Submitted:
21 June 2024
Posted:
25 June 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Results
Discussion
Conclusions and Limitations
Authors contribution
Conflict of interest:
Funding
References
- Depression and Other Common Mental Disorders Available online: https://www.who.int/publications/i/item/depression-global-health-estimates.
- Global Burden of Disease Study 2019 (GBD 2019) Data Resources | GHDx Available online: https://ghdx.healthdata.org/gbd-2019.
- Di Cesare, M.; Magliocchietti, N.; Romanelli, M.; Santori, E. Rapporto Salute Mentale. Analisi Dei Dati Del Sistema Informativo per La Salute Mentale (SISM). Anno 2022. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=3369 (accessed on 17 December 2023).
- Mahli, G.S.; Mann JJ, G.S. Depression. The Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Sengupta, S. Diagnosis of Depression in General Practice. Indian J Med Sci 2005, 59, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Hellmann-Regen, J.; Piber, D.; Hinkelmann, K.; Gold, S.M.; Heesen, C.; Spitzer, C.; Endres, M.; Otte, C. Depressive Syndromes in Neurological Disorders. Eur Arch Psychiatry Clin Neurosci 2013, 263, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Lai, W.; Long, E.; Zhang, X.; Li, W.; Zhu, Y.; Chen, C.; Zhong, X.; Liu, Z.; et al. Prevalence of Depression and Depressive Symptoms among Outpatients: A Systematic Review and Meta-Analysis. BMJ Open 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Maj, M. Understanding Depression beyond the “Mind-Body” Dichotomy. World Psychiatry 2023, 22, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.; Stein, D.J.; Parker, G.; Zimmerman, M.; Fava, G.A.; De Hert, M.; Demyttenaere, K.; McIntyre, R.S.; Widiger, T.; Wittchen, H.U. The Clinical Characterization of the Adult Patient with Depression Aimed at Personalization of Management. World Psychiatry 2020, 19, 269. [Google Scholar] [CrossRef]
