Submitted:
25 June 2024
Posted:
26 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Media
2.2. S. cohnii isolates
2.3. Genome sequence and assembly
2.4. Bioinformatics
3. Results
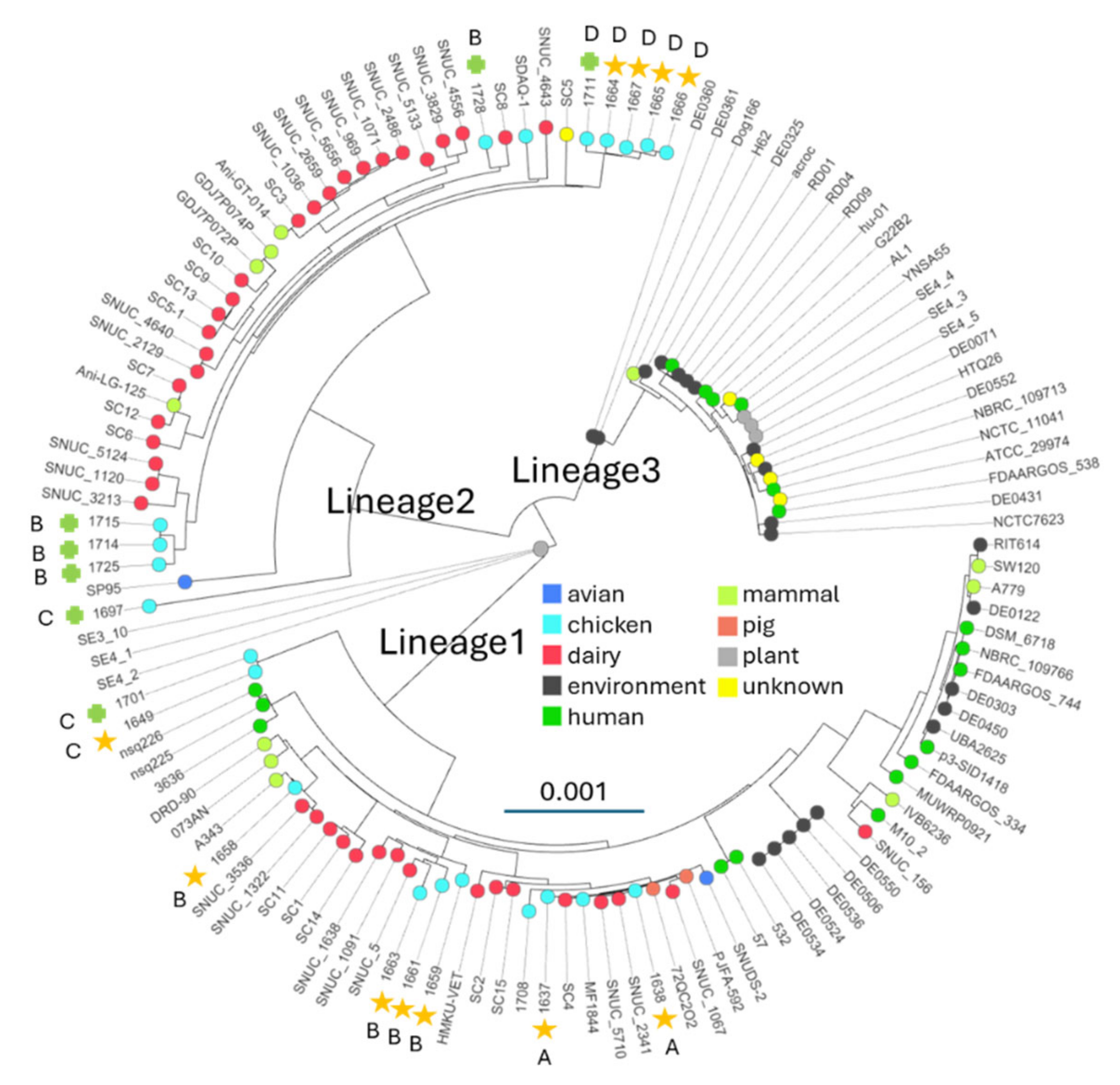
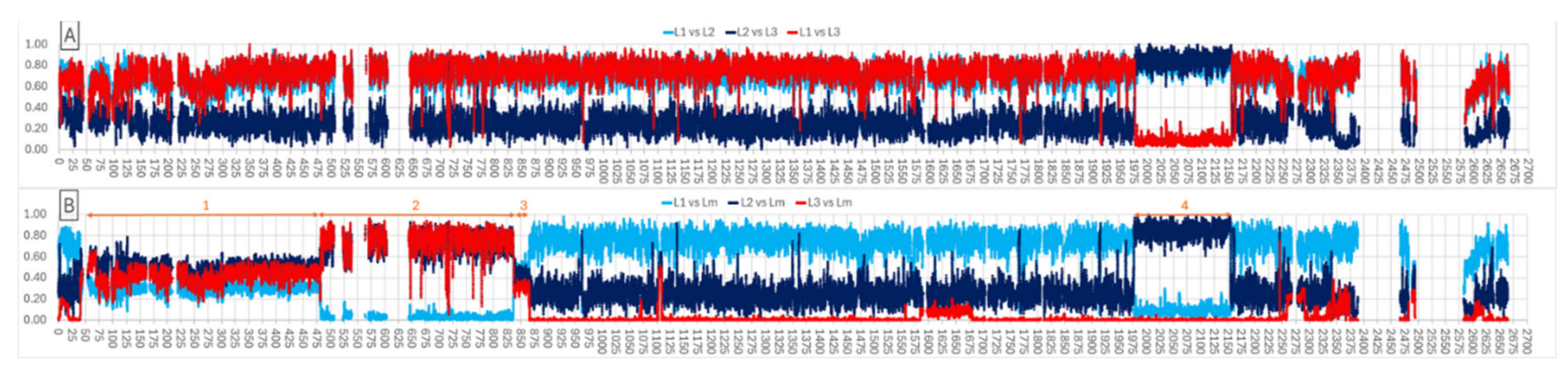
3.1. Phylogenomic analyses identify three lineages
3.2. Lineage association with host and virulence
- YNSA55: China, human, survey of S. aureus, no disease state recorded
- NCTC11041: Homo sapiens skin, no disease
- FDAARGOS-538: Homo sapiens, clinical isolate
- H62: air, environmental
- Acroc: human skin, no disease state recorded
- RD01, RD04, RD09: Bacteria were isolated from multiple surfaces in washrooms on a university campus in Alberta, Canada.
- Hu-01: China Homo sapiens, skin swab, no disease
- G22B2: Homo sapiens, gall bladder
- 57: Homo sapiens, blood; opportunistic pathogen isolated from human patient
- 532: Homo sapiens, catheter; opportunistic pathogen isolated from human patient
- MUWRP0921: human UTI
- FDAARGOS_334: Homo sapiens, clinical isolate
- P3-SID1418: human skin, toe web space
