Submitted:
01 July 2024
Posted:
01 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
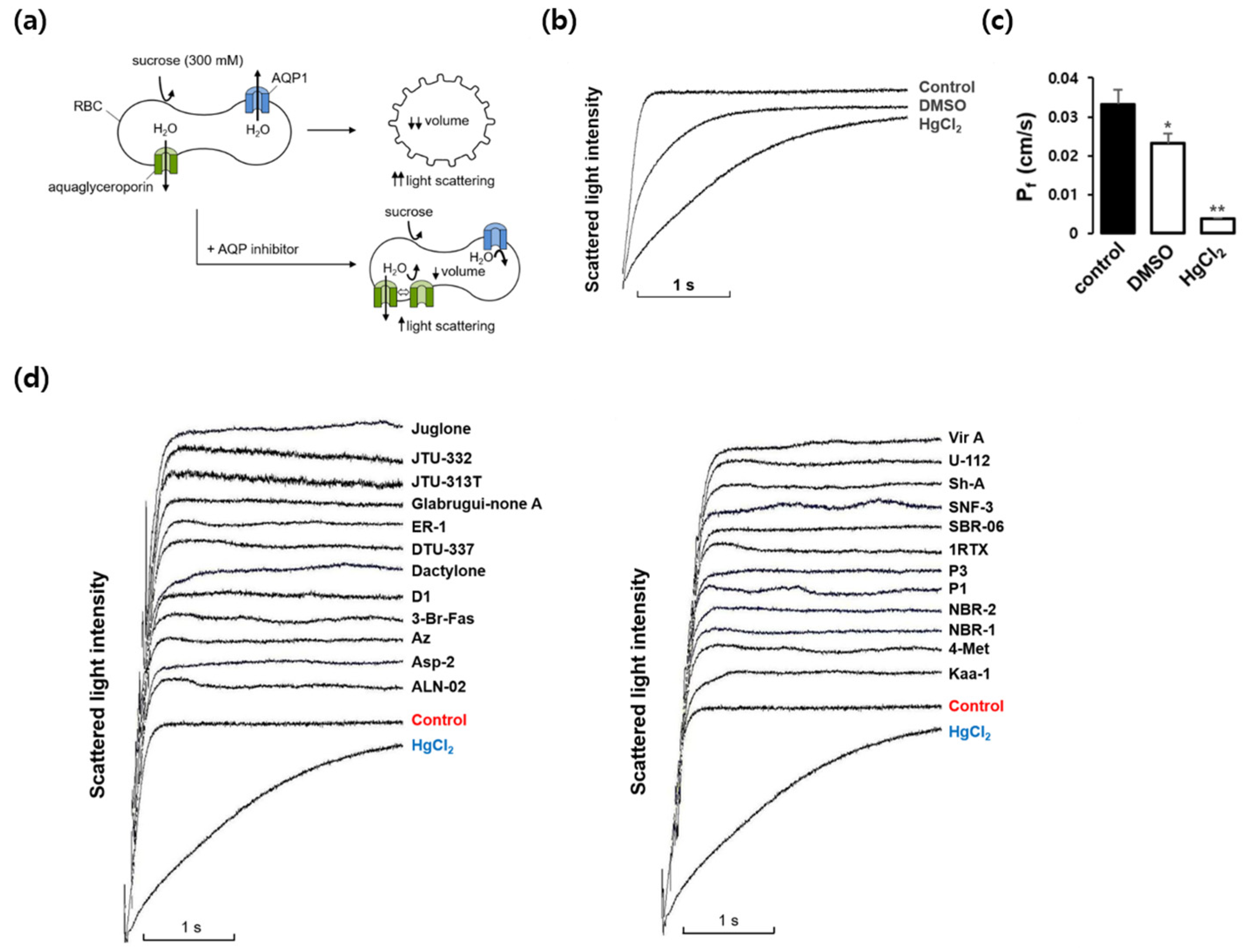
2.1. Effects of marine natural compounds on the osmotic water permeability of the erythrocyte membrane
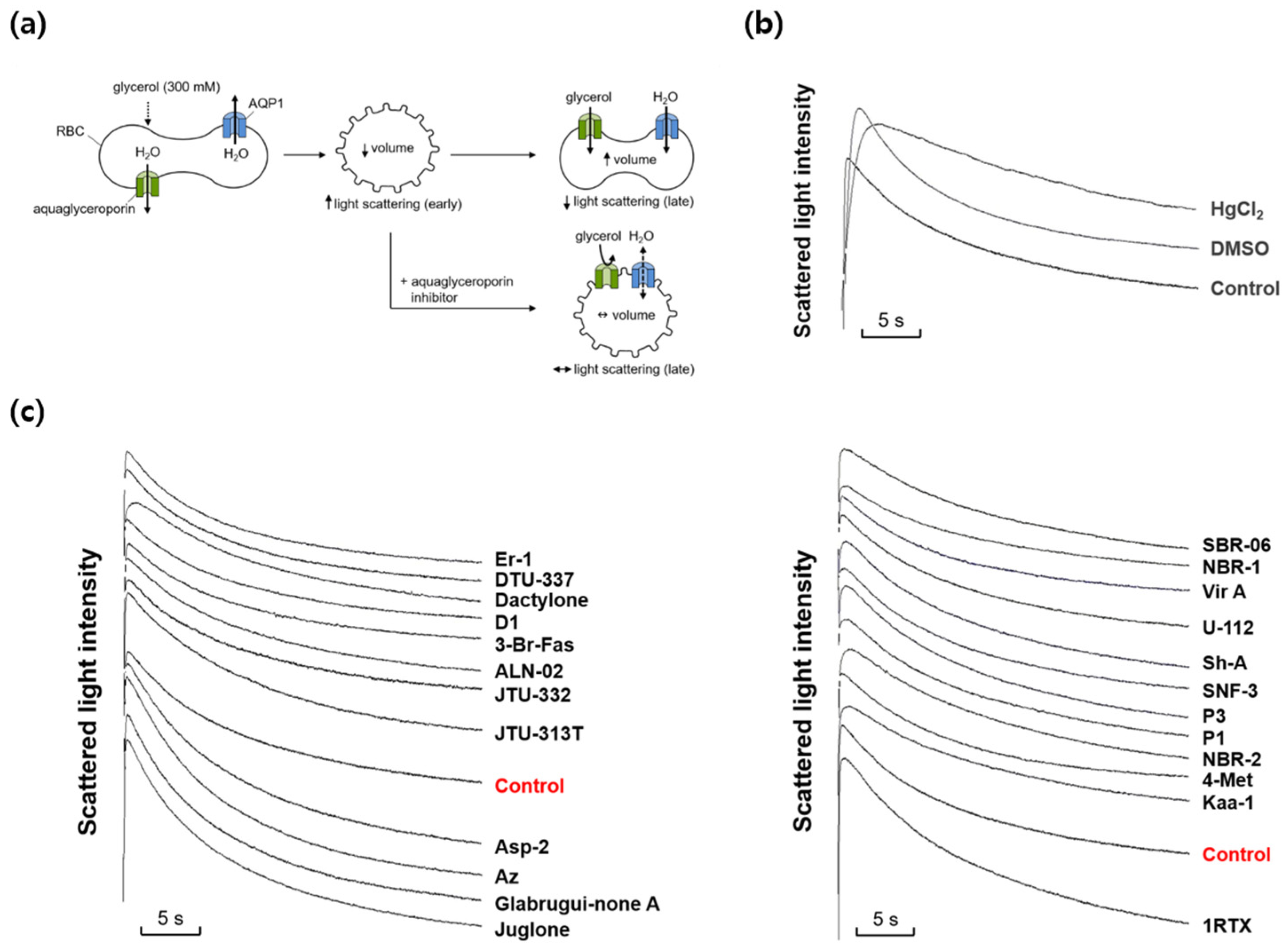
