1. Introduction
In recent years, the use of antibiotics in veterinary medicine has significantly increased, resulting in higher levels of antibiotic residues in animal-derived products such as milk and contributing to antibiotic resistance. The consumption of milk containing antibiotic residues may pose potential health risks to consumers, including allergic reactions and reduced effectiveness in treating infections due to drug resistance [
1]. Antibiotics are naturally occurring low molecular weight metabolites produced by bacteria or fungi that either kill or inhibit the growth of other microorganisms [
2]. They are classified into various groups depending on their chemical structure and mechanism of action, including ß-lactams, aminoglycosides, anthracyclines, (fluoro)quinolones, tetracyclines, lincosamides, and sulfonamides [
3].
Oxytetracycline (OTC) is an antibiotic of the tetracycline group commonly used for treating infections in both animals and humans. To protect consumers and ensure food safety, the European Union has established maximum residue limits (MRLs) for antibiotics in foodstuffs of animal origin; the MRL for OTC is set at 100 ng/mL [
4]. To mitigate the risk of exposure to OTC, several methods for its detection have been developed. Among these, biosensors have emerged as promising lower-cost and more rapid alternatives to conventional detection methodologies, such as chromatography [
5]. Various types of biosensors have been explored which include electrochemical [
6], optical [
7], and colorimetric fluorescent versions [
8]. Among these, electrochemical biosensors have demonstrated significant potential due to their rapid analytical response, emhanced sensitivity, and simplicity [
9]. Specifically, electrochemical aptasensors offer additional advantages, combining high flexibility, sensitivity and selectivity with enhanced stability compared to antibodies, along with their ability to immobilize on diverse surfaces [
10]. Various platforms have been developed for antibiotic detection in milk and especially for detecting oxytetracycline (OTC). Specifically, a limit of detection (LOD) of 5 ng/mL was achieved using thin-film gold electrodes fabricated through metal sputtering [
11]. A LOD of 4.2 ng/mL was observed using nanocomposite-modified electrodes, including multi-walled carbon nanotubes (MWCNTs), gold nanoparticles (AuNPs), reduced graphene oxide (rGO), and chitosan (CS) nanocomposites to modify a glassy carbon electrode (GCE) [
12]. Enhanced sensitivity with a significantly lower LOD of 0.23 ng/mL was obtained by using a GCE grafted with diazonium salt, followed by the attachment of aptamers via carbodiimide binding [
13].
In complex matrices such as milk, the detection of a target molecule can be challenging due to the presence of diverse proteins that may interfere with the performance of the sensor. This interference can decrease the device sensitivity and specificity by causing fouling or non-specific adsorption at the sensor-liquid interface. It is, therefore, essential to incorporate antifouling compounds to hinder the non-specific binding of non-target molecules on the surface [
14].
The main objective of this work was the development of an electrochemical aptamer-based sensor for the sensitive label-free detection of OTC. This was achieved by using a gold electrode modified with α-lipoic acid-NHS [
15], a potentially antifouling compound onto which an amine-terminated aptamer was immobilized via covalent bonding. The interaction between the immobilized aptamer and OTC was probed electrochemically by monitoring changes in the redox peak currents of the Fe(CN)
64-/Fe(CN)
63- redox couple. The fabrication of the aptasensor is described and initial experimental results are presented suggesting that detection of OTC at concentrations lower than the MRL set by EU can be achieved.
2. Materials and Methods
2.1. Materials
Reagents and chemicals were all of analytical grade and provided by Merck (Darmstadt, Germany) and Sigma-Aldrich (Burlington, MA, USA) and used with no more purification. The amine-terminated aptamer sequence was 5′-GGA ATT CGC TAG CAC GTT GAC GCT GGT GCC CGG TTG TGG TGC GAG TGT TGT GTG GAT CCG AGC TCC ACG TG/3AmMO/-3’ and the aptamer was purchased from Integrated DNA Technologies Inc. (Iowa, USA). The linker, α-lipoic acid-NHS, was purchased from MedChemExpress, (New Jersey, USA) and diluted in EtOH 50% (v/v). The aptamers were diluted in phosphate buffer (PB) (10 mM, pH 7.4), containing 1 mM MgCl2. Oxytetracycline (OTC) hydrochloride salt was purchased from Thermo Fisher Scientific (MA, USA) and a stock solution of 100 mg/L was prepared in water and further diluted in PB buffer (0.01 M, pH 7.4). All electrochemical measurements were performed using a solution of 10 mM Fe(CN)64-/Fe(CN)63- containing 0.5 M KCl.
