1. Introduction
Intranasal route of administration of active pharmaceutical ingredients is promising not only for the use of drugs for topical application, but also for drugs of systemic action. This route of administration has become especially important for transportation of therapeutic agents directly to the central nervous system bypassing the blood-brain barrier, which was a traditionally difficult task for other dosage forms. This opens new possibilities for achieving positive therapeutic effects in the treatment of neurological disorders, especially neurodegenerative diseases, acute ischemic stroke, Alzheimer’s disease, as well as after perinatal CNS damage. The intranasal route of administration is often equated with the parenteral route, it differs from other routes by high bioavailability, absence of first pass metabolism of active substances, prospective use in neonatology and in unconscious patients. [
1,
2,
3,
4].
The intranasal route of active substances administration for the treatment of cerebrovascular diseases is based on the peculiarities of the anatomical structure of the nasal cavity. It is currently believed that absorption of active pharmaceutical ingredients occurs via the olfactory and trigeminal nerves. The olfactory pathway allows drugs to directly enter the olfactory bulb, via the olfactory epithelium [
5,
6]. This pathway is critical for substance transport to both anterior and posterior brain regions due to the unique entry points provided by the olfactory and trigeminal nerves. There is also a theory that drug transport is accomplished by both extracellular and intracellular pathways [
7].
Extracellular transport through columnar epithelial cells may occur by paracellular diffusion. Intracellular transport is realized by transcytosis. [
3,
8,
9,
10].
Despite the promise of using the intranasal route of administration to transport active pharmaceutical ingredients to the nasal cavity, there are some factors that may hinder the penetration of active ingredients: poor absorption through the nasal mucosa, possible enzymatic degradation in the nasal cavity, and removal of active substances. To overcome these obstacles, additional strategies have been proposed, including the use of protease inhibitors, adsorption enhancers, mucoadhesive polymers, gel forms, and the use of specific technologies nano- and microemulsions in nasal formulations. [
11,
12,
13].
The development of cerebral insufficiency in children who have undergone pre- and perinatal hypoxia is based on the processes of neurodestruction associated with the activation of transmitter autocoidosis, oxidative and nitrosative stress, disturbance of energy metabolism, mitochondrial dysfunction against the background of inhibition of endogenous neuroprotection systems [
14,
15,
16,
17].A promising direction of neuroprotection after prenatal hypoxia is the use of agents that activate GSH/HSP70-dependent mechanisms of endogenous neuroprotection. In this context, a new molecule (S)-2,6-diaminohexanoic acid 3-methyl-1,2,4-triazolyl-5-trioacetate (Angiolin) is of interest, which demonstrated its ability to regulate HSP70 expression directly or by its influence on the glutathione link of the thiol-disulfide system under conditions of modeling brain and myocardium ischemia, chronic alcoholization [
14,
18]. Angiolin showed neuroprotective activity when it was administered to animals undergoing prenatal hypoxia. The results of the study demonstrated that Angiolin reduced lethality, neurological disorders in animals after intrauterine hypoxia, increased the expression of HSP70, HIF-1a and NO-system parameters in the brain [
19,
20,
21]. Angiolin belongs to class VI of toxicity and has a high safety profile (no cumulation of substances, no immunotoxicity, mutagenicity, carcinogenicity, teratogenicity, embryotoxicity). Absence of toxic effect of the drug at 180-day administration to animals was shown. The first stage of clinical trials of Angiolin as an anti-ischemic agent with a significant effect on cerebral vascular endothelium, myocardium and metabolism (solution for injection) was successfully conducted with the permission of the State Expert Center of the Ministry of Health of Ukraine. The obtained results are justification for further studies and development of new medicinal forms. In view of the above, the development and optimization of a new nasal form with Angiolin for neuroprotection after intrauterine hypoxia is an actual and perspective task [
22].
Aim of this work: to develop and optimize the composition of a new nasal form with the neuroprotective agent Angiolin for use in neonatology after prenatal hypoxia.
2. Materials and Methods
2.1. Materials
Angiolin ((S)-2,6-diaminohexanoic acid 3-methyl-1,2,4-triazolyl-5-thioacetate) (Scientific and Technological Complex “Institute of Monocrystals” NAS) of Ukraine) was used as an active component in the formulation of nasal gel. Adjuvants:D-panthenol, carboxymethylcellulose sodium salt, tween-80, benzalkonium chloride, purified water. The active and adjuvant ingredients of pharmaceutical purity obtained from NPF “SINBIAS” LLC, “Istok-Plus” LLC were used in the experiments.
2.2. Design of the Experiment
For planning the design of the experiment, we used the response surface methodology and Benken’s Box plan-a set of statistical techniques that are used to create a model and conduct an analysis of the responses affected by the optimization factors. The design optimization was performed on three factors (x), three levels (low, medium, high), and three responses (y) (
Table 1).
2.3. Characteristics of the Obtained Gel Samples
2.3.1. pH Test
We weighed 10.0 g of gel, placed it in a measuring cylinder, added it to 100 ml of purified water. The obtained mixture was stirred with a magnetic stirrer. Then we measured the pH using a 15OM pH meter with a glass electrode.
2.3.2. Biopharmaceutical Study of the Kinetics of Substance Release through a Semipermeable Membrane
It was performed by the method of equilibrium dialysis through semipermeable membrane - Cuprophan cellophane film, Type 150 pm, 11±0.5 μm thick in vertical diffusion cells of a nine-position station Franz Cells (PermeGear, Inc., USA). Each cell contains a donor chamber in which the tested nasal gel sample was placed and an acceptor chamber of 25 mL filled with dialysis solution (purified water). In order to stir the dialysis solution uniformly in the acceptor chamber, a magnetic stirrer was added. Stirring speed of dialysis solution was 350 rpm. The temperature regime in the experimental studies was 27±0.2 °C, which was ensured by using a circulating water thermostat (Thermo Scientific HAAKE SC100-S5P).
The percentage of angiolin release was determined after 15 min by quantification of angiolin using spectrophotometric technique on UV 2600 spectrophotometer (Shimadzu, Japan) at 238 nm according to the modified method [
23]. Before measurement, 5 ml of dialysate was taken, placed in a 25 ml volumetric flask and added purified water to the mark. The concentration of active substance in the solution was determined by calibration graph. The results determined from the graph were increased 5 times with dilution and thus calculated the concentration of angiolin in the dialysate.
2.3.3. Rheological Studies
Rheological studies of the experimental gels were performed in oscillation regime on a modular compact rheometer Anton Paar MCR 302. The CP50-1 cone-plate system (diameter 50 mm, cone angle 1 degree) SN71317 was used as measuring devices, which, compared to cylindrical devices, requires significantly less gel sample and can perform the planned tests in oscillatory regime. The temperature in the experiments was provided by an integrated thermostat. (Peltier temperature control for concentric cylinder systems, C-PTD 200).
2.3.4. Viscosity Test of Sample Gels
We weighed 5 g of gel, placed it on a plate, and used the built-in RheoCompass software to place a cone at a distance of 0.1 mm from the plate. The viscosity values (mPa*s) were measured at a shear rate (γ) of 50 1/s. The temperature regime of the experiment was performed at 25 ⁰С.
2.3.5. Thixotropy Test
We weighed out 5 g of gel, placed it on a plate, using the built-in software RheoCompass placed a cone at a distance of 0.1 mm from the plate. The study of the speed of structure recovery of the experimental sample of nasal gel was performed by the method of direct study with three intervals oscillation – rotation - oscillation by DIN SPEC 91143-2:2012 (The three-interval thixotropy test, 3ITT). The experiment was carried out in three stages. 1. The initial measurement interval was carried out at a low shear rate (0.1 s-1) characterizing the resting behavior of the sample. 2. The middle measurement interval was performed at a shear rate of 100-s-1, characterizing the sample behavior at the moment of application. 3. Final interval - the measurement was carried out at a low shear rate of -0.1-s-1. This interval characterizes how quickly the sample restores its structure (in what time).
