Submitted:
29 June 2024
Posted:
01 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
- CDC/FDA
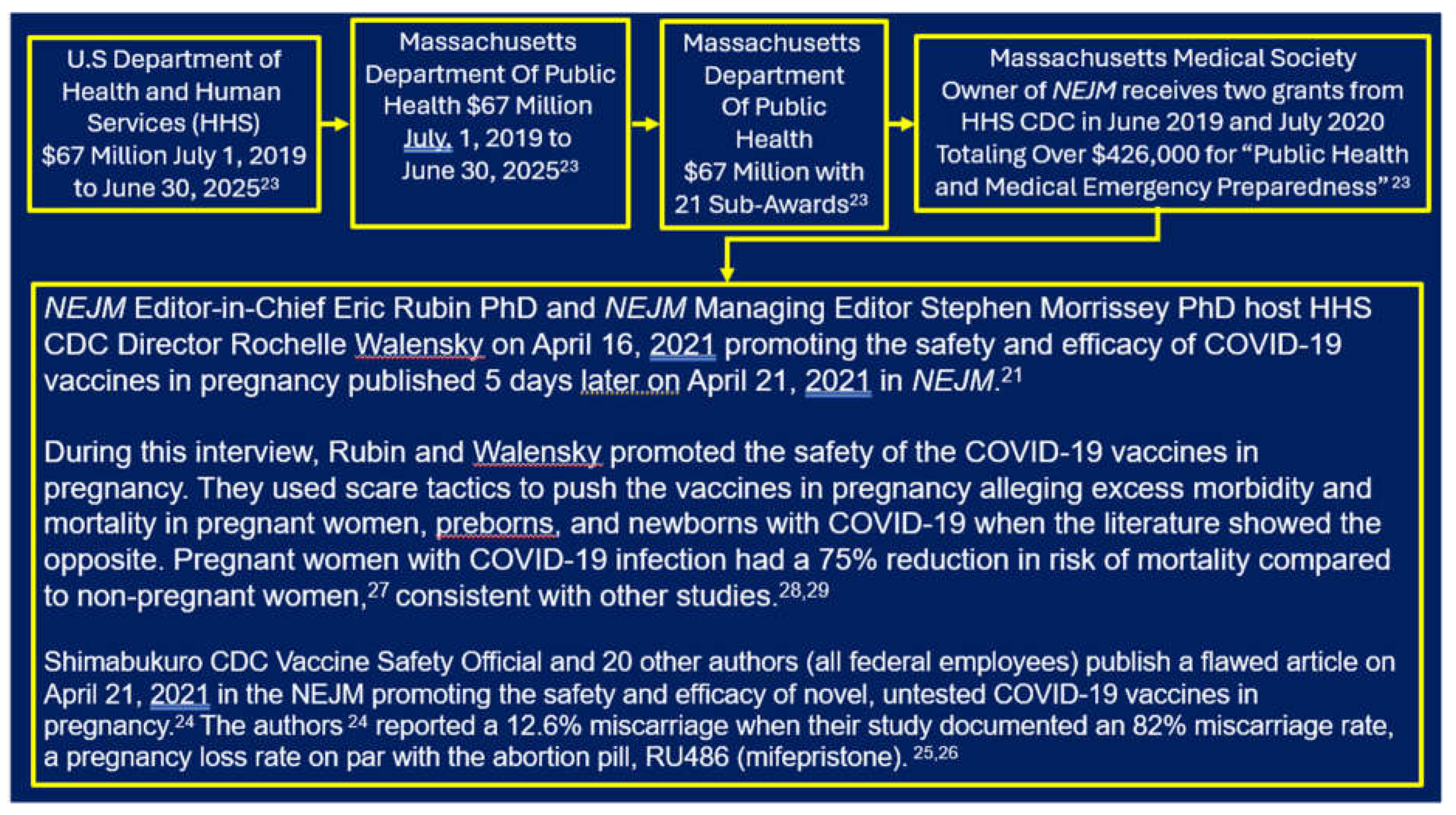
- Then-CDC Director Rochelle Walensky MD MPH
- NEJM’s Editor-in-Chief, Eric Rubin MD PhD
- NEJM’s Managing Editor, Stephen Morrissey PhD
- CDC vaccine safety officer Tom Shimabukuro MD MPH
- Massachusetts Department of Public Health (MDPH)
- Owner of the NEJM – the Massachusetts Medical Society (MMS)
Methods
Results
Discussion
Propaganda: The Government Enlists Private Actors

V-safe Smart Phone Safety Monitoring System Exposed
Pharmaceutical Conflicts of Interest
Grant/Financial Information
Acknowledgements
Conflicts of Interest/Competing Interest
References
- Vargesson N. Thalidomide-induced teratogenesis: History and mechanisms. Wiley Online Library. Birth Defects Research, part C. Embryo Today: Reviews. https://onlinelibrary.wiley.com/doi/full/10.1002/bdrc.21096 (Accessed 6/29/2024). [CrossRef]
- Harold Evans. The Guardian. Thalidomide: How men who blighted lives of thousands evaded justice. November 14, 2014. https://www.theguardian.com/society/2014/nov/14/-sp-thalidomide-pill-how-evaded-justice. (Accessed 6/29/2024).