2.1. Apparatus and Electrodes
A 1200C Series Handheld Potentiostat/Bipotentiostat equipped with the CHI440A Software was used for all the electrochemical measurements (CH Instruments, Austin, TX, USA). The 220AT screen-printed electrodes (SPEs) with a 4 mm diameter gold working electrode (WE), gold counter electrode (CE), and silver reference electrode (RE) were obtained from Metrohm DropSens (Herisau, Switzerland).
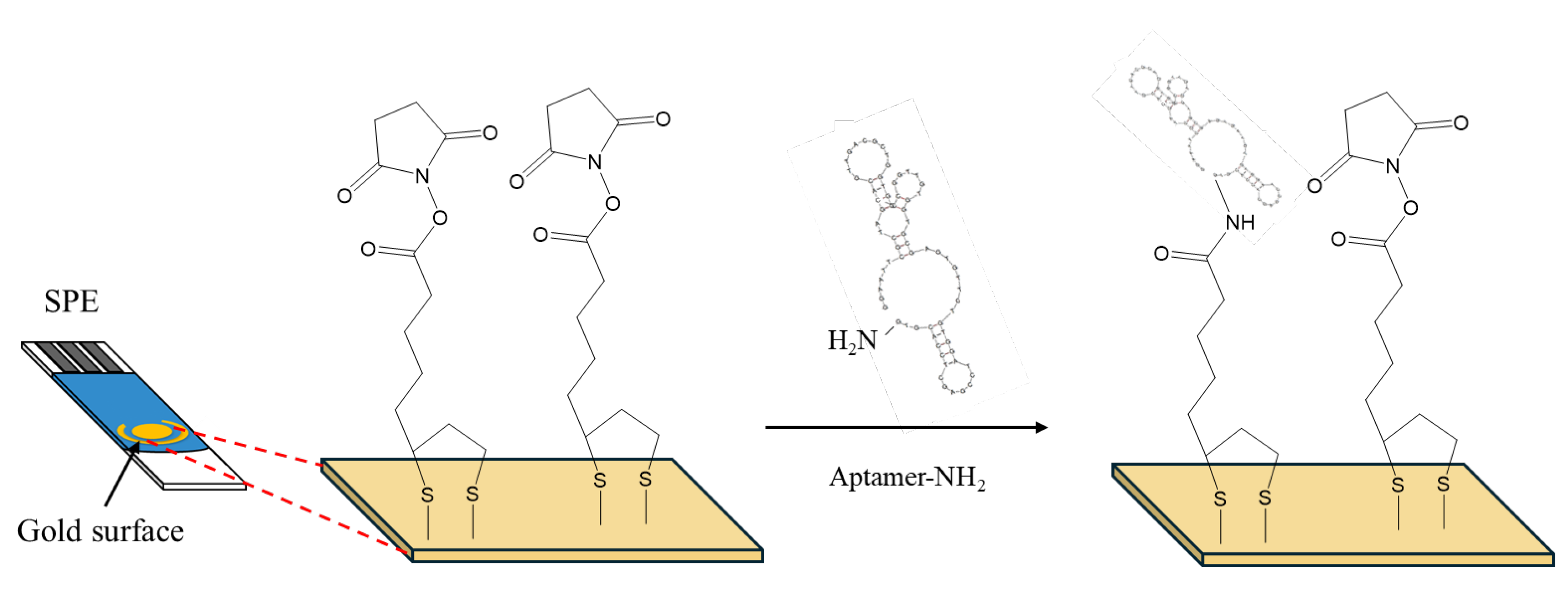
2.1. Preparation of Aptasensors
5 μl of a 2mM α-lipoic acid-NHS solution were drop-casted on the gold working electrode surface of SPEs and incubated for 3 days at 4
oC in a humidity chamber. Then the SPEs were rinsed thoroughly with deionized water and dried under a nitrogen stream. 10 μl of a 100 μM aptamer solution was drop-casted on the working electrode surface and left for 5 h at room temperature (RT) (
Figure 1). Then, the electrode was washed thoroughly with deionized water to remove any excess aptamer molecules and air-dried. Finally, the SPEs were exposed to the OTC solutions ranging from 25 to 500 ng/ml for 30 minutes. The electrochemical measurements were taken in 10 mM Fe(CN)
64-/Fe(CN)
63- containing 0.5 M KCl. The peak currents were recorded using cyclic voltammetry (scan rate: 0.3 V/s) and differential pulse voltammetry (pulse width: 0.05 sec, pulse period: 0.5 sec) in the range of -0.2 to +0.4 V in both in the anodic and the cathodic direction.