2.4. Pharmaco-Toxicological Methods of Research
The studies were performed on a sufficient number of animals, and all manipulations were carried out in accordance with the regulation on the use of animals in biomedical experiments (Strasbourg, 1986, as amended in 2010) and the “General Ethical Principles of Animal Experiments” [
24,
25]. The experiments were performed on 2-month-old white female Wistar rats weighing 95-110 g, obtained from the nursery of the Institute of Pharmacology and Toxicology of the Academy of Medical Sciences of Ukraine. The quarantine (acclimation) period for all animals was 14 days. During the quarantine period, each animal was examined daily (behavior and general condition), and the animals were observed in the cages twice a day (morbidity and mortality). Before the start of the experiment, animals that met the criteria for inclusion in the experiment were randomly assigned to the groups. Animals that did not meet the criteria were excluded from the experiment during quarantine. Cages with animals were placed in separate rooms. Light regime: 12 hours - light, 12 hours - darkness. Air temperature was maintained within 19-25°C, relative air humidity - 50-70%. Air temperature and humidity were registered every day. A ventilation regime was established, providing about 15 room volumes per hour. Experimental animals were kept on the same nutrition, in the usual conditions of the vivarium. Animals were kept in standard cages with 5 animals per cage. The diet consisted of fodder cereals, bread, root crops (beets, carrots).
2.4.1. Acute Toxicity Definition
The tested gels were administered intranasally using a syringe-doser in the maximum allowable volume for this route of administration - 0.4 ml. Within 14 days, the animals were observed for death and changes in the cardiovascular system, respiratory system, CNS, locomotor activity.
2.4.2. Evaluation of Allergizing and skin-Resorptive Activity by the Method of Skin Applications
On the lateral surface of the body of the animal, the hair was clipped in an area of 4 × 4 cm. 0.5 g of gel was applied to this skin area, after which the animals were placed in individual cages for 4 hours to prevent drug licking. Gel application was performed by 20 repeated skin applications 5 times per week. Skin reactions were recorded daily using a skin test grading scale. The first test was performed after 10 applications (if allergy was detected, further application of the gel was stopped). In case of a negative or doubtful result, the number of applications was necessarily increased to 20. Evaluation of the results of the allergic activity of the gel by the method of skin applications was carried out according to the appropriate scale (
Table 2).
2.4.3. Examination of Local Irritant Effect (Conjunctival Test)
The conjunctiva of both eyes of animals of the experimental group was applied with a dosage pipette 0.01 ml of gel. Rats of the control group were injected with distilled water into the conjunctival sac. The observation was carried out for 3 days.
The reaction was evaluated on a scale: 0 points - no changes in conjunctival mucosa; 1 point - slight conjunctival redness; 2 points - conjunctival redness and edema.
2.4.4. Examination of Active Dermal Anaphylaxis
On the lateral surface of the body of the animals, the hair was removed in a 4×4 cm area. 0.5 g of gel was applied to this skin area, after which the animals were placed for 4 hours in individual cages to prevent licking of the drug. Sensitization of animals was detected 5 days after the last application of the preparation. For this 0.3-0.5 g of gel was applied once to the skin of ears. The intensity of anaphylactic shock was recorded after 6, 12 and 24 hours in points according to Weigle index: ++++ - shock with lethal outcome; +++ - shock of severe degree (general convulsions, asphyxia, the animal loses the ability to keep on its feet, falls on the side, does not die); ++ - medium shock (small convulsions, significant symptoms of bronchospasm); + - slight shock (some restlessness, rapid breathing, scratching of the face, spontaneous urination, defecation, hair ruffled); 0 - shock has not developed, its symptoms are absent.
2.5. Statistical Methods
The results of the study were calculated using the standard statistical package of the licensed program “STATISTICA® for Windows 6.0” (StatSoftInc., №AXXR712D83323214FAN5), as well as “SPSS 16.0”, “Microsoft Office Excel 2010”.
3. Results
3.1. Formulation Design
The initial phase of pharmaceutical development using experimental design involves the careful planning and execution of experiments. Experiment design relies on statistical analysis of experimental data, ranging from basic methods such as the t-test for comparing two groups to more complex methods such as analysis of variance (ANOVA). ANOVA is particularly useful for the examination of data in experiments with one or more factors, including situations in which there are interactions between the factors. Statistical evaluation is important for improving the quality of the final product through a comprehensive understanding of the variables which play a role in the final product [
26,
27,
28].
Planning of the optimal formulation of nasal gel with angiolin was performed using Box-Behnken plan, with three levels of factors: Na CMC, Tween-80, D-panthenol and three responses: pH, Viscosity test, and Angiolin Release (
Table 3).
3.2. Organoleptic Characteristics
According to the design of the experiment, we obtained samples of nasal gels, from transparent to slightly yellowish color of different density and consistency, odorless.
3.3. Hydrogen Index (рН)
Many factors contribute to the chemical stability of a formulation, one of which is the hydrogen value. In addition to the chemical stability of the formulation composition, pH can also have a significant effect on oral mucosa and cause additional pathological processes.
Statistical analysis of the obtained results of the effect of variable factors on the pH of the obtained nasal gels is presented in
Table 4.
The obtained results of pH determination of nasal gel samples do not change according to statistical analysis ANOVA for Mean model.
3.4. Rheological Characteristics
Viscosity at a given shear rate, one of the rheological characteristics that allows to compare the consistency properties of experimental gels and the possible influence of formulation excipients on it. The results of the determination of the consistency characteristics of nasal gels are shown in
Table 5.