- National Vaccine Information Center. Search the U.S. Government’s VAERS Data. MedAlerts.org. https://medalerts.org/index.php. (Accessed 6/29/2024).
- Rubin EJ, Baden LR, Walensky Rp, Morrissey S. Audio Interview [April 16, 2021]: Covid-19 Vaccines and pregnancy – A Conversation with CDC Director Rochelle Wallensky. April 21, 2021. N Engl J Med 2021;384:e73. Time Mark for Walensky fear mongering vaccine hesitant pregnant women 16:00-17:10; Time mark for veracity of VAERS and pregnancy surveillance systems by Walensky 7:10-8:20, by Rubin 9:00-9:32, and again by Walensky 11:40-13:20. Time mark for Walensky “very seriously” comment: 7:10-7:22. https://www.nejm.org/doi/full/10.1056/NEJMe2106836 (Accessed 6/29/2024). [CrossRef]
- CDC VAERS Team. Vaccine Adverse Event Reporting System (VAERS) Standard Operating procedures for Covid-19. February 2, 2022. See page 15 of the following document. https://www.cdc.gov/vaccinesafety/pdf/VAERS-COVID19-SOp-02-02-2022-508.pdf. (Accessed 6/29/2024).
- National Vaccine Information Center VAERS database. Listed by year are 138,983 out of 1,640,416 cases with an unknown vaccine date for COVID-19 vaccines. This is from the 5/31/2024 release of VAERS data, MedAlerts.org. https://medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUp1=VACY&EVENTS=ON&VAX%5b%5d=COVID19&VAX%5b%5d=COVID19-2. (Accessed 6/29/2024).
- National Vaccine Information Center VAERS database. Listed by year are 22,834 out of 225,665 cases with an unknown vaccine date for influenza vaccines. This is from the 5/31/2024 release of VAERS data, MedAlerts.org. https://medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUp1=VACY&EVENTS=ON&VAX%5b%5d=FLU(H1N1)&VAX%5b%5d=FLU3&VAX%5b%5d=FLU4&VAX%5b%5d=FLUA3&VAX%5b%5d=FLUA4&VAX%5b%5d=FLUC3&VAX%5b%5d=FLUC4&VAX%5b%5d=FLUN(H1N1)&VAX%5b%5d=FLUN3&VAX%5b%5d=FLUN4&VAX%5b%5d=FLUR3&VAX%5b%5d=FLUR4&VAX%5b%5d=FLUX&VAX%5b%5d=FLUX(H1N1)&VAX%5b%5d=H5N1&VAXTYpES=Influenza (Accessed 6/29/2024).