3. Results and Discussion
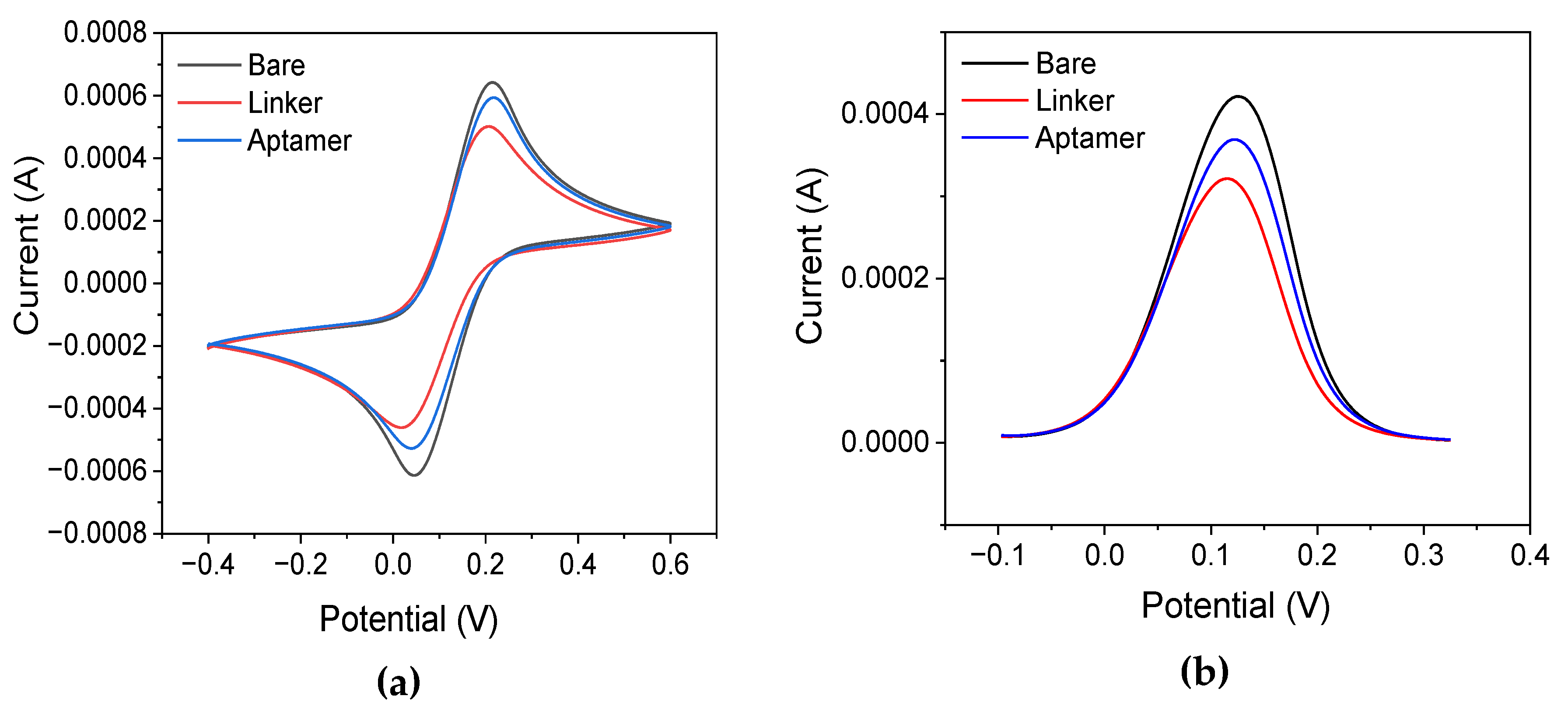
Cyclic voltammetry and differential pulse voltammetry were employed to study the changes in the redox peak currents of ther electrochemical probe at the different steps of the modification precedure. To define the optimum parameters for linker binding on the surface, different concentrations (1, 2, and 4 mM) of the linker and incubation times (1 and 3 days at 4
oC) were investigated. It was found that electrodes incubated with 2 and 4 mM of the linker for 3 days exhibited similar current responses, with a reduction in current observed after the linker was bound to the surface. However, electrodes incubated with a 1 mM concentration of the linker showed lower current reduction. Further experiments indicated inefficient binding with the aptamer, leading to inadequate sensitivity for OTC detection. While current reduction was also observed after one-day incubation with the linker, OTC detection was unsuccessful, as no change in the peak current was observed after employing different concentrations of OTC. Notably, under this condition, the current continued to decrease after modification with the aptamer, in contrast to the 3-day incubation, where the current increased. Upon immobilization of the aptamer on the electrode surface containing the linker, an increase in current was observed. The change in current intensity suggests that immobilization was achieved. In fact, the increase in current after aptamer immobilization has been reported previously in the literature [
