Submitted:
21 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
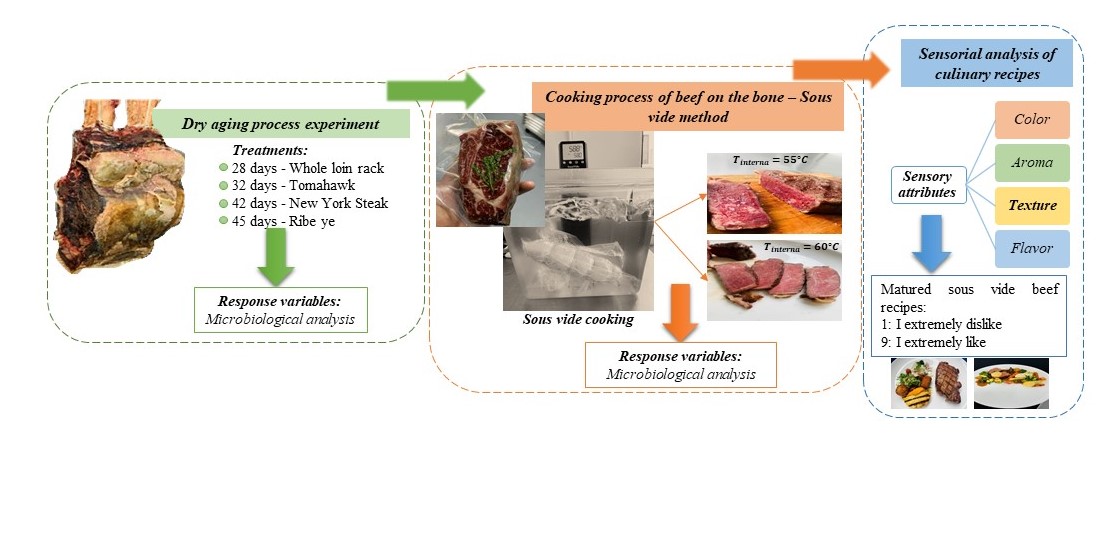
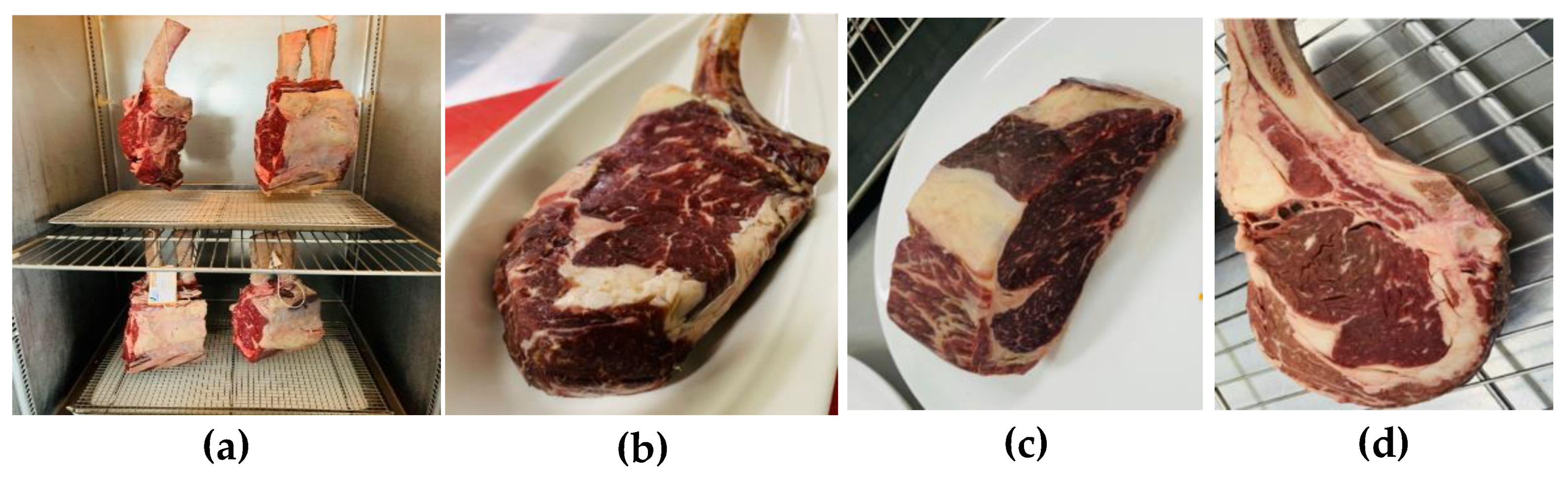
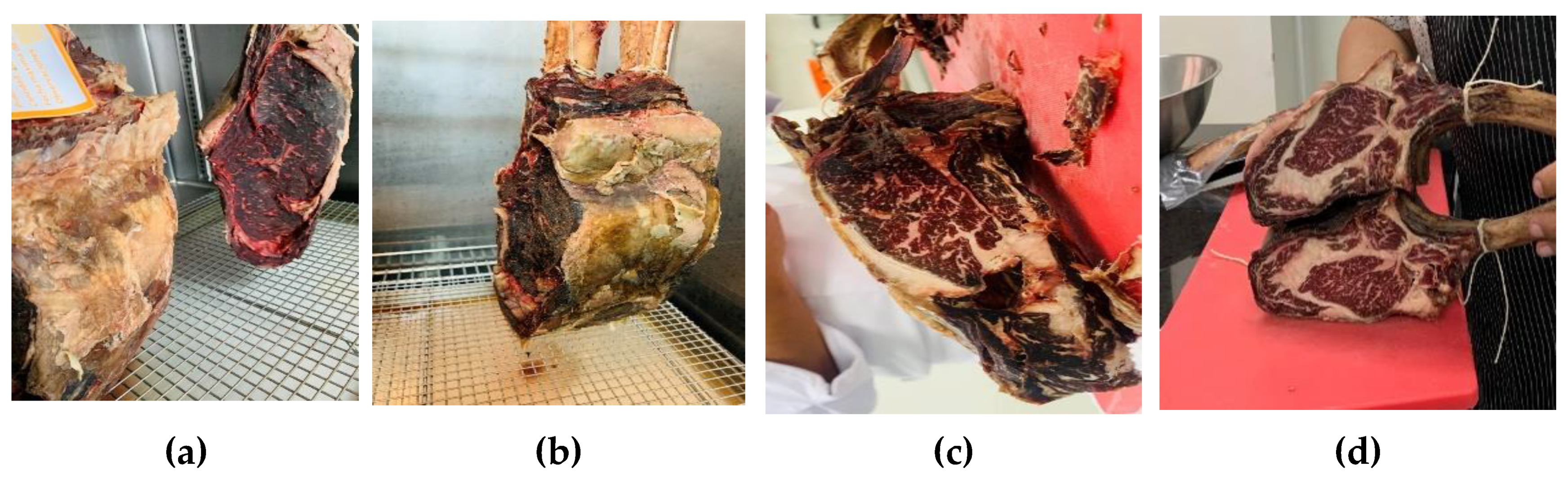
3.1. Dry Aging Process for Bone-In Beef
3.2. Microbiological Tests of the Dry-Aging Process
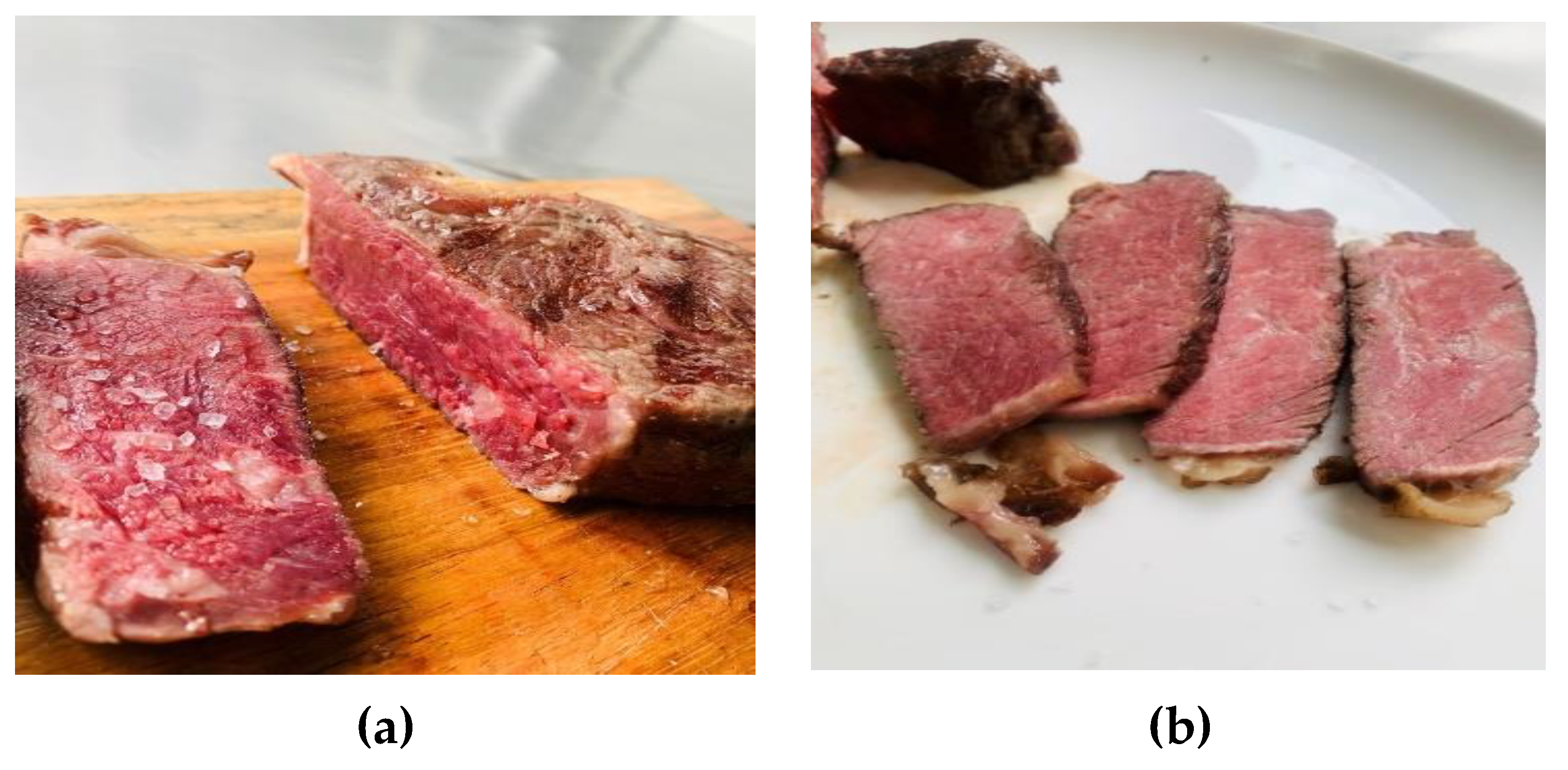
3.3. Cooking Process of Beef on the Bone - Sousvide Method
3.4. Standardization of Matured Sous Vide Beef Recipes
3.5. Sensorial Analysis of Culinary Recipes
- New York Steak on a bed of vegetables with demi-glace sauce and sweet wine
- Grilled dry-aged New York Steak
- Ribe ye in beetroot reduction
- Grilled Toma hawk.
4. Discussion
Funding
Conflicts of Interest
References
- Martínez, H. V. (2021). Mexican Meat Council. Retrieved from Denominación de carne y los procesos fisicoquímicos que ocurren en su maduración: https://comecarne.org/denominacion-de-carne-y-los-procesos-fisicoquimicos-que-ocurren-en-su-maduracion/.