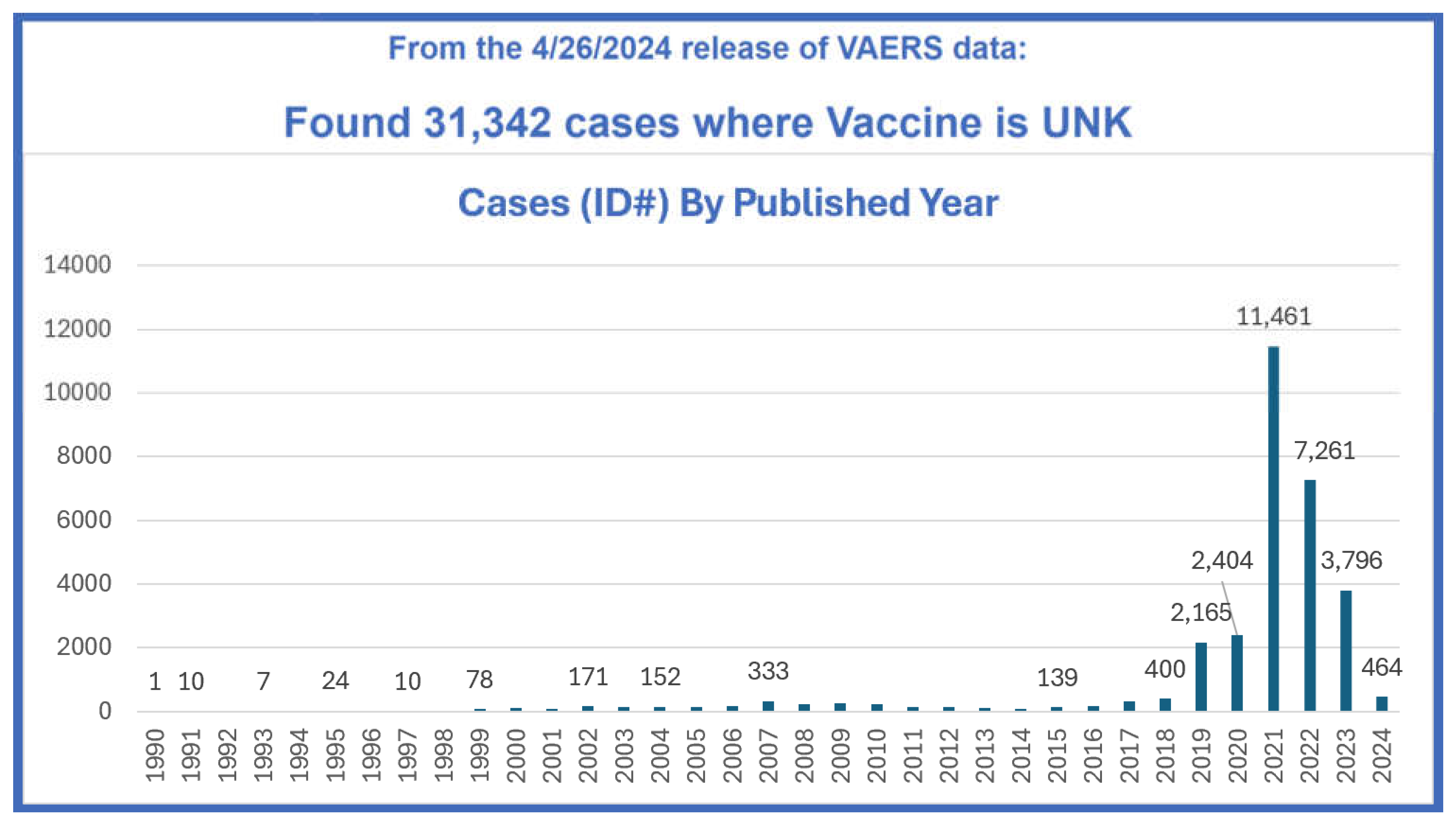
- National Vaccine Information Center. From the 5/31/2024 release of VAERS data. MedAlerts.org. From the 5/31/2024 release of VAERS data. Listed by year are 31,432 cases where the vaccine type is unknown. MedAlerts.org. https://medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUp1=VACY&EVENTS=ON&VAX%5b%5d=FLU(H1N1)&VAX%5b%5d=FLU3&VAX%5b%5d=FLU4&VAX%5b%5d=FLUA3&VAX%5b%5d=FLUA4&VAX%5b%5d=FLUC3&VAX%5b%5d=FLUC4&VAX%5b%5d=FLUN(H1N1)&VAX%5b%5d=FLUN3&VAX%5b%5d=FLUN4&VAX%5b%5d=FLUR3&VAX%5b%5d=FLUR4&VAX%5b%5d=FLUX&VAX%5b%5d=FLUX(H1N1)&VAX%5b%5d=H5N1&VAXTYpES=Influenza (Accessed 6/29/2024).
- WELCOMETHEEAGLE88. VAERS Unprecedented Throttle Job Continues in New Reports This Week Aug 18th. 42.9% (1252) of “new” reports published Aug 18 were submitted to VAERS in 2021. August 19, 2023. https://welcometheeagle.substack.com/p/vaers-unprecedented-throttle-job (Accessed 6/29/2024).
- Peter Halligan. Monthly C19, VAERS and injection uptake report. November 4, 2023. https://peterhalligan.substack.com/p/monthly-c19-vaers-and-injection-uptake (Accessed 6/29/2024).
- Aravind Mohanoor. How v-safe INCREASED the under-reporting rate for COVID19 vaccines. The case for Vaccine Data Science – part 5. March 21, 2023. https://vaccinedatascience.substack.com/p/how-v-safe-increased-the-under-reporting (Accessed 6/29/2024).