16]. We postulate that the current increase can be attributed to a structural reconfiguration of the linker at the electrode surface that facilitates electron flow. To elaborate further, aptamer binding could induce restructuring of the linker in a more linear conformation, exposing a larger area of the gold electrode surface to the redox probe. We also evaluated the electrode modification using different concentrations of aptamer (10, 50, and 100 μΜ), and various incubation times (1 h, 2 h, 5 h, and overnight at 4
oC). Initially, when electrodes were incubated with the aptamer for 1, 2, and 5 h, an increase in the peak current was observed in all cases compared to the peak after linker immobilization. In all cases, the developed sensor was able to detect OTC. Specifically, the 5-hour incubation time at 4
oC exhibited higher sensitivity. However, when the aptamer was incubated overnight, there was a decrease in the current, and the detection of the OTC was unsuccessful. This observation confirms the successful immobilization of the aptamer despite an increase in current. Regarding the optimum aptamer concentration, 100 μM of the aptamer showed the highest sensitivity and wider dynamic range compared to the other concentrations. The respective cyclic voltammograms and differential pulse voltammograms with the selected parameters are presented in
Figure 2a and
Figure 2b, respectively. Finally, the temperature employed during the aptamer incubation was also investigated using incubation at 4
oC and RT. The results suggested that incubation at RT provided better sensitivity and repeatability.
