrome. D) Results of clinical classification in the two modeling groups. The top graph is for 1 week after molding, and the bottom is for 2 weeks after molding. The P value was obtained by Chi-square test. *: 3% NaOH vs 10% NaOH P < 0.05, statistically remarkable difference.
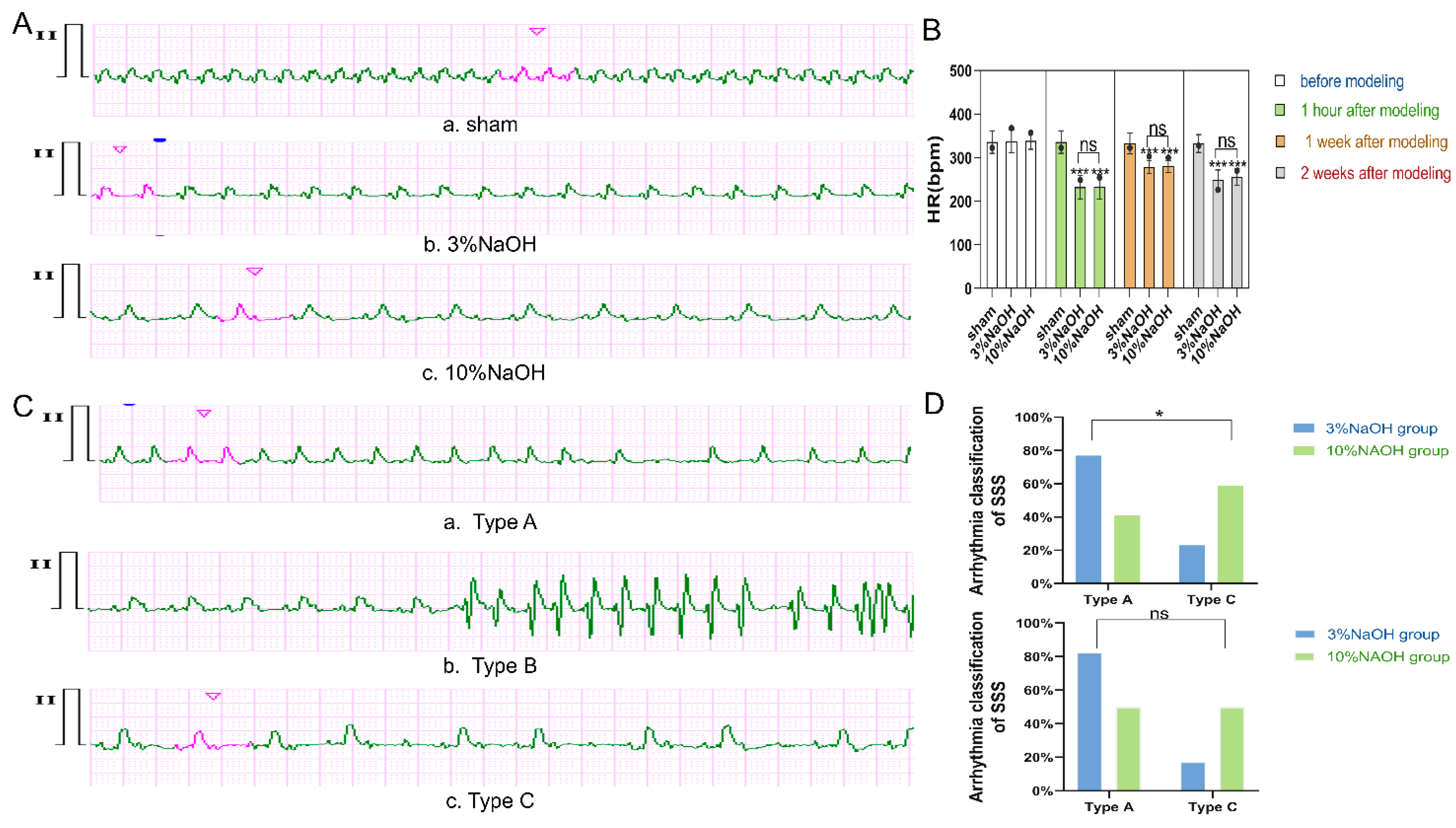
Figure 4.