2.2. Effects of marine natural compounds on glycerol permeability of erythrocyte membrane
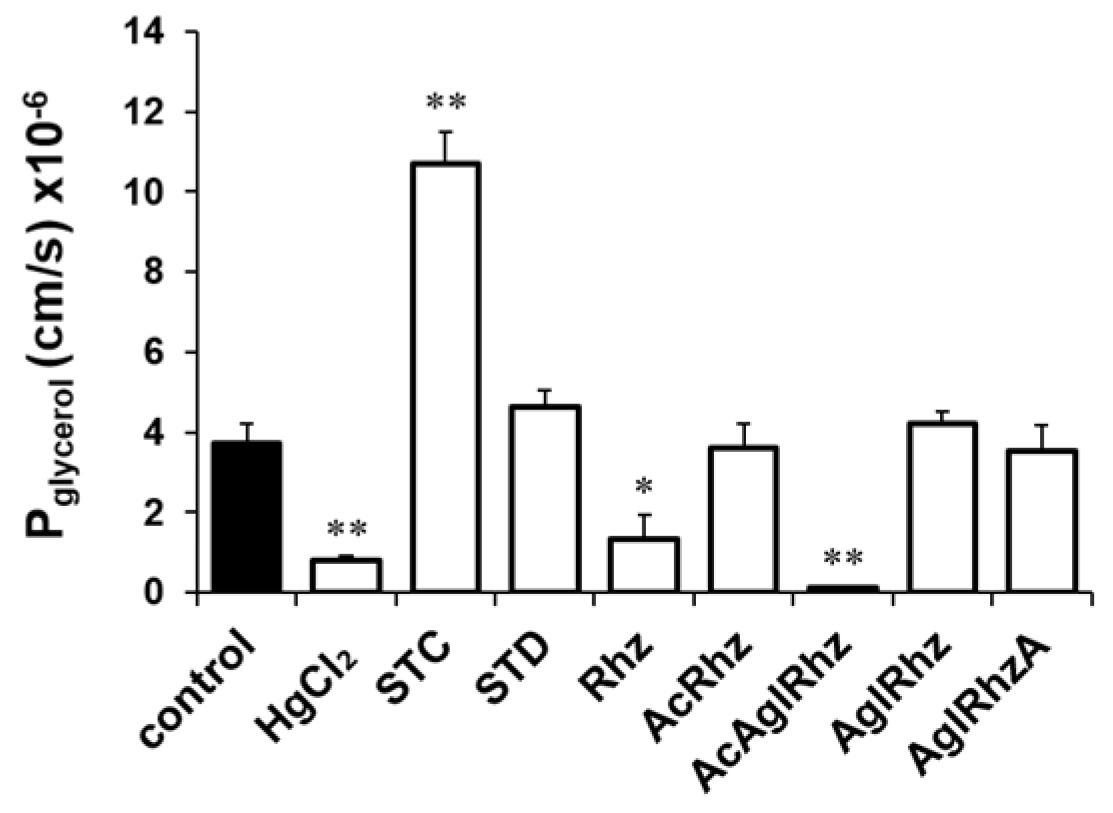
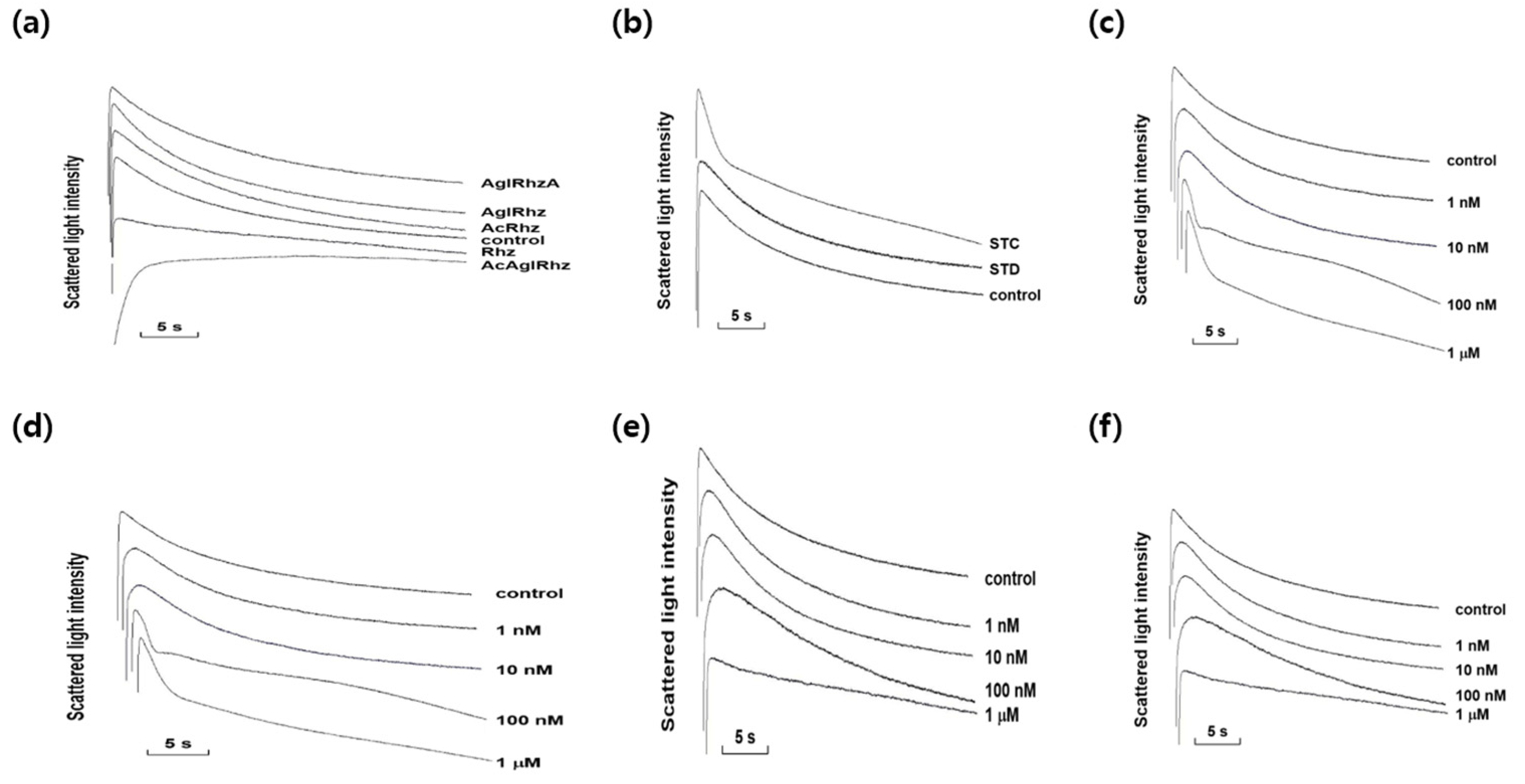
2.3. Effects of stichoposide C, rhizochalin, and their derivatives on glycerol permeability of erythrocyte membrane
2.4. Expression of AQP subtypes in mouse erythrocytes
