Submitted:
21 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
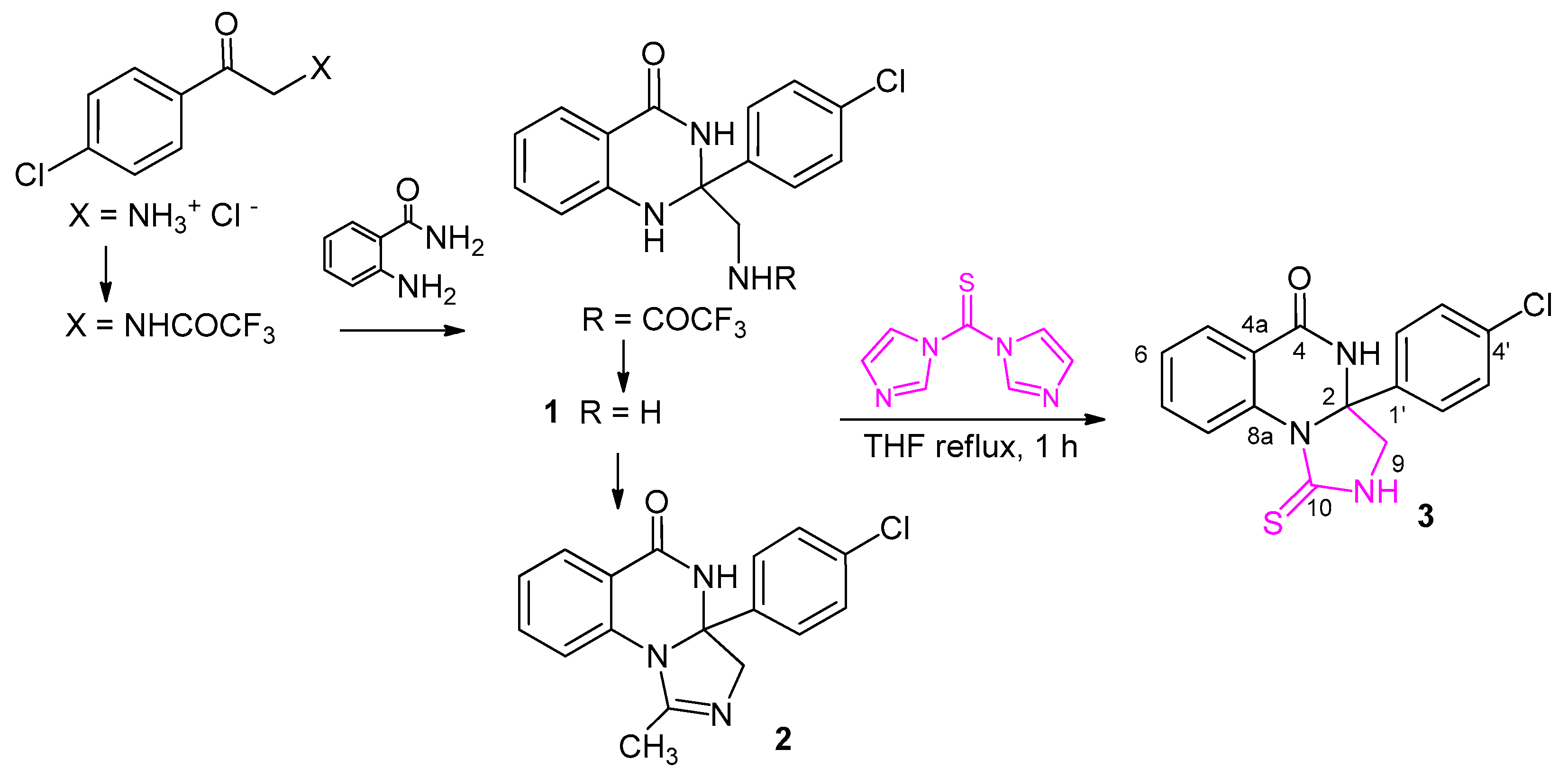
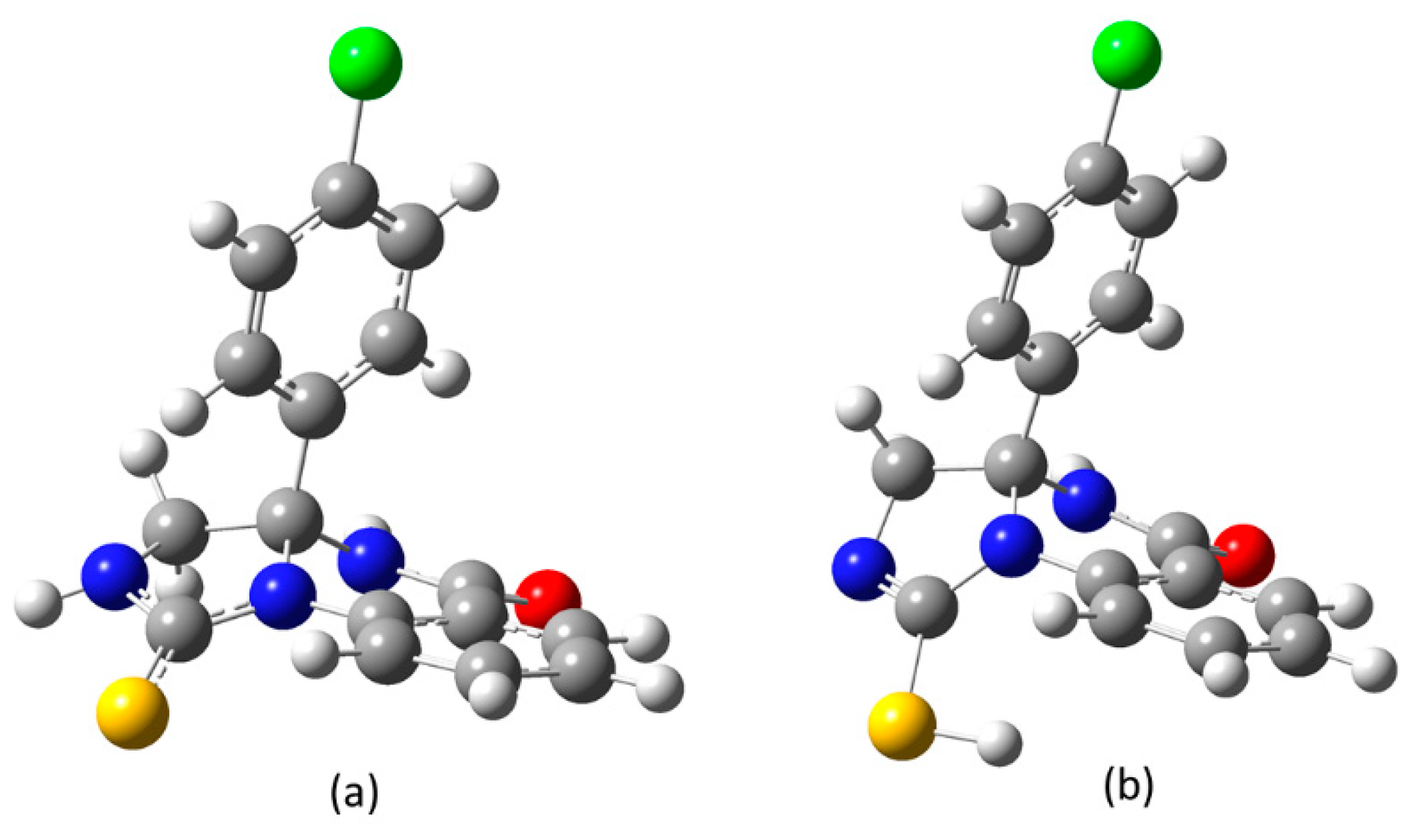
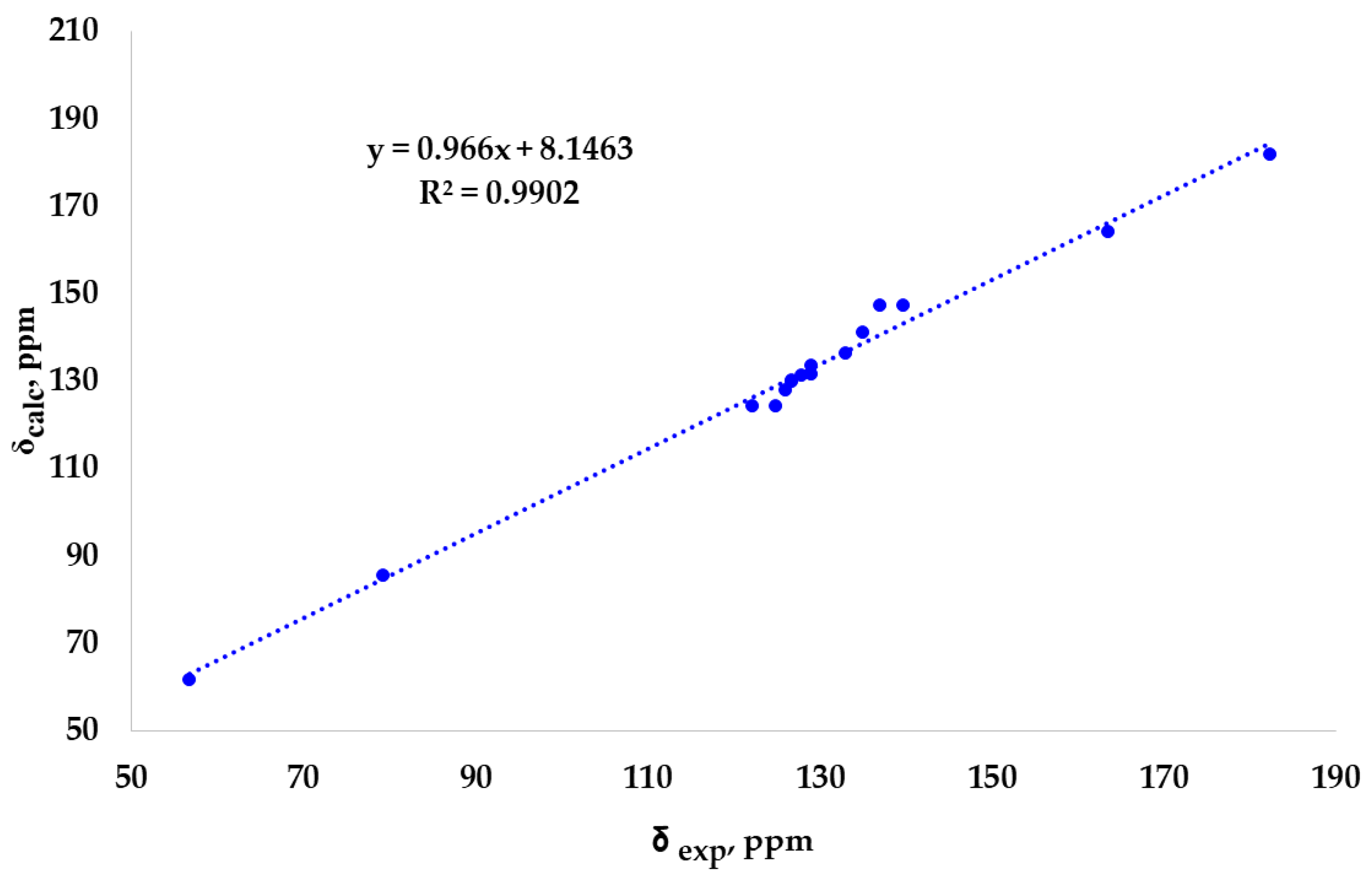
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. Synthesis of 3a-(4-chlorophenyl)-1-thioxo-2,3,3a,4-tetrahydroimidazo[1,5-a] quinazolin-5(1H)-one (3)
3.2. DFT Calculation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riccardo Ronchetti, R.; Moroni, G.; Carotti, A.; Gioiello, A.; Camaioni, E. Recent advances in urea- and thiourea-containing compounds: focus on innovative approaches in medicinal chemistry and organic synthesis. RSC Med. Chem. 2021, 12, 1046–1064. [Google Scholar] [CrossRef] [PubMed]
- Pitta, M.G.R.; Pitta, M.G.R.; Barreto De Melo Rego, J.B.; Galdino, L.S. The Evolution of Drugs on Schistosoma Treatment: Looking to the Past to Improve the Future. Mini-Rev. Med. Chem. 2013, 13, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Savjani, J.K.; Gajjar, A.K. Pharmaceutical Importance and Synthetic Strategies for Imidazolidine-2-thione and Imidazole-2-thione Derivatives. Pakistan J Biol. Sci. 2011, 14, 1076–1089. [Google Scholar] [CrossRef]
- García-Vázquez, J.A.; Sousa-Pedrares, A.; Carabel, M.; Romero, J.; Sousa, A. Electrochemical Synthesis and Structural Characterization of Copper and Silver Complexes of Imidazolidine-2-Thione Ligands. Polyhedron 2005, 24, 2043–2054. [Google Scholar] [CrossRef]