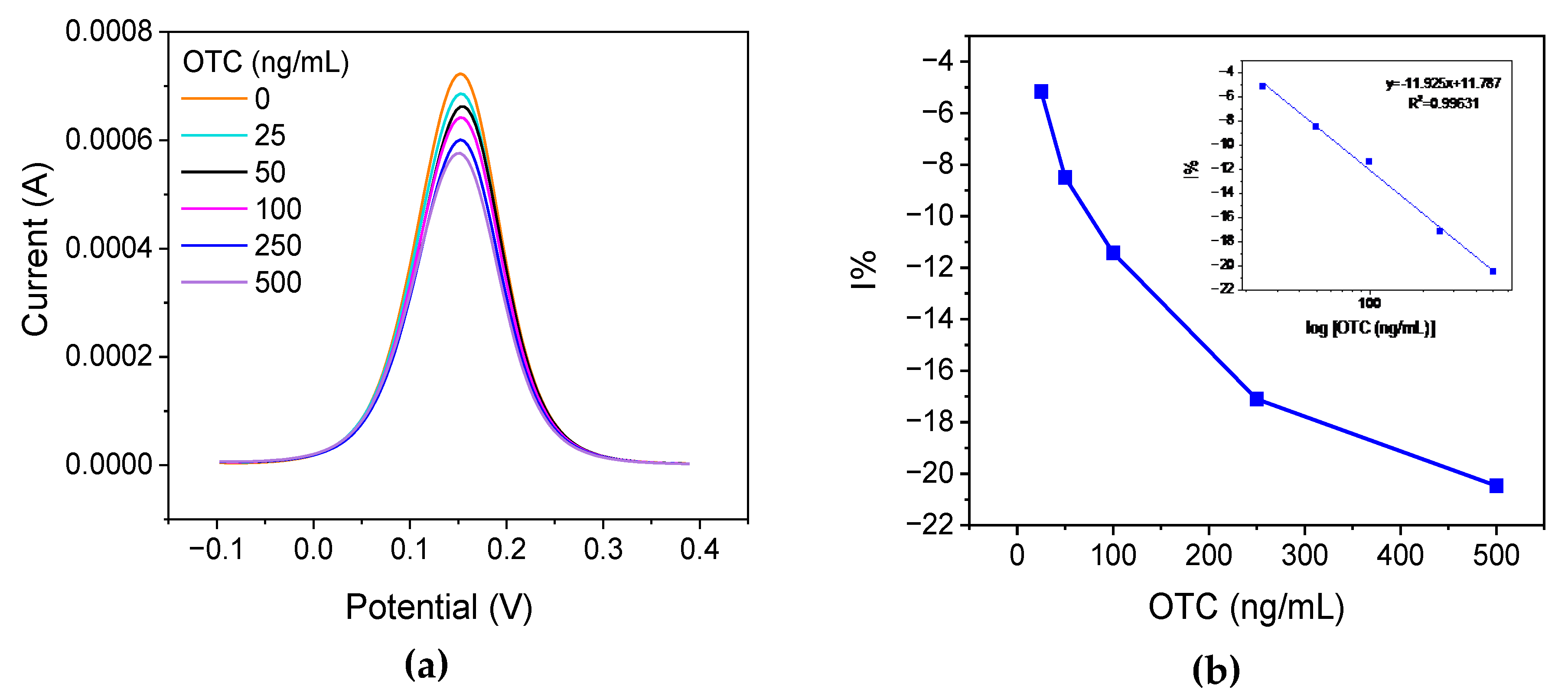
The ability of the developed biosensor to detect OTC was evaluated by exposing the electrodes to different concentrations of OTC (0, 25, 50, 100, 250, and 500 ng/ml) solution for 30 min at RT and at 4
oC. Our results indicate that incubation at RT provides lower LOD and repeatability. The anodic DPVs were recorded and the relative current reduction, I%, was calculated as I% = (i -i
o)/i
o ×100 (where i
o is the DPV current at the aptamer-modified electrode in the absence of OTC and i is the DPV current at the aptamer-modified electrode after exposing to OTC solution). Typical DPVs are presented in
Figure 3a, and the respective calibration curve is presented in
Figure 3b.
A conditional LOD of 14 ng/ml of OTC was calculated for the developed aptasensor using the formula LOD=3*SD
i/S (where SD
i is the standard deviation of the intercept of the calibration plot 3(b) at low concentrations and S is the slope of the calibration plot in
Figure 3(b) at low concentrations).
4. Conclusions
In this work, initial results for the fabrication of an electrochemical label-free aptasensor for OTC detection are presented. By modifying a gold electrode with α-lipoic acid-NHS and an amine-terminated aptamer, proof-of-principle detection of OTC at concentrations lower than the maximum acceptable residue limit set by EU was achieved with a LOD of 14 ng/mL and a dynamic range extending up to 500 ng/mL. Further experiments will be performed to study the ability of the antifouling linker to prevent non-specific adsorption of proteins.