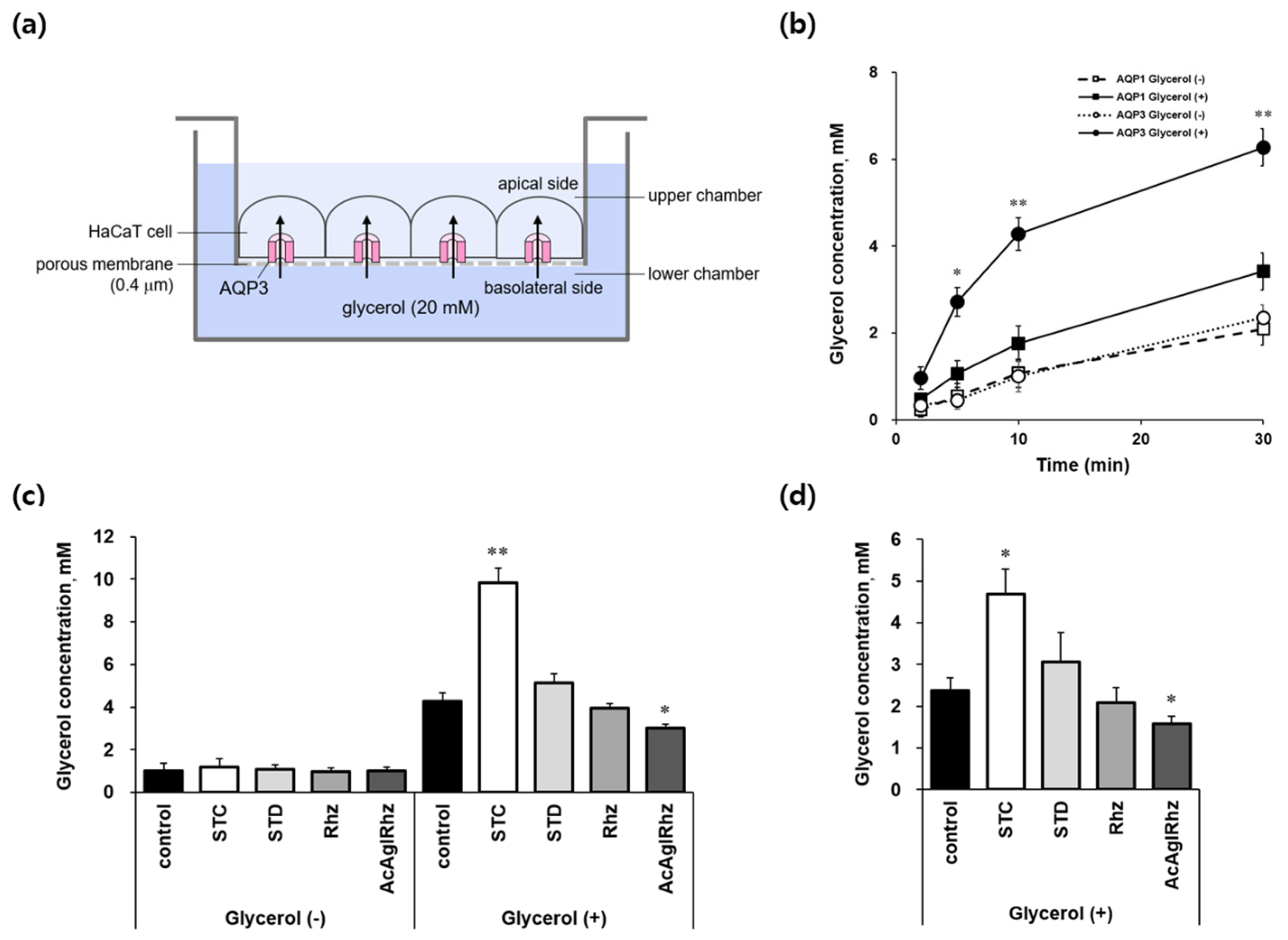
2.5. Effects of stichoposide C, rhizochalin, and their derivatives on AQP3-mediated transepithelial glycerol transport
3. Discussion
4. Materials and Methods
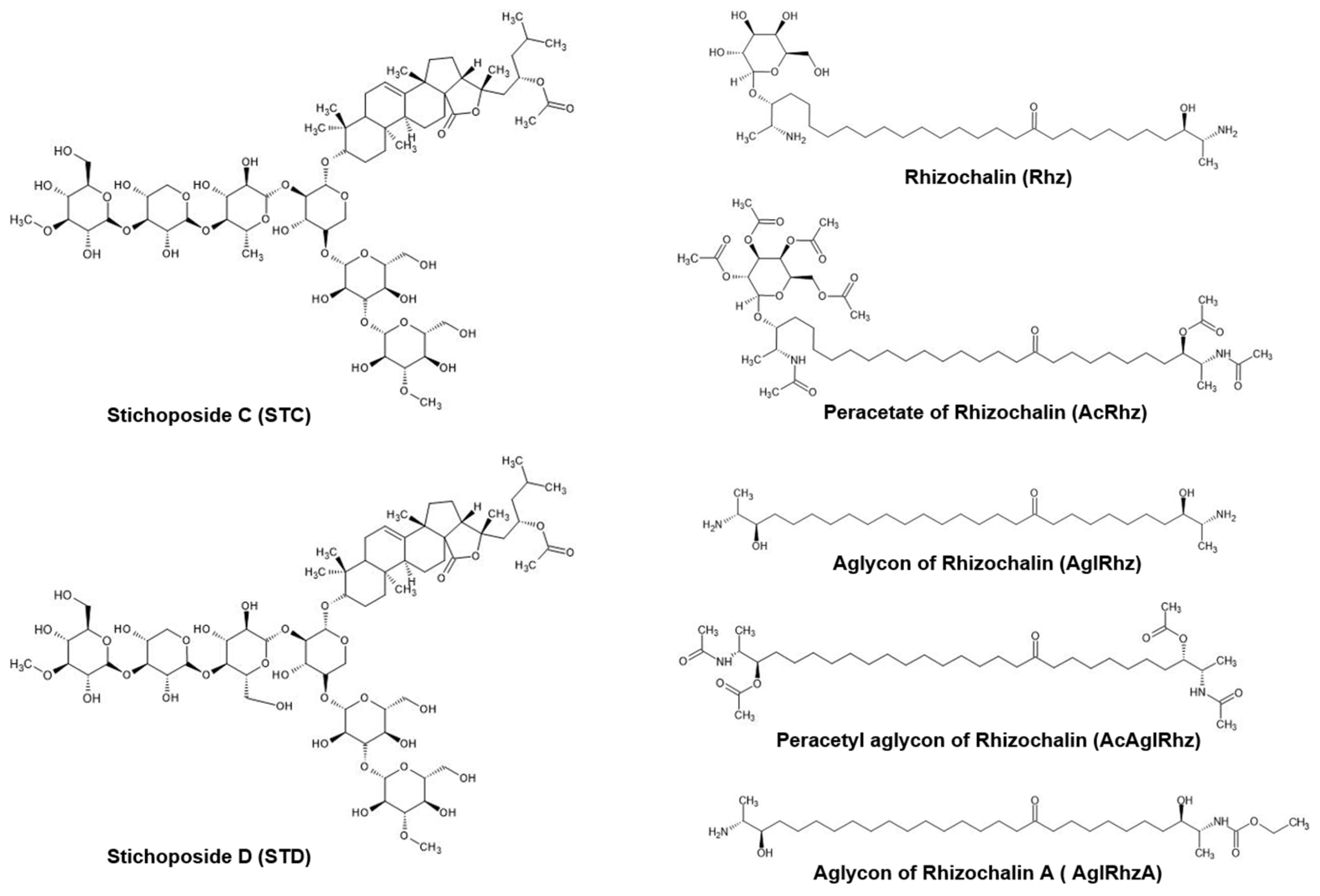
4.1. Marine natural products
4.2. Erythrocyte preparation
4.3. Stopped-flow light scattering measurements
4.4. Tissues preparation and immunohistochemistry
4.5. Cell cultures
4.6. Measurement of transepithelial glycerol transport
4.7. Statistical analysis
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Stonik, V.A. Marine natural products: A way to new drugs. Acta Naturae 2009, 1, 15-25.
- Blunt, J.W.; Copp, B.R.; Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235-294. [CrossRef]
- Jiménez, C. Marine natural products in medicinal chemistry. ACS Med. Chem. Lett. 2018, 9, 959-961. https://doi: 10.1021/acsmedchemlett.8b00368.