- Lobana, T.S.; Aulakh, J.K.; Sood, H.; Arora, D.S.; Garcia-Santos, I.; Kaur, M.; Duff, C.E.; Jasinski, J.P. Synthesis, Structures and Antimicrobial Activity of Copper Derivatives of N -Substituted Imidazolidine-2-Thiones: Unusual Bio-Activity against Staphylococcus epidermidis and Enterococcus faecalis. New J. Chem. 2018, 42, 9886–9900. [Google Scholar] [CrossRef]
- Aulakh, J.K.; Lobana, T.S.; Sood, H.; Arora, D.S.; Smolinski, V.A.; Duff, C.E.; Jasinski, J.P. Synthesis, Structures, and ESI-Mass Studies of Silver(I) Derivatives of Imidazolidine-2-Thiones: Antimicrobial Potential and Biosafety Evaluation. J. Inorg. Biochem. 2018, 178, 18–31. [Google Scholar] [CrossRef]
- Aulakh, J.K.; Lobana, T.S.; Sood, H.; Arora, D.S.; Garcia-Santos, I.; Kaur, M.; Jasinski, J.P. Synthesis, Structures, and Novel Antimicrobial Activity of Silver(I) Halide Complexes of Imidazolidine-2-Thiones. Polyhedron 2020, 175, 114235. [Google Scholar] [CrossRef]
- Espuri, P.F.; Dos Reis, L.L.; De Figueiredo Peloso, E.; Gontijo, V.S.; Colombo, F.A.; Nunes, J.B.; De Oliveira, C.E.; De Almeida, E.T.; Silva, D.E.S.; Bortoletto, J.; Segura, D.F.; Netto, A.V.G.; Marques, M.J. Synthesis and Evaluation of the Antileishmanial Activity of Silver Compounds Containing Imidazolidine-2-Thione. J Biol Inorg Chem 2019, 24, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Ucar, O.; Grześkiewicz, A.M.; Banti, C.; Hadjikakou, S.K.; Ozturk, I.I. Structural Characterization and Biological Evaluation of Antimony(III) and Bismuth(III) Complexes with Imidazolidine-2-Thione. J. Mol. Struct. 2021, 1235, 130270. [Google Scholar] [CrossRef]
