1. Introduction
The virus replication occurs in many consecutive steps to generate many mature viral particles upon the completion of the viral replication in the permissive cells. The virus always hijacks the cellular machinery and directs them to synthesize their viral proteins instead of the cellular proteins [
1,
2]. The viral entry is a crucial step in any viral infection [
3]. The viral entry requires the interaction between many proteins from the viral and host sides [
1]. The presence of viral-specific receptors is considered an important factor in the process of viral entry into the host cells. The host cell is called permissive for certain viruses if they express the specific viral receptors and provide the suitable environments for the virus replication, including any auxiliary receptors, transcription, and translation factors. Coronaviruses are enveloped viruses containing positive sense RNA genomes and belong to the order Nidovirales and are classified into four genera (α, β, λ, and δ) [
4]. The genus β-coronavirus includes five important human coronaviruses the sever acute respiratory syndrome coronavirus-1 (SARS-CoV-1), the Middel East respiratory syndrome coronavirus (MERS-CoV) the SARS-CoV-2, and the human coronavirus-OC43 (HCoV-OC-43). This genus includes some other important viruses affecting animals, particularly the BCoV and the equine coronavirus (ECoV) [
4]. The coronavirus’s genome size ranges from 27-31 kb in length and has a unique organization. The full-length genome is flanked with two untranslated regions at the 5′ and 3′ ends. Coronaviruses are characterized by the production of a set of sub-genomic messenger RNA (mRNA) at their 3′ end [
5]. The 5′ end of the genome of most coronaviruses contains a large gene called gene-1, which consists of two overlapping open reading frames (ORFs) with a ribosomal frameshifting between those two ORFs. However, the 3′ end of the genome is mainly occupied by the common structural proteins interspersed with some small accessory proteins. There are four major structural proteins in most coronaviruses, including the spike glycoprotein (S), the envelope (E), the membrane (M), and the nucleocapsid protein (N). Some members of the genus β-coronavirus, including BCoV and HCoV-OC43, have an additional structural protein called hemagglutinin esterase (HE); thus, their genome is a little larger in size compared to other coronaviruses (31 Kb) [
6]. The S glycoprotein is a key player in all coronavirus replication. There are several proteins, including some cellular receptors, co-receptors, and cellular enzymes are involved in the BCoV/host interaction. The BCoV spike (BCoV/S) and BCoV-HE proteins are important in virus replication and pathogenesis (6). BCoV-S has potential bidding to the 5-N-acetyl-9-O-acetylneuraminic acid, suggesting their possible roles as BCoV receptors (7). On the other hand, the BCoV-HE acts as a receptor-destroying enzyme during BCoV replication [
7]. However, there is a lack of comprehensive understanding of the interplay of the BCoV-S/BCoV-HE and the cellular receptors during BCoV replication. The availability of specific receptors is one of the main factors that make the target cells permissive to coronavirus (CoVs) infection. Each group of CoVs recognizes certain types of receptors and may require the presence of additional auxiliary receptors to facilitate virus attachment and downstream replication. SARS-CoV-2 uses the angiotensinogen-converting enzyme-2 (ACE-2) as the main receptor, and the chaperone GRP78 acts as an auxiliary receptor [
8,
9]. It was also shown that MERS-CoV utilize the dipeptidyl peptidase-4 (DPP4) as receptors in humans and dromedary camels [
10,
11]. The amino-peptidase N (APN) also called cluster of differentiation -13 (CD13) act as a major receptor for the transmissible gastroenteritis virus that mainly cause enteric infections in pigs [
12]. The carcinoembryonic antigen cell adhesion molecule 1 (CEACAM-1) also acts as a major receptor to another coronavirus called murine hepatitis virus (MHV) [
13]. Although the presence of the CoVs receptors and co/receptors is important for the success of viral replication, most coronaviruses require the presence of host cell enzymes that help in the cleavage of the CoV-S and CoV-HE proteins to initiate the process of viral infection. Usually, these host cell enzymes are enriched at the portal of entry of most coronaviruses, particularly the mucosal surfaces of the respiratory and enteric tracts of the affected hosts. It has been recently shown that TMPRSS2 (transmembrane protease serine 2) is a serine protease that plays an important role in SARS-CoV-2 replication, particularly during viral entry to the host cells. TMPRSS2 usually cleaves the viral spike glycoprotein, which activates the virus and facilitates its entrance to the host cells [
14]. Furin is another host cell enzyme that is considered to be a subtilisin-like proprotein convertase. Furin usually cleaves the target proteins at a polybasic amino acid sequence R-X-(K/R)-R (where R is arginine, K is lysine, and X can be any amino acid). The Furin cleavage to the SARS-CoV-2-S protein enhances the pathogenicity transmissibility and increases the virus infectivity in the target host [
15,
16]. Although the roles of the above-mentioned receptors and enzymes were intensively studied in SARS-CoV2, there is a lack of knowledge about the roles of these receptors and enzymes in BCoV infection and replication. The main goals of the current study are to use the in-silico prediction and docking tools to study the roles of these proteins and enzymes in BCoV replication. This study shed light on some unknown aspects of BCoV tissue tropism and pathogenesis. It will also pave the way for the development of some novel vaccines and antiviral therapies for BCoV infection in cattle.
Homology modeling is a well-established method that has been shown to produce quite accurate models for a protein sequence if an X-ray structure of a protein with a sufficient degree of sequence similarity is available [
17]. The method is based on the fact that the structural conformation of a protein is more highly conserved than its amino acid sequence and that small or medium changes in sequence normally result in little variation in the 3D structures [
18]. The quality of the model is directly linked to the identity between template and target sequences. As a rule, models built with over 50% sequence similarities are accurate enough for drug discovery applications [
19].
4. Discussion
The tropism of coronaviruses is a complicated process that requires the availability of some factors from the viral side, including some attachment proteins, particularly the spike glycoprotein. This process also involves some cellular factors, including the receptors and other transcription and translation factors. It also requires some factors from the infected host, particularly the availability of some host enzymes that help activate some essential proteins [
32,
33,
34]. BCoV possesses multiple tissue tropism in cattle. The virus mainly affects the digestive and respiratory tract of the affected animals; this pattern is called pneumoenteritis [
35]. The viral tropism primarily depends on the availability of specific viral receptors, some other transcription translation factors, and some host cell enzymes [
36]. Members of the family coronaviridae utilize many host cell receptors to attach to their target cells [
37].
Meanwhile, most coronaviruses are inert outside the host. Coronaviruses require activation by some host cell proteases to initiate the viral infection inside the host. Usually, the coronaviruses spike glycoproteins cleaved by some host cell proteases of different classes to initiate the viral infection [
32,
33,
34]. Although BCoV was discovered long ago, little is still known about viral tropism, especially the roles of the host cell receptors and the host enzymes in fine-tuning the viral tissue tropism [
35]. The main aims of the current study are to explore the possibilities of identifying some novel receptors of BCoV and to predict the interaction of some key viral proteins (S and HE) with some host proteases, particularly the Furin and the TMPRSS2. The BCoV/S glycoprotein is the main viral protein involved in the process of viral attachment to the host cells [
38]. The S protein comprises two subunits (S1 and S2): the N-terminal domain (NTD) and BCoV_S1_CTD are in the S1 subunit, whereas the fusion peptide (FP) and heptad repeat (HR.) domains 1 and 2 are located in the S2 subunit of the BCoV/S glycoprotein. The BCoV/ S1 protein usually attaches to the cell membrane by interacting with viral receptors on the surface of the target cells, initiating the viral infection. Spike protein S2 mediates the fusion of the virion and cellular membranes by acting as a class I viral fusion protein. Also, it acts as a viral fusion peptide, which is unmasked following the S2 cleavage site occurring upon virus endocytosis [
39,
40]. The distal S1 subunit of the coronavirus spike protein is responsible for receptor binding. Either the S1-NTD or the S1-RBD at C-terminal domain of the BCoV-S1 protein chain, or occasionally both, are involved in the binding to the host receptors [
41,
42]. The observed binding between Neu5,9Ac2 and the BCoV NTD is particularly interesting because BCoV haemagglutinin esterase (HE) also utilizes this O-acylated sugar molecule as a substrate. While our study identified interacting residues like Asp-187, Gly-189, His-185, and Lys-196, another report [
43] proposed a slightly similar set of critical residues for Neu5,9Ac2 binding (Tyr-162, Glu-182, Trp-184, and His-185).
BCoV/S and BCoV/HE proteins act synergistically and harmoniously to orchestrate the BCoV infection in the target cell [
7,
44]. The BCoV/S is mainly involved in the initial attachment of the virus to the host cells, while the BCoV/HE destroys the sialic acid in the cel
l’s surface, promoting the viral release from the cell [
7].
Most Betacoroanviruses, including the BCoV isolates, use the 9-O-acetyl-Sas as receptors; however, during the evolution of these viruses, some isolates started to recognize other forms of sialic acid (the 4-O-acetyl-SA isoform) [
45]. It was recently shown that the HE gene of the SARS-CoV-2 recognizes and binds to the 9-O-acetyl-Sas in contrast to the type-II HE protein of SARS-CoV-2 uses the other isoform; the 4-O-acetyl-SA [
45]. The ligand-interaction sites of the BCoV HE and the subset of coronavirus S glycoproteins evolved to recognize 9-O-Ac-Sia via hydrogen bonding [
46] specifically.