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69-85. [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111-129. [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200-216. [CrossRef]
- The International Transporter Consortium.; Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215-236. [CrossRef]
- Alam, S.; Doherty, E.; Ortega-Prieto, P.; Arizanova, J.; Fets, L. Membrane transporters in cell physiology, cancer metabolism and drug response. Dis. model. mech. 2023, 16. [CrossRef]
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A Guide to Plasma Membrane Solute Carrier Proteins. FEBS J. 2021, 288, 2784–2835. [CrossRef]
- Thomas, C.; Tampé, R. Structural and Mechanistic Principles of ABC Transporters. Annu. Rev. Biochem. 2020, 89, 605–636. [CrossRef]
- Kass, R.S. The Channelopathies: Novel Insights into Molecular and Genetic Mechanisms of Human Disease. J. Clin. Invest. 2005, 115, 1986–1989. [CrossRef]
- Hediger, M.A.; Clémençon, B.; Burrier, R.E.; Bruford, E.A. The ABCs of Membrane Transporters in Health and Disease (SLC Series): Introduction. Molecular Aspects of Medicine 2013, 34, 95–107. [CrossRef]
- Harraz, O.F.; Delpire, E. Recent Insights into Channelopathies. Physiol. Rev. 2024, 104, 23–31. [CrossRef]
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC Transporters as Therapeutic Targets: Emerging Opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560. [CrossRef]
- César-Razquin, A.; Snijder, B.; Frappier-Brinton, T.; Isserlin, R.; Gyimesi, G.; Bai, X.; Reithmeier, R.A.; Hepworth, D.; Hediger, M.A.; Edwards, A.M.; et al. A Call for Systematic Research on Solute Carriers. Cell 2015, 162, 478–487. [CrossRef]
- Mizuno, N.; Niwa, T.; Yotsumoto, Y.; Sugiyama, Y. Impact of Drug Transporter Studies on Drug Discovery and Development. Pharmacol. Rev. 2003, 55, 425–461. [CrossRef]
- Shugarts, S.; Benet, L.Z. The Role of Transporters in the Pharmacokinetics of Orally Administered Drugs. Pharm. Res. 2009, 26, 2039–2054. [CrossRef]
- Galetin, A.; Brouwer, K.L.R.; Tweedie, D.; Yoshida, K.; Sjöstedt, N.; Aleksunes, L.; Chu, X.; Evers, R.; Hafey, M.J.; Lai, Y.; et al. Membrane Transporters in Drug Development and as Determinants of Precision Medicine. Nat. Rev. Drug Discov. 2024, 23, 255–280. [CrossRef]
- Ishibashi, K.; Morishita, Y.; Tanaka, Y. The Evolutionary Aspects of Aquaporin Family. Adv. Exp. Med. Biol. 2017, 969, 35–50. [CrossRef]
- Verkman, A.S. Aquaporins at a Glance. J. Cell Sci. 2011, 124, 2107–2112. [CrossRef]
- Verkman, A.S. Aquaporins. Curr. Biol. 2013, 23, R52–R55. [CrossRef]
- Moeller, H.B.; Rittig, S.; Fenton, R.A. Nephrogenic Diabetes Insipidus: Essential Insights into the Molecular Background and Potential Therapies for Treatment. Endocr. Rev. 2013, 34, 278–301. [CrossRef]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 Deletion in Mice Reduces Brain Edema after Acute Water Intoxication and Ischemic Stroke. Nat. Med. 2000, 6, 159–163. [CrossRef]
- Maeda, N.; Hibuse, T.; Funahashi, T. Role of Aquaporin-7 and Aquaporin-9 in Glycerol Metabolism; Involvement in Obesity. In Handb. Exp. Pharmacol.; Beitz, E., Ed.; Springer Naure: Berlin, Germany, 2009; Volume 190, pp. 233–249. [CrossRef]
- Berry, V.; Francis, P.; Kaushal, S.; Moore, A.; Bhattacharya, S. Missense Mutations in MIP Underlie Autosomal Dominant ‘polymorphic’ and Lamellar Cataracts Linked to 12q. Nat. Genet. 2000, 25, 15–17. [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin 4 and Neuromyelitis Optica. Lancet Neurol. 2012, 11, 535–544. [CrossRef]
- Verkman, A.S. Aquaporins in Clinical Medicine. Annu. Rev. Med. 2012, 63, 303–316. [CrossRef]
- Tradtrantip, L.; Jin, B.J.; Yao, X.; Anderson, M.O.; Verkman, A.S. Aquaporin-Targeted Therapeutics: State-of-the-Field. Adv. Exp. Med. Biol. 2017, 969, 239–250. [CrossRef]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but Elusive Drug Targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [CrossRef]
- Salman, M.M.; Kitchen, P.; Yool, A.J.; Bill, R.M. Recent Breakthroughs and Future Directions in Drugging Aquaporins. Trends Pharmacol. Sci. 2022, 43, 30–42. [CrossRef]
- Yang, B.; Fukuda, N.; van Hoek, A.; Matthay, M.A.; Ma, T.; Verkman, A.S. Carbon Dioxide Permeability of Aquaporin-1 Measured in Erythrocytes and Lung of Aquaporin-1 Null Mice and in Reconstituted Proteoliposomes. J. Biol. Chem. 2000, 275, 2686–2692. [CrossRef]
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2023: An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2024, 29, 585. [CrossRef]
- Montaser, R.; Luesch, H. Marine Natural Products: A New Wave of Drugs? Future Med. Chem. 2011, 3, 1475–1489. [CrossRef]
- Romano, G.; Almeida, M.; Varela Coelho, A.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs. 2022, 20, 219. [CrossRef]
- Stonik, V.A.; Kalinin, V.I.; Avilov, S.A. Toxins from Sea Cucumbers (Holothuroids): Chemical Structures, Properties, Taxonomic Distribution, Biosynthesis and Evolution. J. Nat. Toxins 1999, 8, 235–248.