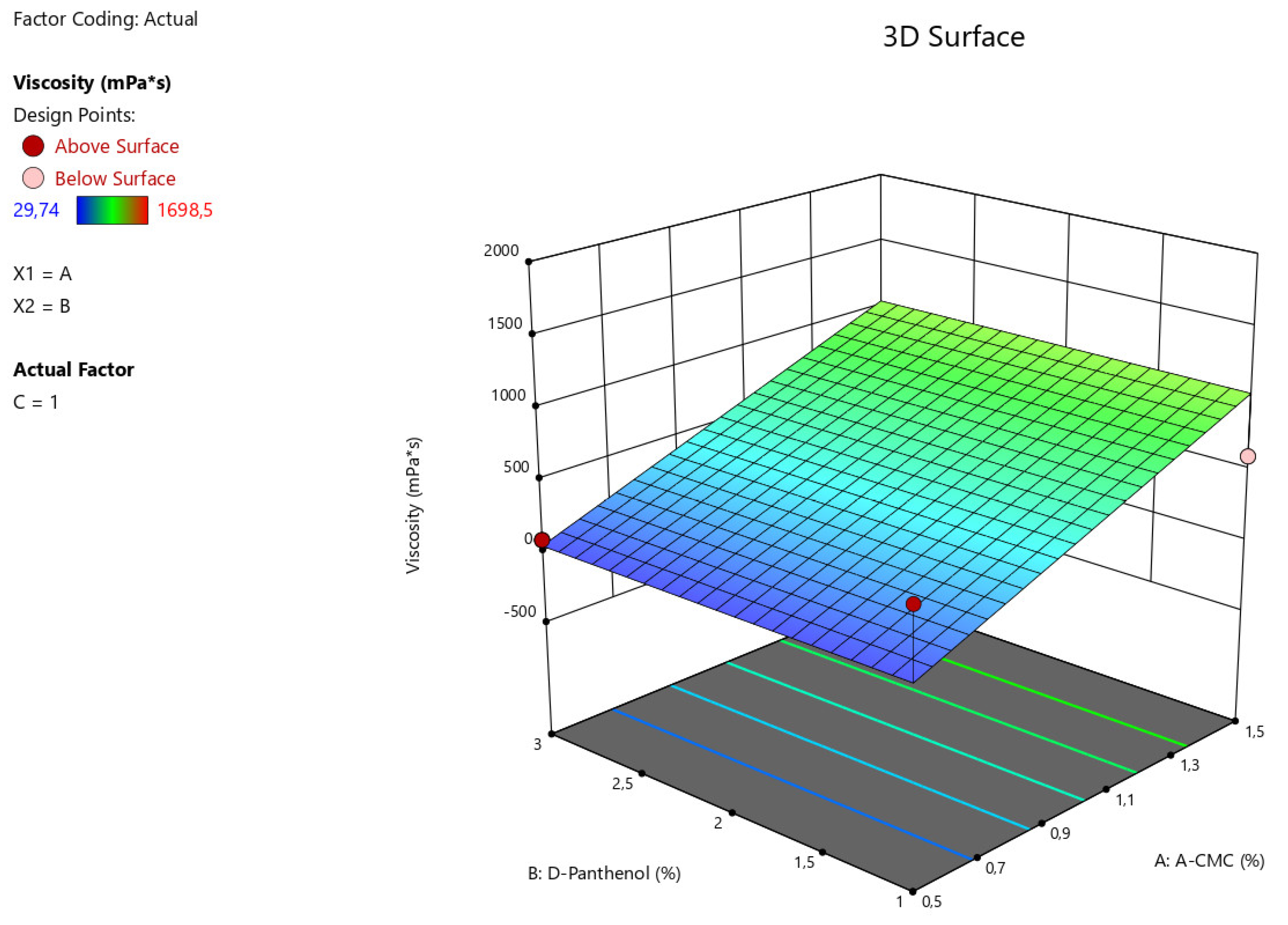
The obtained data from statistical analysis ANOVA for Reduced Linear model demonstrates the reliable influence of factors A - Na CMC on the viscosity characteristics of the manufactured nasal gel samples (F-value > P-value). The relationship between the viscosity value of gel preparation with factors (x) considering the coefficients is presented in the equation:
Viscosity = 545,888+499,59875*A
Figure 1.
3D illustration of the relationship between the variable factors (Na CMC, D-panthenol) and viscosity characteristics of nasal gels.
Figure 1.
3D illustration of the relationship between the variable factors (Na CMC, D-panthenol) and viscosity characteristics of nasal gels.
The therapeutic effectiveness of a nasal local-acting drug depends on the active pharmaceutical ingredients included in the drug formulation as well as the rate of drug release in the oral cavity (