- Covid-19 vaccine 30% Unknown Ages. https://medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUp1=AGE&EVENTS=ON&VAX[]=COVID19&VAX[]=COVID19-2 (Accessed 6/29/2024).
- Unknown state location 16%. https://medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUp1=STA&EVENTS=ON&VAX[]=COVID19&VAX[]=COVID19-2&STATE=NOTFR (Accessed 6/29/2024).
- Covid-19 as concomitant medication, ~282 reports: https://medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUp1=STA&EVENTS=ON&VAX[]=COVID19&VAX[]=COVID19-2&STATE=NOTFR (Accessed 6/29/2024).
- Deleted Reports Dashboard. https://www.vaersaware.com/deleted-reports-2007-2022 (Accessed 6/29/2024).
- Unpublished ID Numbers Dashboard. https://www.vaersaware.com/unpublished-reports-id-s (Accessed 6/29/2024).
- BNT162b2 5.3.6 Cumulative Analysis of post-authorization Adverse Event Reports. https://phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf (Accessed 6/29/2024).
- Celia Farber. Court-Ordered Pfizer Documents They Tried To Have Sealed For 55 years Show 1223 Deaths, 158,000 Adverse Events in 90 Days post EUA Release. The Most Shocking Document Release Of The Last 100 years. December 5, 2021. https://celiafarber.substack.com/p/court-ordered-pfizer-documents-they (Accessed 6/29/2024).
- Michelle Maluske. CTV News. ‘Data is power’: Experts weigh-in on court-ordered release of Pfizer vaccine documents. March 11, 2022. https://windsor.ctvnews.ca/data-is-power-experts-weigh-in-on-court-ordered-release-of-pfizer-vaccine-documents-1.5816089 (Accessed 6/29/2024) Maggie M.
- Thorp MM. Jim Thorp MD. Tentacles of a Covert and Exploitative Propaganda Machine Compliments of the US Government America Out Loud October 28, 2022. Tentacles of a Covert and Exploitative Propaganda Machine Compliments of the US Government - America Out Loud (Accessed 6/29/2024).
- Thorp MM, Thorp JA. Government Curated ‘Science’: Corporate Lies, Greed and Human Destruction. America Out Loud December 11, 2022. Government Curated 'Science': Corporate Lies, Greed and Human Destruction - America Out Loud.
- Thorp MM, Thorp JA. FOIA Reveals Troubling Relationship between HHS/CDC & The American College of Obstetricians and Gynecologists. May 7, 2023. https://www.americaoutloud.news/foia-reveals-troubling-relationship-between-hhs-cdc-the-american-college-of-obstetricians-and-gynecologists/ (Accessed 6/29/2024).
- Thorp MM, Thorp JA. COVID-19 Government Relief Funds Turned the Healthcare Industry on Its Head. December 10, 2023. America Out Loud. https://www.americaoutloud.news/covid-19-government-relief-funds-turned-the-healthcare-industry-on-its-head/ (Accessed 6/29/2024).
- Thorp MM, Thorp JA. The Government Cartel paid billions to Walgreens and CVS not to fill Ivermectin – the question is why. May 20, 2024. America Out. https://www.americaoutloud.news/the-government-cartel-paid-billions-to-walgreens-and-cvs-not-to-fill-ivermectin-the-question-is-why/ (Accessed 6/29/2024).
- Thorp MM, Thorp JA. US Government coerced leaders of faith to push COVID-19 vaccines on Americans. January 14, 2024. America Out Loud https://www.americaoutloud.news/us-government-coerced-leaders-of-faith-to-push-covid-19-vaccines-on-americans/ (Accessed 6/29/2024).
- The NEW ENGLAND JOURNAL of MEDICINE. About NEJM: Policies and Practices. https://www.nejm.org/about-nejm/about-nejm#:~:text=Our%20mission%20is%20to%20publish,practice%20and%20improve%20patient%20outcomes (Accessed 6/29/2024).
- AWARD PROFILE Grant Summary Cooperative Agreement FAIN NU90TP92206. Awarding Agency Department of Health and Human Services (HHS) and Recipient: MASSACHUSETTS DEPARTMENT OF PUBLIC HEALTH. Total Awarded Amount. $329.0 Million. https://www.usaspending.gov/award/ASST_NON_NU90TP922006_7523.
