Submitted:
22 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Method
3. Results
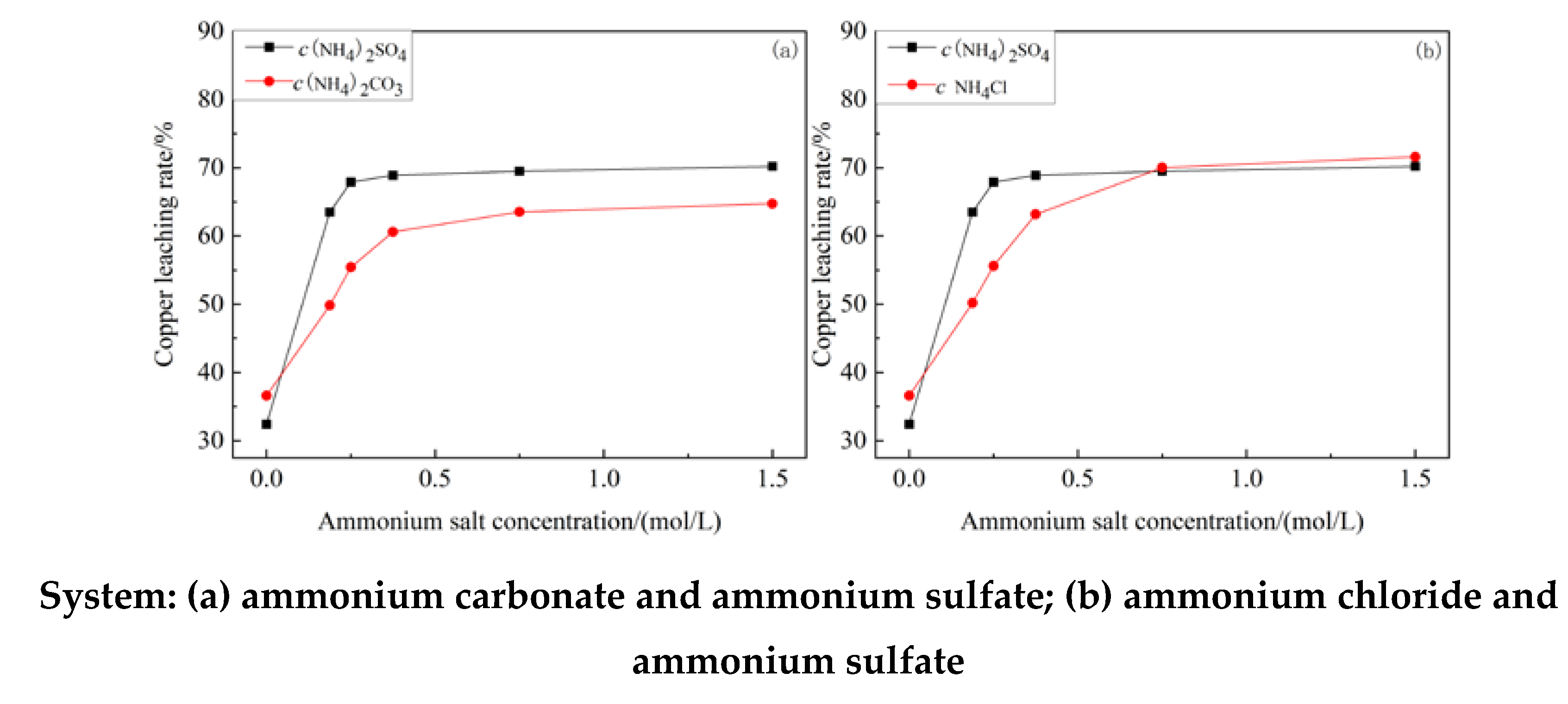
3.1. Influence of Ammonia Concentration on Copper Leaching Rate
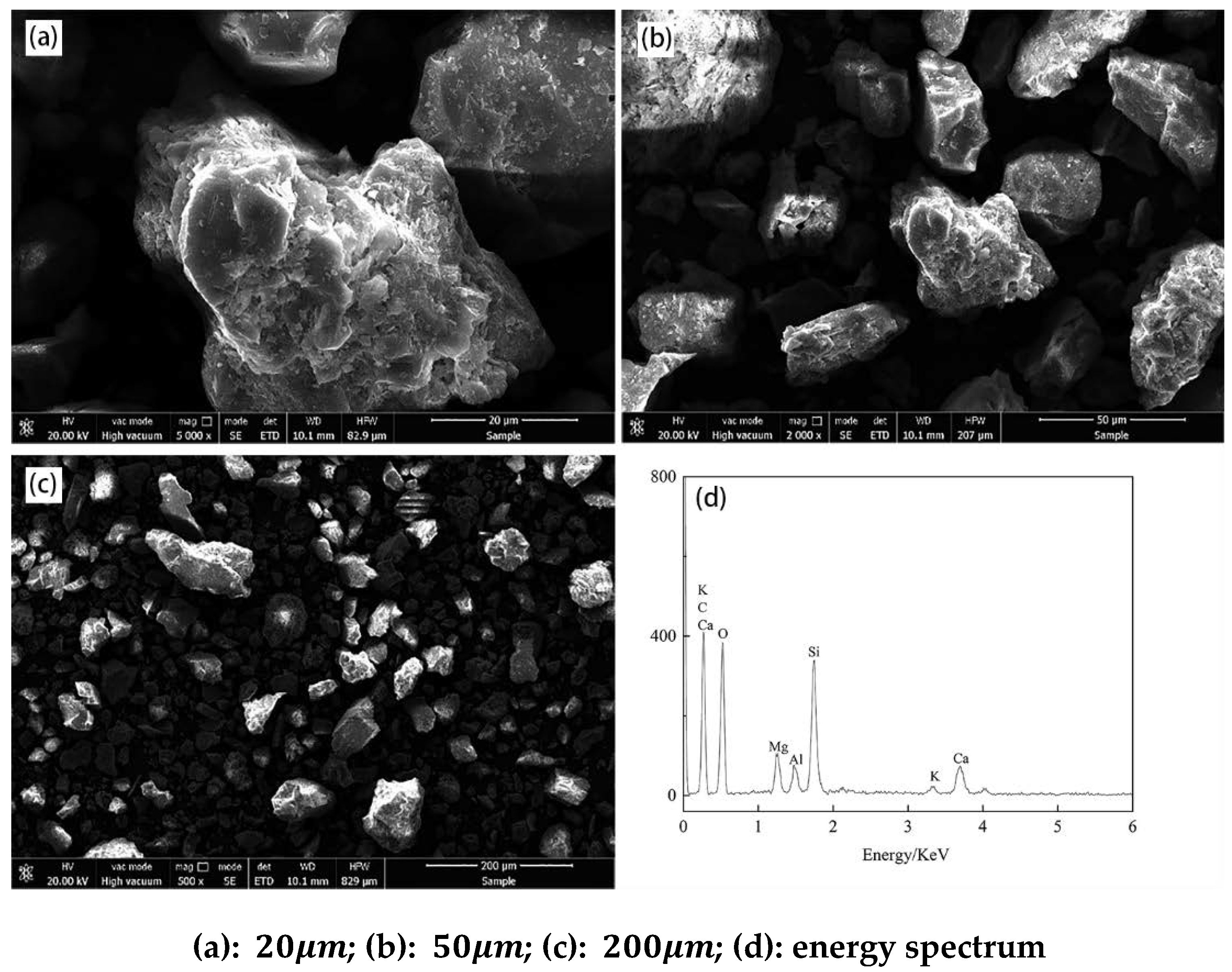
3.2. Structure Characterization of Leaching Residue
4. Discussion
4.1. Leaching Principle
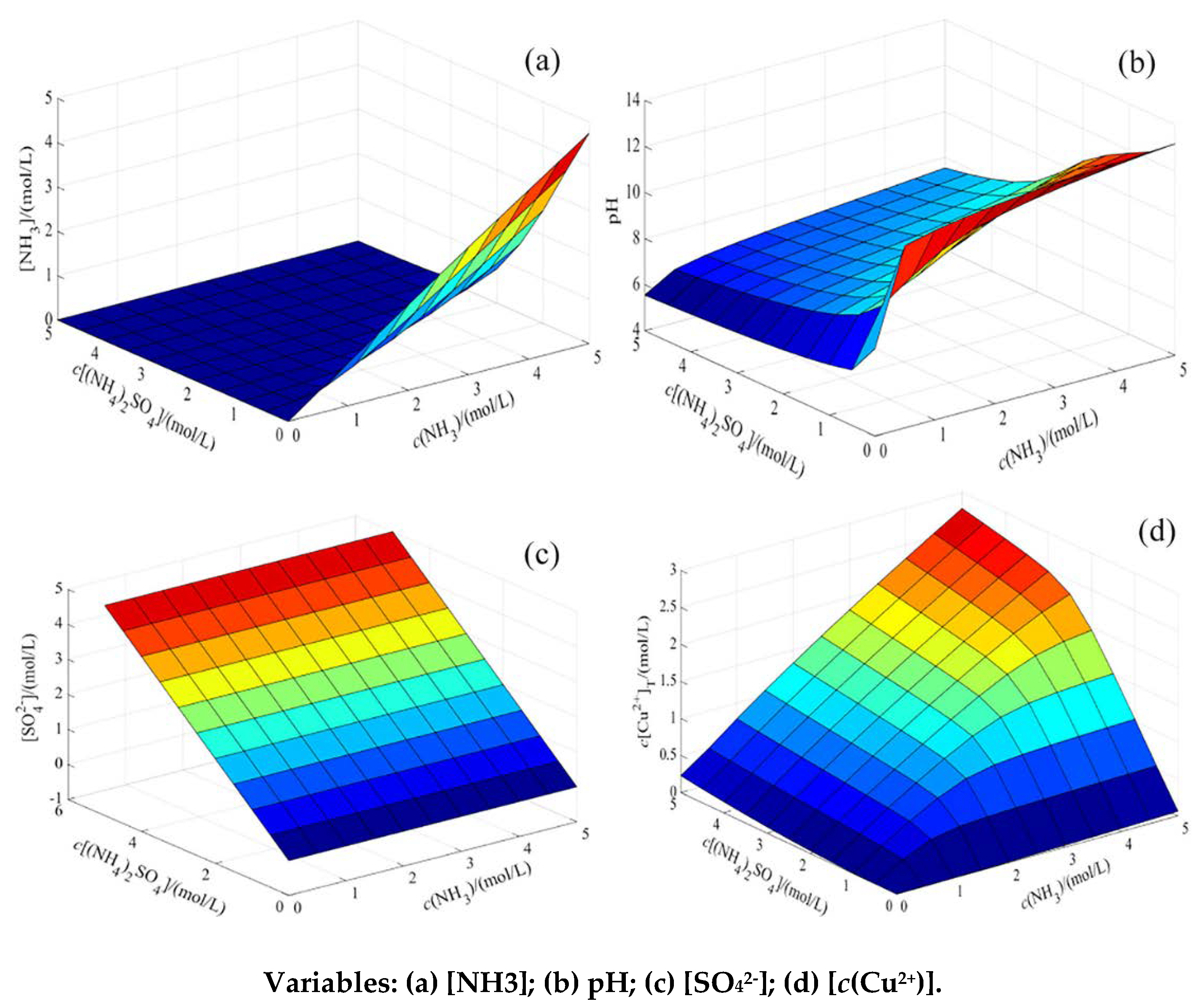
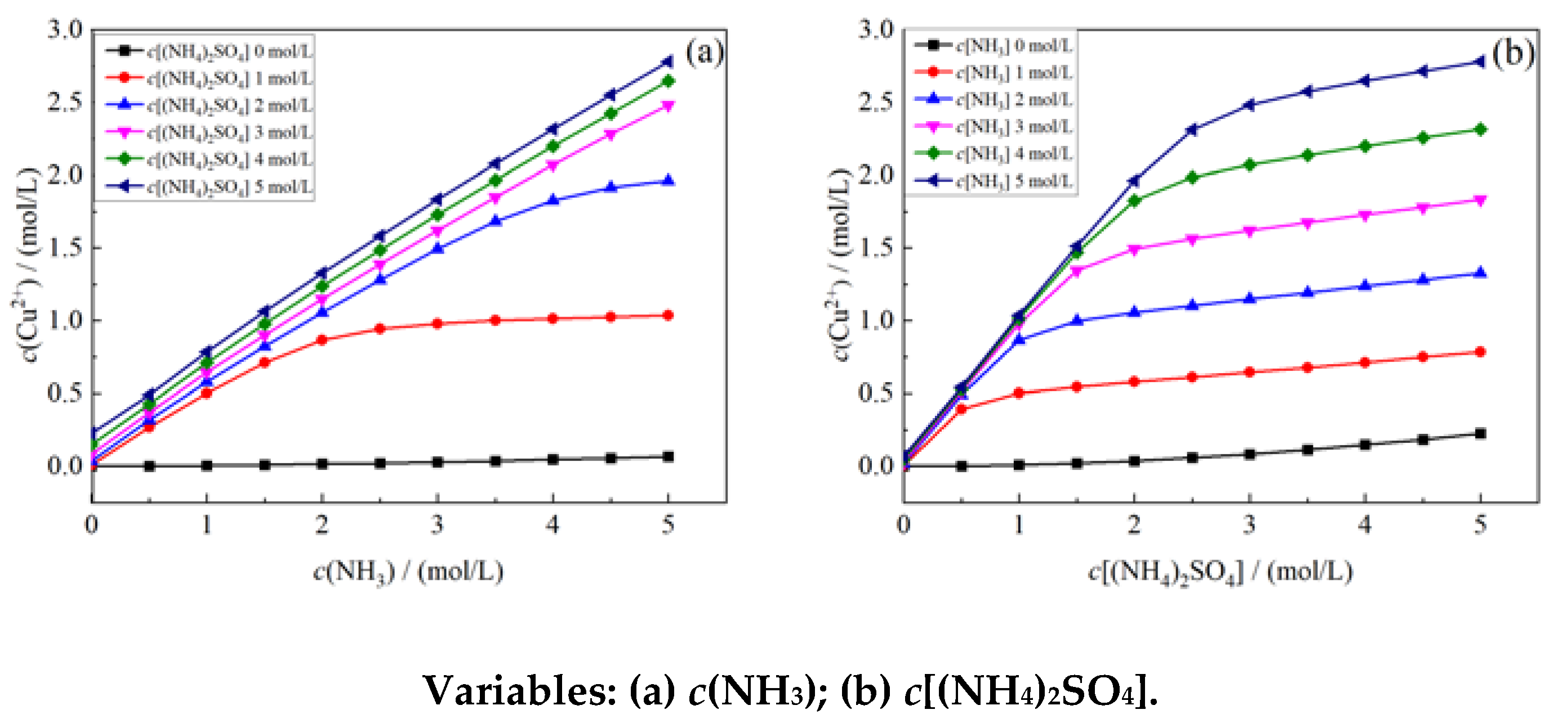
4.2. Thermodynamic Analysis
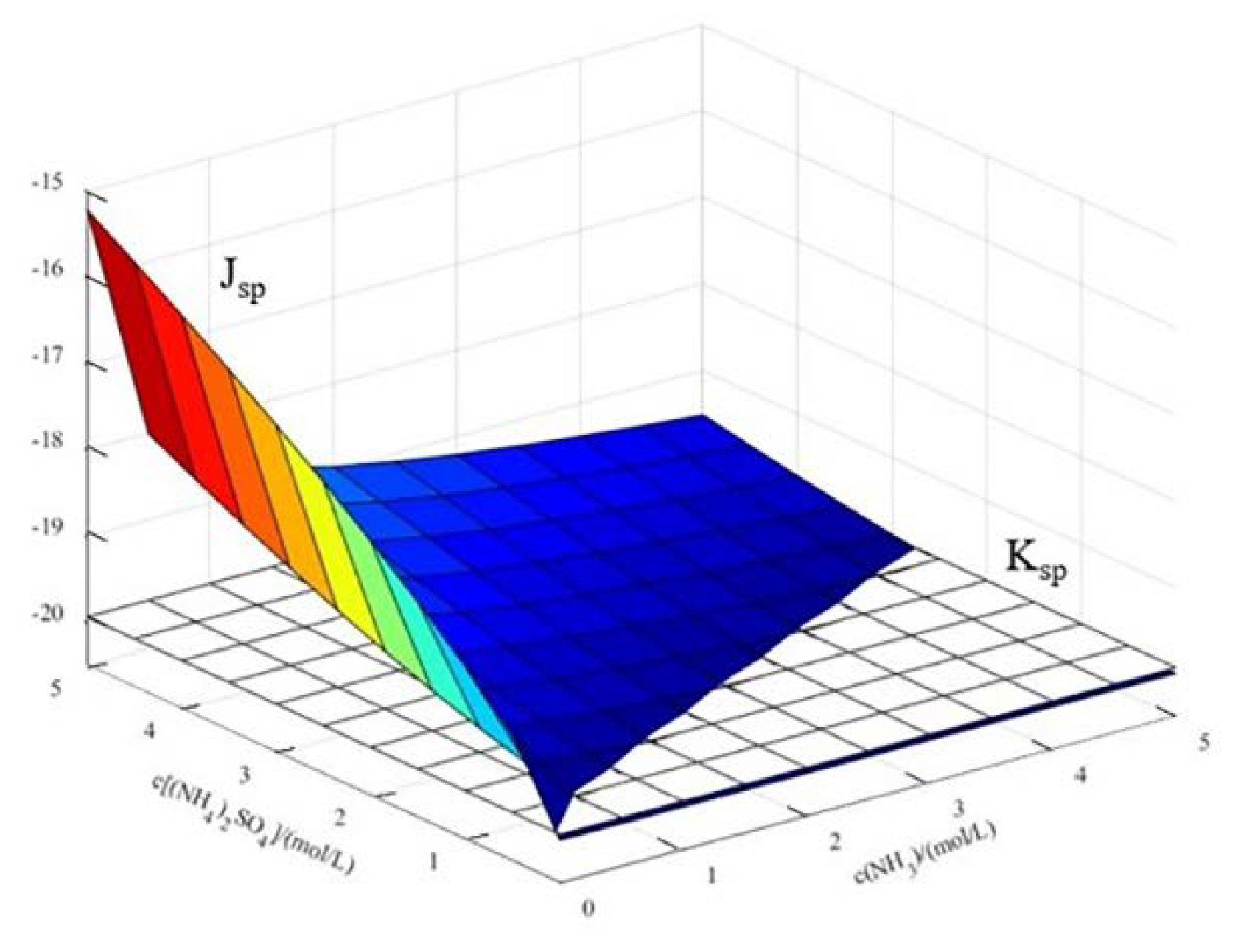
4.1.1. Establishment of Thermodynamic Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, T.; Xu, J.Y. Review of development status of copper-cobalt mining in Congo. Chinese Journal of Nonferrous Metals 2021, 31, 3422. [Google Scholar]