Table 6).
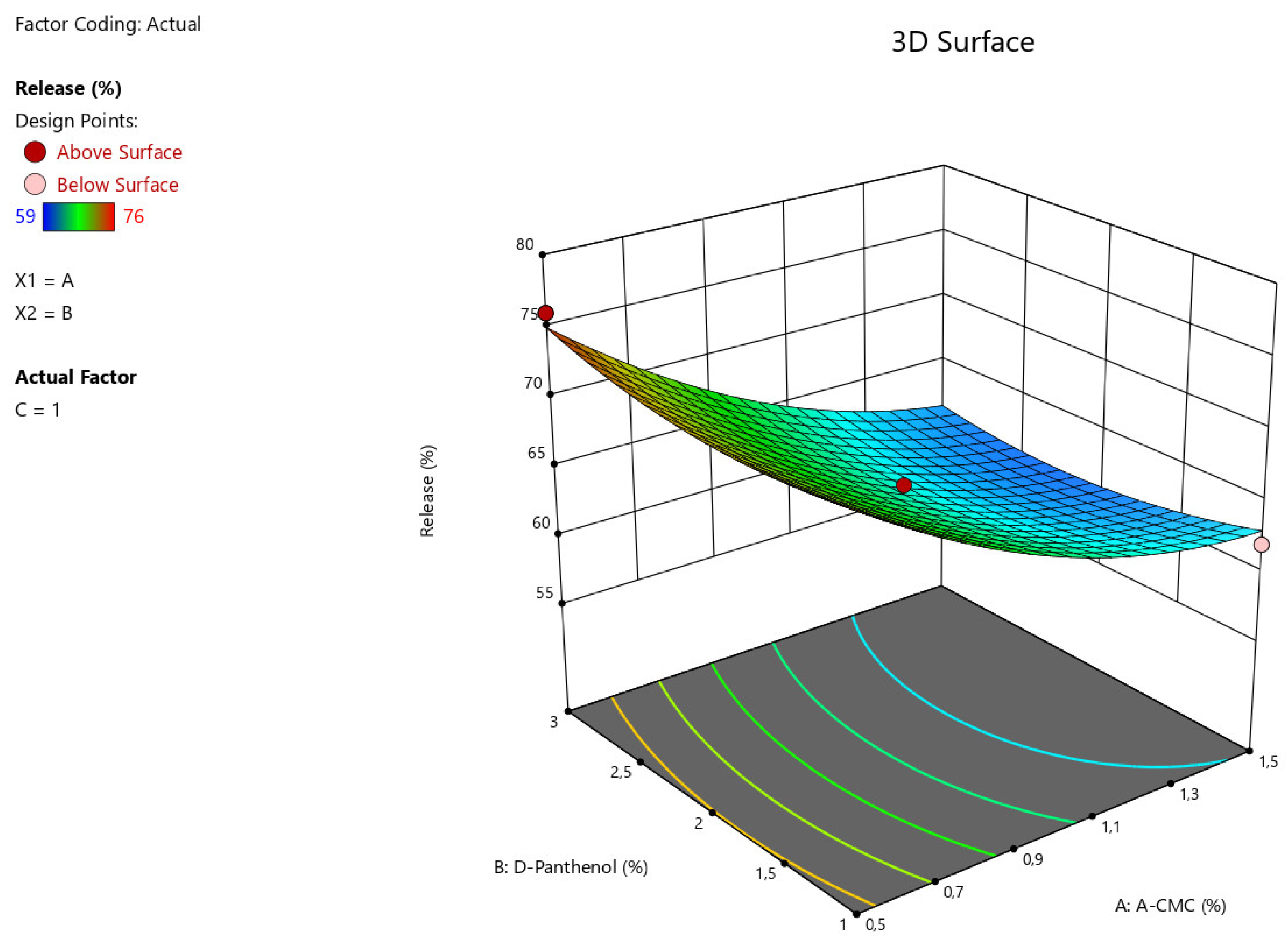
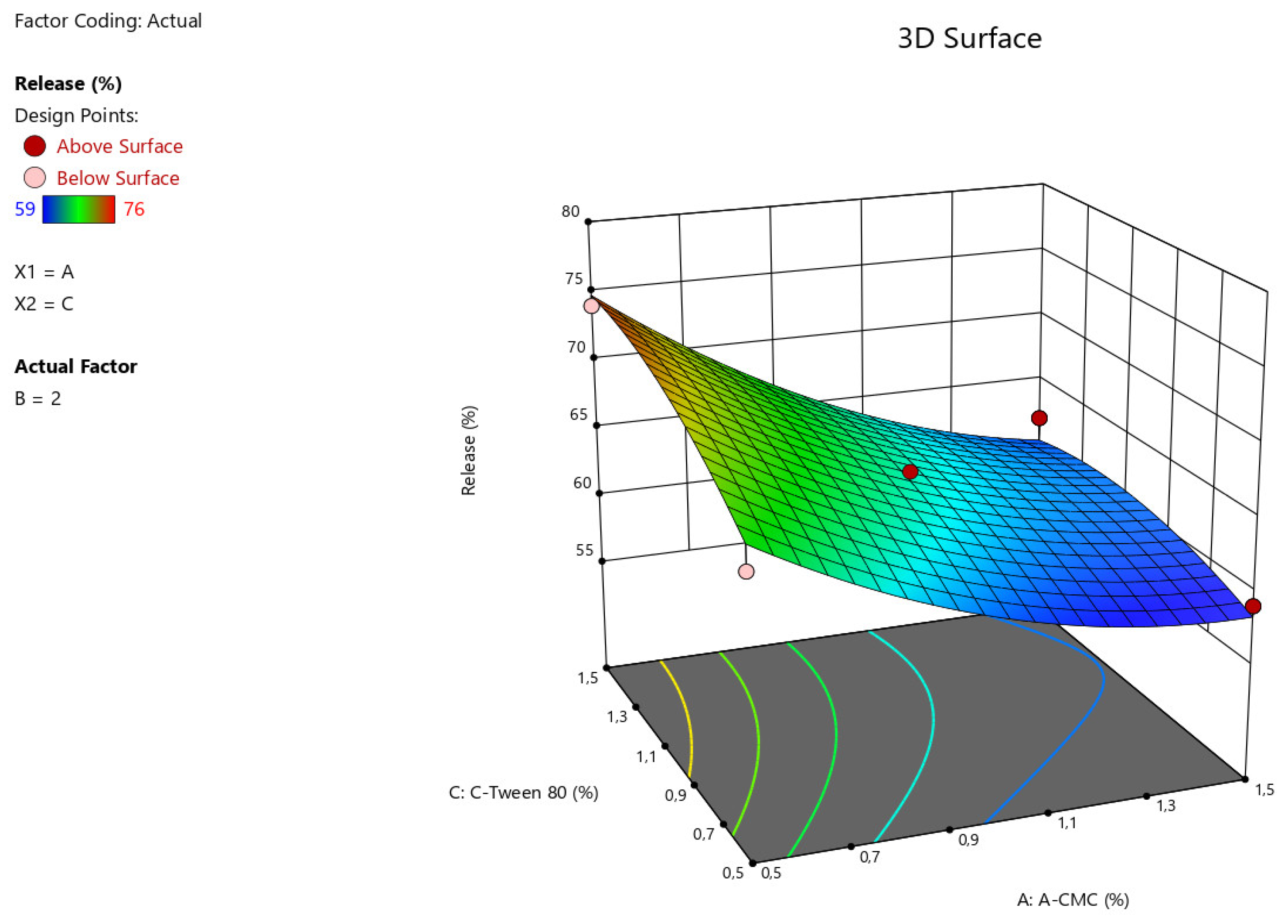
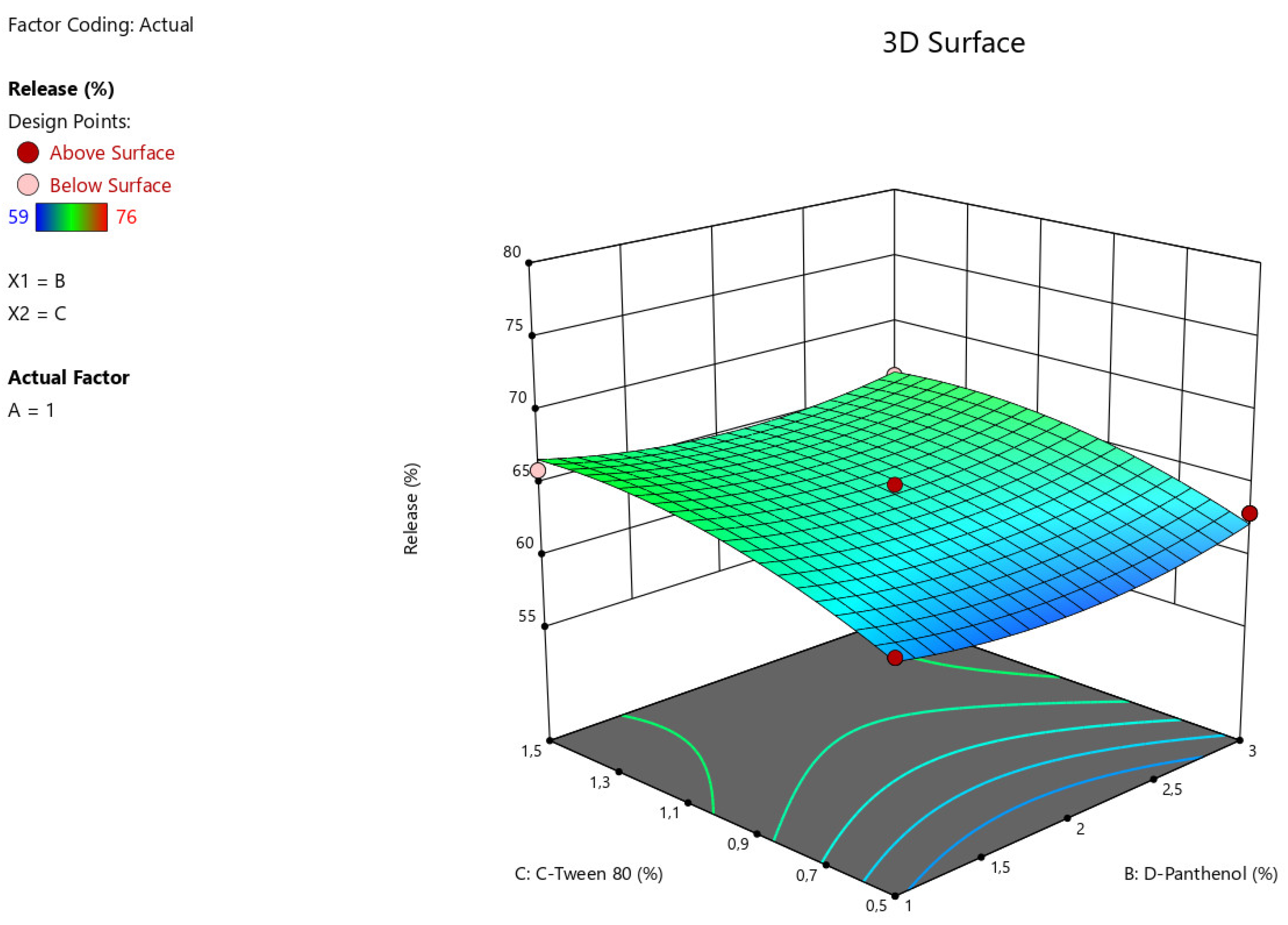
The results of statistical analysis of ANOVA for Quadratic model demonstrate a significant effect of factors A:Na CMC, C: Tween-80 on the intensity of angiolin release from nasal gels (F-value > P-value). The relationship between the percentage of angiolin release intensity and research factors is shown in the equation:
Release = 64,33-6*A+0*B+2,25*C-0,7499*AB-1,25*AC-0,25*BC+2,45*A²+1,45*B²-1,54*C²
Figure 2.
3D illustration of the relationship between the variable factors (Na CMC, D-Panthenol) and the percentage of angiolin release intensity from nasal gels.
Figure 2.
3D illustration of the relationship between the variable factors (Na CMC, D-Panthenol) and the percentage of angiolin release intensity from nasal gels.
Figure 3.