In the case of most betacoronaviruses, the RBD of CTD or domain B of the spike glycoproteins showed the highest variability within S1 subunits across various members of the coronaviruses, including betacoronaviruses. This phenomenon allows coronaviruses to bind to various types of host cell receptors [
46]. It has been proved that SARS-CoV-2 uses the ACE-2 as a valid receptor for the viral entry into the target host cells [
47]. However, there are no records about the potential roles of ACE2 as receptors for BCoV. In our study, based on the interaction between bovine ACE2 and BCoV /S interaction with its CTD, the bovine cell surface ACE2 could act as a putative receptor for BCoV/S (
Figure 13). This finding is based on the BCoV spike binding to the ACE2 recepto
r’s tendency to show lower interaction energy [
48]. This claim is also supported by the nature of the interaction energy, which is consistent with the structure of the virus receptor interface [
49].
We also investigated the potential use of the Neuropilin-1 (NRP1) as a receptor for the BCoV. Our ZDock method result suggested the high interaction affinity (-22.99 kcal/mol) of NRP1 with BCoV/S-NTD and BCoV/S-CTD region (
Figure 5 and
Figure 13 and
Table 2). This raises the possibility that BCoV could potentially use the NRP receptors for viral entry into the host cell. According to some previous reports, the higher expression of NRP1 will facilitate virus-host cell interactions, especially in cells that do not express other potential BCoV receptors [
50,
51]. Previous research indicates that Neuropilin-1 (NRP-1) exhibits stronger binding affinity to the CTD region of S1, and this interaction stabilizes the folded conformation of the S protein [
52]. Conversely, in the absence of NRP-1, the SARS-CoV-2 S protein tends to stretch and unfold.
It is week known that MHV utilizes the CEACAM1 protein for a dual function. The CEACAM-1 acts as a functional receptor for MHV in addition to enhancing the activation of the MHV-S glycoprotein by inducing some conformational changes, allowing the fusion of the virus with the target cells
[53]. However, there is no data about the potential roles of CEACAM-1 in the BCoV replication. Our data showing low binding affinity interaction between BCoV/S and CEACAM-1 suggested that CEACAM-1 interacted with low affinity to the NTD of BCoV/S protein. The variations in binding of coronaviruses to CEACAM-1 can be directly attributed to the structural differences between their N-terminal domains, particularly those of BCoV and MHV. Unlike MHVs sugar-binding ancestors, contemporary MHVs utilize their NTDs for specific interaction with the host cell protein CEACAM-1 (
Figure 13) [
54].
The aminopeptidase N (APN), a widely found enzyme on cell surfaces, is involved in various cellular processes like survival, migration, blood pressure regulation, and even virus uptake [
55]. PDCoV can utilize APN as a receptor to enter cells, highlighting AP
N’s potential role as a viral infection gateway from different host species [
56]. Aminopeptidase N from porcine functions as a receptor for the enveloped RNA virus TGEV [
12]. This underscores the wide variety of membrane-bound proteins viruses exploit to infiltrate cells. The APN is a common receptor for the members of the alpha coronaviruses, particularly the HCoV-229E and the TGEV in pigs [
57,
58,
59]. Based on our protein-protein interaction (E_RDock), the interaction energy of the best pose of APN interaction with BCoV/S shows low-affinity interaction (-2.96 kcal/mol). This result confirms that the binding interaction of BCOV/S is not strong, and it shows a putative binding with the APN. The RBD of CTD from BCoV/S is involved in interaction with Bovine APN (
Figure 13).
The DPP4 is a cell surface protease that exhibits exopeptidase activity and is expressed on the surface of various cell types, including those found in the human airways [
60]. Researchers have compared the potential binding interactions between the receptor binding domain (RBD) of different spike variants of SARS-CoV-2 and DPP4 with the interactions observed in the experimentally determined structure of the MERS-CoV complex with DPP4 [
61]. Members of the Betacoroanviruses, especially the SARS-CoV-2 and the MERS-CoV, utilize the ACE2 and DPP4 as receptors, respectively [
62]. The pathogenicity of MERS-CoV is caused by the specific binding of its S1B domain or S1-CTD to the human DPP4 receptor [
63]. In our study, docking of the BCoV spike with the DPP4 shows low interaction affinity of DPP4 towards BCoV/S (E_R Dock score -6.23 kcal/mol). However, the binding of DPP4 with BCoV/S1-CTD region or S1B domain is correlated with MERS-CoV binding of its S1B domain or S1-CTD to the human DPP4 (
Figure 13).
For virus entry and infections, proteolytic cleavage is widely used to activate the fusion machinery of viral glycoproteins. Furin binding to RRSRR (Arginine-Arginine-Serine-Arginine-Arginine) site of BCoV spike protein, which is related to the conserved cleavage site of SARS-CoV-2 cleavable PRRAR|S residues at receptor-binding (S1) and fusion (S2) domains of the spike protein [
64]. Such a motif may allow Spikes to be cut into S1 and S2 by Furin and TMPRSS2-like proteases before maturity, which provides S1 with the flexibility to change the conformation to better fit the host receptor. The arginine residues at the SARS-CoV-2 spike protein catalytic site are popped out of the closed state of the S protein to form multiple non-covalent interactions with the Furin [
65]. In our study, the BCoV/S interaction with the Furin, shows that Furin binds near the spike protein S1/S2 cleavage RRSRR site specific for Furin and TMPRSS2 proteases (
Figure 13).
TMPRSS2 has been shown to proteolytically activate the S glycoprotein of many coronaviruses, including SARS-CoV-2, SARS-CoV-1, MERS-CoV, 229E, as well as influenza virus [
66,
67,
68]. TMPRSS2 triggers HKU1-mediated cell-cell fusion and viral entry and binds with high affinity to both HKU1A and HKU1B RBDs.[
69].
The protease furin binds firmly with the S protein RRSRR site, which is conserved in BCoV as RRSRR at the S1/S2 site, whereas the TMPRSS2 cleaves the S protein in the lungs of SARS-CoV-2 infected person and promotes pathogenicity [
70]. SARS-CoV-2 S protein S1/S2 is cleaved by furin protease, and subsequently, TMPRSS2 mediates the cleavage and activation of the S2 region on the S2 protein [
71]. TMPRSS2, which colocalizes with ACE2 at the cell membrane, has been identified as the dominant proteolytic driver of S protein activation and SARS-CoV-2 infection of the aerodigestive tract [
72]. TMPRSS2 protease can be opted as a potential therapeutic target for bovine host-specific viruses. In this study, TMPRSS2 binds near the S1/S2 junction RRSRR protease cleavage-specific region for furin and TMPRSS2 proteases. Therefore, there is limited knowledge about the bovine-specific virus-host interactions that determine cellular entry of SARS-CoV-2. Viruses display considerable redundancy and flexibility because they can exploit weak multivalent interactions to enhance affinity. To date, studies of BCoV entry have focused almost entirely on ACE2 and Neu5,9Ac2. In this study, we aimed to see the interaction of bovine cell surface molecules as receptors for BCoV/S, including Nue5,9Ac2, NRP1, DPP4, CEACAM-1, APN ACE2, TMPRSS2, and Furin shown in
Figure 13. The results from our study suggested that NRP1 showed greater binding affinity towards BCoV/S other than receptor Neu5,9Ac2. However, bovine HE also showed a stronger binding affinity with Neu5,9Ac2. Our in silico structural interaction data provide a blueprint for understanding the BCoV specificity for the different bovine receptors.
Author Contributions
Conceptualization, MGH., and MC.; methodology, MGH, YMK, ND, AS, RN.; software, YMK, ND, AS, RNE.; validation, YMK., ND., and AS.; formal analysis, YMK., ND., and AS.; investigation, MGH, MC, RNE.; resources, MGH, RNE, MC.; data curation, MGH, YMK, ND, AS, RNE.; writing—original draft preparation, YMK, MGH.; writing—review and editing, YMK, ND, AS, RNE, MC, MGH.; visualization, YMK, ND, AS, RNE, MC, MGH.; supervision, MGH.; project administration, MGH, RNE, MC.; funding acquisition, MGH, RNE, MC All authors have read and agreed to the published version of the manuscript.