- Pan, C.C.; Gaur, A.P.S. Enhanced electrical conductivity in graphene-copper multilayer composite. Aip Adv 2022, 12, 6. [Google Scholar] [CrossRef]
- Liu, C.H.; Fan, F.Y. Copper resources supply and consumption patterns and trend in the Belt and Road Region. Conservation and Utilization of Mineral Resources 2018, 2, 44. [Google Scholar]
- Han, J.; Xia, P. An analysis of China’s copper resources supply situation in the Post-COVID-19Era. Acta Geoscientica Sinica 2021, 42, 223. [Google Scholar]
- Pietrzyk, S.; Tora, B. Trends in global copper mining – a review. IOP Conference Series: Materials Science and Engineering, Zawiercie, Poland, September 2018. [CrossRef]
- Chen, L.; He, H.P. Experimental study on benefication of one low-grade copper oxide in Xinjiang. Yunnan Metallurgy 2021, 50, 18. [Google Scholar]
- Jiang, S.Q. Distribution of copper resources in the word. World Nonferrous Metals 2018, 2, 1. [Google Scholar]
- Wang, C.Y. Situation and developing trend of copper hydrometallurgy in China. Xinjiang Geology 2001, 19, 281. [Google Scholar]
- Hao, M.; Wang, P. Spatial distribution of copper in-use stocks and flows in China: 1978–2016. J. Clean Prod. 2020, 261, 8. [Google Scholar] [CrossRef]
- Han, J.W.; Liu, W. Influence of NH4HF2 activation on leaching of low-grade complex copper ore in NH3-NH4Cl solution. Sep. Purif. Technol. 2017, 181, 29. [Google Scholar] [CrossRef]