3D illustration of the relationship between the variable factors (Na CMC, Tween-80) and the percentage of angiolin release intensity from nasal gels.
Figure 3.
3D illustration of the relationship between the variable factors (Na CMC, Tween-80) and the percentage of angiolin release intensity from nasal gels.
Figure 4.
3D illustration of the relationship between modifying factors (Tween-80, D-Panthenol) and the percentage of angiolin release intensity from nasal gels.
Figure 4.
3D illustration of the relationship between modifying factors (Tween-80, D-Panthenol) and the percentage of angiolin release intensity from nasal gels.
Then, optimization of nasal gel formulation was carried out using Box-Behnken design with number characteristics to predict the optimal characteristics of nasal gel formulation with lysozyme hydrochloride. The optimization procedure was set up in Design Expert 13.0 software on the following objectives: Release - goal maximize, Viscosity - goal maximize. The obtained results of prediction variants are summarized in
Table 7.
For further studies, we selected formulation No. 1 (
Table 8) which had the highest desirability - 0.39, % release - 63.25, viscosity - 1045.480 of the following formulation (
Table 8):
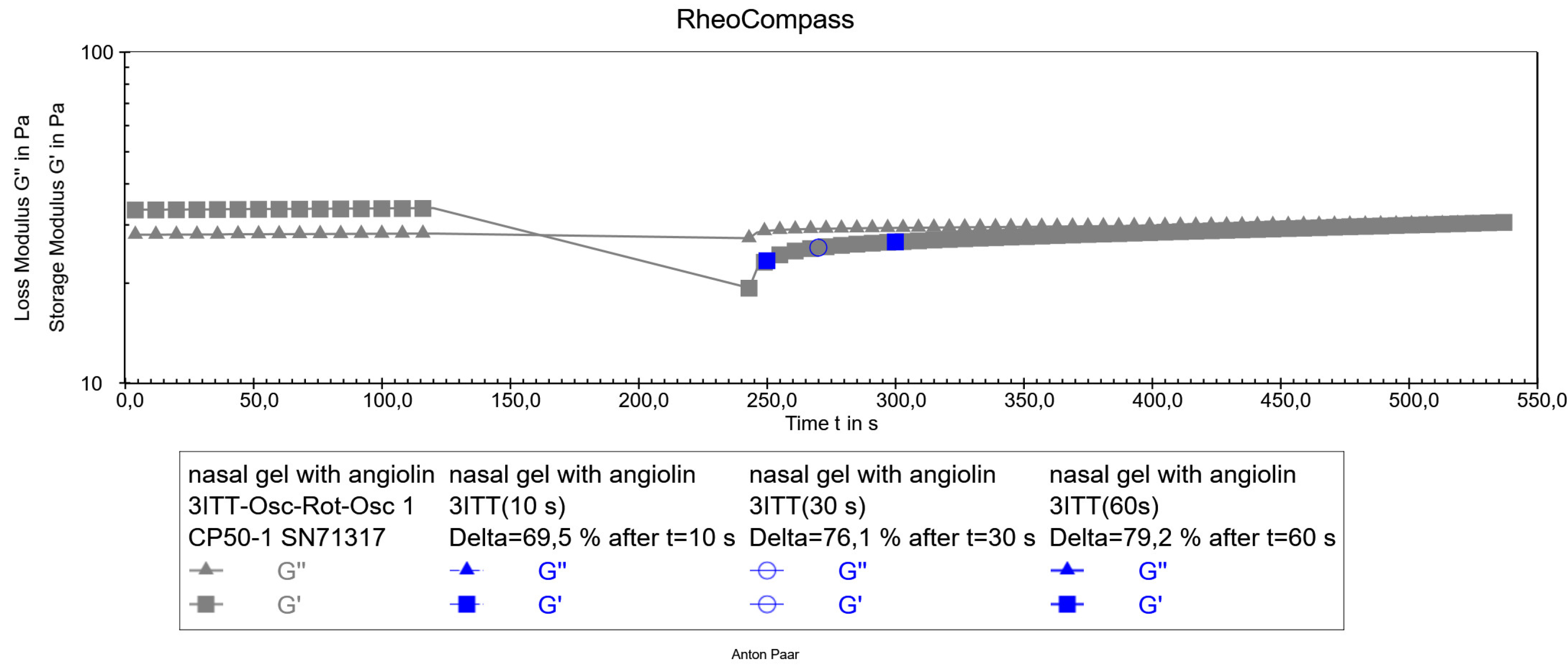
After preparation of the optimized formulation of the nasal gel with Angiolin, its thixotropic properties were studied (
Figure 5).
Figure 5.
Thixotropy test of optimized nasal gel formulation with angiolin.
Figure 5.
Thixotropy test of optimized nasal gel formulation with angiolin.
3.5. Pharmaco-toxicological research.
The obtained results of thixotropy test show that the experimental nasal gel has satisfactory thixotropic properties. Its structure is restored after the applied force, namely the restoration of the structure after 10 seconds was 69%, after 30 seconds was 76%, after 60 seconds was 79%, which allows to predict the stability of the dosage form both after preparation and after use. As a result of pharmaco-toxicological tests it was found that administration of nasal gel in appropriate volume did not cause death of animals during the whole observation period (
Table 9). Also, the use of the gel did not cause any behavioral or visual abnormalities in the animals.
The obtained results demonstrate that nasal gel with angiolin belongs to class VI of toxicity. There were no visible pathological changes in the appearance and behavior of experimental animals on the 1st, 7th and 14th days after a single intranasal administration of Angiolin gel. In the study of a possible local irritant effect of Angiolin gel, mild conjunctival redness was observed in 1 of 10 animals immediately after drug administration. No conjunctival changes were observed in the remaining 9 animals (
Table 10). On days 2 and 3 after application of the drug, no positive reaction from the eye conjunctiva was detected in all animals, which shows the absence of irritative action of this dosage form.
Thus, nasal gel with Angiolin has no local irritant effect. As the results of the studies showed, daily application of 0.5 g of Angiolin gel to the clipped area of the lateral surface of the body of animals (4x4 cm) for 5 days and following single application of 0.3 g of this gel did not cause the development of anaphylactic shock (
Table 11). There were no signs of anaphylactic shock at 6, 12, and 24 hours after application of 0.3 g of gel.
Thus, 1% gel with Angiolin at 5-day administration does not cause allergic reactions of anaphylactic type.
4. Discussion
The modern view of the pathogenesis of CNS damage after prenatal hypoxia determines the use of a promising direction, namely, the use of drugs with neuroprotective effect. Creation of new dosage forms for neonatology determines the use of software and mathematical technologies in pharmaceutical development, which allows not only to reduce the time and resources of researchers, but also to create cerebroprotective drugs with controlled pharmaco-technological properties. Our results are consistent with our preliminary studies on the development of dosage forms with neuroprotective agents - Ademol, Noopept and IL-1β antagonist. Experimental results of these dosage forms confirmed their high neuroprotective activity and good safety profile [
26,
29,
30,
31,
32]. These results justify further preclinical study of 1% nasal gel with Angiolin as a neuroprotective agent after intrauterine hypoxia.
5. Conclusions
1. We performed formulation development of a novel nasal gel formulation with Angiolin using Box-Benken experimental design for the therapy of prenatal CNS damage.
2. It was found that the consistency properties of the nasal gel were significantly influenced by the gelling agent and mucoadhesive component - sodium salt of carboxymethylcellulose.
3. We have optimized the composition of nasal gel formulation with Angiolin using the formed models and relationships between the factors. The optimized composition of the obtained nasal gel has satisfactory parameters of thixotropic properties.