- IPES-Food. (2022). The politics of protein: Examining claims about livestock, fish, ‘alternative proteins’ and sustainability. IPES-Food. Recuperado de http://www.ipes-food.org.
- Di Paolo, M.; Ambrosio, R.L.; Lambiase, C.; Vuoso, V.; Salzano, A.; Bifulco, G.; Barone, C.M.A.; Marrone, R. (2023). Effects of the Aging Period and Method on the Physicochemical, Microbiological and Rheological Characteristics of Two Cuts of Charolais Beef. Foods 2023, 12, 531. [Google Scholar] [CrossRef]
- Ayala Vargas, C. (2018). Nutritional importance of meat. Instituto de Investigaciones Agropecuarias y de recursos naturales, 54 - 61 pp.
- Bureš, D; Needham, T; Lebedová, N; Bartoň, L. (2023). Effects of Wet and Dry Ageing on the Physical, Chemical and Sensory Quality of Fleckvieh Cattle Meat. 69th International Congress of Meat Science and Technology, August 20-25, Padova, Italy. Available at: https://www.researchgate.net/publication/373900683.
- Ribeiro, J. C.; Cunha, I.G.; Pereira, B.; Augusto, W. F.; Rodrigues, E. M.; Chaves, F. R. (2022). Influencia de la maduración seca y húmeda de la carne de vacuno en la calidad microbiológica y seguridad. Semina: Cienc. Agrár. Londrina, v. 42, n. 1, p. 155-166.
- Ding, Z.; Wei, Q.; Liu, C.; Zhang, H.; Huang, F. (2022). The Quality Changes and Proteomic Analysis of Cattle Muscle Postmortem during Rigor Mortis. Foods, 11, 217. [CrossRef]
- Voitsekhivska., L; Franko., O; Verbytskyi., S; Okhrimenko., Y. (2022). Fermentation Process Of Beef Effected By Its Physical And Chemical Traits. Food Resources Vol. 10 (2022) № 18. [CrossRef]
- Terjung, N. , Witte, F., Volker, H. (2021). The dry aged beef paradox: Why dry aging is sometimes not better than wet aging. Meat Science 172: 108355.