- Kalinin, V.I.; Ivanchina, N.V.; Krasokhin, V.B.; Makarieva, T.N.; Stonik, V.A. Glycosides from Marine Sponges (Porifera, Demospongiae): Structures, Taxonomical Distribution, Biological Activities and Biological Roles. Mar. Drugs. 2012, 10, 1671–1710. [CrossRef]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Anticancer Activity of Sea Cucumber Triterpene Glycosides. Mar. Drugs. 2015, 13, 1202–1223. [CrossRef]
- Yun, S.H.; Sim, E.H.; Han, S.H.; Kim, T.R.; Ju, M.H.; Han, J.Y.; Jeong, J.S.; Kim, S.H.; Silchenko, A.S.; Stonik, V.A.; et al. In Vitro and in Vivo Anti-Leukemic Effects of Cladoloside C2 Are Mediated by Activation of Fas/Ceramide Synthase 6/P38 Kinase/c-Jun NH2-Terminal Kinase/Caspase-8. Oncotarget 2017, 9, 495–511. [CrossRef]
- Menchinskaya, E.S.; Dyshlovoy, S.A.; Venz, S.; Jacobsen, C.; Hauschild, J.; Rohlfing, T.; Silchenko, A.S.; Avilov, S.A.; Balabanov, S.; Bokemeyer, C.; et al. Anticancer Activity of the Marine Triterpene Glycoside Cucumarioside A2-2 in Human Prostate Cancer Cells. Mar. Drugs. 2024, 22, 20. [CrossRef]
- Kitagawa, I.; Kobayashi, M.; Inamoto, T.; Yasuzawa, T.; Kyogoku, Y. The Structures of Six Antifungal Oligoglycosides, Stichlorosides A1, A2, B1, B2, C1, and C2, from the Sea Cucumber Stichopus Chloronotus (BRANDT). Chem. Pharm. Bull. 1981, 29, 2387–2391. [CrossRef]
- Stonik, V.A.; Mal’tsev, I.I.; Kalinovskii, A.I.; Conde, C.; Elyakov, G.B. Glycosides of Marine Invertegrates. XI. Two New Triterpene Glycosides from Holothurians of the family Stichopadidae. Chem. Nat. Compd. 1982, 18, 177–182. [CrossRef]
- Yun, S.H.; Park, E.S.; Shin, S.W.; Na, Y.W.; Han, J.Y.; Jeong, J.S.; Shastina, V.V.; Stonik, V.A.; Park, J.I.; Kwak, J.Y. Stichoposide C Induces Apoptosis through the Generation of Ceramide in Leukemia and Colorectal Cancer Cells and Shows in Vivo Antitumor Activity. Clin. Cancer Res. 2012, 18, 5934–5948. [CrossRef]
- Fedorov, S.N.; Dyshlovoy, S.A.; Kuzmich, A.S.; Shubina, L.; Avilov, S.A.; Silchenko, A.S.; Bode, A.M.; Dong, Z.; Stonik, V.A. In Vitro Anticancer Activities of Some Triterpene Glycosides from Holothurians of Cucumariidae, Stichopodidae, Psolidae, Holothuriidae and Synaptidae Families. Nat. Prod. Commun. 2016, 11, 1239–1242.
- Park J.I.; Bae H.R.; Kim C.G.; Stonik V.A.; Kwak J.Y. Relationships between chemical structures and functions of triterpene glycosides isolated from sea cucumbers. Front Chem. 2014, 9, 2:77. doi: 10.3389/fchem.2014.00077.
- Bahrami, Y.; Franco, C.M.M. Acetylated Triterpene Glycosides and Their Biological Activity from Holothuroidea Reported in the Past Six Decades. Mar. Drugs. 2016, 14, 147. [CrossRef]
- Makarieva, T.N.; Denisenko, V.A.; Stonik, V.A.; Milgrom, Yu.M.; Rashkes, Ya.V. Rhizochalin, a Novel Secondary Metabolite of Mixed Biosynthesis from the Sponge Rhizochalina Incrustata. Tetrahedron Lett. 1989, 30, 6581–6584. [CrossRef]
- Makarieva, T.N.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Santalova, E.A.; Pokanevich, E.V.; Molinski, T.F.; Stonik, V.A. Rhizochalin A, a Novel Two-Headed Sphingolipid from the Sponge Rhizochalina Incrustata. J. Nat. Prod. 2005, 68, 255–257. [CrossRef]
- Molinski, T.F.; Makarieva, T.N.; Stonik, V.A. (-)-Rhizochalin Is a Dimeric Enantiomorphic (2R)-Sphingolipid: Absolute Configuration of Pseudo-C(2v)-Symmetric Bis-2-Amino-3-Alkanols by CD. Angew. Chem., Int. Ed. Engl. 2000, 39, 4076–4079.