- Li, Q.; Gao, W. Direct reduction leaching of a low-grade copper-cobalt oxide ore. Mining and Metallurgy 2019, 28, 60. [Google Scholar]
- Deng, J.S.; Wen, S.M. Extracting copper from copper oxide ore by a zwitterionic reagent and dissolution kinetics. INT J MIN MET MATER 2015, 22, 241. [Google Scholar] [CrossRef]
- Bingöl, D.; Canbazogˇlu, M. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching. HYDROMETALLURGY 2005, 76, 55. [Google Scholar] [CrossRef]
- Gargul, K. Ammonia leaching of slag from direct-to-blister copper smelting technology. AIMS Materials Science 2020, 7, 565. [Google Scholar] [CrossRef]
- Çalban, T.; Çolak, S. Optimization of leaching of copper from oxidized copper ore in NH3-(NH4)2SO4 medium. CHEM ENG COMMUN 2005, 192, 1515. [Google Scholar] [CrossRef]
- Cao, C.F.; Wang, X. Thermodynamic analysis of leaching malachite in CuO-CO2-NH3-H2O system. Nonferr.Met.Sci.Eng. 2014, 5, 82. [Google Scholar]
- Liu, W.; Tang, M.T.; Tang, C.B.; He, J.; Yang, S.H.; Yang, J.G. Thermodynamics of solubility of Cu2(OH)2CO3 in ammonia-ammonium chloride-ethylenediamine(En)-water system. T NONFERR METAL SOC. 2010, 20, 336. [Google Scholar] [CrossRef]
- Robert, M.S.; Arthur, E.M. Critical Stability Constants, 1th ed.; Plenum Press: New York and London, 1976; p. 31. [Google Scholar]
- Li, Y.; Kawashima, N. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. ADV COLLOID INTERFAC 2013, 197, 1. [Google Scholar] [CrossRef]

| SiO2 | CaO | MgO | Al2O3 | K2O | Fe2O3 | CuO |
| 56.1202 | 18.3875 | 11.6775 | 8.5244 | 1.6679 | 1.3900 | 1.2818 |
| TiO2 | P2O5 | Co2O3 | MnO | SO3 | ZrO2 | Na2O |
| 0.5054 | 0.1725 | 0.1199 | 0.1054 | 0.0308 | 0.0141 | 0.0023 |
| Type | Stability constant lgβ |
| Cu(NH3)2+ | 4.24 |
| Cu(NH3)22+ | 7.83 |
| Cu(NH3)32+ | 10.80 |
| Cu(NH3)42+ | 13.00 |
| Cu(NH3)52+ | 12.43 |
| Cu(OH)+ | 6.30 |
| Cu(OH)2(aq) | 12.80 |
| Cu(OH)3- | 14.50 |
| Cu(OH)42- | 15.60 |
| Cu2(OH)22+ | 17.28 |
| Cu(NH3)3(OH)+ | 14.90 |
| Cu(NH3)2(OH)2(aq) | 15.70 |
| Cu(NH3)(OH)3- | 16.30 |
| HCO3- | 9.56 |
| H2CO3 | 15.98 |
| NH4+ | 9.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




