Submitted:
20 June 2024
Posted:
21 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
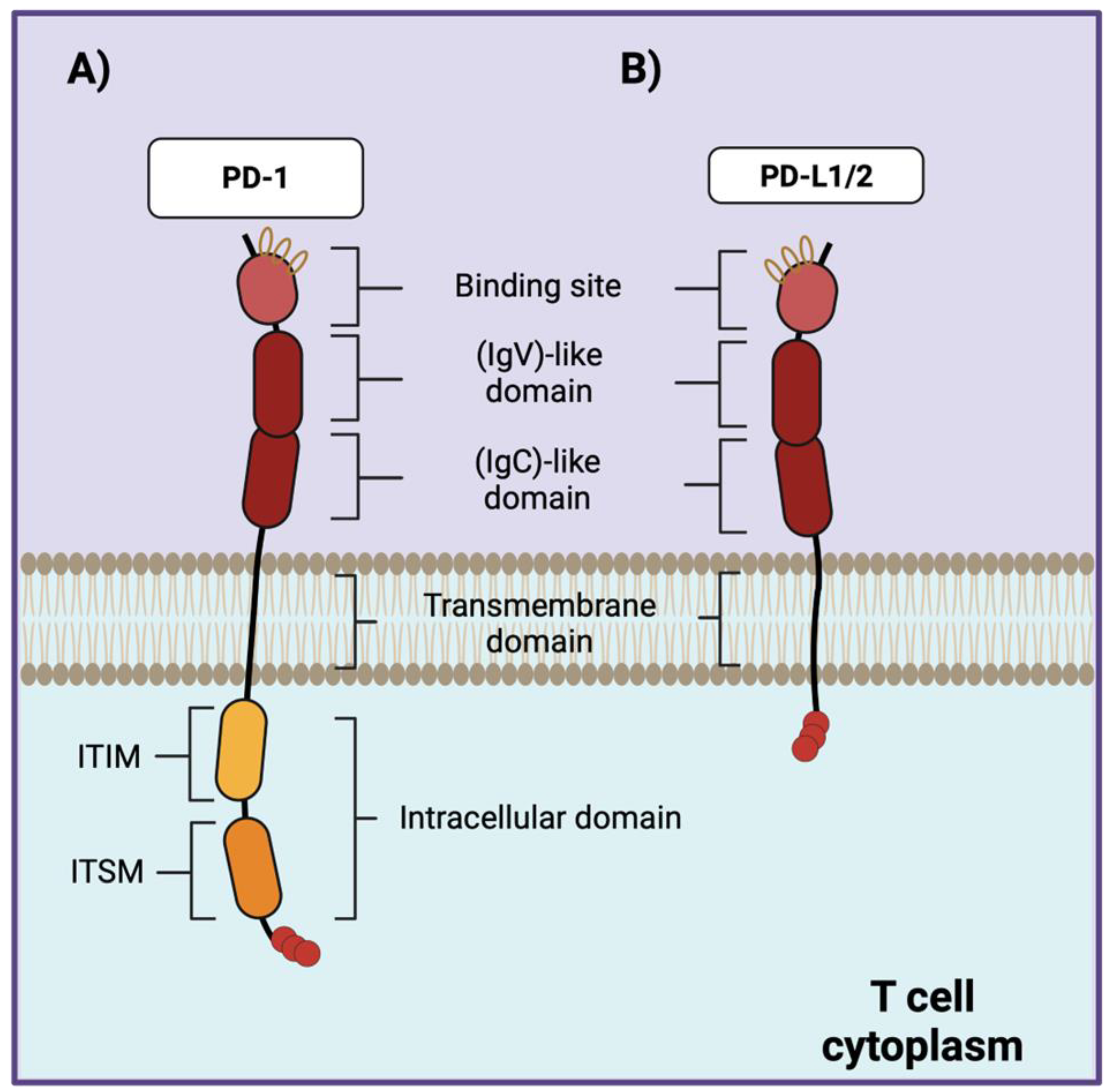