- Renyu, Z.; Michelle J.Y., Y.; Alastair B., R.; Mustafa M., F. (2022). Mechanisms and strategies to tailor dry-aged meat flavour. Trends in Food Science & Technology, Volumen 119, Páginas 400-411, ISSN 0924-2244. [CrossRef]
- Guatava Redondo, C. , & Trujillo Trujillo, L. (2011). Standardization of maturation conditions at laboratory level of buffalo (Bubalus Bubalis) meat in second (flank) and third (lizard) quality cuts. Bogotá, Colombia: Universidad de la Salle.
- Ilze, G.; Raitis, K.; Liga, S.; Sanita, S. (2019). Changes of Physical Parameters of Meat During Wet Ageing. Foodbalt. Department of Food Technology, Faculty of Food Technology, Latvia University of Life Science and Technologies. p. 61-65.
- Kim, M; Choe, J; Jung Lee, H; Yoon, Y; Yoon, S; Jo, C. (2019). Efectos del envejecimiento y del método de envejecimiento sobre los rasgos fisicoquímicos y sensoriales de diferentes cortes de carne de vacuno. Food Sci. Anim. Resour. 2019 Febrero 39(1):54~64. [CrossRef]
- Zavadlav, Sandra, Marijana Blažić, Franco Van de Velde, Charito Vignatti, Cecilia Fenoglio, Andrea M. Piagentini, María E. Pirovani, Cristina M. Perotti, Danijela Bursać Kovačević, and Predrag Putnik. 2020. “Sous-Vide as a Technique for Preparing Healthy and High-Quality Vegetable and Seafood Products”. Foods 9, no. 11: 1537. [CrossRef]
- Urbani, V; Biolatto, A; Palladino, M. (2022). Influencia de la dieta con taninos y del almacenamiento refrigerado sobre la calidad sensorial del músculo semitendinosus cocido sous-vide de vaca de refugio. RIA Vol. 48 n. º 3 Noviembre 2022, Argentina.
- Kurp, L.; Danowska-Oziewicz, M.; Kebukowska, L. (2022). Sous Vide Cooking Effects on Physicochemical, Microbiological and Sensory Characteristics of Pork Loin. Appl. Sci. 2022, 12, 2365. [Google Scholar] [CrossRef]
- Gil, M.; Rudy, M.; Stanisławczyk, R.; Duma-Kocan, P. (2022). Effect of Traditional Cooking and Sous Vide Heat Treatment, Cold Storage Time and Muscle on Physicochemical and Sensory Properties of Beef. Molecules. 2022, 27, 7307. [Google Scholar] [CrossRef]
- ZhenKun Cui, Han Yan, Tatiana Manoli, Haizhen Mo, JiCai BI, and Hao Zhang. (2021). Review Advantages and challenges of sous vide cooking. Food Science and Technology Research, 27 (1), 25–34, 2021. [CrossRef]
- Geileskey, A. ; Rey, RD; Corte, D. ; Pinto, P.; Ledward, DA. (1998). The kinetics of hemoprotein formation of cooked meat hemoproteins in meat and model systems. Meat Science. 1998, 48, 189–199. [Google Scholar]
- King, N.J.; Whyte, R. (2006). ¿Parece cocinado? Una revisión de los factores que influyen en el color de la carne cocinada. J. Food Sci. 2006, 71, R31–R40. [Google Scholar]
- Coria H, J.; Meléndez P, R.; Méndez A, A.; & Arjona R, J. (2020). Changes in myoglobin content in porcine Longissimus thoracis muscle during frozen storage. Revista mexicana de ciencias pecuarias, 11(3), 651-668. 05 February 2021. [CrossRef]
- Xargayó, M. , Fernández, E., Borrisser, F., Trenchs, O., & Lagares, J. (2021). Sous vide: Una revolución en la cocción industrial [PDF]. Metalquimia. Recuperado de /mnt/data/sous-vide-una-revolucion-en-la-coccion-industrial.pdf.
- Lound, L. (2020). Design and development of food products with Sous Vide technology. National University of Entre Rios. ISSN 2250-4559. Eva Perón 24; 3260 FIB Concepción del Uruguay, Entre Ríos, Argentina. 83-96.
- Pacheco P, W. A. , Colorado A, Z. D., Agudelo C, E. L., Verbel M, M. L., Ruíz L, R., Palacio P, J. C., & Vélez A, L. M. (2023). Effect of two cooking systems on heat transfer and microbial lethality during cooking of hams. Agricultural Science and Technology, 24(1), e2834. [CrossRef]
- Perry, N. (2011). Dry aging beef. International Journal of Gastronomy and Food Science 1 (2012) 78-80.