- World Health Organization UN Decade of Healthy Ageing: Plan of Action 2021-2030. World Health Organization 2020, 1–26.
- Cai, H.; Jin, Y.; Liu, R.; Zhang, Q.; Su, Z.; Ungvari, G.S.; Tang, Y.L.; Ng, C.H.; Li, X.H.; Xiang, Y.T. Global Prevalence of Depression in Older Adults: A Systematic Review and Meta-Analysis of Epidemiological Surveys. Asian J Psychiatr 2023, 80, 103417. [Google Scholar] [CrossRef] [PubMed]
- Clouston, S.A.P.; Brewster, P.; Kuh, D.; Richards, M.; Cooper, R.; Hardy, R.; Rubin, M.S.; Hofer, S.M. The Dynamic Relationship Between Physical Function and Cognition in Longitudinal Aging Cohorts. Epidemiol Rev 2013, 35, 33–50. [Google Scholar] [CrossRef]
- Birk, J.L.; Kronish, I.M.; Moise, N.; Falzon, L.; Yoon, S.; Davidson, K.W. Depression and Multimorbidity: Considering Temporal Characteristics of the Associations between Depression and Multiple Chronic Diseases. Health Psychology 2019, 38, 802–811. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Buckwalter, K.; Olin, J.; Martinez, R.; Wainscott, C.; Krishnan, K.R.R. Comorbidity of Late Life Depression: An Opportunity for Research on Mechanisms and Treatment. Biol Psychiatry 2002, 52, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S. Depression in the Elderly. The Lancet 2005, 365, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Enache, D.; Winblad, B.; Aarsland, D. Depression in Dementia: Epidemiology, Mechanisms, and Treatment. Curr Opin Psychiatry 2011, 24, 461–472. [Google Scholar] [CrossRef]