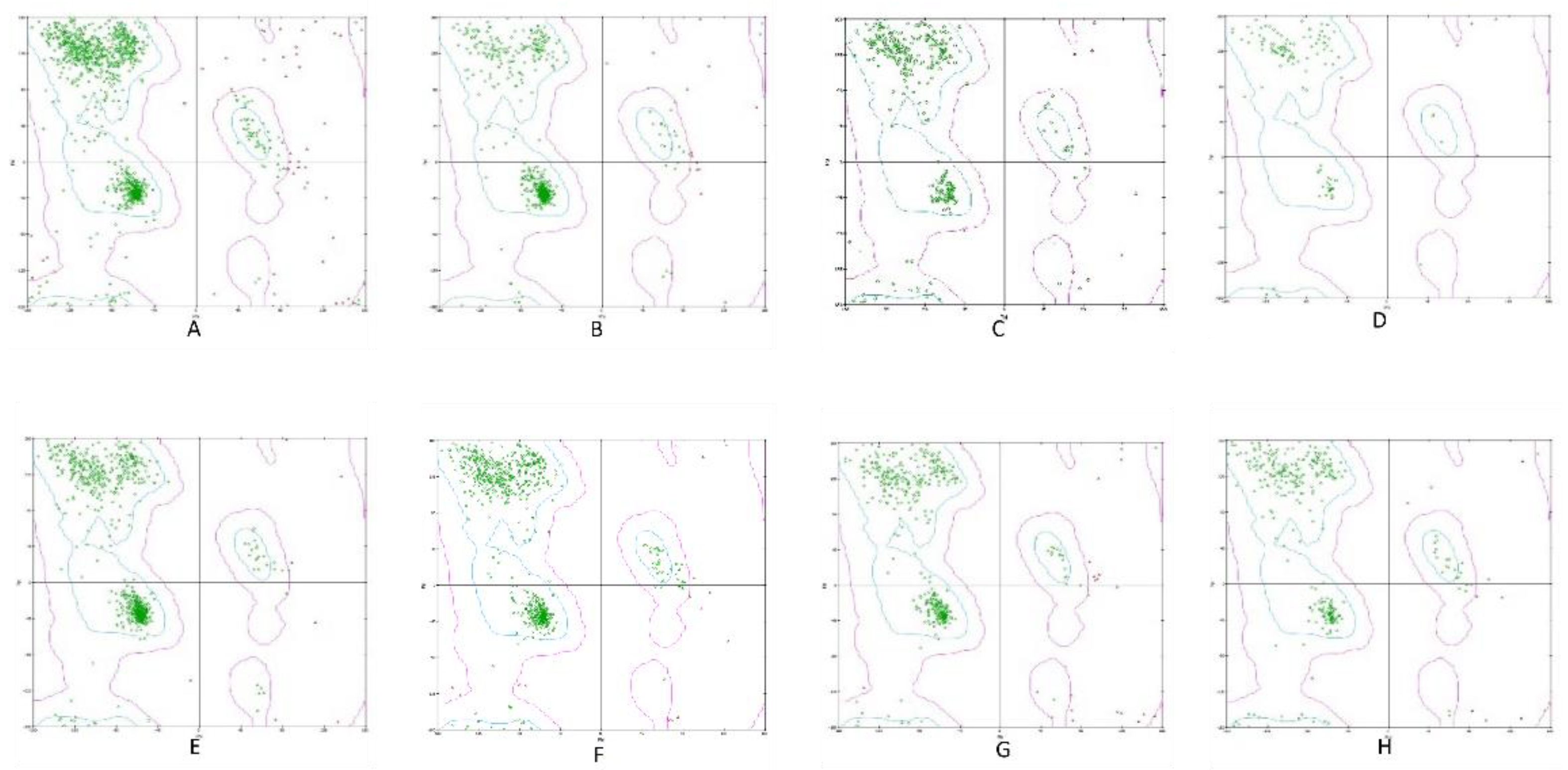
Figure 1.
The validity and stability of the Homology modeled protein structure using the Ramachandran plot (A) BCoV-Spike (B) Bovine ACE2 (C) Bovine NRP1 (D) Bovine CEACAM-1 (E) Bovine APN (F) Bovine DPP4 (G) Bovine Furin (H) Bovine TMPRSS2.
Figure 1.
The validity and stability of the Homology modeled protein structure using the Ramachandran plot (A) BCoV-Spike (B) Bovine ACE2 (C) Bovine NRP1 (D) Bovine CEACAM-1 (E) Bovine APN (F) Bovine DPP4 (G) Bovine Furin (H) Bovine TMPRSS2.
Figure 2.
Schematic representation of the BCoV S Protein: S1 and S2 Chains (A) The listed domain boundaries are mostly defined as S-NTD, N-terminal Domain; RBD, Receptor Binding Domain; S1-CTD, C-Terminal domain; S2-HR1, Heptat Repeat 1; S2-HR2, Heptad Repeat 2; S2-CD, Cytoplasm Domain. (B) Schematic drawing of the three-dimensional Structure of BCoV Spike protein showing different domains on S1 and S2 Chains.
Figure 2.
Schematic representation of the BCoV S Protein: S1 and S2 Chains (A) The listed domain boundaries are mostly defined as S-NTD, N-terminal Domain; RBD, Receptor Binding Domain; S1-CTD, C-Terminal domain; S2-HR1, Heptat Repeat 1; S2-HR2, Heptad Repeat 2; S2-CD, Cytoplasm Domain. (B) Schematic drawing of the three-dimensional Structure of BCoV Spike protein showing different domains on S1 and S2 Chains.
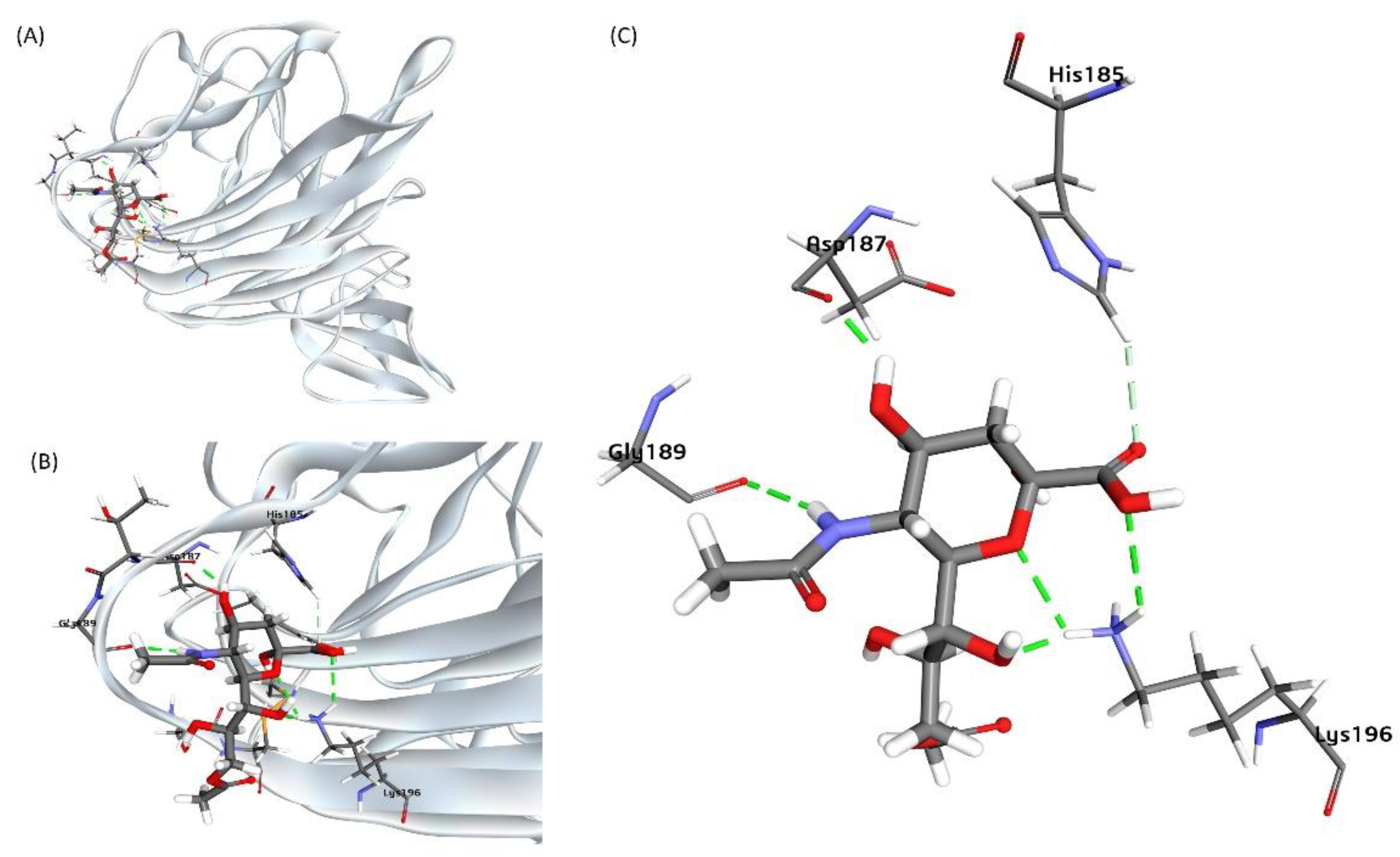
Figure 3.
Sialic acid (Neu5,9Ac2) as the receptor for BCoV Spike protein at the NTD site. (A) Neu5,9Ac2 binding to the N-terminal domain (NTD) of the BCoV Spike protein is illustrated. (B) and (C) Detailed views of the interactions between Neu5,9Ac2 and the specific amino acid residues at the NTD site of the BCoV Spike protein: His185, Asp187, Gly189, and Lys196.
Figure 3.
Sialic acid (Neu5,9Ac2) as the receptor for BCoV Spike protein at the NTD site. (A) Neu5,9Ac2 binding to the N-terminal domain (NTD) of the BCoV Spike protein is illustrated. (B) and (C) Detailed views of the interactions between Neu5,9Ac2 and the specific amino acid residues at the NTD site of the BCoV Spike protein: His185, Asp187, Gly189, and Lys196.