- Shimabukuro TT, Kim SY, Myers TR, Moro PL. et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. April 21, 2021, N Engl J Med 2021;384:2273-2282. https://www.nejm.org/doi/full/10.1056/nejmoa2104983 (Accessed 6/29/2024). [CrossRef]
- Thorp KE, Thorp MM, Thorp EM, Thorp JA. COVID-19 & Disaster Capitalism – Part I. G Med Sci. 2022; 3(1):159-178. See pages 170-171 documenting the data manipulation revealing 82% miscarriage rate in the Shimabukuro article. https://www.doi.org/10.46766/thegms.medethics.22071901 (Accessed 6/29/2024). [CrossRef]
- Thorp MM, Thorp JA. Pushing COVID-19 Shots in Pregnancy: The Greatest Ethical Breach in the History of Medicine. America Out Loud News. February 12, 2023. https://www.americaoutloud.news/pushing-covid-19-shots-in-pregnancy-the-greatest-ethical-breach-in-the-history-of-medicine/ (Accessed 6/29/2024).
- Pineles BL, Goodman KE, Pineles L, O'Hara LM; et al. In-Hospital Mortality in a Cohort of Hospitalized Pregnant and Nonpregnant Patients With COVID-19. Ann Intern Med. 2021 Aug;174(8):1186-1188. Epub 2021 May 11. PMID: 33971101; PMCID: PMC8251936. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8251936/ (Accessed 6/29/2024). [CrossRef]
- Knight M, Ramakrishnan R, Bunch K; et al. Females in hospital with SARS-CoV-2 infection, the association with pregnancy and pregnancy outcomes: A UKOSS/ISARIC/CO-CIN investigation. Scientific Advisory Group for Emergencies; 2021. https://pubmed.ncbi.nlm.nih.gov/32513659/ (Accessed June 29, 2024).
- Metz TD, Clifton RG, Hughes BL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2021;137:571-580. [PMID:] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7984765/ (Accessed 6/29/2024). [CrossRef]
- Food and Drug Administration (FDA). FDA Docket Number FDA-2022-N-2810. “178th Meeting of the Vaccines and Related Biological Products Advisory Committee (VRBPAC)” (recorded live broadcast). Posted on YouTube, January 26, 2023. Accessed February 6, 2023 (cited material located at 6:58:32 – 6:59:42). https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-january-26-2023-meeting-announcement (Accessed 6/29/2024).
- CDC Newsroom. FDA and CDC Response to the Florida Surgeon General. March 10, 2023. https://www.cdc.gov/media/releases/2023/p0313-letter.html (Accessed 6/16/2024). (Accessed 6/29/2024).
- CDC Vaccine Safety. Clinical Immunization Safety Assessment (CISA) Project. Vaccine Safety Monitoring – CISA. https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html#anchor_1580313832826 (Accessed 6/29/2024).
- Johns Hopkins BLOOMBERG SCHOOL of PUBLIC HEALTH. What VAERS Is [And Isn’t]. May 3, 2022. https://publichealth.jhu.edu/2022/what-vaers-is-and-isnt (Accessed 6/29/2024).
- Nietzel MT. Top 20 Universities For NIH Funding; Johns Hopkins Ranks First Again. February 10, 2024. https://www.forbes.com/sites/michaeltnietzel/2024/02/10/top-20-universities-for-nih-funding-johns-hopkins-ranks-first-again/?sh=2ea1fcb4e25d (Accessed 6/29/2024).
- Clinical Immunization Safety Assessment (CISA) Current CISA Project Sites. https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html#anchor_1580313832826 (Accessed 6/29/2021).
- USASPENDING.gov. RECIPIENT PROFILE THE JOHNS HOPKINS UNVERSITY. https://www.usaspending.gov/recipient/e895f676-c226-223f-b5ed-dda80c2ec73a-C/latest (Accessed 6/29/2024).
- Thorp MM, Thorp JA. VAERS – The Last Bastion of Vaccine Free Speech? June 9, 2023. https://www.americaoutloud.news/vaers-the-last-bastion-of-vaccine-free-speech-2/ (Accessed 6/29/2024).
- Vaccine Safety Database (VSD). 2021. “Rapid Cycle Analysis (RCA) to Monitor the Safety of COVID-19 Vaccines in Near Real-Time with the Vaccine Safety Data Link,” Project #1342, Version 1.1, March 3, 2021, p. 14. Accessed May 31, 2023. https://www.cdc.gov/vaccinesafety/pdf/VSD-1342-COVID19-RCA-Protocol_FinalV1.1_508.pdf. (Accessed 6/29/2024).