- Bradley, B.; Backus, D.; Gray, E. Depression in the Older Adult: What Should Be Considered? Ment Health Clin 2016, 6, 222. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, A.; Grover, S. Clinical Practice Guidelines for Management of Depression in Elderly. Indian J Psychiatry 2018, 60, S341. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S. The STROBE Guidelines. Saudi J Anaesth 2019, 13, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Albert, U.; Tomasetti, C.; Marra, C.; Neviani, F.; Pirani, A.; Taddeo, D.; Zanetti, O.; Maina, G. Treating Depression in Clinical Practice: New Insights on the Multidisciplinary Use of Trazodone. Front Psychiatry 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Srifuengfung, M.; Pennington, B.R.T.; Lenze, E.J. Optimizing Treatment for Older Adults with Depression. Ther Adv Psychopharmacol 2023, 13. [Google Scholar] [CrossRef]
- Stecher, C.; Cloonan, S.; Domino, M.E. The Economics of Treatment for Depression. Annu Rev Public Health 2023, 45. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; George, C.; Lo, S.; Tan, E.; Bordia, A.; Hilmer, S. The Outcomes of Considering Goals of Care in Medication Reviews for Older Adults: A Systematic Review. Expert Rev Clin Pharmacol 2023, 1–24. [Google Scholar] [CrossRef]
- Montorio, I.; Izal, M. The Geriatric Depression Scale: A Review of Its Development and Utility. Int Psychogeriatr 1996, 8, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Halli-Tierney, A.D.; Scarbrough, C.; Carroll, D. Polypharmacy: Evaluating Risks and Deprescribing. Am Fam Physician 2019, 100, 32–38. [Google Scholar] [PubMed]