- Oliván, M. , Sierra, V., & García, P. (2013). Effect of maturation time on the organoleptic quality of beef [PDF]. Regional Service for Agri-Food Research and Development. Retrieved from /mnt/data/olivanetal2013SERIDA.pdf.
- Meat technology update. Cutting edge technology for the meat processing industry (2010). Dry ageing of beef.
- Galletly, J. (2016). Design of dry-aged beef and review of good manufacturing practices. Project funded by donor company MLA.
- Dashdorj, D; Tripathi, V; Cho, S; Kim, Y; Hwang, I. Dry aging of beef; Review. Journal of Animal Science and Technology (2016) 58:20. [CrossRef]
- Lee, H; Jang, M; Park, S; Jeong, J; Shim, Y; Kim, J. (2016). Determination of indicators for dry-aged beef. Quality. Food Sci. Anim. Resour. 2019 Dec. 39(6):934~942. [CrossRef]
- Habtu, E. , Mekonnen, B., Kiros, H., Getachew, B., & Fesseha, H. (2020). Meat Tenderization of Efficiency of Papain, Bromelain and Zingiber officinale on Old Aged Beef Carcass of local Zebu cattle. Trends in Technical & Scientific Research, 4(1), 10-16. Recuperado de https://ideas.repec.org/a/adp/oattsr/v4y2020i1p10-16.html.
- Pierre, E.M.; Benoit, G.; Muriel, D.; Sandrine, P.; Patrick, S.; Jean-François, H.; Emmanuel, A. (2022). Evolution of Sensory Properties of Beef during Long Dry Ageing. Foods 2022, 11, 2822. [Google Scholar] [CrossRef]
- Zhang, R; Yoo, M; Ross, A; Farouk, M. (2022). Mechanisms and strategies to tailor dry-aged meat flavour. Trends in Food Science & Technology 119 (2022) 400–411. [CrossRef]
- Lancaster, J; Smart, J; Van Buren, J; Buseman, B; Weber, T; Insausti, K; Nasados, J; Glaze, B; Prince, W; Colle, M; Bass, P. (2022). Assessment of dry-aged beef from commercial aging locations across the United States. International Journal of Gastronomy and Food Science 27 (2022) 100466.
- Ryu, S; Shin, M; Cho, S; Hwang, I; Kim, Y; Oh, S. Molecular Characterization of Microbial and Fungal Communities on Dry-Aged Beef of Hanwoo Using Metagenomic Analysis. (2020). Foods 2020, 9, 1571; [CrossRef]
- Ryu, S.; Park, M.R.; Maburutse, B.E. ; Lee,W. J.; Park, D.-J.; Cho, S.; Hwang, I.; Oh, S.; Kim, Y. (2018). Diversidad y Características de la Comunidad Microbiológica de la Carne en la Carne de Res Envejecida en Seco. J. Microbiol. Biotechnol. 2018, 28, 105–108. [Google Scholar]
- Ribeiro, A.; Oliveira, I.; Soares, K.; Silva, F.; Teixeira, P.; Saraiva, C. (2023). Microbial, Physicochemical Profile and Sensory Perception of Dry-Aged Beef Quality: A Preliminary Portuguese Contribution to the Validation of the Dry Aging Process. Foods 2023, 12, 4514. [Google Scholar] [CrossRef]
- Gowda, T; Zutter, L; Royen, G; Damme, I. (2022). Exploring the microbiological quality and safety of dry-aged beef: A cross-sectional study of loin surfaces during ripening and dry-aged beef steaks from commercial meat companies in Belgium. Food Microbiology 102 (2022) 103919.
- da Silva, A.C.M.; Pena, P.d.O.; Pflanzer, S.B.; Nascimento, M.d.S.D. (2019). Effect of different dry aging temperatures on Listeria innocuaas surrogate for Listeria monocytogenes. Meat Sci. 2019, 157, 107884. [Google Scholar]
- Matos, L; Silva, A; Perez, V; Gonçalves, J; Silva, M; Bertelli, S; Rezende, J; Gini, C; Murad, N; Mendes, M; Girone, N. (2024). Comparison of bacterial diversity in wet- and dry-aged beef using traditional microbiology and next generation sequencing. The Microbe 2 (2024) 100035.
- Capouya, R. , Mitchell, T., Clark, D.I., Clark, D.L., Bass, P., (2020). A survey of microbial communities on dry-aged beef in commercial meat processing facilities. Meat Muscle Biol. 4. [CrossRef]
- Przybylski, W.; Jaworska, D.; Płecha, M.; Dukaczewska, K.; Ostrowski, G.; Sałek, P.; Sawicki, K.; Pawłowska, J. Fungal Biostarter. (2023). Effect on the Quality of Dry-Aged Beef. Foods 2023, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- ACMSF. Recommendations for the Production of Prepackaged Chilled Food. Advisory Committee on the Microbial Safety of Foods, 1995.