Results of pathological morphology and verifications of ions related to spontaneous electrical activity. A) H&E staining (scale 20µm). a. normal tissue structure; b. cells in 3% NaOH disordered and swollen, with lightly stained cytoplasm, indicating edema-like degeneration, nuclear fragmentation, and dissolution; c. similar changes in 10% NaOH. The five-pointed star indicates SANA, and the arrow indicates the P cell. B) membrane ion channel mRNA transcription results. The circle indicates each value of repeated independent biological samples (n = 3), and the error bar indicates the standard deviation. Corrected P value obtained through one-way ANOVA and post-Bonferroni. *, **, ***: vs sham P<0.05, P<0.01, P<0.001, statistically significant difference. C) HCN4 protein expression change (n=3). ***: vs sham P<0.001, statistically significant. D calcium ions content change in SAN tissue of each group(n = 3).*, **: vs sham P<0.05, P<0.01, *: 3% NaOH vs 10% NaOH P<0.05, statistically significant.
Figure 4.
Results of pathological morphology and verifications of ions related to spontaneous electrical activity. A) H&E staining (scale 20µm). a. normal tissue structure; b. cells in 3% NaOH disordered and swollen, with lightly stained cytoplasm, indicating edema-like degeneration, nuclear fragmentation, and dissolution; c. similar changes in 10% NaOH. The five-pointed star indicates SANA, and the arrow indicates the P cell. B) membrane ion channel mRNA transcription results. The circle indicates each value of repeated independent biological samples (n = 3), and the error bar indicates the standard deviation. Corrected P value obtained through one-way ANOVA and post-Bonferroni. *, **, ***: vs sham P<0.05, P<0.01, P<0.001, statistically significant difference. C) HCN4 protein expression change (n=3). ***: vs sham P<0.001, statistically significant. D calcium ions content change in SAN tissue of each group(n = 3).*, **: vs sham P<0.05, P<0.01, *: 3% NaOH vs 10% NaOH P<0.05, statistically significant.

Table 1.
Reference Criteria for Arrhythmia Classification of Sick Sinus Syndrome.
Table 1.
Reference Criteria for Arrhythmia Classification of Sick Sinus Syndrome.
| Type |
Lesion Range |
ECG Manifestations |
type A
(simple SSS type) |
confined to the SAN |
sinus bradycardia, sinus arrest, and sinoatrial block, without rapid arrhythmias and atrioventricular(AV) or bundle branch conduction block |
type B
(bradycardia-tachycardia syndrome type) |
in addition to involving the SAN, atrial or perinodal area is also affected. |
sinus bradycardia, sinus arrest, and sinoatrial block, by atrial tachycardia, paroxysmal atrial fibrillation, and supraventricular tachycardia. |
type C
(double node lesion type or total conduction system lesion) |
involving the SAN and atrioventricular node(AVN), with intra-atrial or bundle branch conduction block |
sinus bradycardia, sinoatrial block accompanied by AV conduction block or bundle branch conduction block |
Table 2.
The amount of surviving rats pre-molding and post-molding.
Table 2.
The amount of surviving rats pre-molding and post-molding.
| Group |
Pre-Molding(n) |
1 hour Post-Molding |
1 week Post-Molding(n) |
2 weeks Post-Molding |
| sham |
12 |
12 |
12 |
9 |
| 3%NaOH |
30 |
26 |
26 |
23 |
| 10%NaOH |
30 |
17 |
17 |
14 |
Table 3.
Possible influencing factors in the model establishment process between the modeled groups.
Table 3.
Possible influencing factors in the model establishment process between the modeled groups.
| Variable |
3%NaOH (n=31) |
10%NaOH (n=31) |
Z/X2
|
P-Value |
| Amount of NaOH(g) |
0.011(0.008,0.012) |
0.009(0.007,0.012) |
Z=0.97 |
>0.05 |
| number of injections(n) |
4.5(4.0,6.0) |
2.5(2.0,3.0) |
Z=5.09 |
<0.001 |
| hematuria (n%) |
26(86.7) |
18(60) |
X2=5.46 |
<0.05 |
Table 4.
The outcomes of logistic regression analysis affecting the occurrence of model establishment events.
Table 4.
The outcomes of logistic regression analysis affecting the occurrence of model establishment events.
| Variable |
B Value |
Standard Error |
Wald X2
|
P-Value |
OR |
95%CI |
| number of injections |
0.46 |
0.27 |
2.98 |
>0.05 |
1.58 |
0.94-2.66 |
| hematuria |
3.73 |
0.89 |
17.44 |
<0.001 |
41.46 |
7.22-238.15 |