- Mayer, T.; Haefeli, W.E.; Seidling, H.M. Different Methods, Different Results - How Do Available Methods Link a Patient’s Anticholinergic Load with Adverse Outcomes? Eur J Clin Pharmacol 2015, 71, 1299–1314. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Feng, L.; Fam, J.; Mahendran, R.; Kua, E.H.; Ng, T.P. Coexisting Medical Comorbidity and Depression: Multiplicative Effects on Health Outcomes in Older Adults. Int Psychogeriatr 2014, 26, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Harpole, L.H.; Williams, J.W.; Olsen, M.K.; Stechuchak, K.M.; Oddone, E.; Callahan, C.M.; Katon, W.J.; Lin, E.H.; Grypma, L.M.; Unützer, J. Improving Depression Outcomes in Older Adults with Comorbid Medical Illness. Gen Hosp Psychiatry 2005, 27, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Albert, U.; Tomasetti, C.; Marra, C.; Neviani, F.; Pirani, A.; Taddeo, D.; Zanetti, O.; Maina, G. Treating Depression in Clinical Practice: New Insights on the Multidisciplinary Use of Trazodone. Front Psychiatry 2023, 14. [Google Scholar] [CrossRef]
- Bruce, M.L.; Sirey, J.A. Integrated Care for Depression in Older Primary Care Patients. Can J Psychiatry 2018, 63, 439. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, N. Introduction: Stigma and Discrimination against Older People with Mental Disorders. Int J Geriatr Psychiatry 2003, 18, 669. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.L.; Lyberg, A.; Severinsson, E. Living with Stigma: Depressed Elderly Persons’ Experiences of Physical Health Problems. Nurs Res Pract 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Polenick, C.A.; Martire, L.M. Caregiver Attributions for Late-Life Depression and Their Associations with Caregiver Burden. Fam Process 2013, 52, 709. [Google Scholar] [CrossRef]