- FDAARGOS_744: Homo sapiens, clinical isolate
- NBRC_109766: unknown
- RIT614: smartphone
- Nsq225: human, linezolid-resistant clinical isolate
- Nsq226: human, linezolid-resistant clinical isolate
- 3636: human oral infection
3.3. Horizontal transfer between lineages
3.4. Lineage specific polypeptides
3.5. Lineage diagnostic qPCR
3.6. S. cohnii plasmids
3.7. Genetic diversity in BCO infections
4. Discussion
Author Contributions
Acknowledgments
Research Assurance
References
- Becker, K., C. Heilmann, and G. Peters, Coagulase-negative Staphylococci. Clin Microbiol Rev 2014. 27 870.
- Cheung, G.Y.C. and M. Otto, Virulence Mechanisms of Staphylococcal Animal Pathogens. Int J Mol Sci 2023. 24 14587.
- Cox, H.U., S.S. Newman, A.F. Roy, and J.D. Hoskins, Species of Staphylococcus isolated from animal infections. The Cornell veterinarian 1984. 74 124-135.
- Mathema, B., J. Mediavilla, L. Chen, and B. Kreiswirth, Evolution and taxonomy of staphylococci, in Staphylococci in Human Disease, 2nd Edition, K. Crossley, et al., Editors. 2009, John Wiley & Sons: Oxford, UK. p. 31-64.