- Centers for Disease Control and prevention (CDC). (2024.) “Historical Vaccine Safety Concerns: Swine Flu and Guillain Barré Syndrome – 1976” (following small increased risk of Guillain Barré Syndrome (GBS), which was 1 case for every 100,000 people who received the vaccine, federal officials determined that the possibility of an association of GBS with the vaccine necessitated pausing immunization) https://www.cdc.gov/vaccinesafety/concerns/concerns-history.html (Accessed 6/29/2024).
- Centers for Disease Control and prevention (CDC). (2024.) “Historical Vaccine Safety Concerns: Rotovirus Vaccine and Intussusception – 1988-1999” (following some infants experiencing a rare type bowel obstruction know as intussusception following vaccination, CDC recommended use of the vaccine be suspended in lieu of emergency investigations to determine whether the vaccine was the actual mechanism of causation). https://www.cdc.gov/vaccinesafety/concerns/concerns-history.html (Accessed 6/29/2024).
- Aaron Siri. V-Safe part 1: After 464 Days, CDC Finally Coughed up Covid-19 Vaccine Safety Data Showing 7.7% of people Reported Needing Medical Care. First part of an incredible story that shows just how broken our public “health” apparatus is: Very, very broken. https://aaronsiri.substack.com/p/v-safe-part-1-after-464-days-cdc?utm_source=profile&utm_medium=reader2 (Accessed 6/29/2024).
- Comingled V-Safe and VAERS Reports. https://medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUp1=AGE&EVENTS=ON&CUSTOMSQL=SpLIT+%3D+VSAFE (Accessed 6/29/2024).
- VAERS children age 0-17years. https://medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUp1=AGE&EVENTS=ON&VAX[]=COVID19&VAX[]=COVID19-2&WhichAge=range&LOWAGE=0&HIGHAGE=17.99 (Accessed 6/29/2024).
- WelcomeTheEagle Substack. VaersAware.com. VAERS Dashboard age 0-17 https://www.vaersaware.com/entire-vaers-1990-current (Accessed 6/19/2024) ICAN Informed Consent Action Network. Fourth VSAFE PRODUCTION REVEALS A DISTURBING TREND IN MISCARRIAGES AND MENSTRUAL DISTURBANCES FOLLOW COVID-19 VACCINATION. June 18, 2024. https://icandecide.org/press-release/fourth-v-safe-production-reveals-a-disturbing-trend-in-miscarriages-and-menstrual-disturbances-following-covid-19-vaccination/ (Accessed 6/29/2024).
- Aaron Siri. V-Safe part 10: Federal Judge Orders CDC to Make public 7.8 Million V-safe Free-Text Entries within 12 Months. Tenth part of an incredible story that shows just how broken our public “health” apparatus is: Very, very broken. https://aaronsiri.substack.com/p/v-safe-part-10-federal-judge-orders (Accessed 6/29/2024).
- ICAN Informed Consent Action Network. FOURTH V-SAFE PRODUCTION REVEALS ADISTURBING TREND IN MISCARRIAGES AND MENSTRUAL DISTURBANCES FOLLOWING COVID-19 VACCINATIOIN. Juine 18, 2024 https://icandecide.org/press-release/fourth-v-safe-production-reveals-a-disturbing-trend-in-miscarriages-and-menstrual-disturbances-following-covid-19-vaccination/ (Accessed 6/29/2024).
- New Tranche of VSAFE DROPPED Today (4th Installment of Free-text) Many deaths now documented in the “Free Text” field of the new V-safe data. June 19, 2024. https://welcometheeagle.substack.com/p/new-tranche-of-vsafe-dropped-today (Accessed 6/29/2024).
- ICAN. CDC STACKS ITS VACCINE COMMITTEE WITH PHARMA-AFFILIATED MEMBERS AHEAD OF JUNE 2024 VOTE ON COVID-19 VACCINES. June 14, 2024. https://icandecide.org/press-release/cdc-stacks-its-vaccine-committee-with-pharma-affiliated-members-ahead-of-june-2024-vote-on-covid-19-vaccines/ (Accessed 6/29/2024).