- Krishnamoorthy, Y.; Rajaa, S.; Rehman, T. Diagnostic Accuracy of Various Forms of Geriatric Depression Scale for Screening of Depression among Older Adults: Systematic Review and Meta-Analysis. Arch Gerontol Geriatr 2020, 87. [Google Scholar] [CrossRef] [PubMed]
- Crowther, G.; Ninan, S. Managing Depression in Frail Older People; Too Little Too Late or Pathologising Loss? Future Healthc J 2023, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.F.; Hamm, M.; Cameron, F.; Lightfoot, M.; Maher, R.; Kincman, J.; Reynolds, C. Improving Effective Mental Health Consultation for Rural Older Adults Living With Depression and Pain: Learning From the Experiences of Rural Primary Care Physicians. Prim Care Companion CNS Disord 2021, 23, 29380. [Google Scholar] [CrossRef]
- Voros, V.; Fekete, S.; Tenyi, T.; Rihmer, Z.; Szili, I.; Osvath, P. Untreated Depressive Symptoms Significantly Worsen Quality of Life in Old Age and May Lead to the Misdiagnosis of Dementia: A Cross-Sectional Study. Ann Gen Psychiatry 2020, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T.; Fordyce, S.W. Geriatric Psychiatry- An Emerging Specialty. Mo Med 2010, 107, 401. [Google Scholar]
- Chen, Y. jing; Li, X. xia; Pan, B.; Wang, B.; Jing, G. zhuang; Liu, Q. qian; Li, Y. fei; Bing, Z. tong; Yang, K. hu; Han, X. mei; et al. Non-Pharmacological Interventions for Older Adults with Depressive Symptoms: A Network Meta-Analysis of 35 Randomized Controlled Trials. Aging Ment Health 2021, 25, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Gramaglia, C.; Gattoni, E.; Marangon, D.; Concina, D.; Grossini, E.; Rinaldi, C.; Panella, M.; Zeppegno, P. Non-Pharmacological Approaches to Depressed Elderly With No or Mild Cognitive Impairment in Long-Term Care Facilities. A Systematic Review of the Literature. Front Public Health 2021, 9, 685860. [Google Scholar] [CrossRef]