Author Contributions
Conceptualization, A, E and M.T.; methodology, D.K, G.G, L.N. and S.A.; investigation, D.K, G.G, L.N. and S.A.; resources, A.E. and M.T.; data curation, D.K, G.G, L.N. and S.A. writing—original draft preparation, D.K, G.G, L.N. and S.A..; writing—review, A.E. and M.T.; supervision, A.E. and M.T.; project administration, A.E, M.T. and S.A.; funding acquisition, A.E. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a European Union Horizon 2020 Research and Innovation Program through the Marie Sklodowska-Curie grant agreement No. 101007299 (SAFEMILK) and by Thompson Surface Innovations, Inc. of Toronto.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The relevant data are available by the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hebbal, M.A.; Latha, C.; Menon, K.V.; Deepa, J. Occurrence of oxytetracycline residues in milk samples from Palakkad, Kerala, India. Vet World 2020, 13, 1056–1064. [Google Scholar] [CrossRef]
- Gualerzi, C.O.; Brandi, L.; Fabbretti, A.; Pon, C.L. (Eds.) Antibiotics: Targets, Mechanisms and Resistance; Wiley-VCH Verlag: Weinheim, Germany, 2014. [Google Scholar] [CrossRef]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-Based Biosensors for Antibiotic Detection: A Review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Union 2010, 15, 1–72.
- Tasci, F.; Canbay, H.S.; Doganturk, M. Determination of antibiotics and their metabolites in milk by liquid chromatography-tandem mass spectrometry method. Food Control 2021, 127, 108147. [Google Scholar] [CrossRef]
- Lin, J.; Qian, J.; Wang, Y.; Yang, Y.; Zhang, Y.; Chen, J. ; Chen, X,; Chen, Z. Quantum dots@ porous carbon platform for the electrochemical sensing of oxytetracycline. Microchem J, 1063. [Google Scholar] [CrossRef]
- Marquez, M.J.; Roncales, C.J.; Tigcal, R.A.; Quinto, E. Development of optical detection for antibiotic residues: oxytetracycline in freshwater aquaculture. In MATEC Web of conferences 2019, 268, 6013–6018. [Google Scholar] [CrossRef]
- Zhao, Y.; Ong, S.; Chen, Y.; Jimmy Huang, P.J.; Liu, J. Label-Free and Dye-Free Fluorescent Sensing of Tetracyclines Using a Capture-Selected DNA Aptamer. Anal Chem 2022, 94, 10175–10182. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Tsekenis, G.; Oravczova, V.; Hianik, T. Electrochemical Aptasensors for Antibiotics Detection: Recent Achievements and Applications for Monitoring Food Safety. Sensors 2022, 22, 3684. [Google Scholar] [CrossRef] [PubMed]
- Machairas, V.; Anagnostoupoulos, A.; Soulis, D.; Economou, A.; Jakab, K.; Melios, N.; Keresztes, Z.; Tsekenis, G.; Wang, J.; Speliotis, T. Microfabricated Gold Aptasensors for the Label-Free Electrochemical Assay of Oxytetracycline Residues in Milk. Eng. Proc. 2023, 58, 1. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Khajehsharifi, H.; Hajihosseini, S. Detection of Oxytetracycline Using an Electrochemical Label-Free Aptamer-Based Biosensor. Biosensors 2022, 12, 468. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yan, W.; Guo, Y.; Wang, X.; Zhang, F.; Yu, L.; Guo, C.; Fang, G. Sensitive and selective electrochemical aptasensor via diazonium-coupling reaction for label-free determination of oxytetracycline in milk samples. Sens. Actuators Rep. 2020, 2, 100009. [Google Scholar] [CrossRef]
- Spagnolo, S.; De La Franier, B.; Hianik, T.; Thompson, M. Surface probe linker with tandem anti-fouling properties for application in biosensor technology. Biosensors 2020, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Martić, S.; Beheshti, S.; Rains, M.K. , Kraatz, H.B. Electrochemical investigations into Tau protein phosphorylations. Analyst 2012, 137, 2042–2046. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, Z.; Fan, H.; Ai, S.; Han, R. Electrochemical detection of avian influenza virus H5N1 gene sequence using a DNA aptamer immobilized onto a hybrid nanomaterial-modified electrode. Electrochim. Acta. 2011, 56, 6266–6270. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).