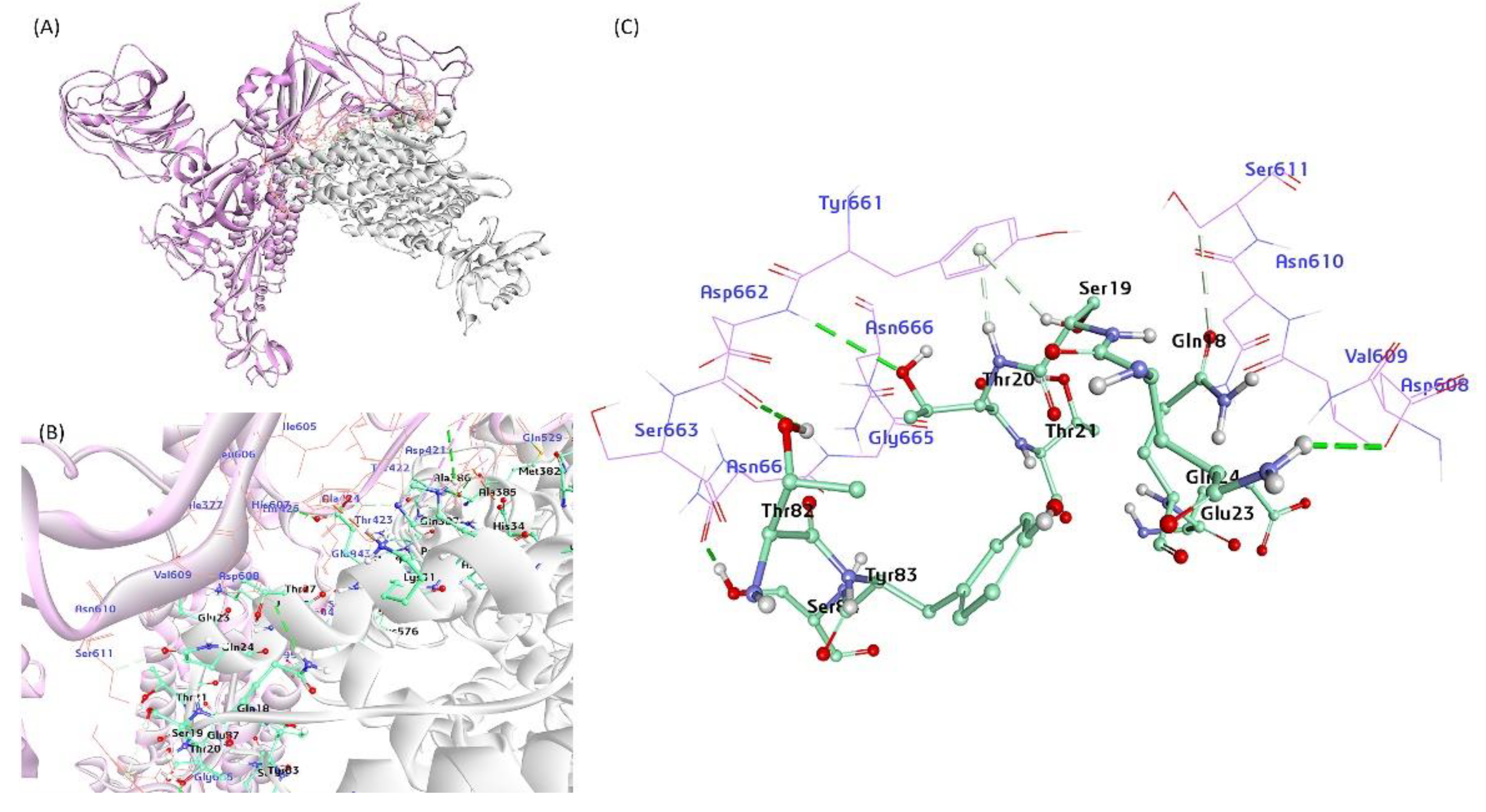
Figure 4.
A proposed Model for the BCoV/S glycoprotein interaction with the bovine ACE2 protein. (A) The BCoV Spike protein interacting with ACE2, showing possible binding interactions at the receptor-binding domain (RBD) of the C-terminal domain (CTD) of the S1 protein chain. (B) and (C) Detailed views of the protein-protein interaction interface, highlighting the amino acid residues involved in the interaction between ACE2 and the N-terminal domain (NTD) of the BCoV Spike protein. ACE2 interaction residues: Ser19, Thr20, Thr21, Glu23, Gln24, Thr27, Glu30, Lys81, Thr82, and other residues. BCoV Spike NTD interaction residues: Tyr661, Asp662, Ser663, Gly665, Asn666, Ser611, Thr425, Arg419, Ala527, and other residues.
Figure 4.
A proposed Model for the BCoV/S glycoprotein interaction with the bovine ACE2 protein. (A) The BCoV Spike protein interacting with ACE2, showing possible binding interactions at the receptor-binding domain (RBD) of the C-terminal domain (CTD) of the S1 protein chain. (B) and (C) Detailed views of the protein-protein interaction interface, highlighting the amino acid residues involved in the interaction between ACE2 and the N-terminal domain (NTD) of the BCoV Spike protein. ACE2 interaction residues: Ser19, Thr20, Thr21, Glu23, Gln24, Thr27, Glu30, Lys81, Thr82, and other residues. BCoV Spike NTD interaction residues: Tyr661, Asp662, Ser663, Gly665, Asn666, Ser611, Thr425, Arg419, Ala527, and other residues.
Figure 5.
The proposed model for the interaction between the bovine NRP1 and the BCoV/S glycoprotein. (A) NRP1 interactions with the C-terminal domain (CTD) and N-terminal domain (NTD) of the S1 protein, illustrating possible binding conformations. (B) and (C) Specific NRP1 residues (Leu322, Leu333, Val269, Arg307, Tyr138, Gln266) and their binding affinities to amino acid residues in the NTD and CTD of the S1 protein.
Figure 5.
The proposed model for the interaction between the bovine NRP1 and the BCoV/S glycoprotein. (A) NRP1 interactions with the C-terminal domain (CTD) and N-terminal domain (NTD) of the S1 protein, illustrating possible binding conformations. (B) and (C) Specific NRP1 residues (Leu322, Leu333, Val269, Arg307, Tyr138, Gln266) and their binding affinities to amino acid residues in the NTD and CTD of the S1 protein.
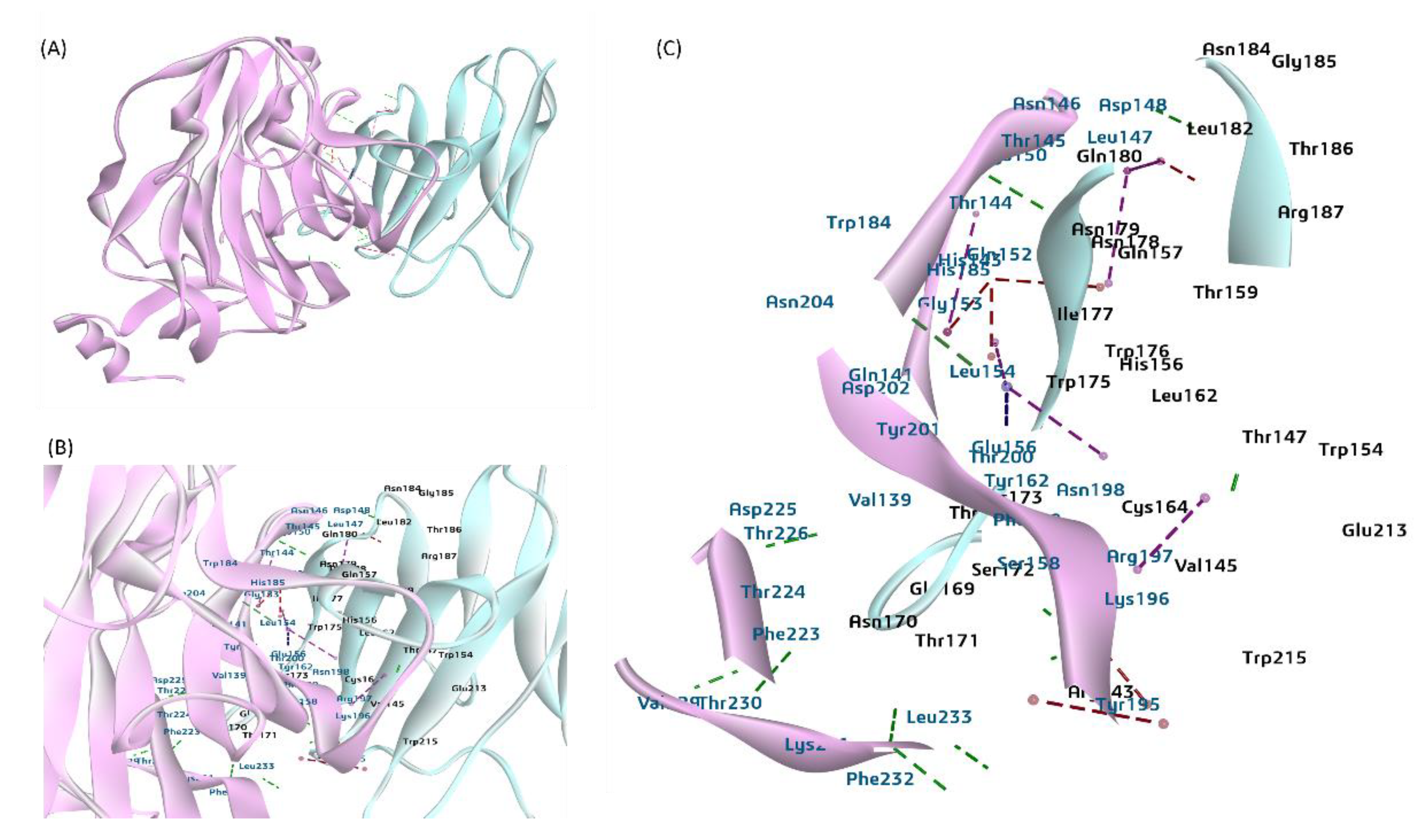
Figure 6.