- Shang, W.; Guo, L.; Liu, Y.; Li, Y.; Wei, Q.; Guo, K.; Yang, M.; Wei, L.; Xu, Z.; Niu, J.; et al. PROTOCOL: Non-Pharmacological Interventions for Older People with a Diagnosis of Depression: An Evidence and Gap Map. Campbell Systematic Reviews 2023, 19, e1354. [Google Scholar] [CrossRef]
- Srifuengfung, M.; Pennington, B.R.T.; Lenze, E.J. Optimizing Treatment for Older Adults with Depression. 2023; 13. [Google Scholar] [CrossRef]
- O’mahony, D.; O’sullivan, D.; Byrne, S.; O’connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START Criteria for Potentially Inappropriate Prescribing in Older People: Version 2. Age Ageing 2015, 44, 213–218. [Google Scholar] [CrossRef]
- Gers, L.; Petrovic, M.; Perkisas, S.; Vandewoude, M. Antidepressant Use in Older Inpatients: Current Situation and application of the Revised STOPP Criteria. Ther Adv Drug Saf 2018, 9, 373. [Google Scholar] [CrossRef]
- Pazan, F.; Weiss, C.; Wehling, M. The FORTA (Fit fOR The Aged) List 2021: Fourth Version of a Validated Clinical Aid for Improved Pharmacotherapy in Older Adults. Drugs Aging 2022, 39, 245–247. [Google Scholar] [CrossRef]
- Richardson, K.; Bennett, K.; Maidment, I.D.; Fox, C.; Smithard, D.; Kenny, R.A. Use of Medications with Anticholinergic Activity and Self-Reported Injurious Falls in Older Community-Dwelling Adults. J Am Geriatr Soc 2015, 63, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Novella, A.; Elli, C.; Ianes, A.; Pasina, L. Anticholinergic Burden and Cognitive Impairment in Nursing Homes: A Comparison of Four Anticholinergic Scales. Drugs Aging 2023, 40, 1017–1026. [Google Scholar] [CrossRef]