- Pruett, S.T.; Bushnev, A.; Hagedorn, K.; Adiga, M.; Haynes, C.A.; Sullards, M.C.; Liotta, D.C.; Merrill, A.H. Biodiversity of Sphingoid Bases (“sphingosines”) and Related Amino Alcohols. J. Lipid Res. 2008, 49, 1621–1639. [CrossRef]
- Nicholas, G.M.; Hong, T.W.; Molinski, T.F.; Lerch, M.L.; Cancilla, M.T.; Lebrilla, C.B. Oceanapiside, an Antifungal Bis-Alpha,Omega-Amino Alcohol Glycoside from the Marine Sponge Oceanapia Phillipensis. J. Nat. Prod. 1999, 62, 1678–1681. [CrossRef]
- Jin, J.O.; Shastina, V.; Park, J.I.; Han, J.Y.; Makarieva, T.; Fedorov, S.; Rasskazov, V.; Stonik, V.A.; Kwak, J.Y. Differential Induction of Apoptosis of Leukemic Cells by Rhizochalin, Two Headed Sphingolipids from Sponge and Its Derivatives. Biol. Pharm. Bull. 2009, 32, 955–962. [CrossRef]
- Fedorov, S.N.; Makarieva, T.N.; Guzii, A.G.; Shubina, L.K.; Kwak, J.Y.; Stonik, V.A. Marine Two-Headed Sphingolipid-like Compound Rhizochalin Inhibits EGF-Induced Transformation of JB6 P+ Cl41 Cells. Lipids 2009, 44, 777–785. [CrossRef]
- Dyshlovoy, S.A.; Hauschild, J.; Venz, S.; Krisp, C.; Kolbe, K.; Zapf, S.; Heinemann, S.; Fita, K.D.; Shubina, L.K.; Makarieva, T.N.; et al. Rhizochalinin Exhibits Anticancer Activity and Synergizes with EGFR Inhibitors in Glioblastoma In Vitro Models. Mol. Pharm. 2023, 20, 4994–5005. [CrossRef]
- Hara-Chikuma, M.; Verkman, A.S. Physiological Roles of Glycerol-Transporting Aquaporins: The Aquaglyceroporins. Cell. Mol. Life Sci. 2006, 63, 1386–1392. [CrossRef]
- Rojek, A.; Praetorius, J.; Frøkiaer, J.; Nielsen, S.; Fenton, R.A. A Current View of the Mammalian Aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [CrossRef]
- Calamita, G.; Delporte, C. Involvement of Aquaglyceroporins in Energy Metabolism in Health and Disease. Biochim. 2021, 188, 20–34. [CrossRef]
- Sohara, E.; Rai, T.; Miyazaki, J.; Verkman, A.S.; Sasaki, S.; Uchida, S. Defective Water and Glycerol Transport in the Proximal Tubules of AQP7 Knockout Mice. Am. J. Physiol. Renal Physiol. 2005, 289, F1195-1200. [CrossRef]
- Calamita, G.; Perret, J.; Delporte, C. Aquaglyceroporins: Drug Targets for Metabolic Diseases? Front. Physiol. 2018, 9, 851. [CrossRef]
- Pimpão, C.; Wragg, D.; da Silva, I.V.; Casini, A.; Soveral, G. Aquaglyceroporin Modulators as Emergent Pharmacological Molecules for Human Diseases. Front. Mol. Biosci. 2022, 9, 845237. [CrossRef]
- Yang, B.; Kim, J.K.; Verkman, A.S. Comparative Efficacy of HgCl2 with Candidate Aquaporin-1 Inhibitors DMSO, Gold, TEA+ and Acetazolamide. FEBS Lett. 2006, 580, 6679–6684. [CrossRef]
- Martins, A.P.; Marrone, A.; Ciancetta, A.; Galán Cobo, A.; Echevarría, M.; Moura, T.F.; Re, N.; Casini, A.; Soveral, G. Targeting Aquaporin Function: Potent Inhibition of Aquaglyceroporin-3 by a Gold-Based Compound. PLoS One 2012, 7, e37435. [CrossRef]
- Nave, M.; Castro, R.E.; Rodrigues, C.M.; Casini, A.; Soveral, G.; Gaspar, M.M. Nanoformulations of a Potent Copper-Based Aquaporin Inhibitor with Cytotoxic Effect against Cancer Cells. Nanomedicine (Lond.) 2016, 11, 1817–1830. [CrossRef]
- Jelen, S.; Wacker, S.; Aponte-Santamaría, C.; Skott, M.; Rojek, A.; Johanson, U.; Kjellbom, P.; Nielsen, S.; de Groot, B.L.; Rützler, M. Aquaporin-9 Protein Is the Primary Route of Hepatocyte Glycerol Uptake for Glycerol Gluconeogenesis in Mice. J. Biol. Chem. 2011, 286, 44319–44325. [CrossRef]
- Sonntag, Y.; Gena, P.; Maggio, A.; Singh, T.; Artner, I.; Oklinski, M.K.; Johanson, U.; Kjellbom, P.; Nieland, J.D.; Nielsen, S.; et al. Identification and Characterization of Potent and Selective Aquaporin-3 and Aquaporin-7 Inhibitors. J. Biol. Chem. 2019, 294, 7377–7387. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