- NSW Food Authority. Sous Vide-Food Safety Precautions for Restaurants; NSW Food Authority: Silverwater, NSW, Australia, 2022; pp. 3-33; Available at: https://www.foodauthority.nsw.gov.au/sites/default/files/_Documents/scienceandtechnical/sousvide_food_safey_precautions.pdf.
- Cho, D.K.; Lee, B.; Oh, H.; Lee, J.S.; Kim, Y.S.; Choi, Y.M. (2020). Effect of searing process on quality characteristics and storage stability of sous-vide cooked pork patties. Foods 2020, 9, 1011. [Google Scholar]
- Xiong, Y.L. (2004). Chemical and physical characteristics of meat—Protein Functionality. In Encyclopedia of Meat Sciences; Jensen, W.K., Devine, C., Dikeman, M., Eds.; Academic Press: Cambridge, UK, 2004; pp. 218–225. [Google Scholar]
- Trees, E; Skytta, E; Mokkila, M; Kinnunen, A; Lindstro, M; Lahteenma, L; Ahvenainen, R; Korkeala, H. (2000). Safety Evaluation of Sous Vide-Processed Products with Respect to Nonproteolytic Clostridium botulinum by Use of Challenge Studies and Predictive Microbiological Models. Applied And Environmental Microbiology, Jan. 2000, p. 223 – 229.
- Diaz, M, P. (2009). Quality and Deterioration of Sous Vide Dishes Prepared From Meat and Fish and Stored in Refrigeration. Doctoral thesis. University of Murcia. Department of Food Technology, Nutrition and Bromatology.
- Rinaldi, M.; Dall’Asta, C.; Paciulli, M.; Cirlini, M.; Manzi, C.; Chiavaro, E. (2014). A novel time/temperature approach to sous vide cooking of beef muscle. Food Bioprocess Technol. 2014, 7, 2969–2977. [Google Scholar]
- Latoch, A.; Głuchowski, A.; Czarniecka-Skubina, E. (2023). Sous-Vide as an Alternative Method of Cooking to Improve the Quality of Meat: A Review. Foods 2023, 12, 3110. [Google Scholar] [CrossRef]
- Dry aging: Some guidelines (2020). An essential guide to approach meat maturation with INOX BIM Climatic Cabinets. Wirtex (Professional catering equipment).
- Kim, S.; Kim, J. C.; Park, S.; Kim, J.; Yoon, Y.; Lee, H. (2021). Identification of Microbial Flora in Dry Aged Beef to Evaluate the Rancidity during Dry Aging. Processes 2021, 9, 2049. [Google Scholar] [CrossRef]



| Factor | Level | Response variables |
|---|---|---|
| Dry aging of meat | 28 days 32 days 42 days 45 days |
Microbiological analysis |
| Factor | Level | Response variables |
|---|---|---|
| Cooking | Sous vide | Sensory evaluation Microbiological analysis |
| Treatment | Microbiological analysis * | Result | ||
|---|---|---|---|---|
| Analysis | Method | Specification | ||