4. The developed 1% gel for neuroprotection with Angiolin meets all the requirements for harmlessness and safety to drug forms of this group - low toxicity, absence of local irritants, and allergic effect.
6. Perspectives for Further Research
Prospective further studies of the new nasal gel to investigate its specific activity.
Author Contributions
Conceptualization, I.B. and B.B.; methodology, B.B.; software, B.B. and O.K.; validation, O.K., and K.B.; formal analysis, K.B.; investigation, O.K.; resources, O.A., V.O.; data curation, D.S.; writing—original draft preparation, S.B. and B.B.; writing—review and editing, O.A., V.O.; visualization, K.B.; supervision, I.B., V.O.; project administration, I.B., V.O.; funding acquisition, I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded as part of the research work of the Department of Pharmacology and Medical Formulation of Zaporizhzhia State Medical and Pharmaceutical University on the theme “The role of thiol-disulfide system in the realization of mechanisms of neurodestruction/neuroprotection and the development of ways of pharmacological modulation after prenatal hypoxia” (State registration No. 0123U101110).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Zaporizhzhia State Medical and Pharmaceutical University Commission on Bioethics (protocol No. 2 of 18.05.2023) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated during this research are included in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gomez, D.; Martinez, J.A.; Hanson, L.R.; Frey, W.H.; Toth, C.C. Intranasal treatment of neurodegenerative diseases and stroke. Front. Biosci. (Schol Ed.) 2012, 4, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Madden, S.; Carrazana, E.; Rabinowicz, A.L. Optimizing Absorption for Intranasal Delivery of Drugs Targeting the Central Nervous System Using Alkylsaccharide Permeation Enhancers. Pharmaceutics 2023, 15, 2119. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Hsu, W.H. Evaluation of Recent Intranasal Drug Delivery Systems to the Central Nervous System. Pharmaceutics 2022, 14, 629. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, M.C.; Alhamami, M.; Miedema, S.B.; Yun, Y.; Ruiz-Cardozo, M.; Vannier, M.W. Imaging of intranasal drug delivery to the brain. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 1–31. [Google Scholar] [PubMed]
- Dwibhashyam, V.S.; Nagappa, A.N. Strategies for enhanced drug delivery to the central nervous system. Indian J. Pharm. Sci. 2008, 70, 145–53. [Google Scholar] [PubMed]
- Trevino, J.T.; Quispe, R.C.; Khan, F.; Novak, V. Non-Invasive Strategies for Nose-to-Brain Drug Delivery. J. Clin. Trials 2020, 10, 439. [Google Scholar] [PubMed]
- Kousalya, S.; Kuppusamy, G.; Veera, V.S.R.K. Nose to brain transport pathways an overview: potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.D.; Frey, W.H.; Craft, S.; Danielyan, L.; Hallschmid, M.; Schiöth, H.B.; Benedict, C. Intranasal treatment of central nervous system dysfunction in humans. Pharm. Res. 2013, 30, 2475–2484. [Google Scholar] [CrossRef]
- Chung, S.; Peters, J.M.; Detyniecki, K.; Tatum, W.; Rabinowicz, A.L.; Carrazana, E. The nose has it: Opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters. Epilepsy Behav. Rep. 2022, 21, 100581. [Google Scholar] [CrossRef]
- Shrewsbury, S.B. The Upper Nasal Space: Option for Systemic Drug Delivery, Mucosal Vaccines and “Nose-to-Brain”. Pharmaceutics 2023, 15, 1720. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tao, J.; Wang, J. Design and Application in Delivery System of Intranasal Antidepressants. Front. Bioeng. Biotechnol. 2020, 8, 626882. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. In Situ-Based Gels for Nose to Brain Delivery for the Treatment of Neurological Diseases. Pharmaceutics 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Belenichev, I.F.; Aliyeva, O.G.; Popazova, O.O.; Bukhtiyarova, N.V. Involvement of heat shock proteins HSP70 in the mechanisms of endogenous neuroprotection: the prospect of using HSP70 modulators. Front. Cell. Neurosci. 2023, 17, 1131683. [Google Scholar] [CrossRef] [PubMed]
- Cerio, F.G.; Lara-Celador, I.; Alvarez, A.; Hilario, E. Neuroprotective therapies after perinatal hypoxic-ischemic brain injury. Brain Sci. 2013, 3, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Andelius, T.C.K.; Bøgh, N.; Pedersen, M.V.; Omann, C.; Andersen, M.; Andersen, H.B.; Hjortdal, V.E.; Pedersen, M.; Rasmussen, M.B.; Kyng, K.J.; Henriksen, T.B. Early changes in cerebral metabolism after perinatal hypoxia-ischemia: a study in normothermic and hypothermic piglets. Front. Pediatr. 2023, 11, 1167396. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.J.; Ling, E.A.; Li, F. Immunomodulatory Mechanism and Potential Therapies for Perinatal Hypoxic-Ischemic Brain Damage. Front. Pharmacol. 2020, 11, 580428. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Feroz, S.; Chekman, I.S.; Nagornaya, E.A.; Gorbacheva, S.V.; Gorchakova, N.A. Thiol-disulfide system: role in endogenous cyto - and organoprotection, pathways of pharmacological modulation. Yuston Publishing House: Kyiv, Ukraine, 2020; pp. 57–88.
- Belenichev, I.F.; Cherniy, V.I.; Nagornaya, E.A.; Pavlov, S.V.; Cherniy, T.V. Neuroprotection and neuroplasticity. Logos: Kyiv, Ukraine, 2015; pp. 167–186.
- Chekman, I.S.; Kazakova, O.A.; Mazur, I.A.; Nagornaya, E.A.; Belenichev, I. F. , Gorchakova, N.A. New original metabolitotropic endothelioprotector “Angiolin”: Quantum-chemical parameters and peculiarities of pharmacological action. Rep. NAS Ukraine 2017, 8, 86–93. [Google Scholar]
- Belenichev, I.; Popazova, O.; Bukhtiyarova, N.; Savchenko, D.; Oksenych, V.; Kamyshnyi, O. Modulating Nitric Oxide: Implications for Cytotoxicity and Cytoprotection. Antioxidants 2024, 13, 504. [Google Scholar] [CrossRef]
- Kolesnik, Y.M. Report on preclinical study of specific biological activity (anti-ischemic, endothelioprotective) of the drug Lisinium (Angiolin) at parenteral administration. Zaporozhye, Ukraine 2018, 11, 2584. [Google Scholar]
- Kucherenko, L.I.; Bidnenko, O.S.; Tkachenko, G.I. Regarding L-lysine 3-methyl-1. 2.4-triazole-5-thioacetate Standardization Pharm. review 2017, 1, 28–31. [Google Scholar]
- Council Directive 86/609/EEC of 24 November 1986 on the Approximation of Laws, Regulations and Administrative Provisions of the Member States Regarding the Protection of Animals Used for Experimental and Other Scientific Purposes. Off. J. Eur. Union 1986, 358, 1–28.
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 276, 33–79.