A putative model for the interaction between the bovine CEACAM-1 protein and the BCoV-S glycoprotein and mapping the interactive amino acid residues. (A) The bovine CEACAM-1 protein interacts with the BCoV/S glycoprotein NTD of the BCoV spike. (B) and (C) CEACAM-1 binds through LEU233, PHE232, VAL229, Phe232, THR145, ASP148 and other amino acids with N-terminal domain (NTD) of Spike glycoprotein with residues ARG143, ASN170, THR171, ASN178, LYS196, LYS196 with hydrogen and Pi bond interactions.
Figure 6.
A putative model for the interaction between the bovine CEACAM-1 protein and the BCoV-S glycoprotein and mapping the interactive amino acid residues. (A) The bovine CEACAM-1 protein interacts with the BCoV/S glycoprotein NTD of the BCoV spike. (B) and (C) CEACAM-1 binds through LEU233, PHE232, VAL229, Phe232, THR145, ASP148 and other amino acids with N-terminal domain (NTD) of Spike glycoprotein with residues ARG143, ASN170, THR171, ASN178, LYS196, LYS196 with hydrogen and Pi bond interactions.
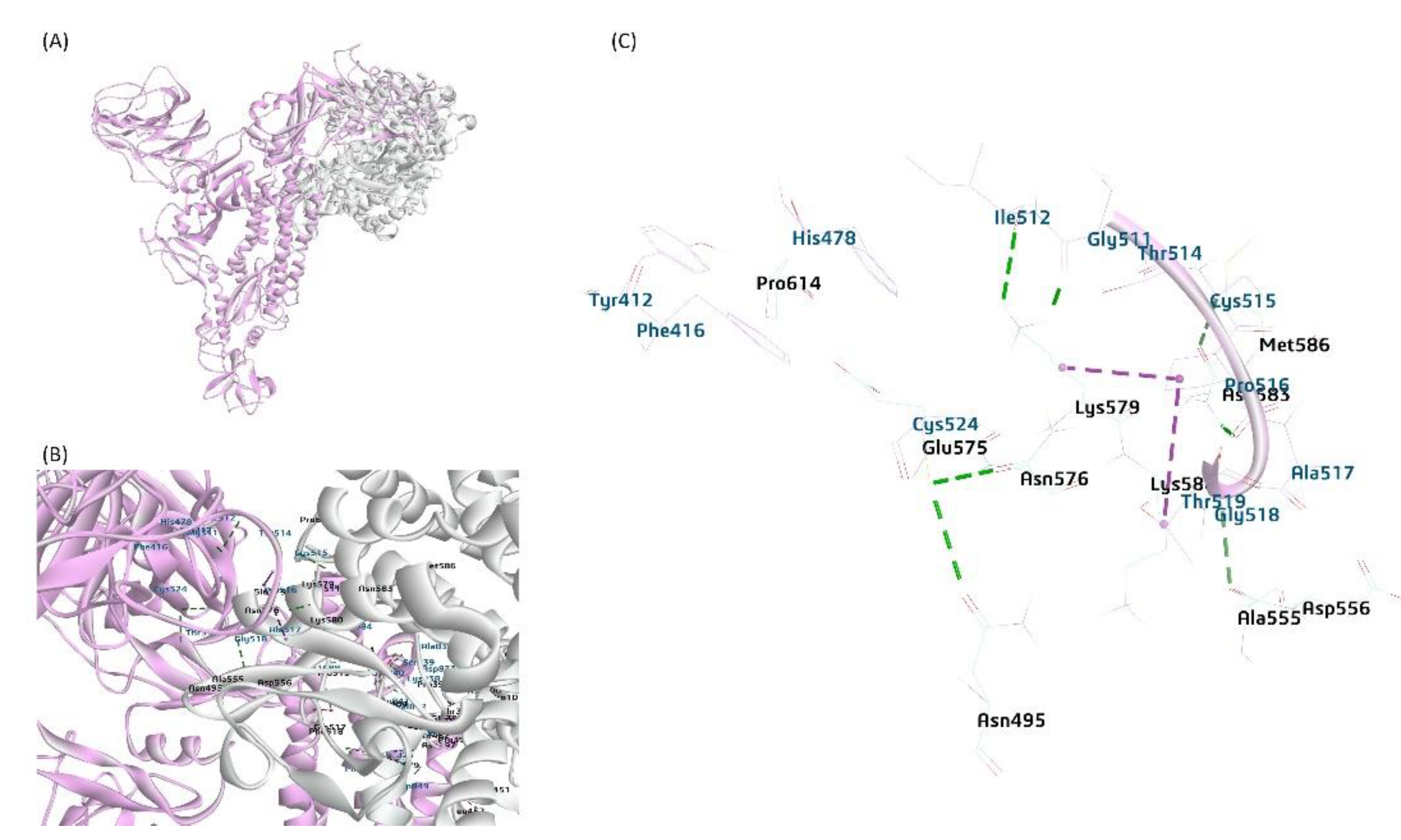
Figure 7.
A putative model representing the interaction of BCoV/S glycoprotein and the bovine aminopeptidase N (APN) protein. (A) The APN interactions with BCoV/S1 RBD and CTD (B) (C) BCoV/S1 chain CTD residues binding to the bovine APN through the residues (TYR412, PHE416, HIS478, CYS524, ILE512, GLY511, THR514, CYS515, PRO516, THR518, ALA517 and GLY518 to amino acid residues ASN495, ALA555, ASP565, GLY575, ASP576, ARG614 and other amino acid residues of APN by hydrogen bond, Pi bond and salt bridge interactions).
Figure 7.
A putative model representing the interaction of BCoV/S glycoprotein and the bovine aminopeptidase N (APN) protein. (A) The APN interactions with BCoV/S1 RBD and CTD (B) (C) BCoV/S1 chain CTD residues binding to the bovine APN through the residues (TYR412, PHE416, HIS478, CYS524, ILE512, GLY511, THR514, CYS515, PRO516, THR518, ALA517 and GLY518 to amino acid residues ASN495, ALA555, ASP565, GLY575, ASP576, ARG614 and other amino acid residues of APN by hydrogen bond, Pi bond and salt bridge interactions).
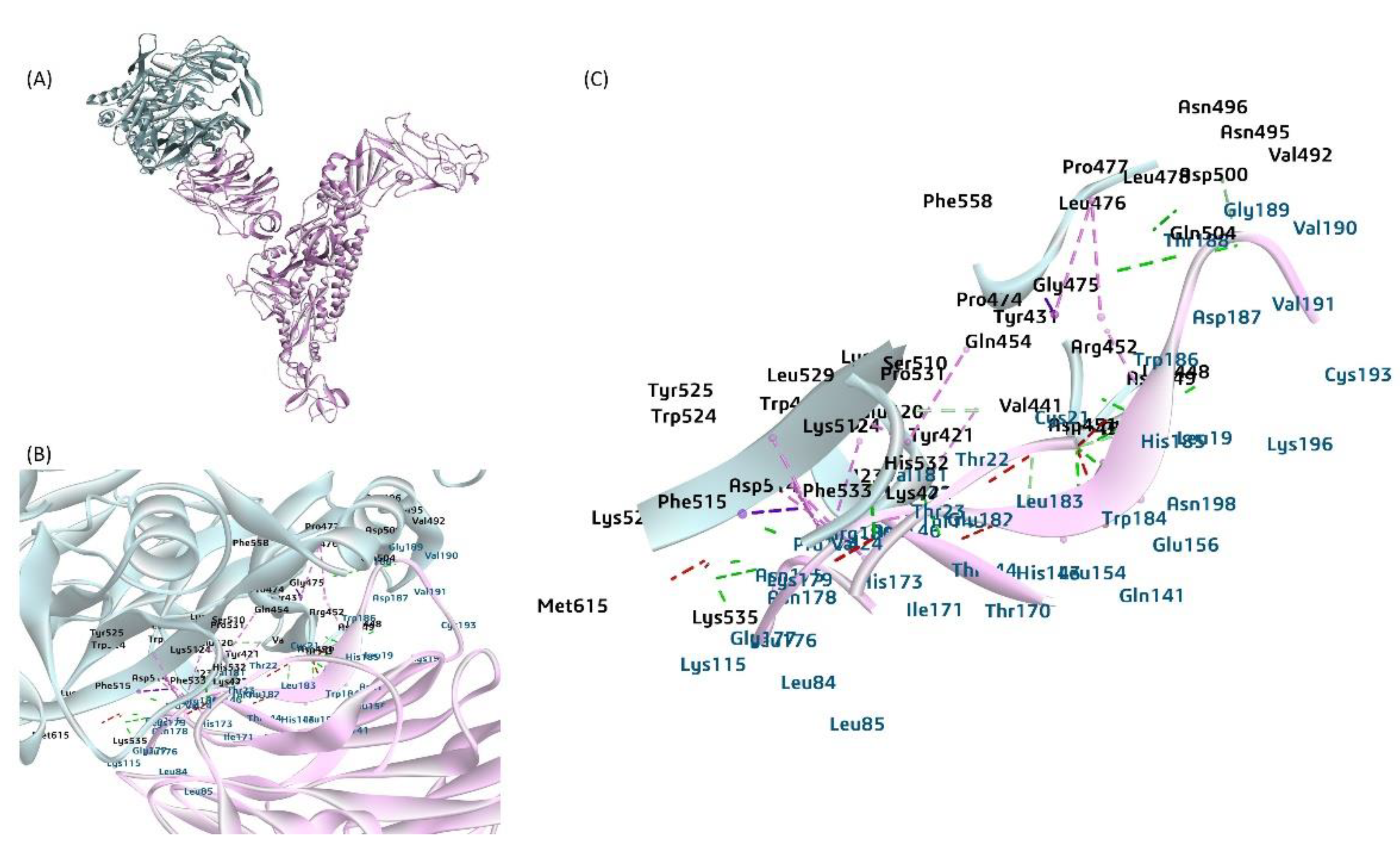
Figure 8.