- Grossi, C.M.; Richardson, K.; Savva, G.M.; Fox, C.; Arthur, A.; Loke, Y.K.; Steel, N.; Brayne, C.; Matthews, F.E.; Robinson, L.; et al. Increasing Prevalence of Anticholinergic Medication Use in Older People in England over 20 Years: Cognitive Function and Ageing Study I and II. BMC Geriatr 2020, 20. [Google Scholar] [CrossRef]
- Herrero-Zazo, M.; Berry, R.; Bines, E.; Bhattacharya, D.; Myint, P.K.; Keevil, V.L. Anticholinergic Burden in Older Adult Inpatients: Patterns from Admission to Discharge and Associations with Hospital Outcomes. Ther Adv Drug Saf 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Kayahan Satış, N.; Naharcı, M.İ. Investigating the Association of Anticholinergic Burden with Depression in Older Adults: A Cross-Sectional Study. Psychogeriatrics 2024. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, A.; Bianchetti, A.; Cagnin, A.; De Berardis, D.; Di Fazio, I.; Incalzi, R.A.; Marra, C.; Neviani, F.; Laurenzi, P.F.; Nicoletti, F. Trazodone: A Multifunctional Antidepressant. Evaluation of Its Properties and Real-World Use. JOURNAL OF GERONTOLOGY AND GERIATRICS 2021, 69, 120–129. [Google Scholar] [CrossRef]
- Zheng, Y.; Lv, T.; Wu, J.; Lyu, Y. Trazodone Changed the Polysomnographic Sleep Architecture in Insomnia Disorder: A Systematic Review and Meta-Analysis. Scientific Reports 2022 12:1 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Gonçalo, A.M.G.; Vieira-Coelho, M.A. The Effects of Trazodone on Human Cognition: A Systematic Review. Eur J Clin Pharmacol 2021, 77, 1623. [Google Scholar] [CrossRef]
- Srifuengfung, M.; Pennington, B.R.T.; Lenze, E.J. Optimizing Treatment for Older Adults with Depression. Ther Adv Psychopharmacol 2023, 13. [Google Scholar] [CrossRef]