- Siegel, P.B., K. Barger, and F. Siewerdt, Limb Health in Broiler Breeding: History Using Genetics to Improve Welfare. J Appl Poult Res 2019. 28 785-790.
- Wideman, R.F. and R.D. Prisby, Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: A translational model for the pathogenesis of femoral head necrosis. Front Endocrinol (Lausanne) 2013. 3 183.
- Wideman, R.F., Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult. Sci. 2016. 95 325-344.
- Wideman, R.F., K.R. Hamal, J.M. Stark, J. Blankenship, H. Lester, K.N. Mitchell, G. Lorenzoni, and I. Pevzner, A wire-flooring model for inducing lameness in broilers: Evaluation of probiotics as a prophylactic treatment. Poult. Sci. 2012. 91 870-883.
- Gilley, A.D., H. Lester, I.Y. Pevzner, N.B. Anthony, and R.F. Wideman, Evaluating portable wire-flooring models for inducing bacterial chondronecrosis with osteomyelitis in broilers. Poult. Sci. 2014. 93 1354-1367.
- Harry, E.G., The Characteristics of Staphylococcus aureus Isolated from Cases of Staphylococcosis in Poultry. Res Vet Sci 1967. 8 479-489.
- Kibenge, F.S., G. Wilcox, and D. Pass, Pathogenicity of four strains of Staphylococci isolated from chickens with clinical tenosynovitis. Avian Pathol 1983. 12 213-220.
- Mutalib, A., C. Riddell, and A.D. Osborne, Studies on the pathogenesis of Staphylococcal osteomyelitis in chickens. II. Role of the respiratory tract as a route of infection. Avian Dis 1983. 27 157-160.
- Mutalib, A., C. Riddell, and A.D. Osborne, Studies on the Pathogenesis of Staphylococcal Osteomyelitis in Chickens. I. Effect of Stress on Experimentally Induced Osteomyelitis. Avian Dis 1983. 27 141-156.
- McNamee, P.T. and J.A. Smyth, Bacterial chondronecrosis with osteomyelitis ('femoral head necrosis') of broiler chickens: a review. Avian Pathol 2000. 29 477-495.
- Butterworth, A., N.A. Reeves, D. Harbour, G. Werrett, and S.C. Kestin, Molecular typing of strains of Staphylococcus aureus isolated from bone and joint lesions in lame broilers by random amplification of polymorphic DNA. Poult. Sci. 2001. 80 1339-1343.
- Lowder, B.V., C.M. Guinane, N.L. Ben Zakour, L.A. Weinert, A. Conway-Morris, R.A. Cartwright, A.J. Simpson, A. Rambaut, U. Nübel, and J.R. Fitzgerald, Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. PNAS USA 2009. 106 19545-19550.
- Ekesi, N.S., B. Dolka, A. Alrubaye, and D. Rhoads, Analysis of Genomes of Bacterial Isolates from Lameness Outbreaks in Broilers. Poult. Sci. 2021. 100 101148.
- Fitzgerald, J.R., Livestock-associated Staphylococcus aureus: origin, evolution and public health threat. Trends Microbiol 2012. 20 192-198.
- Al-Rubaye, A.A.K., M.B. Couger, S. Ojha, J.F. Pummill, J.A. Koon, II , R.F. Wideman, Jr., and D.D. Rhoads, Genome analysis of Staphylococcus agnetis, an agent of lameness in broiler chickens. PLoS One 2015. 10 e0143336.
- Poulsen, L.L., I. Thøfner, M. Bisgaard, R.H. Olsen, J.P. Christensen, and H. Christensen, Staphylococcus agnetis, a potential pathogen in broiler breeders. Vet Microbiol 2017. 212 1-6.
- Shwani, A., P.R.F. Adkins, N.S. Ekesi, A. Alrubaye, M.J. Calcutt, J.R. Middleton, and D.D. Rhoads, Whole genome comparisons of Staphylococcus agnetis isolates from cattle and chickens. Appl Environ Microbiol 2020. 86 e00484-20.
- Adkins, P.R.F., J.R. Middleton, M.J. Calcutt, G.C. Stewart, and L.K. Fox, Species identification and strain typing of Staphylococcus agnetis and Staphylococcus hyicus isolates from bovine milk by use of a novel multiplex PCR assay and pulsed-field gel electrophoresis. J Clin Microbiol 2017. 55 1778-1788.