A proposed model for the interaction between the bovine DPP4 protein and the BCoV-S glycoprotein. (A) The DPP4 protein interacts with the BCoV/S1 protein NTD. (B) and (C) The BCoV/ S1 chain N-terminal domain (NTD) residues bind to the DPP4 through TRP184, HIS185, TRP186, ASP187, THR188, GLY189, LYS196 which is core site for Neu5, 9Ac2 binding to BCoV-NTD. The binding amino acid residues from DPP4 to the NTD site are TYR43, VAL441 ARG552 GLY475, LEU476, LYS-512, PHE515, LYS520, HIS532, PHE533, LYS535, MET615, and other amino acid residues by hydrogen bond, Pi bond and salt bridge interactions. .
Figure 8.
A proposed model for the interaction between the bovine DPP4 protein and the BCoV-S glycoprotein. (A) The DPP4 protein interacts with the BCoV/S1 protein NTD. (B) and (C) The BCoV/ S1 chain N-terminal domain (NTD) residues bind to the DPP4 through TRP184, HIS185, TRP186, ASP187, THR188, GLY189, LYS196 which is core site for Neu5, 9Ac2 binding to BCoV-NTD. The binding amino acid residues from DPP4 to the NTD site are TYR43, VAL441 ARG552 GLY475, LEU476, LYS-512, PHE515, LYS520, HIS532, PHE533, LYS535, MET615, and other amino acid residues by hydrogen bond, Pi bond and salt bridge interactions. .
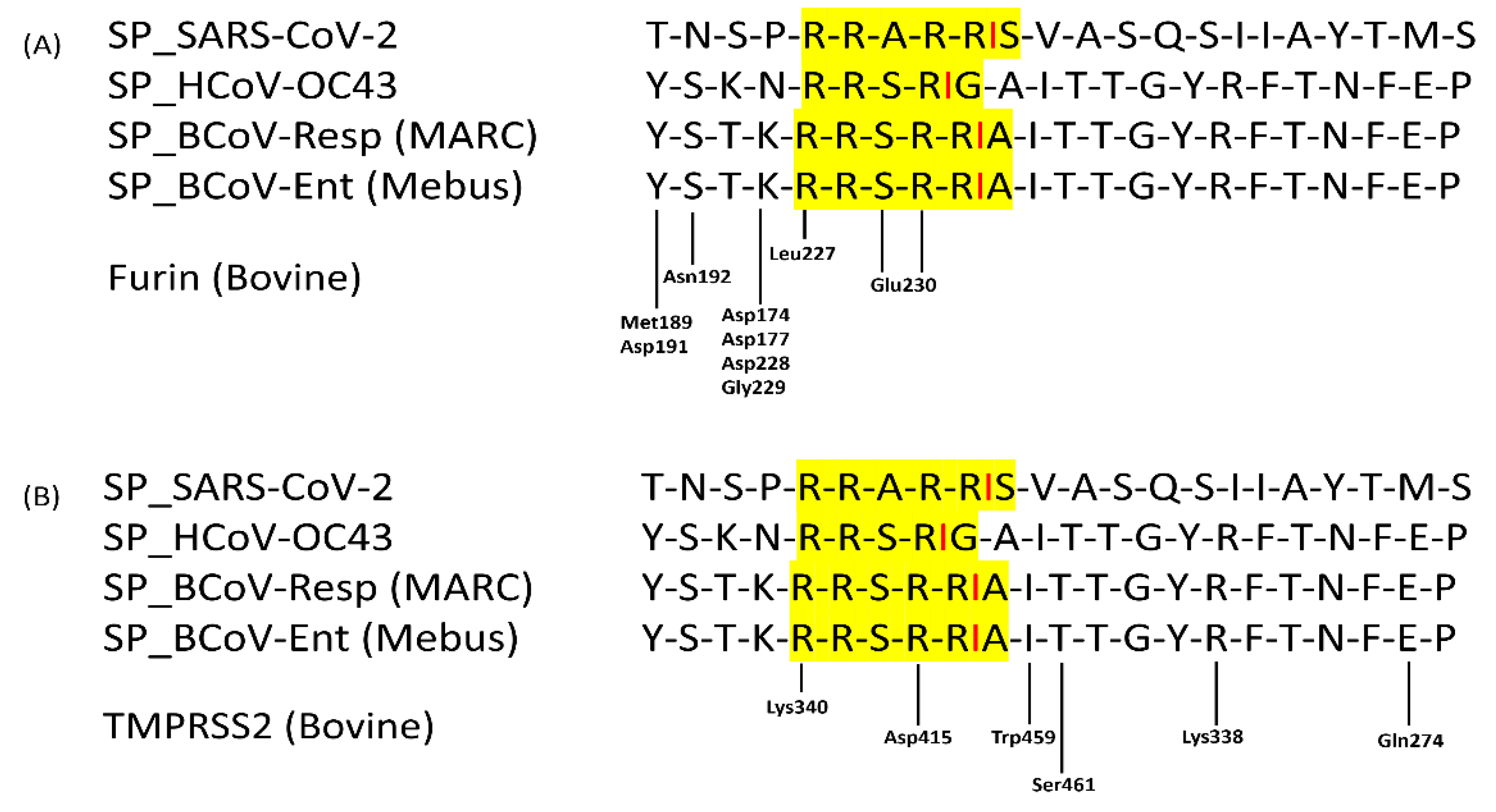
Figure 9.
Sequence alignment of the BCoV/S enteric (Mebus) strain with spike protein sequences of BCoV-Respiratory (MARC), Human Coronavirus (HCoV)-OC43 and SARS-CoV-2 strains. The yellow highlighted region contains polybasic residues and its specific cleavage site for furin and TMPRSS2 proteases. (A) showing the furin protease binding residues near its cleavage (RRSRR) at the S1/S2 site of S protein. (B) showing the TMPRSS2 protease binding residues near its cleavage (RRSRR) at the S1/S2 site of S protein.
Figure 9.
Sequence alignment of the BCoV/S enteric (Mebus) strain with spike protein sequences of BCoV-Respiratory (MARC), Human Coronavirus (HCoV)-OC43 and SARS-CoV-2 strains. The yellow highlighted region contains polybasic residues and its specific cleavage site for furin and TMPRSS2 proteases. (A) showing the furin protease binding residues near its cleavage (RRSRR) at the S1/S2 site of S protein. (B) showing the TMPRSS2 protease binding residues near its cleavage (RRSRR) at the S1/S2 site of S protein.
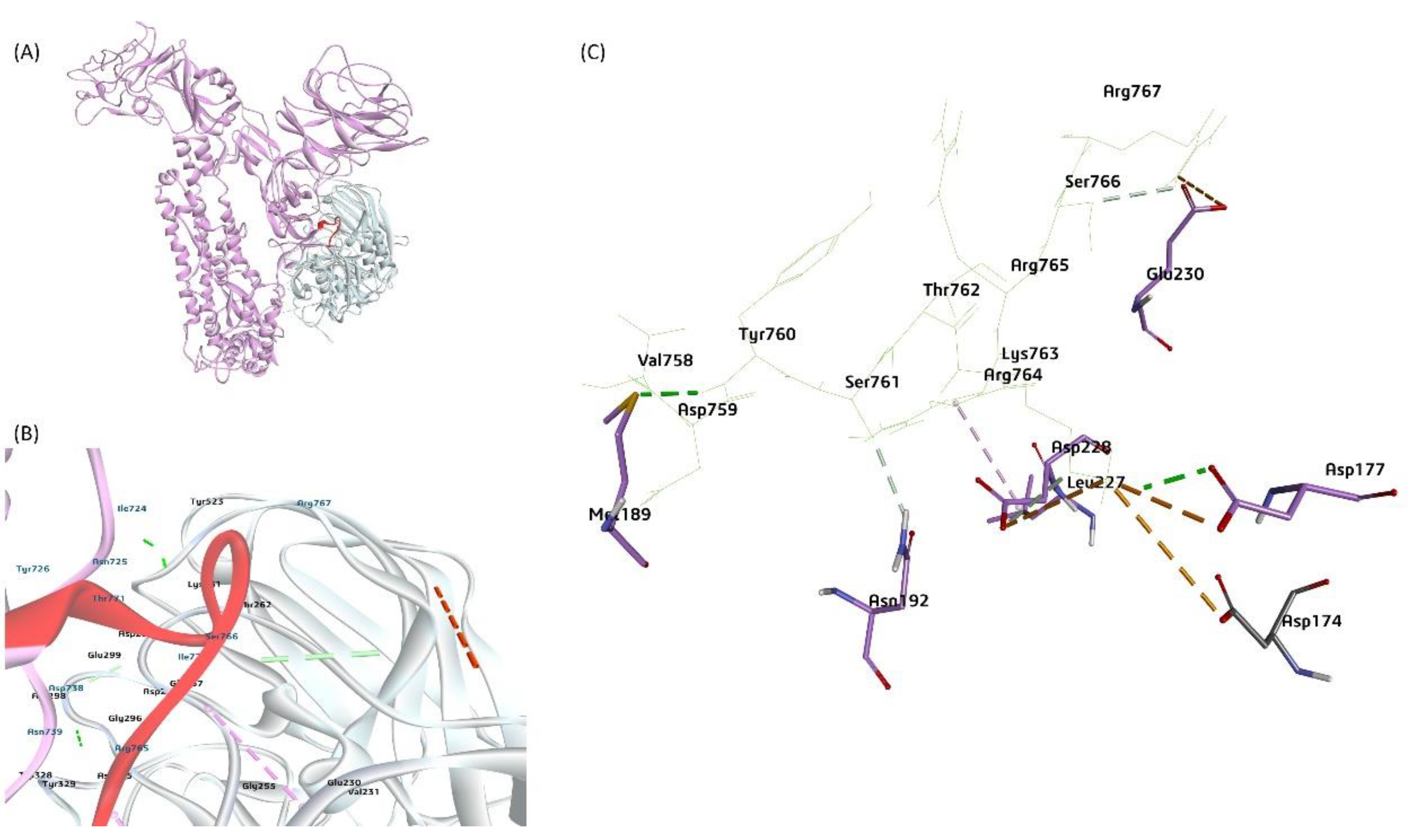
Figure 10.