- Kuptsova, A.O.; Vinogradova, E.E.; Kravchenko, A.N.; Gazieva, G.A. Methods for Substitution of the Thioxo Group with the Oxo Group in Imidazolidine-2-Thione Derivatives. Russ. Chem. Bul.l 2022, 71, 885–904. [Google Scholar] [CrossRef]
- Trzhtsinskaya, B.V.; Abramova, N.D. Imidazole-2-Thiones: Synthesis, Structure, Properties. Sulfur Rep. 1991, 10, 389–421. [Google Scholar] [CrossRef]
- Öğretir, C.; Öztürk, İ.İ.; Tay, N.F. Quantum Chemical Studies on Tautomerism, Isomerism and Deprotonation of Some 5(6)-Substituted Benzimidazole-2-Thiones. Arkivoc 2007, 75–99. [Google Scholar] [CrossRef]
- Altun, A.; Azeez, N. Structural Elucidation of Sulfur Derivatives of Benzimidazoles by Density Functional Calculations, and Vibrational and NMR Spectral Analyses. Pharm. Anal. Acta 2015, 7, 459–462. [Google Scholar] [CrossRef]
- Defant, A.; Innocenti, N.; Mancini, I. 3a-(4-Chlorophenyl)-1-Methyl-3a,4-Dihydroimidazo[1,5-a]Quinazolin-5(3H)-One: Synthesis and In Silico Evaluation as a Ligand in the µ-Opioid Receptor. Molbank 2023, 2023, M1622. [Google Scholar] [CrossRef]
- Dwarakanath, K.; Sathyanarayana, D.N. Vibrational Spectra and Assignments for the Fundamental Vibrations of Imidazolidine-2-thione and -2-selenone Bull. Chem. Soc. Jpn. 1979, 52, 2699–2704. [Google Scholar] [CrossRef]
- Sifferlen, T.; Rueping, M.; Gademann, K.; Jaun, B.; Seebach, D. β-Thiopeptides: Synthesis, NMR Solution Structure, CD Spectra, and Photochemistry. Helv. Chim. Acta 1999, 82, 2067–2093. [Google Scholar] [CrossRef]