- Adkins, P.R.F., S. Dufour, J.N. Spain, M.J. Calcutt, T.J. Reilly, G.C. Stewart, and J.R. Middleton, Molecular characterization of non-aureus Staphylococcus spp. from heifer intramammary infections and body sites. J Dairy Sci 2018. 101 5388-5403.
- Adkins, P.R.F., S. Dufour, J.N. Spain, M.J. Calcutt, T.J. Reilly, G.C. Stewart, and J.R. Middleton, Cross-sectional study to identify staphylococcal species isolated from teat and inguinal skin of different-aged dairy heifers. J Dairy Sci 2018. 101 3213-3225.
- Akarsu, H., A. Liljander, M. Younan, I. Brodard, G. Overesch, I. Glücks, F. Labroussaa, P. Kuhnert, V. Perreten, S. Monecke, J.F. Drexler, V.M. Corman, L. Falquet, and J. Jores, Genomic Characterization and Antimicrobial Susceptibility of Dromedary-Associated Staphylococcaceae from the Horn of Africa. Appl Environ Microbiol 2022. 88 e01146-22.
- Naseem, M.N., C. Turni, R. Gilbert, A. Raza, R. Allavena, M. McGowan, C. Constantinoiu, C.T. Ong, A.E. Tabor, and P. James, Role of Staphylococcus agnetis and Staphylococcus hyicus in the Pathogenesis of Buffalo Fly Skin Lesions in Cattle. Microbiol Spectr 2022. 10 e00873-22.
- Ayala, D.I., D.S. Grum, N.P. Evans, K.N. Russo, E.A. Kimminau, B.R. Trible, M.M. Lahoti, C.L. Novak, and T.P. Karnezos, Identification and characterization of the causative agents of Focal Ulcerative Dermatitis in commercial laying hens. Front Vet Sci 2023. 10.
- Alrubaye, A., N.S. Ekesi, A. Hasan, D.A. Koltes, R. Wideman Jr, and D. Rhoads, Chondronecrosis with osteomyelitis in broilers: Further defining a bacterial challenge model using standard litter flooring and protection with probiotics. Poult. Sci. 2020. 99 6474-6480.
- Rhoads, D.D., J. Pummill, N.S. Ekesi, and A.A.K. Alrubaye, Horizontal transfer of probable chicken-pathogenicity chromosomal islands between Staphylococcus aureus and Staphylococcus agnetis. PLoS One 2023. 18 e0283914.
- Richardson, E.J., R. Bacigalupe, E.M. Harrison, L.A. Weinert, S. Lycett, M. Vrieling, K. Robb, P.A. Hoskisson, M.T.G. Holden, E.J. Feil, G.K. Paterson, S.Y.C. Tong, A. Shittu, W. van Wamel, D.M. Aanensen, J. Parkhill, S.J. Peacock, J. Corander, M. Holmes, and J.R. Fitzgerald, Gene exchange drives the ecological success of a multi-host bacterial pathogen. Nat Ecol Evol 2018. 2 1468-1478.
- Asnayanti, A., A.D.T. Do, K. Alharbi, and A. Alrubaye, Inducing experimental bacterial chondronecrosis with osteomyelitis lameness in broiler chickens using aerosol transmission model. Poult. Sci. 2024. 103 103460.
- Kloos, W.E. and J.F. Wolfshohl, Staphylococcus cohnii Subspecies: Staphylococcus cohnii subsp. cohnii subsp. nov. and Staphylococcus cohnii subsp. urealyticum subsp. nov. Int J Syst Evol Microbiol 1991. 41 284-289.
- Lavecchia, A., M. Chiara, C. De Virgilio, C. Manzari, C. Pazzani, D. Horner, G. Pesole, and A. Placido, Comparative Genomics Suggests a Taxonomic Revision of the Staphylococcus cohnii Species Complex. Genome Biol Evol 2021. 13.
- Soldera, J., W.L. Nedel, P.R.C. Cardoso, and P.A. d'Azevedo, Bacteremia due to Staphylococcus cohnii ssp. urealyticus caused by infected pressure ulcer: case report and review of the literature. Sao Paulo Med J 2013. 131 59-61.