- The ACIP voting members voted YES unanimously on June 27, 2024 to recommend 2024-2025 COVID-19 vaccines as authorized or approved by FDA in persons ≥ 6 months of age. Advance to Timestamp 8:47:40. https://mail.proton.me/u/0/inbox/5OQkV3P7hA3c-280S3lCKWyoQBXhRXaRAuiaOGxKH2z2TB3I2x6B4wewTZlbGfz7TI6O2IV-6iQX_vcYMfeUXA== (Accessed 6/29/2024).
| Adverse Event | MedAlerts “Symptoms” Utilized | Adverse Events Raw Data Expressed as COVID-19 Vaccines / Influenza Vaccines / All Other Vaccines |
|---|---|---|
| Miscarriage (spontaneous abortion) | “abortion missed” or “abortion spontaneous” or “abortion spontaneous complete” or “abortion spontaneous complicated” or “abortion spontaneous incomplete” | 3494 / 315 / 936 |
| Fetal chromosome abnormality | “foetal chromosome abnormality” | 17 / 0 / 2 |
| Fetal malformation | “foetal cystic hygroma” or “foetal malformation” or “foetal megacystis” | 40 / 2 / 8 |
| Cervical insufficiency | “cervical incompetence” | 10 / 2 / 9 |
| Premature rupture of membranes | “premature rupture of membranes” | 114 / 14 / 53 |
| Premature labor | “premature labour” | 189 / 53 / 223 |
| Premature delivery | “premature baby” or “premature delivery” |
404 / 142 / 356 |
| Placental calcification | “placental calcification” | 5 / 0 /2 |
| Placental infarction | “placental infarction” | 8 / 0 / 2 |
| Placental thrombosis | “foetal placental thrombosis” | 6 / 0 / 0 |
| Placenta accreta | “placenta accreta” | 3 / 0 / 0 |
| Placental abruption | “premature separation of placenta” | 90 / 14 / 44 |
| Placental insufficiency | “placental insufficiency” | 24 / 0 / 2 |
| Placental disorder | “placental disorder” | 41 / 17 / 55 |
| Fetal-maternal hemorrhage | “foetal maternal hemorrhage” | 7 / 1 / 1 |
| Fetal growth restriction | “foetal growth abnormality” | 21 / 0 / 1 |
| Reduced amniotic fluid volume | “amniotic fluid index decreased” or “amniotic fluid volume decreased” | 17 / 1 / 11 |
| Preeclampsia | “pre-eclampsia” | 147 / 26 / 96 |
| Fetal heart rate abnormality | “foetal heart rate abnormal” or “foetal heart rate acceleration abnormal” or “foetal heart rate deceleration” or “foetal heart rate deceleration abnormal” or “foetal heart rate decreased” or “foetal heart rate disorder” or “foetal heart rate increased” or “foetal heart rate indeterminate” | 228 / 48 / 94 |
| Fetal cardiac disorder | “foetal cardiac disorder” | 22 / 4 / 8 |
| Fetal vascular malperfusion | “foetal vascular malperfusion | 19 / 0 / 0 |
| Fetal arrhythmia | “foetal arrhythmia” | 10 / 0 / 0 |
| Fetal distress | “foetal distress syndrome” | 18 / 6 / 27 |
| Fetal biophysical profile abnormal | “foetal biophysical profile abnormal” | 4 / 0 / 0 |
| Hemorrhage in pregnancy | “haemorrhage in pregnancy” | 164 / 7 / 25 |
| Fetal cardiac arrest | “foetal cardiac arrest” | 21 / 2 / 2 |
| Fetal death (stillbirth) | “foetal death” or “stillbirth” | 477 / 68 / 175 |
| Prematue infant death | “premature baby death” | 12 / 0 / 1 |
| Neonatal asphyxia | “neonatal asphyxia” | 8 / 0 / 3 |
| Neonatal dyspnea | “neonatal dyspnea” | 13 / 0 / 1 |
| Neonatal infection | “neonatal infection” | 5 / 0 / 4 |
| Neonatal hemorrhage | “haemorrhage neonatal” | 4 / 0 / 0 |
| Neonatal insufficient breast milk | “neonatal insufficient breast milk” | 10 / 0 / 0 |