| Dry Aging (28 Days) | *NMP fecal coliforms 45 | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | 120(m) - < 1.100(M) | 150 |
| *Coagulase-Positive Staphylococcus Count (CFU/g-mL) | UNE EN ISO 6888 – 1 2000 | 100(m) - 1.000(M) | <100 (+/- 1 CFU**) | |
| Clostridium Sulphite-Reducing Spore Count (CFU/g-mL) | INVIMA:1998. Cap. 2, Num. 10. | 100(m) - 1.000(M) | <10 | |
| Detection of Salmonella in 25g | AOAC OMA 2016.01 ED 21: 2019. | Absence | Absence | |
| Dry Aging (32 Days) | *NMP fecal coliforms 45 | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | 120(m)- < 1.100(M) | 180 |
| *Coagulase-Positive Staphylococcus Count (CFU/g-mL) | UNE EN ISO 6888 – 1 2000 | 100(m) - 1.000(M) | <100 (+/- 1CFU**) | |
| Clostridium Sulphite-Reducing Spore Count (CFU/g-mL) | INVIMA:1998. Cap. 2, Num. 10 | 100(m) - 1.000(M) | <10 | |
| Detection of Salmonella in 25g | AOAC OMA 2016.01 ED 21: 2019. | Absence | Absence | |
| Dry Aging (42 days) | *NMP fecal coliforms 45 | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | 120(m) - < 1.100(M) | 4 |
| *Coagulase-Positive Staphylococcus Count (CFU/g-mL) | UNE EN ISO 6888 – 1 2000 | 100(m) - 1.000(M) | <100 (+/- 1CFU**) | |
| Clostridium Sulphite-Reducing Spore Count (CFU/g-mL) | INVIMA:1998. Cap. 2, Num. 10 | 100(m) - 1.000(M) | <10 | |
| Detection of Salmonella in 25g | AOAC OMA 2016.01 ED 21: 2019. | Absence | Absence | |
| Dry Aging (45 days) | *NMP fecal coliforms 45 | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | 120(m) - < 1.100(M) | 10 |
| *Coagulase-Positive Staphylococcus Count (CFU/g-mL) | UNE EN ISO 6888 – 1 2000 | 100(m) - 1.000(M) | <100 (+/- 1CFU**) | |
| Clostridium Sulphite-Reducing Spore Count (CFU/g-mL) | INVIMA:1998. Cap. 2, Num. 10 | 100(m) - 1.000(M) | <10 | |
| Detection of Salmonella in 25g | AOAC OMA 2016.01 ED 21: 2019 | Absence | Absence | |
| Treatment | Microbiological analysis * | Result | ||
|---|---|---|---|---|
| Analysis | Method | Specification | ||
| Dry Aging (28 Days) | Total Mesophilic Aerobic Count (CFU/g-mL) | AOAC 966.23 ED 21:2019 | <10.000 | (l) 85.000 |
| *NMP total coliform /g-mL | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | <3 | (l) 9 | |
| *NMP fecal coliforms 45/g-mL | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | <3 | (l) 9 | |
| *Coagulase-Positive Staphylococcus Count (CFU/g-mL) | UNE EN ISO 6888 – 1:2000 | <100 | <100 (+/- 1CFU**) | |
| *Bacillus aereus count CFU/g-mL L | UNE EN ISO 7932:2005 | <100 | <100 (+/- 1CFU**) | |
| * Detection of Salmonella in 25g | ISO 6579 – 1:2017 | Absence | Absence | |