- Marek, A., D. Stepień-Pyśniak, E. Pyzik, Ł. Adaszek, J. Wilczyński, and S. Winiarczyk, Occurrence and characterization of Staphylococcus bacteria isolated from poultry in Western Poland. Berl tierarztl Wschr 2016. 129 147-152.
- Al-Rubaye, A.A.K., N.S. Ekesi, S. Zaki, N.K. Emami, R.F. Wideman, and D.D. Rhoads, Chondronecrosis with osteomyelitis in broilers: Further defining a bacterial challenge model using the wire flooring model. Poult. Sci. 2017. 96 332-340.
- Shwani, A., B. Zuo, A. Alrubaye, J. Zhao, and D.D. Rhoads, A Simple, Inexpensive Alkaline Method for Bacterial DNA Extraction from Environmental Samples for PCR Surveillance and Microbiome Analyses. Appl Sci 2024. 14 141.
- Shwani, A. Bacterial Chondronecrosis with Osteomyelitis in broilers: genomics, phylogenomics, and methods to detect specific pathogens during outbreaks., in Cell and Molecular Biology. 2022, University of Arkansas. p. 287.
- Dyer, D.W. and J.J. Iandolo, Rapid isolation of DNA from Staphylococcus aureus. Appl Environ Microbiol 1983. 46 283-28.
- Olson, R.D., R. Assaf, T. Brettin, N. Conrad, C. Cucinell, James J. Davis, Donald M. Dempsey, A. Dickerman, Emily M. Dietrich, Ronald W. Kenyon, M. Kuscuoglu, Elliot J. Lefkowitz, J. Lu, D. Machi, C. Macken, C. Mao, A. Niewiadomska, M. Nguyen, Gary J. Olsen, Jamie C. Overbeek, B. Parrello, V. Parrello, Jacob S. Porter, Gordon D. Pusch, M. Shukla, I. Singh, L. Stewart, G. Tan, C. Thomas, M. VanOeffelen, V. Vonstein, Zachary S. Wallace, Andrew S. Warren, Alice R. Wattam, F. Xia, H. Yoo, Y. Zhang, Christian M. Zmasek, Richard H. Scheuermann, and Rick L. Stevens, Introducing the Bacterial and Viral Bioinformatics Resource Center (BV-BRC): a resource combining PATRIC, IRD and ViPR. Nucl. Acid Res. 2022. 51 D678-D689.
- Lees, J.A., S.R. Harris, G. Tonkin-Hill, R.A. Gladstone, S.W. Lo, J.N. Weiser, J. Corander, S.D. Bentley, and N.J. Croucher, Fast and flexible bacterial genomic epidemiology with PopPUNK. Genome Research 2019. 29 304-316.
- Treangen, T.J., B.D. Ondov, S. Koren, and A.M. Phillippy, The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol 2014. 15 524.
- Pritchard, L., R.H. Glover, S. Humphris, J.G. Elphinstone, and I.K. Toth, Genomics and taxonomy in diagnostics for food security: soft-rotting enterobacterial plant pathogens. Anal Methods 2016. 8 12-24.
- Hölzer, M., POCP-nf: an automatic Nextflow pipeline for calculating the percentage of conserved proteins in bacterial taxonomy. Bioinformatics 2024. 40.
- Han, M.V. and C.M. Zmasek, phyloXML: XML for evolutionary biology and comparative genomics. BMC Bioinformatics 2009. 10 356.
- Argimón, S., K. Abudahab, R.J.E. Goater, A. Fedosejev, J. Bhai, C. Glasner, E.J. Feil, M.T.G. Holden, C.A. Yeats, H. Grundmann, B.G. Spratt, and D.M. Aanensen, Microreact: visualizing and sharing data for genomic epidemiology and phylogeography. Microb Genom 2016. 2.
- Grant, J.R., E. Enns, E. Marinier, A. Mandal, E.K. Herman, C.Y. Chen, M. Graham, G. Van Domselaar, and P. Stothard, Proksee: in-depth characterization and visualization of bacterial genomes. Nucleic Acids Res 2023. 51 W484-w492.