- Semenenko, S.; Semenenko, I.; Burlaka, B.; Samura, I.; Bukhtiyarova, N.; Ryzhenko, V.; Khromylova, O. Evaluation of the Cerebroprotective Properties of Ademol-gel in the Analysis of Specific Indicators in the Open Field Test. Biomed. Pharmacol. J. 2023, 16, 1219–1227. [Google Scholar] [CrossRef]
- Ashton, J. ANOVA and the analysis of drug combination experiments. Nat. Methods 2015, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, I.M.; Pinto, C.F.F.; Moreira, C.S.; Saviano, A.M.; Lourenço, F.R. Design of Experiments (DoE) applied to Pharmaceutical and Analytical Quality by Design (QbD). Braz. J. Pharm. Sci. 2018, 54, e01006. [Google Scholar] [CrossRef]
- Semenenko, S.I.; Miedviedieva, K.P.; Vasiuk, S.O.; Burlaka, B.S. Development of a spectrophotometric technique for the quantitative determination of ademol. Current issues in pharmacy and medicine: science and practice 2023, 16, 28–32. [Google Scholar]
- Belenichev, I.F.; Burlaka, B.S.; Ryzhenko, O.I.; Ryzhenko, V.P.; Aliyeva, O.G.; Makyeyeva, L.V. Neuroprotective and anti-apoptotic activity of the IL-1 antagonist RAIL-gel in rats after ketamine anesthesia. Pharmakeftiki, 2021, 33, 97–106. [Google Scholar]
- Dagda, R.K.; Dagda, R.Y.; Vazquez-Mayorga, E.; Martinez, B.; Gallahue, A. Intranasal Administration of Forskolin and Noopept Reverses Parkinsonian Pathology in PINK1 Knockout Rats. Int. J. Mol. Sci. 2023, 24, 690. [Google Scholar] [CrossRef]
- Burlaka, B.S.; Belenichev, I.F.; Ryzhenko, O.I.; Ryzhenko, V.P.; Aliyeva, O.G.; Makyeyeva, L.V.; Popazova, O.O.; Bak, P.G. The effect of intranasal administration of an IL-1b antagonist (RAIL) on the state of the nitroxydergic system of the brain during modeling of acute cerebrovascular accident. Pharmacia 2021, 68, 665–670. [Google Scholar] [CrossRef]
Table 1.
Factors and their levels used in the experiment design of the nasal gel with Angiolin.
Table 1.
Factors and their levels used in the experiment design of the nasal gel with Angiolin.
| Factor |
Parameter |
Levels |
| Low (-) |
Medium (0) |
High (+) |
| x1 |
Carboxymethylcellulose sodium salt, Na CMC, % |
0,5 |
1 |
1,5 |
| x2 |
D-panthenol % |
1 |
2 |
3 |
| x3 |
Tween-80, % |
0,5 |
1 |
1,5 |
Table 2.
Scale for evaluation of application skin tests.
Table 2.
Scale for evaluation of application skin tests.
| Reaction designation |
Symbols |
Reaction description |
| negative |
- |
no change in the skin |
| doubtful |
± |
small erythema without edema |
| weak-positive |
+ |
erythema and edema at the application site |
| positive |
++ |
erythema, edema, papules |
| strong positive |
+++ |
erythema, edema, papules, isolated vesicles |
Table 3.
Formulation design, factors, and responses received.
Table 3.
Formulation design, factors, and responses received.
| Run |
Factor 1
A:CMC
% |
Factor 2
B: D-Panthenol,
% |
Factor 3
C:Tween 80,
% |
Response 1,
рН |
Response 1,
рН |
Response 3
Release, % |
| 1 |
1,5 |
3 |
1 |
6,41 |
782,15 |
60 |
| 2 |
0,5 |
2 |
1,5 |
6,41 |
80,01 |
74 |
| 3 |
1 |
2 |
1 |
6,41 |
432,57 |
65 |
| 4 |
1,5 |
2 |
0,5 |
6,41 |
1657,2 |
59 |
| 5 |
1 |
3 |
0,5 |
6,41 |
258,08 |
63 |
| 6 |
1 |
3 |
1,5 |
6,42 |
392,88 |
66 |
| 7 |
1 |
1 |
0,5 |
6,41 |
573,06 |
62 |
| 8 |
0,5 |
3 |
1 |
6,41 |
88,28 |
76 |
| 9 |
0,5 |
1 |
1 |
6,42 |
547,31 |
75 |
| 10 |
0,5 |
2 |
0,5 |
6,41 |
29,74 |
66 |
| 11 |
1,5 |
2 |
1,5 |
6,42 |
1698,5 |
62 |
| 12 |
1,5 |
1 |
1 |
6,41 |
604,28 |
62 |
| 13 |
1 |
1 |
1,5 |
6,41 |
443,38 |
66 |
| 14 |
1 |
2 |
1 |
6,41 |
161,23 |
64 |
| 15 |
1 |
2 |
1 |
6,42 |
439,65 |
64 |
Table 4.
Effect of variable factors on the pH value of nasal gels.
Table 4.
Effect of variable factors on the pH value of nasal gels.
| Source |
Sum of Squares |
df |
Mean Square |
F-value |
p-value |
Test result |
| Model |
0,0000 |
0 |
|
|
|
|
| Residual |
0,0003 |
14 |
0,0000 |
|
|
|
| Lack of Fit |
0,0002 |
12 |
0,0000 |
0,5667 |
0,7871 |
not significant |
| Pure Error |
0,0001 |
2 |
0,0000 |
|
|
|
| Cor Total |
0,0003 |
14 |
|
|
|
|
Table 5.
Effect of variable factors (x) on the value of viscosity characteristics of nasal gels.
Table 5.
Effect of variable factors (x) on the value of viscosity characteristics of nasal gels.
| Source |
Sum of Squares |
df |
Mean Square |
F-value |
p-value |
Test result |
| Model |
1,997E+06 |
1 |
1,997E+06 |
16,14 |
0,0015 |
significant |
| A-A-CMC |
1,997E+06 |
1 |
1,997E+06 |
16,14 |
0,0015 |
|
| Residual |
1,608E+06 |
13 |
1,237E+05 |
|
|
|
| Lack of Fit |
1,558E+06 |
11 |
1,416E+05 |
5,62 |
0,1606 |
not significant |
| Pure Error |
50397,74 |
2 |
25198,87 |
|
|
|
| Cor Total |
3,605E+06 |
14 |
|
16,14 |
0,0015 |
|
Table 6.
Effect of variable factors (x) on the intensity of angiolin release from nasal gels.
Table 6.
Effect of variable factors (x) on the intensity of angiolin release from nasal gels.
| Source |
Sum of Squares |
df |
Mean Square |
F-value |
p-value |
Test result |
| Model |
377,93 |
9 |
41,99 |
13,40 |
0,0053 |
significant |
| A-A-CMC |
288,00 |
1 |
288,00 |
91,91 |
0,0002 |
|
| B-D-Panthenol |
5,684E-14 |
1 |
5,684E-14 |
1,814E-14 |
1,0000 |
|
| C-C-Tween 80 |
40,50 |
1 |
40,50 |
12,93 |
0,0156 |
|
| AB |
2,25 |
1 |
2,25 |
0,7181 |
0,4354 |
|
| AC |
6,25 |
1 |
6,25 |
1,99 |
0,2170 |
|
| BC |
0,2500 |
1 |
0,2500 |
0,0798 |
0,7889 |
|
| A² |
22,31 |
1 |
22,31 |
7,12 |
0,0444 |
|
| B² |
7,85 |
1 |
7,85 |
2,51 |
0,1743 |
|
| C² |
8,78 |
1 |
8,78 |
2,80 |
0,1551 |
|
| Residual |
15,67 |
5 |
3,13 |
|
|
|
| Lack of Fit |
15,00 |
3 |
5,00 |
15,00 |
0,0631 |
not significant |
| Pure Error |
0,6667 |
2 |
0,3333 |
|
|
|
| Cor Total |
393,60 |
14 |
|
|
|
|
Table 7.
Effect of variable factors (x) on the intensity of angiolin release from nasal gels.
Table 7.