- Jayasekara, R.; Procter, N.; Harrison, J.; Skelton, K.; Hampel, S.; Draper, R.; Deuter, K. Cognitive Behavioural Therapy for Older Adults with Depression: A Review. J Ment Health 2015, 24, 168–171. [Google Scholar] [CrossRef] [PubMed]
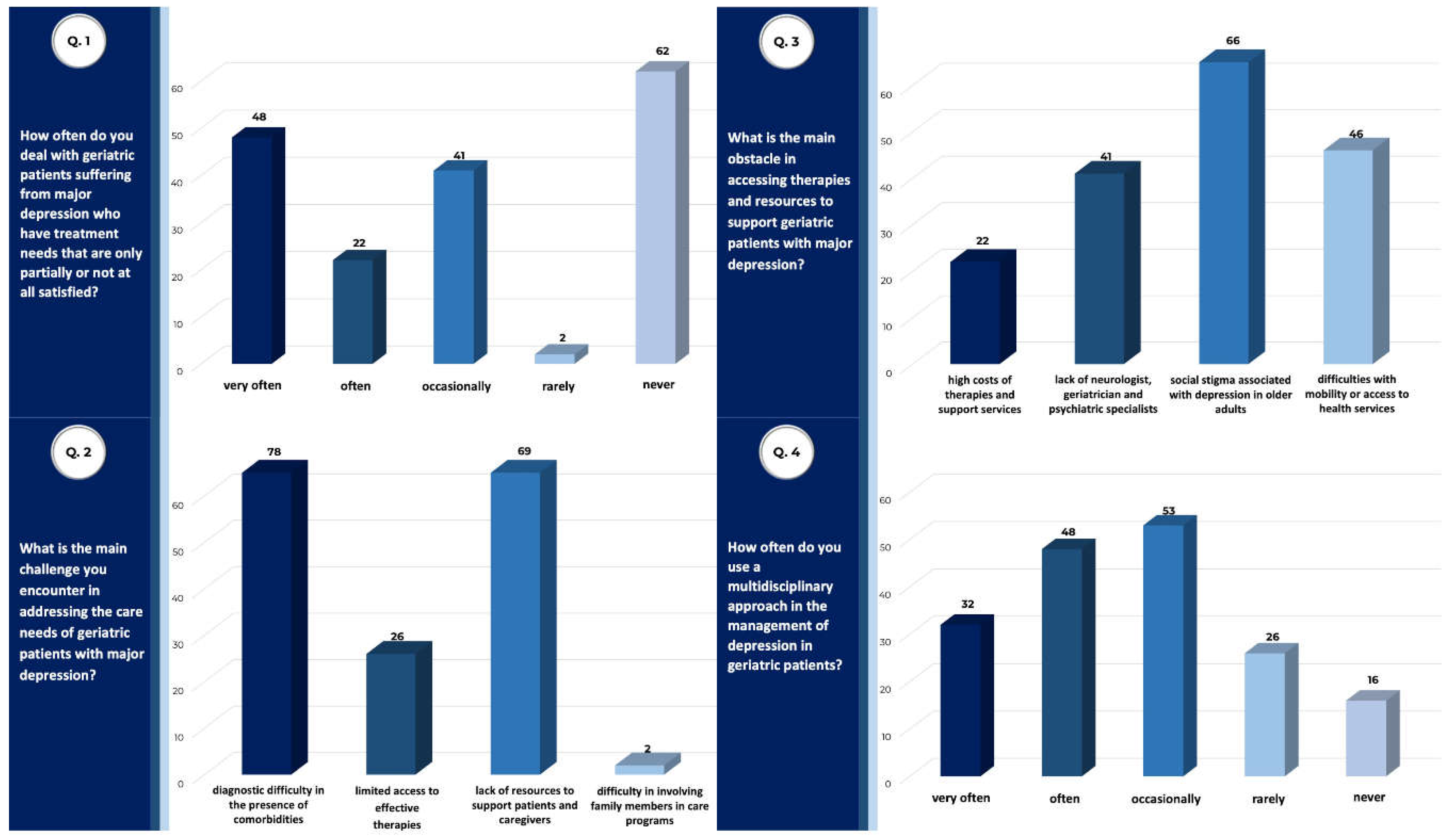
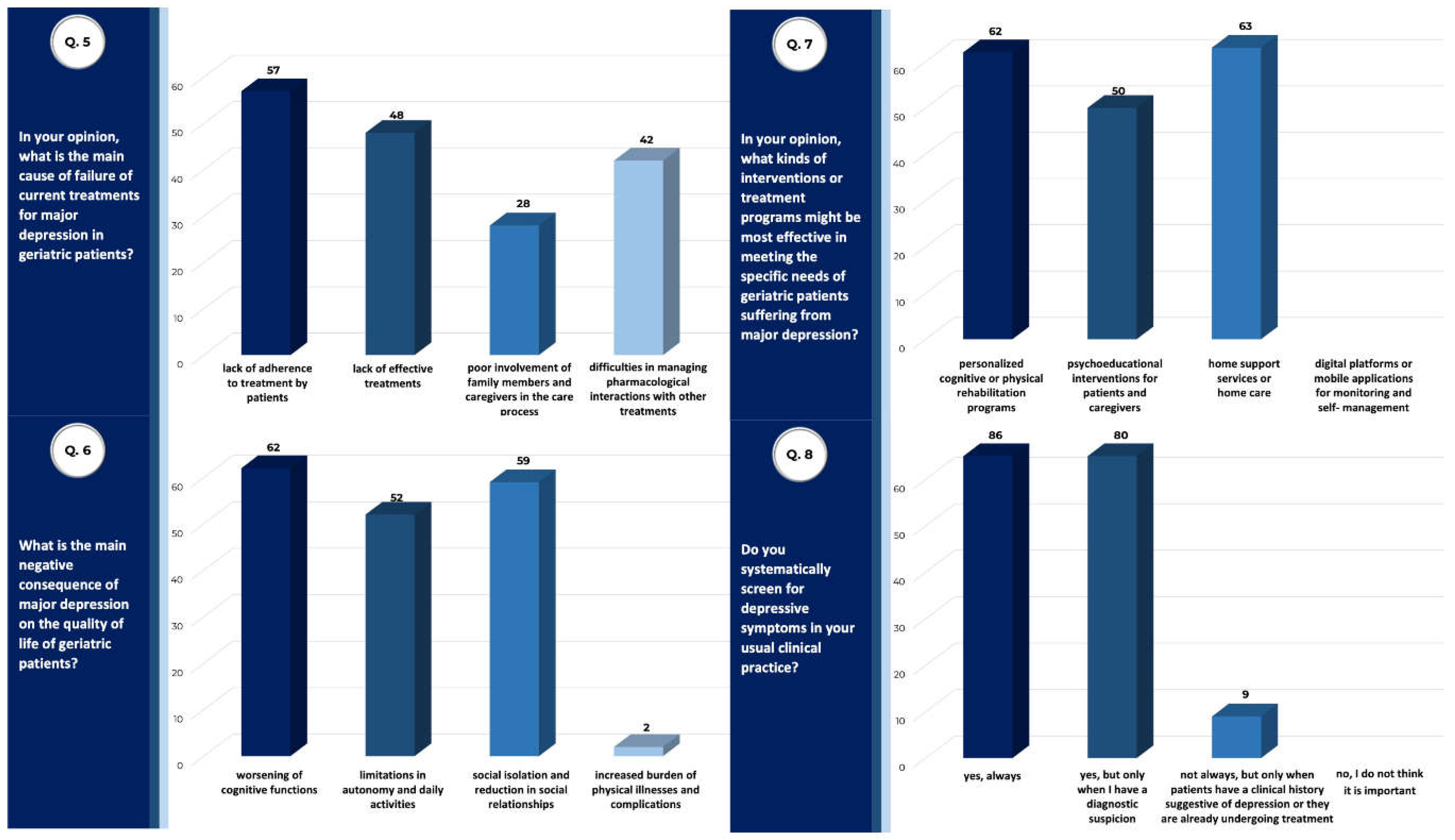
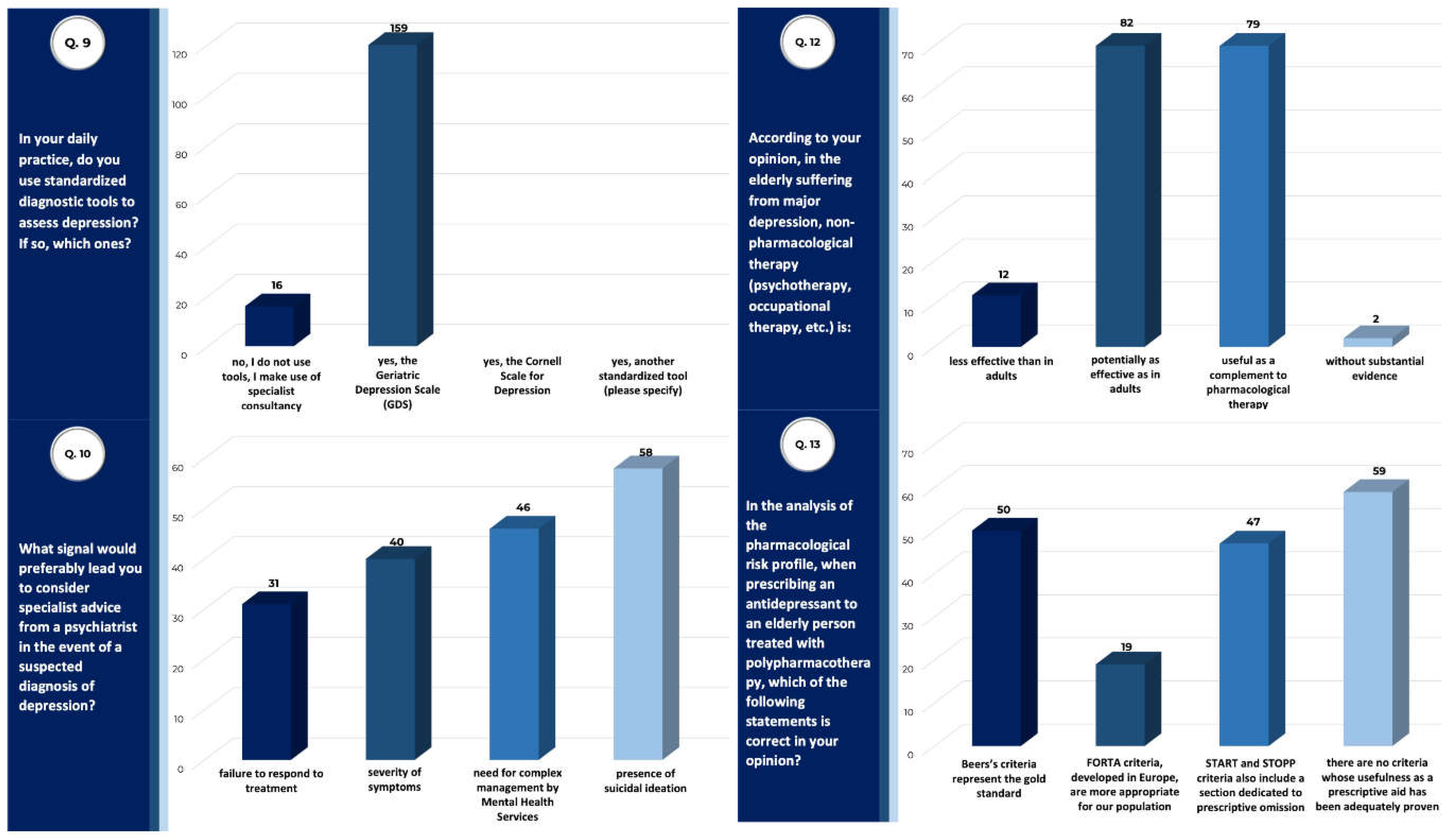
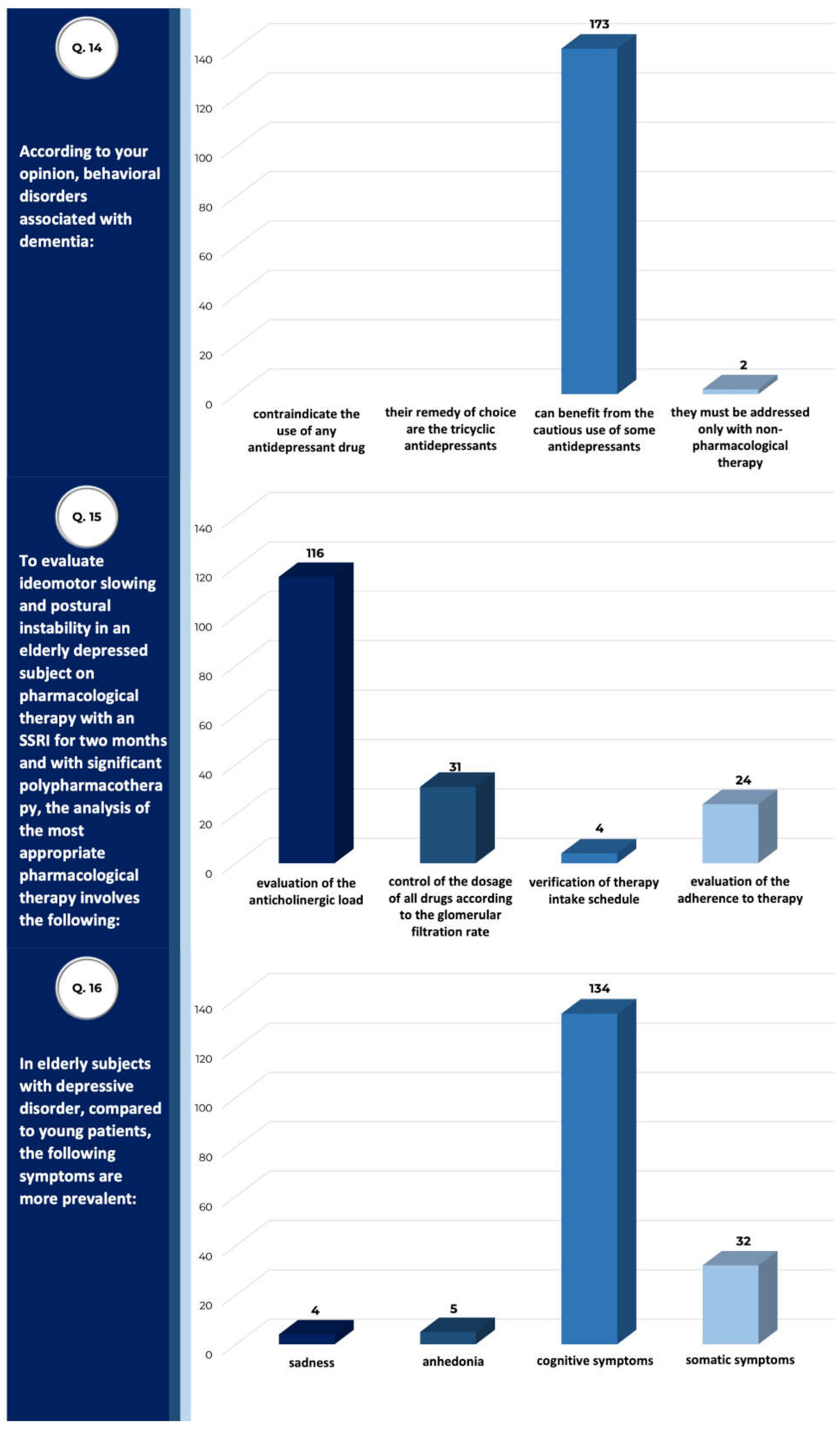
| Total Sample (n) | 175 |
|---|---|
| Age | |
| 30-35 | 50 (28.6%) |
| 36-40 | 24 (13.7%) |
| 41-45 | 14 (8%) |
| 46-50 | 16 (9.1%) |
| 51-55 | 25 (14.3%) |
| 56-60 | 12 (6.9%) |
| 61-65 | 20 (11.4%) |
| 66-70 | 10 (5.7%) |
| >70 | 4 (2.3%) |
| Gender | |
| Male | 63 (36%) |
| Female | 112 (64%) |
| Years of experience | |
| 1-5 | 47 (26.9%) |
| 6-10 | 19 (10.9%) |
| 11-15 | 20 (11.4%) |
| 16-20 | 23 (13%) |
| 21-25 | 24 (13.7%) |
| 26-30 | 8 (4.6%) |
| 31-35 | 12 (6.9%) |
| 36-40 | 10 (5.7%) |
| 41-45 | 8 (4.6%) |
| 46-50 | 4 (2.3%) |
| Specialization | |
| Geriatrics | 165 (94.3%) |
| Internal medicine | 8 (4.6%) |
| General medicine | 2 (1.1%) |
| Work structure | |
| Public structure | 104 (59.4%) |
| Private structure | 26 (14.9%) |
| University hospital | 40 (22.9%) |
| Third sector organisation | 2 (1.1%) |
| Freelancer | 3 (1.7%) |
| Working environment | |
| RSA | 25 (14.3%) |
| Acute care unit | 68 (38.8%) |
| Rehabilitation ward | 18 (10.3%) |
| Long-term care | 6 (3.4%) |
| Home care | 19 (10.9%) |
| Outpatient clinic | 33 (18.9%) |
| Emergency room | 4 (2.3%) |
| Research institute | 2 (1.1%) |
| Geographical distribution | |
| Northern Italy | 114 (65.1%) |
| Central Italy | 33 (18.9%) |
| Southern Italy | 28 (16%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