- Brown, C.L., J. Mullet, F. Hindi, J.E. Stoll, S. Gupta, M. Choi, I. Keenum, P. Vikesland, A. Pruden, and L. Zhang, mobileOG-db: a Manually Curated Database of Protein Families Mediating the Life Cycle of Bacterial Mobile Genetic Elements. Appl Environ Microbiol 2022. 88 e0099122.
- Madden, T. BLAST® Command Line Applications User Manual. 2008 9/25/2020 [cited 2020]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279691/.
- Seemann, T., Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [CrossRef] [PubMed]
- Page, A.J., C.A. Cummins, M. Hunt, V.K. Wong, S. Reuter, M.T.G. Holden, M. Fookes, D. Falush, J.A. Keane, and J. Parkhill, Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015. 31 3691-3693.
- Bayliss, S.C., H.A. Thorpe, N.M. Coyle, S.K. Sheppard, and E.J. Feil, PIRATE: A fast and scalable pangenomics toolbox for clustering diverged orthologues in bacteria. GigaScience 2019. 8.
- Brynildsrud, O., J. Bohlin, L. Scheffer, and V. Eldholm, Rapid scoring of genes in microbial pan-genome-wide association studies with Scoary. Genome Biol 2016. 17 238.
- Shi, Z.J., S. Nayfach, and K.S. Pollard, Maast: genotyping thousands of microbial strains efficiently. Genome Biol 2023. 24 186.
- Shen, W., S. Le, Y. Li, and F. Hu, SeqKit: A Cross-Platform and Ultrafast Toolkit for FASTA/Q File Manipulation. PLoS One 2016. 11 e0163962.
- Lalucat, J., M. Mulet, M. Gomila, and E. García-Valdés, Genomics in Bacterial Taxonomy: Impact on the Genus Pseudomonas. Genes 2020. 11 139.
- Graña-Miraglia, L., C. Arreguín-Pérez, G. López-Leal, A. Muñoz, A. Pérez-Oseguera, E. Miranda-Miranda, R. Cossío-Bayúgar, and S. Castillo-Ramírez, Phylogenomics picks out the par excellence markers for species phylogeny in the genus Staphylococcus. PeerJ 2018. 6 e5839.
- Varghese, N.J., S. Mukherjee, N. Ivanova, K.T. Konstantinidis, K. Mavrommatis, N.C. Kyrpides, and A. Pati, Microbial species delineation using whole genome sequences. Nucl. Acid Res. 2015. 43 6761-6771.
- Richter, M. and R. Rosselló-Móra, Shifting the genomic gold standard for the prokaryotic species definition. PNAS USA 2009. 106 19126-19131.
- Chen, J., N. Quiles-Puchalt, Y.N. Chiang, R. Bacigalupe, A. Fillol-Salom, M.S.J. Chee, J.R. Fitzgerald, and J.R. Penadés, Genome hypermobility by lateral transduction. Science 2018. 362 207-212.
- Lan, R. and P.R. Reeves, Escherichia coli in disguise: molecular origins of Shigella. Microbes and Infection 2002. 4 1125-1132.
- Abram, K., Z. Udaondo, C. Bleker, V. Wanchai, T.M. Wassenaar, M.S. Robeson, and D.W. Ussery, Mash-based analyses of Escherichia coli genomes reveal 14 distinct phylogroups. Commun Biol 2021. 4 117.
- Yang, T. and F. Gao, High-quality pan-genome of Escherichia coli generated by excluding confounding and highly similar strains reveals an association between unique gene clusters and genomic islands. Brief Bioinform 2022. 23.
- Gonzalez-Alba, J.M., F. Baquero, R. Cantón, and J.C. Galán, Stratified reconstruction of ancestral Escherichia coli diversification. BMC Genomics 2019. 20 936.