Effect of variable factors (x) on the intensity of angiolin release from nasal gels.
| Number |
A-CMC,% |
D-Panthenol,% |
C-Tween 80,% |
рН |
Viscosity, mPa*s |
Release,% |
Desirability |
Selected |
| 1 |
1,500 |
1,000 |
1,203 |
6,413 |
1045,480 |
63,253 |
0,390 |
Selected |
| 2 |
1,500 |
1,000 |
1,199 |
6,413 |
1045,483 |
63,252 |
0,390 |
|
| 3 |
1,500 |
1,000 |
1,156 |
6,413 |
1045,484 |
63,240 |
0,390 |
|
| 4 |
1,500 |
1,000 |
1,267 |
6,413 |
1045,485 |
63,228 |
0,389 |
|
| 5 |
1,500 |
1,000 |
1,317 |
6,413 |
1045,485 |
63,173 |
0,387 |
|
| 6 |
1,147 |
1,000 |
1,346 |
6,413 |
693,050 |
65,195 |
0,381 |
|
| 7 |
1,147 |
1,000 |
1,347 |
6,413 |
693,083 |
65,195 |
0,381 |
|
| 8 |
1,145 |
1,000 |
1,351 |
6,413 |
690,815 |
65,216 |
0,381 |
|
| 9 |
1,149 |
1,000 |
1,352 |
6,413 |
695,111 |
65,176 |
0,381 |
|
| 10 |
1,141 |
1,000 |
1,343 |
6,413 |
686,560 |
65,256 |
0,381 |
|
| 11 |
1,158 |
1,000 |
1,337 |
6,413 |
703,316 |
65,101 |
0,381 |
|
| 12 |
1,128 |
1,000 |
1,364 |
6,413 |
673,421 |
65,382 |
0,381 |
|
| 13 |
1,111 |
1,000 |
1,356 |
6,413 |
656,933 |
65,547 |
0,380 |
|
| 14 |
1,173 |
1,000 |
1,364 |
6,413 |
718,592 |
64,959 |
0,380 |
|
| 15 |
1,097 |
1,000 |
1,361 |
6,413 |
642,599 |
65,694 |
0,380 |
|
| 16 |
1,098 |
1,000 |
1,377 |
6,413 |
643,747 |
65,682 |
0,380 |
|
| 17 |
1,221 |
1,000 |
1,318 |
6,413 |
766,358 |
64,569 |
0,380 |
|
| 18 |
1,321 |
1,000 |
1,289 |
6,413 |
867,085 |
63,898 |
0,380 |
|
| 19 |
1,238 |
1,000 |
1,295 |
6,413 |
783,634 |
64,437 |
0,380 |
|
| 20 |
1,071 |
1,000 |
1,371 |
6,413 |
616,924 |
65,969 |
0,380 |
|
| 21 |
0,982 |
3,000 |
1,331 |
6,413 |
527,538 |
66,722 |
0,368 |
|
| 22 |
0,982 |
3,000 |
1,337 |
6,413 |
527,577 |
66,721 |
0,368 |
|
| 23 |
0,978 |
3,000 |
1,334 |
6,413 |
523,881 |
66,779 |
0,368 |
|
| 24 |
0,980 |
3,000 |
1,327 |
6,413 |
525,567 |
66,752 |
0,368 |
|
| 25 |
0,983 |
3,000 |
1,327 |
6,413 |
528,520 |
66,706 |
0,368 |
|
| 26 |
0,978 |
3,000 |
1,339 |
6,413 |
523,890 |
66,778 |
0,368 |
|
| 27 |
0,982 |
3,000 |
1,315 |
6,413 |
528,122 |
66,711 |
0,368 |
|
| 28 |
0,981 |
3,000 |
1,351 |
6,413 |
526,517 |
66,735 |
0,368 |
|
| 29 |
0,994 |
3,000 |
1,355 |
6,413 |
539,887 |
66,527 |
0,368 |
|
| 30 |
0,974 |
3,000 |
1,383 |
6,413 |
519,721 |
66,829 |
0,368 |
|
| 31 |
0,959 |
3,000 |
1,286 |
6,413 |
505,317 |
67,054 |
0,367 |
|
| 32 |
0,982 |
3,000 |
1,428 |
6,413 |
528,014 |
66,657 |
0,367 |
|
| 33 |
1,027 |
3,000 |
1,260 |
6,413 |
572,949 |
66,021 |
0,367 |
|
| 34 |
0,970 |
3,000 |
1,451 |
6,413 |
516,307 |
66,816 |
0,366 |
|
| 35 |
0,970 |
3,000 |
1,109 |
6,413 |
516,073 |
66,581 |
0,361 |
|
| 36 |
0,975 |
3,000 |
1,067 |
6,413 |
521,297 |
66,378 |
0,358 |
|
Table 8.
Optimized formulation of nasal gel with angiolin.
Table 8.
Optimized formulation of nasal gel with angiolin.
| Name |
Quantity (g) |
| Angiolin |
1,0 |
| Sodium CMC |
1,5 |
| D-panthenol |
1 |
| Benzalkonium chloride |
0,02 |
| Tween-80 |
1,2 |
| Purified water |
Up to 100 |
Table 9.
Study of acute toxicity of 1% nasal gel with Angiolin in rats by intranasal administration.
Table 9.
Study of acute toxicity of 1% nasal gel with Angiolin in rats by intranasal administration.
| Volume, ml /100 g |
Dose,
mg/kg |
Number of rats |
Lethality, % |
| Total |
Dead |
Survived |
| 0.4 |
4 |
6 |
0 |
6 |
0 |
Table 10.
Results of the study of local irritant effect of 1% gel with Angiolin.
Table 10.
Results of the study of local irritant effect of 1% gel with Angiolin.
| No of animal |
Period of the study, day |
| 1 |
2 |
3 |
| Control |
Angiolin gel |
Control |
Angiolin gel |
Control |
Angiolin gel |
| 1 |
0 |
1 |
0 |
0 |
0 |
0 |
| 2 |
0 |
0 |
0 |
0 |
0 |
0 |
| 3 |
0 |
0 |
0 |
0 |
0 |
0 |
| 4 |
0 |
0 |
0 |
0 |
0 |
0 |
| 5 |
0 |
0 |
0 |
0 |
0 |
0 |
| 6 |
0 |
0 |
0 |
0 |
0 |
0 |
| 7 |
0 |
0 |
0 |
0 |
0 |
0 |
| 8 |
0 |
0 |
0 |
0 |
0 |
0 |
| 9 |
0 |
0 |
0 |
0 |
0 |
0 |
| 10 |
0 |
0 |
0 |
0 |
0 |
0 |
Table 11.
Evaluation of active skin anaphylaxis of 1% gel with Angiolin in Weigle index score.
Table 11.
Evaluation of active skin anaphylaxis of 1% gel with Angiolin in Weigle index score.
| No of animal |
Period of the study, hours |
| 6 |
12 |
24 |
| Control |
Angiolin gel |
Control |
Angiolin gel |
Control |
Angiolin gel |
| 1 |
0 |
0 |
0 |
0 |
0 |
0 |
| 2 |
0 |
0 |
0 |
0 |
0 |
0 |
| 3 |
0 |
0 |
0 |
0 |
0 |
0 |
| 4 |
0 |
0 |
0 |
0 |
0 |
0 |
| 5 |
0 |
0 |
0 |
0 |
0 |
0 |
| 6 |
0 |
0 |
0 |
0 |
0 |
0 |
| 7 |
0 |
0 |
0 |
0 |
0 |
0 |
| 8 |
0 |
0 |
0 |
0 |
0 |
0 |
| 9 |
0 |
0 |
0 |
0 |
0 |
0 |
| 10 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).