The proposed model of furin protease interactions with BCoV/S cleavage site (A) Furin protease interaction at the furin-specific recognition site (RRSRR region) at the BCoV/S1/S2 junction. (B) and (C) Conformation of the furin residues Glu230, Leu227, Asp228, Asp174, Asp177, Asn192, Met189 with binding affinity to BCoV/S1 protein amino acid residues including Val758, Asp759, Tyr760 Ser761, Thr762 Lys763, Arg764, Arg765, Ser766, Arg767 at RRSRR|A (S1/S2) cleavage site.
Figure 10.
The proposed model of furin protease interactions with BCoV/S cleavage site (A) Furin protease interaction at the furin-specific recognition site (RRSRR region) at the BCoV/S1/S2 junction. (B) and (C) Conformation of the furin residues Glu230, Leu227, Asp228, Asp174, Asp177, Asn192, Met189 with binding affinity to BCoV/S1 protein amino acid residues including Val758, Asp759, Tyr760 Ser761, Thr762 Lys763, Arg764, Arg765, Ser766, Arg767 at RRSRR|A (S1/S2) cleavage site.
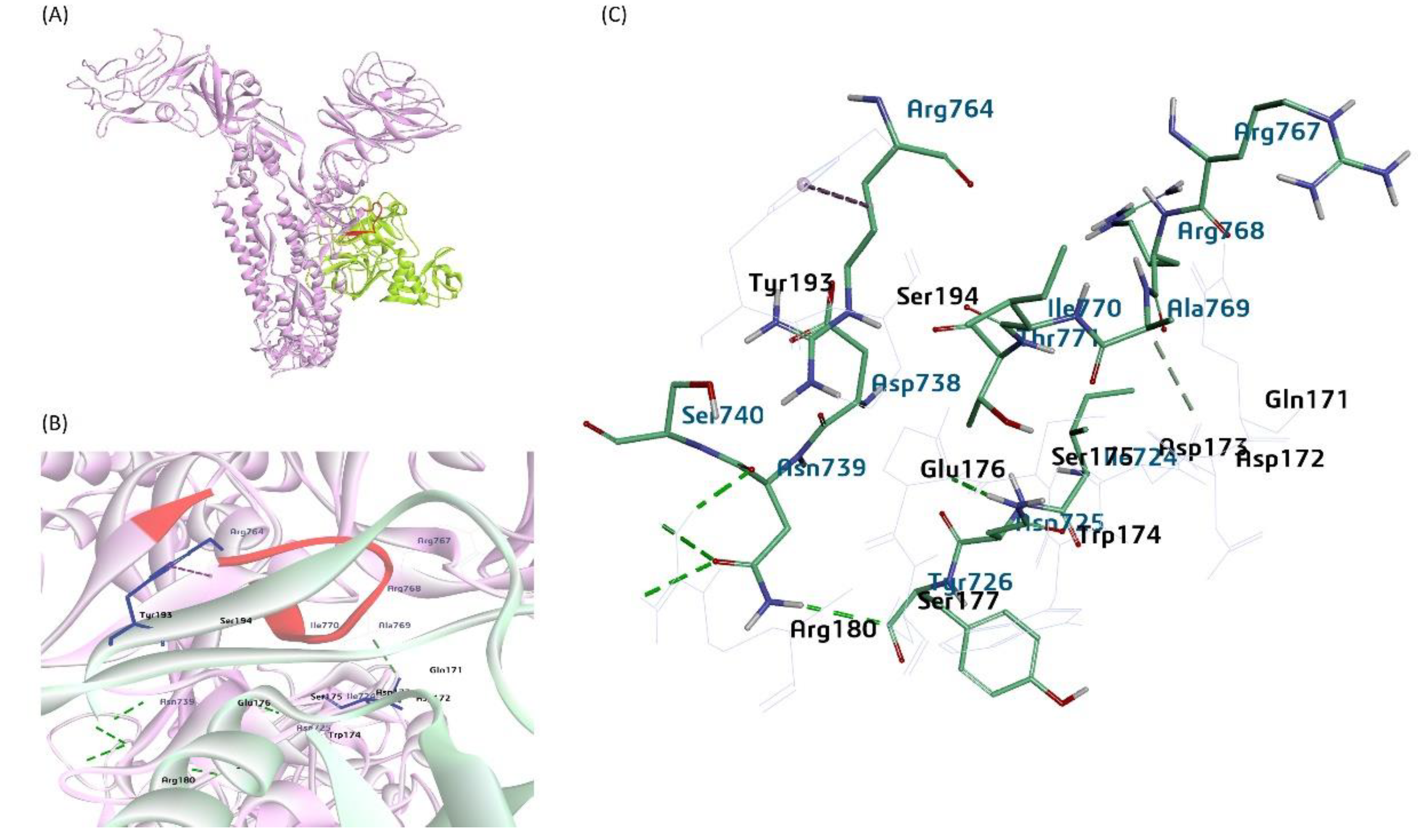
Figure 11.
A proposed model representing the TMPRSS2 serin protease binding upstream and downstream the BCoV/S protein polybasic cleavage site RRSRR|A. (B) and (C) Mapping the TMPRSS2 residues (Val278, His294, Thr339, Asp415, Asn416, Trp459, Gly460, Ser461, Gly46)2 binding affinity to the BCoV/S1/S2 site (Asp738, Ser740, Thr741, Ser742, Ser743, Ser761, Arg764, Thr762, Lys763, Arg767, Thr771 and Ile779).
Figure 11.
A proposed model representing the TMPRSS2 serin protease binding upstream and downstream the BCoV/S protein polybasic cleavage site RRSRR|A. (B) and (C) Mapping the TMPRSS2 residues (Val278, His294, Thr339, Asp415, Asn416, Trp459, Gly460, Ser461, Gly46)2 binding affinity to the BCoV/S1/S2 site (Asp738, Ser740, Thr741, Ser742, Ser743, Ser761, Arg764, Thr762, Lys763, Arg767, Thr771 and Ile779).