- Alrubaye, A.A.K., N.S. Ekesi, A. Hasan, E. Elkins, S. Ojha, S. Zaki, S. Dridi, R.F. Wideman, M.A. Rebollo, and D.D. Rhoads, Chondronecrosis with Osteomyelitis in Broilers: Further Defining Lameness-Inducing Models with Wire or Litter Flooring, to Evaluate Protection with Organic Trace Minerals. Poult. Sci. 2020. 99 5422-5429.
| Isolate | Colony Color | |||||
| Number | Exp | Bird | WGS | Lineage | CO | CS |
| 1637 | 29 | 10A | yes | 1 | p | l |
| 1649 | 29 | 10A | yes | 1 | t | t |
| 1662 | 29 | 10B | 1 | p | l | |
| 1667 | 29 | 10B | yes | 2 | w | l |
| 1670 | 29 | 10B | 1 | t | t | |
| 1638 | 29 | 11A | yes | 1 | p | l |
| 1650 | 29 | 11A | 1 | t | t | |
| 1651 | 29 | 11B | 1 | t | t | |
| 1652 | 29 | 12B | 1 | t | t | |
| 1653 | 29 | 13A | 1 | t | t | |
| 1654 | 29 | 13B | 1 | t | t | |
| 1663 | 29 | 13B | yes | 1 | p | l |
| 1655 | 29 | 14A | 1 | t | t | |
| 1656 | 29 | 14B | 1 | t | t | |
| 1657 | 29 | 2A | 1 | t | t | |
| 1658 | 29 | 2A | yes | 1 | p | l |
| 1659 | 29 | 3B | yes | 1 | p | l |
| 1664 | 29 | 3B | yes | 2 | w | l |
| 1644 | 29 | 5B | 1 | t | t | |
| 1645 | 29 | 6A | yes | 1 | t | t |
| 1636 | 29 | 6B | 1 | p | l | |
| 1646 | 29 | 6B | 1 | t | t | |
| 1661 | 29 | 9A | yes | 1 | p | l |
| 1665 | 29 | 9A | yes | 2 | w | l |
| 1669 | 29 | 9A | 1 | t | t | |
| 1639 | 29 | 9B | 1 | p | l | |
| 1648 | 29 | 9B | 1 | t | t | |
| 1666 | 29 | 9B | yes | 2 | w | l |
| 1697 | 30 | 2 | yes | 2 | t | t |
| 1725 | 30 | 3 | yes | 2 | t | t |
| 1711 | 30 | 4 | yes | 2 | w | l |
| 1701 | 30 | 6 | yes | 1 | t | t |
| 1728 | 30 | 8 | yes | 2 | p | l |
| 1714 | 30 | 10 | yes | 2 | p | l |
| 1715 | 30 | 10 | yes | 2 | p | l |
| lineage | n | 1 | 2 | 3 | m |
|---|---|---|---|---|---|
| 1 | 56 | 0.99012±0.00771 | 0.92023±0.00566 | 0.92526±0.00333 | 0.94591±0.00196 |
| 2 | 40 | 0.99290±0.00591 | 0.96082±0.00171 | 0.94935±0.00025 | |
| 3 | 23 | 0.99558±0.00368 | 0.97667±0.00216 | ||
| m | 5 | 0.99996±0.00004 |
| Sn | 1 | 2 | 3 | ||
|---|---|---|---|---|---|
| n | 4 | 4 | 1 | 4 | |
| POCP | Sn | 93.9±1.8 | 84.2±1.4 | 85.4±1.6 | 84.0±0.7 |
| 1 | 90.4±2.3 | 88.5±1.5 | 89.2±1.6 | ||
| 2 | NA | 88.2±0.6 | |||
| 3 | 95.3±2.0 | ||||
| ANI | Sn | 0.997±0.0015 | 0.857±0.0007 | 0.879±0.0459 | 0.859±0.0012 |
| 1 | 0.990±0.0036 | 0.919±0.0003 | 0.925±0.0015 | ||
| 2 | NA | 0.961±0.0004 | |||
| 3 | 0.997±0.0017 |
| Trait | Gene Count | |||
| Bonferroni P < 0.05 | Odds Ratio | 100% Specificity and Sensitivity | ||
| > 4.4 | < 0.24 | |||
| Lineage 1 | 1756 | 857 | 899 | 84 |
| Lineage 2 | 1716 | 867 | 849 | 14 |
| Lineage 3 | 1311 | 707 | 604 | 13 |
| Human | 333 | 195 | 138 | 0 |
| Mammal | 7 | 0 | 7 | 0 |
| Avian | 7 | 7 | 0 | 0 |
| Chicken | 17 | 17 | 0 | 0 |
| Animal | 166 | 37 | 129 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