| Dry Aging (32 Days) | Total Mesophilic Aerobic Count (CFU/g-mL) | AOAC 966.23 ED 21:2019 | <10.000 | (l) 80.000 |
| *NMP total coliform /g-mL | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | <3 | (l) 10 | |
| *NMP fecal coliforms 45/g-mL | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | <3 | (l) 10 | |
| *Coagulase-Positive Staphylococcus Count (CFU/g-mL) | UNE EN ISO 6888 – 1:2000 | <100 | <100 (+/- 1CFU**) | |
| *Bacillus aereus count CFU/g-mL L | UNE EN ISO 7932:2005 | <100 | <100 (+/- 1CFU**) | |
| * Detection of Salmonella in 25g | ISO 6579 – 1:2017 | Absence | Absence | |
| Dry Aging (42 days) | Total Mesophilic Aerobic Count (CFU/g-mL) | AOAC 966.23 ED 21:2019 | <10.000 | (l) 90.000 |
| *NMP total coliform /g-mL | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | <3 | (l) 23 | |
| *NMP fecal coliforms 45/g-mL | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | <3 | (l) 23 | |
| *Coagulase-Positive Staphylococcus Count (CFU/g-mL) | UNE EN ISO 6888 – 1:2000 | <100 | <100 (+/- 1CFU**) | |
| *Bacillus aereus count CFU/g-mL L | UNE EN ISO 7932:2005 | <100 | <100 (+/- 1CFU**) | |
| * Detection of Salmonella in 25g | ISO 6579 – 1:2017 | Absence | Absence | |
| Dry Aging (45 days) | Total Mesophilic Aerobic Count (CFU/g-mL) | AOAC 966.23 ED 21:2019 | <10.000 | (l) 90.000 |
| *NMP total coliform /g-mL | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | <3 | (l) 23 | |
| *NMP fecal coliforms 45/g-mL | ICMSF:2000 Method 1, Volume 1, Ed. 2:2000 | <3 | (l) 23 | |
| *Coagulase-Positive Staphylococcus Count (CFU/g-mL) | UNE EN ISO 6888 – 1:2000 | <100 | <100 (+/- 1CFU**) | |
| *Bacillus aereus count CFU/g-mL L | UNE EN ISO 7932:2005 | <100 | <100 (+/- 1CFU**) | |
| * Detection of Salmonella in 25g | ISO 6579 – 1:2017 | Absence | Absence | |
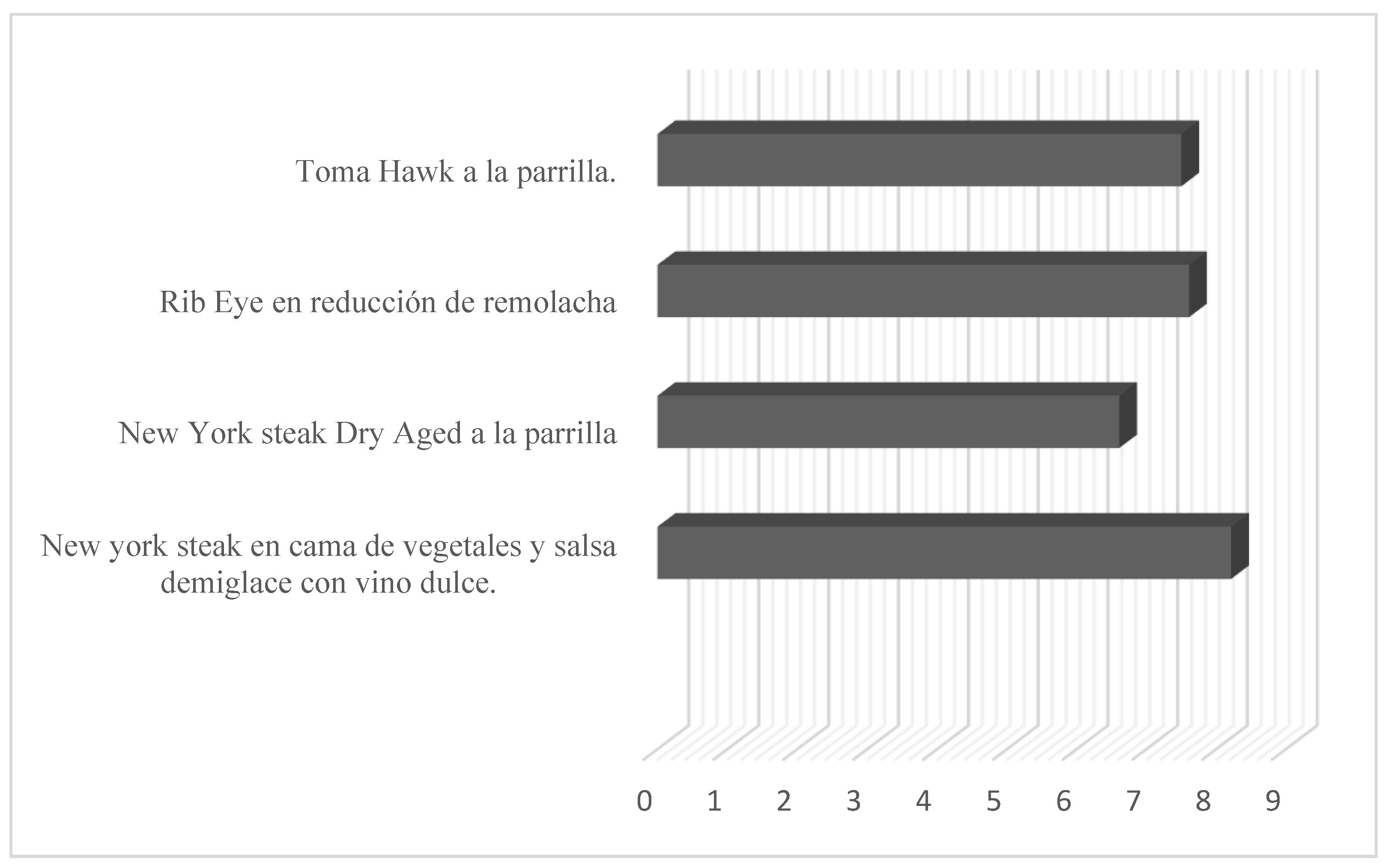
| Culinary recipes | Sensory Attributes | General acceptability | |||
|---|---|---|---|---|---|
| Aroma | Colour | Flavour | Texture | ||
| New York Steak on a bed of vegetables and demiglace sauce with sweet wine | 7,94 ± 1,05 | 8,29 ± 1,01 | 8,37 ± 0,85 | 8,26 ± 1,05 | 8,215 ± 0,99 |
| Grilled dry-aged New York Steak | 6,50± 1,57 | 6,47 ± 1,59 | 6,6 ± 1,97 | 6,86 ± 2,09 | 6,61 ± 1,805 |
| Ribe ye in beetroot reduction | 7,21 ± 1,48 | 7,60 ± 1,16 | 7,77 ± 1,41 | 7,87 ± 1,05 | 7,612 ± 1,275 |
| Grilled Toma hawk | 7,29 ± 1,46 | 7,21 ± 1,40 | 7,21 ± 1,48 | 8,29 ± 1,01 | 7.5 ± 1,34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