- Kozyra, P.; Kaczor, A.; Karczmarzyk, Z.; Wysocki, W.; Pitucha, M. Experimental and Computational Studies of Tautomerism Pyridine Carbonyl Thiosemicarbazide Derivatives. Struct. Chem. 2023, 34, 1973–1984. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T., Jr.; Kudin, K.N.; Burant, J.C.; et al. Gaussian; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Becke, A.D. Density-Functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. 4. A new dynamical correlation functional and implications for exact exchange mixing. J. Chem. Phys. 1996, 104, 1040–1046. [Google Scholar] [CrossRef]
- Gauss, J. Effects of electron correlation in the calculation of nuclear magnetic resonance chemical shifts. J. Chem. Phys. 1993, 99, 3629–3643. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to K. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comp.Chem., 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 194101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- London, F. The quantic theory of inter-atomic currents in aromatic combinations. J.Phys.Radium 1937, 8, 397–409. [Google Scholar] [CrossRef]
- McWeeny, R. Perturbation Theory for Fock-Dirac Density Matrix. Phys.Rev. 1962, 126, 1028–1034. [Google Scholar] [CrossRef]
- Ditchfield, R. Molecular Orbital Theory of Magnetic Shielding and Magnetic Susceptibility. J. Chem. Phys. 1972, 56, 5688–5691. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism. 1. Gauge-invariant LCAO method for N.M.R. chemical shifts. Mol. Phys, 1974; 27, 789–807. [Google Scholar] [CrossRef]
- Wolinski, K.; Hilton, J.F.; Pulay, P. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A Comparison of Models for Calculating Nuclear Magnetic Resonance Shielding Tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