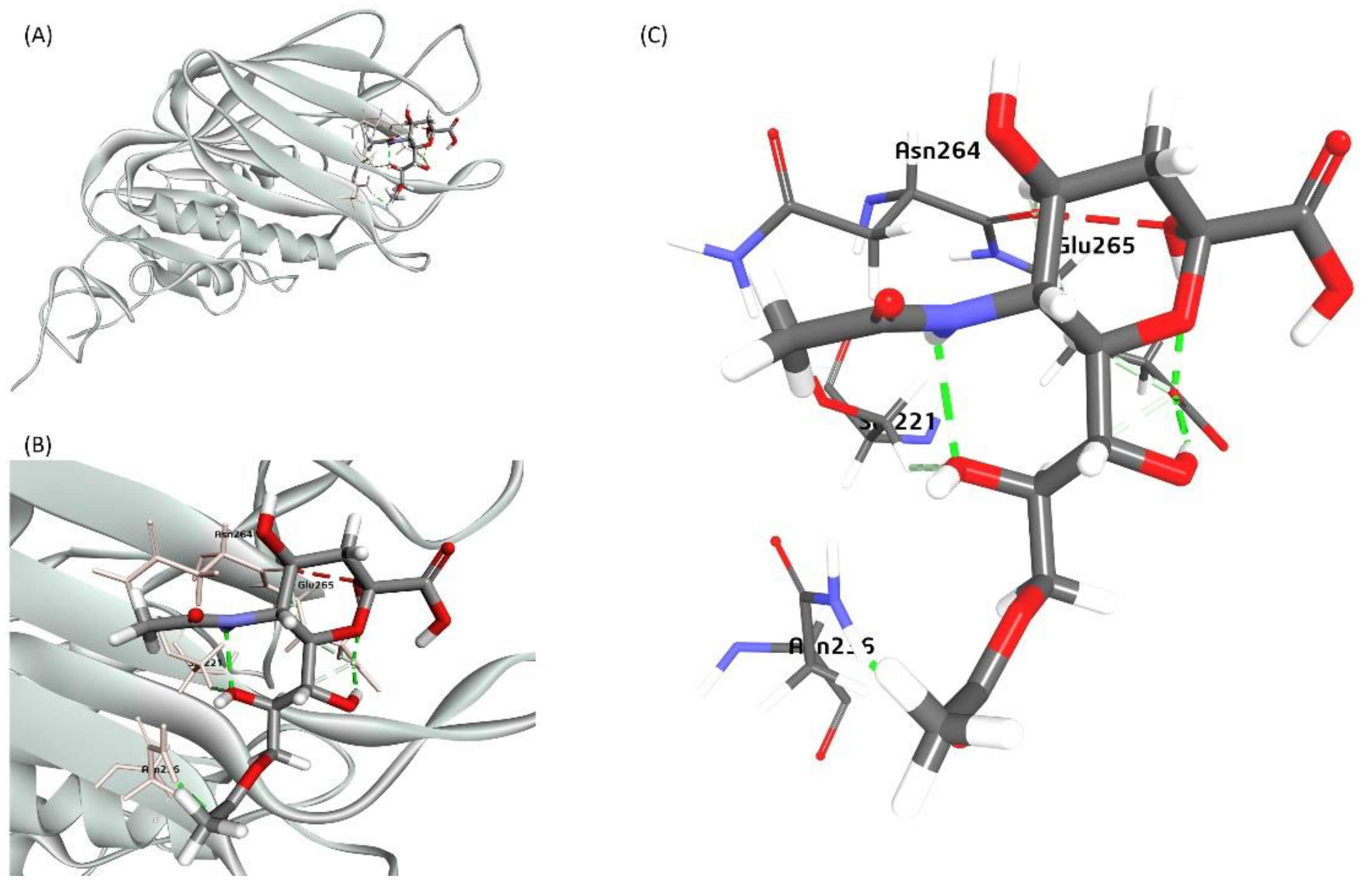
Figure 12.
A proposed model for the bovine sialic acid (Neu5,9Ac2) interaction with the BCoV/HE protein (A) shows the binding of Neu5,9Ac2 as a Ligand for the BCoV/HE protein receptor. (B) and (C) showing ligand binding to the interacted amino acid residues- (Asn-264, Glu-265, Ser-221, and Asn-236) of the BCoV/HE protein.
Figure 12.
A proposed model for the bovine sialic acid (Neu5,9Ac2) interaction with the BCoV/HE protein (A) shows the binding of Neu5,9Ac2 as a Ligand for the BCoV/HE protein receptor. (B) and (C) showing ligand binding to the interacted amino acid residues- (Asn-264, Glu-265, Ser-221, and Asn-236) of the BCoV/HE protein.
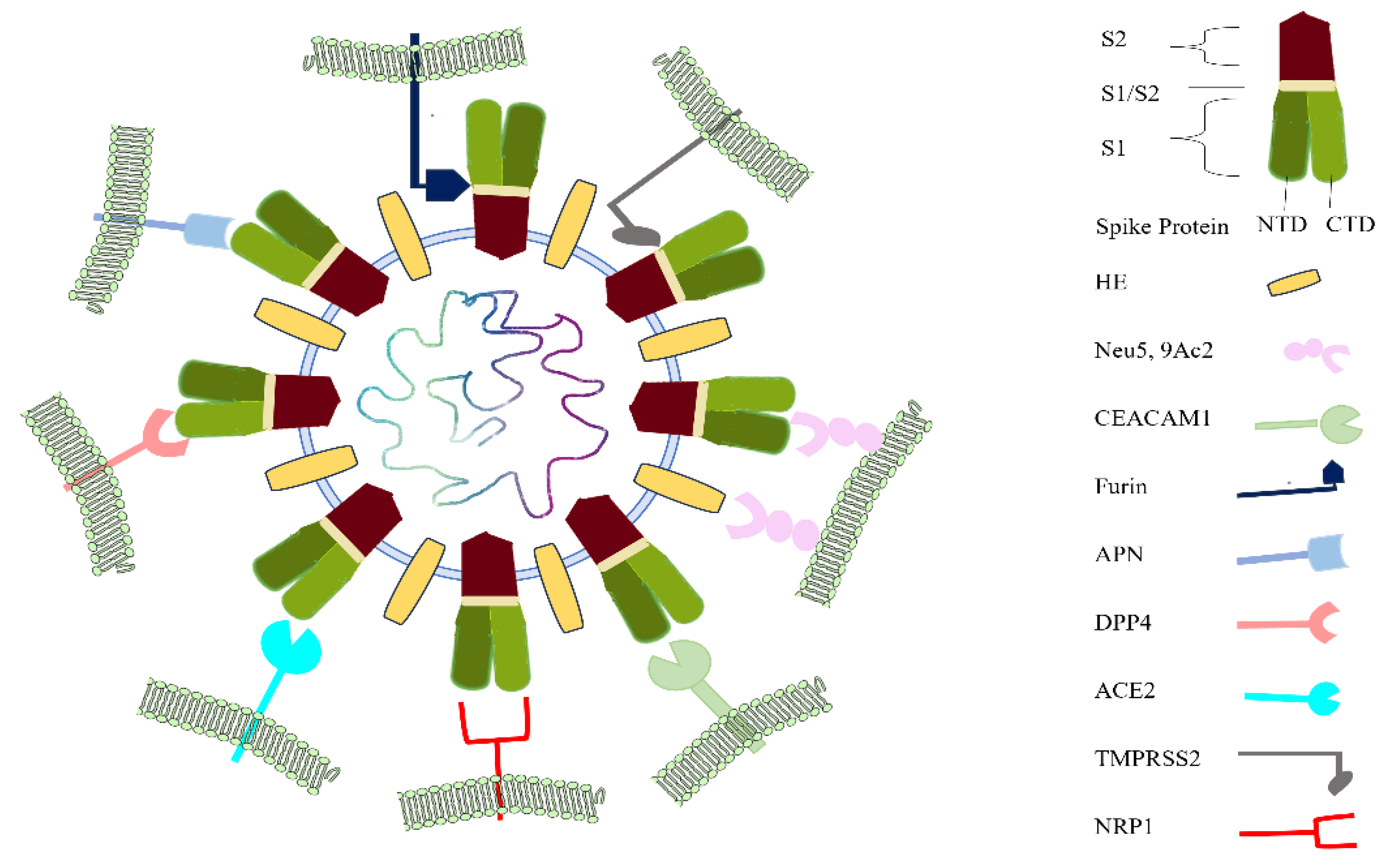
Figure 13.
Schematic representation showing the interaction between the BCoV/S and BCoV/HE proteins with the potential cellular receptors, including host cell proteases (Furin and TMPRSS2). With most of the receptors, BCoV/S interacted with the NTD and CTD. The NTD is an N-terminal domain present in the BCoV/S1 protein. The CTD is the C-terminal domain of the BCoV/S1 chain.
Figure 13.
Schematic representation showing the interaction between the BCoV/S and BCoV/HE proteins with the potential cellular receptors, including host cell proteases (Furin and TMPRSS2). With most of the receptors, BCoV/S interacted with the NTD and CTD. The NTD is an N-terminal domain present in the BCoV/S1 protein. The CTD is the C-terminal domain of the BCoV/S1 chain.
Table 1.
Homology Model Verification Using Verify-3D Server in BIOVIA Discovery Studio.
Table 1.
Homology Model Verification Using Verify-3D Server in BIOVIA Discovery Studio.
| Homology model (low- DOPE Score) |
Verify Score |
Verify Expected High Score |
Verify Expected Low Score |
|
BCoV-Spike
|
476.141 |
561.414 |
252.636 |
|
ACE2
|
308.35 |
358.11 |
161.153 |
|
Furin
|
227.99 |
216.48 |
97.4159 |
|
TMPRSS2
|
142.08 |
152.573 |
68.6579 |
|
NRP1
|
125.32 |
159.001 |
71.5506 |
|
DPP4
|
308.11 |
333.627 |
150.132 |
|
APN
|
385.36 |
415.465 |
186.959 |
|
CEACAM-1
|
32.41 |
58.3052 |
26.2373 |
Table 2.
Energetics and bonding interaction of the BCoV/ S glycoprotein with bovine receptor proteins.
Table 2.
Energetics and bonding interaction of the BCoV/ S glycoprotein with bovine receptor proteins.
Complex
(Best Pose) |
ZDock score |
E_R Dock Score |
Total hydrogen bonds |
Total Pi bonds |
Salt Bridge |
| BCoV/S-ACE2 |
22.58 |
-6.26 |
7 |
28 |
0 |
| BCoV/S-NRP1 |
26 |
-21.22 |
29 |
9 |
0 |
| BCoV/S-CEACAM-1 |
16.8 |
-6.29 |
12 |
9 |
1 |
| BCoV/S-APN |
17.6 |
-2.95 |
19 |
2 |
1 |
| BCoV/S-DPP4 |
22.44 |
-6.23 |
20 |
9 |
1 |
| BCoV/S-Furin |
16.6 |
-13.14 |
30 |
1 |
0 |
| BCoV/S-TMPRSS2 |
20 |
0.641 |
15 |
6 |
0 |