| Neonatal pneumonia | “neonatal pneumonia” | 4 / 0 / 1 |
| Neonatal respiratory distress | “neonatal respiratory distress” | 12 / 0 / 7 |
| Neonatal respiratory distress syndrome | “neonatal respiratory distress syndrome” | 12 / 0 / 23 |
| Neonatal seizure | “neonatal seizure” | 7 / 0 / 6 |
| Adverse Event | Original Adverse Events Raw Data COVID-19 / Influenza / All Other Vaccines | Unconcealed Adverse Events Raw Data COVID-19 / Influenza / All Other Vaccines | Percent of Original Raw Data Concealed COVID-19 / Influenza / All Other Vaccines |
|---|---|---|---|
| Miscarriage (spontaneous abortion) | 3494 / 315 / 936 | 393 / 64 / 280 | 11% / 20% / 30% |
| Fetal chromosome abnormality | 17 / 0 / 2 | 1 / 0 / 2 | 6% / 0% / 100% |
| Fetal malformation | 40 / 2 / 8 | 6 / 0 / 3 | 15% / 0% / 38% |
| Cervical insufficiency | 10 / 2 / 2009 | 10 / 2 / 2011 | 0% / 0% / 22% |
| Premature rupture of membranes | 114 / 14 / 53 | 2 / 5 / 13 | 2% / 36% / 25% |
| Premature labor | 189 / 53 / 223 | 10 / 7 / 44 | 5% / 13% / 20% |
| Premature delivery | 404 / 142 / 356 | 36 / 74 / 245 | 9% / 52% / 69% |
| Placental calcification | 5 / 0 /2 | 5 / 0 / 2 | 0% / 0% / 0% |
| Placental infarction | 8 / 0 / 2 | 10 / 0 / 2 | 25% / 0% / 0% |
| Placental thrombosis | 6 / 0 / 0 | 6 / 0 / 0 | 0% / 0% / 0% |
| Placenta accreta | 3 / 0 / 0 | 3 / 0 / 0 | 0% / 0% / 0% |
| Placental abruption | 90 / 14 / 44 | 116 / 19 / 66 | 29% / 36% / 50% |
| Placental insufficiency | 24 / 0 / 2 | 27 / 0 / 3 | 13% / 0% / 50% |
| Placental disorder | 41 / 17 / 55 | 44 / 17 / 74 | 7% / 0% / 35% |
| Fetal-maternal hemorrhage | 7 / 1 / 1 | 7 / 1 / 1 | 0% / 0% / 0% |
| Fetal growth restriction | 21 / 0 / 1 | 23 / 0 / 2 | 10% / 0% / 100% |
| Reduced amniotic fluid volume | 17 / 1 / 11 | 18 / 3 / 13 | 6% / 200% / 18% |
| Preeclampsia | 147 / 26 / 96 | 160 / 29 / 123 | 9% / 12% / 28% |
| Fetal heart rate abnormality | 228 / 48 / 94 | 240 / 49 / 112 | 5% / 2% / 19% |
| Fetal cardiac disorder | 22 / 4 / 8 | 28 / 4 / 14 | 27% / 0% / 75% |
| Fetal vascular malperfusion | 19 / 0 / 0 | 21 / 0 / 0 | 11% / 0% / 0% |
| Fetal arrhythmia | 10 / 0 / 0 | 10 / 0 / 1 | 0% / 0% / 1 |
| Fetal distress | 18 / 6 / 27 | 20 / 9 / 39 | 11% / 50% / 44% |
| Fetal biophysical profile abnormal | 4 / 0 / 0 | 4 / 0 / 1 | 0% / 0% / NA |
| Hemorrhage in pregnancy | 164 / 7 / 25 | 10 / 2 / 5 | 6% / 29% / 20% |
| Fetal cardiac arrest | 21 / 2 / 2 | 1 / 0 / 0 | 5% / 0% / 0% |
| Fetal death (stillbirth) | 477 / 68 / 175 | 562 / 107 / 287 | 18% / 57% / 64% |
| Premature infant death | 12 / 0 / 1 | 12 / 0 / 1 | 0% / 0% / 0% |
| Neonatal asphyxia | 8 / 0 / 3 | 8 / 1 / 7 | 0% / 0% / 133% |
| Neonatal dyspnea | 13 / 0 / 1 | 18 / 0 / 1 | 38% / 0% / 0% |
| Neonatal infection | 5 / 0 / 4 | 5 / 0 / 8 | 0% / 0% / 100% |
| Neonatal hemorrage | 4 / 0 / 0 | 4 / 0 / 0 | 0% / 0% / 0% |
| Neonatal insufficient breast milk | 10 / 0 / 0 | 12 / 0 / 0 | 20% / 0% / 0% |
| Neonatal pneumonia | 4 / 0 / 1 | 4 / 1 / 2 | 0% / 1 / 100% |
| Neonatal respiratory distress | 12 / 0 / 7 | 14 / 0 / 8 | 17% / 0% / 14% |
| Neonatal respiratory distress syndrome | 12 / 0 / 23 | 12 / 0 / 30 | 0% / 0% / 30% |
| Neonatal seizure | 7 / 0 / 6 | 7 / 0 / 6 | 0% / 0% / 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