2. PD-1 Expression and Function. What Do We know?
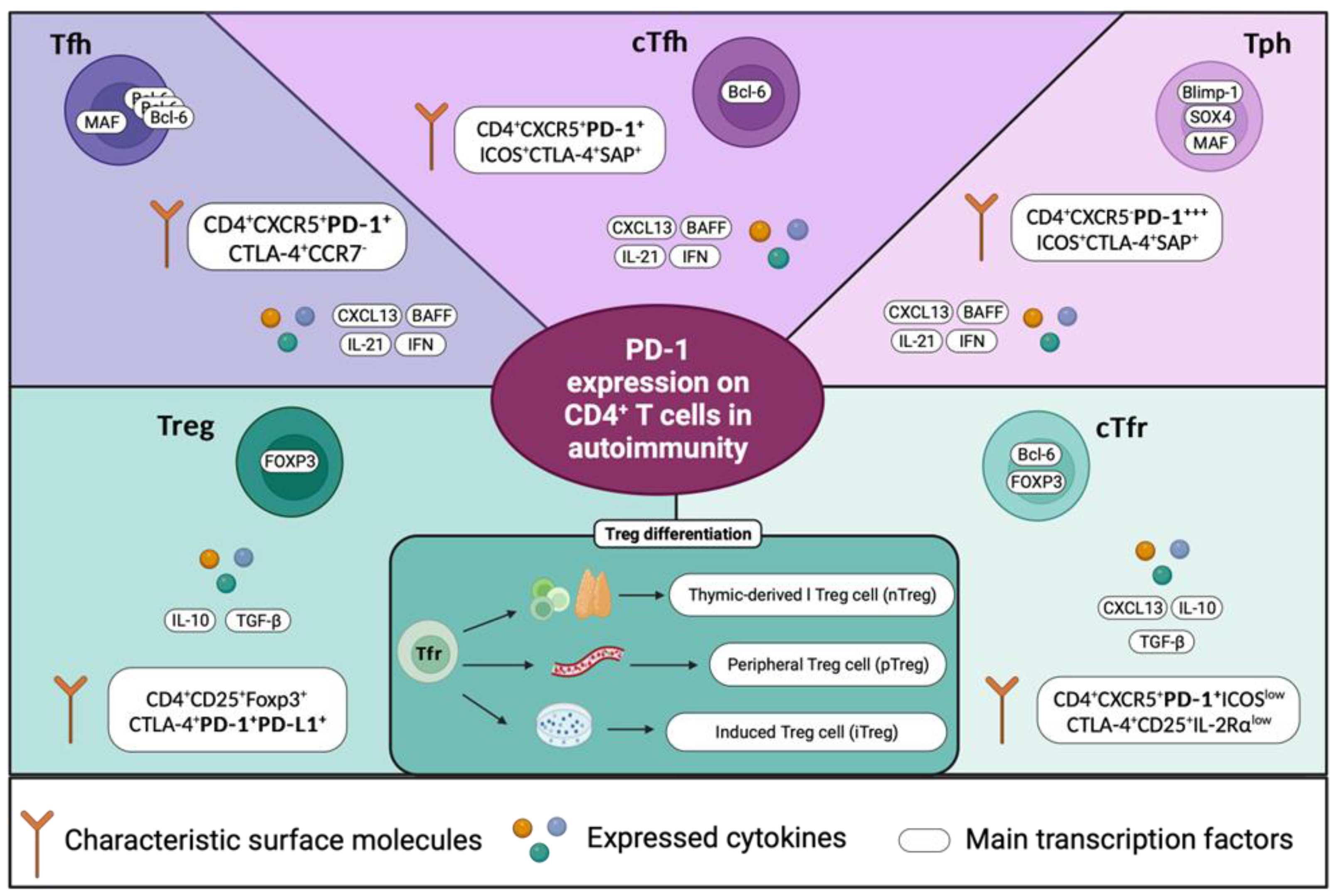
3. PD-1/PD-L1/PD-L2 in Central and Peripheral Tolerance
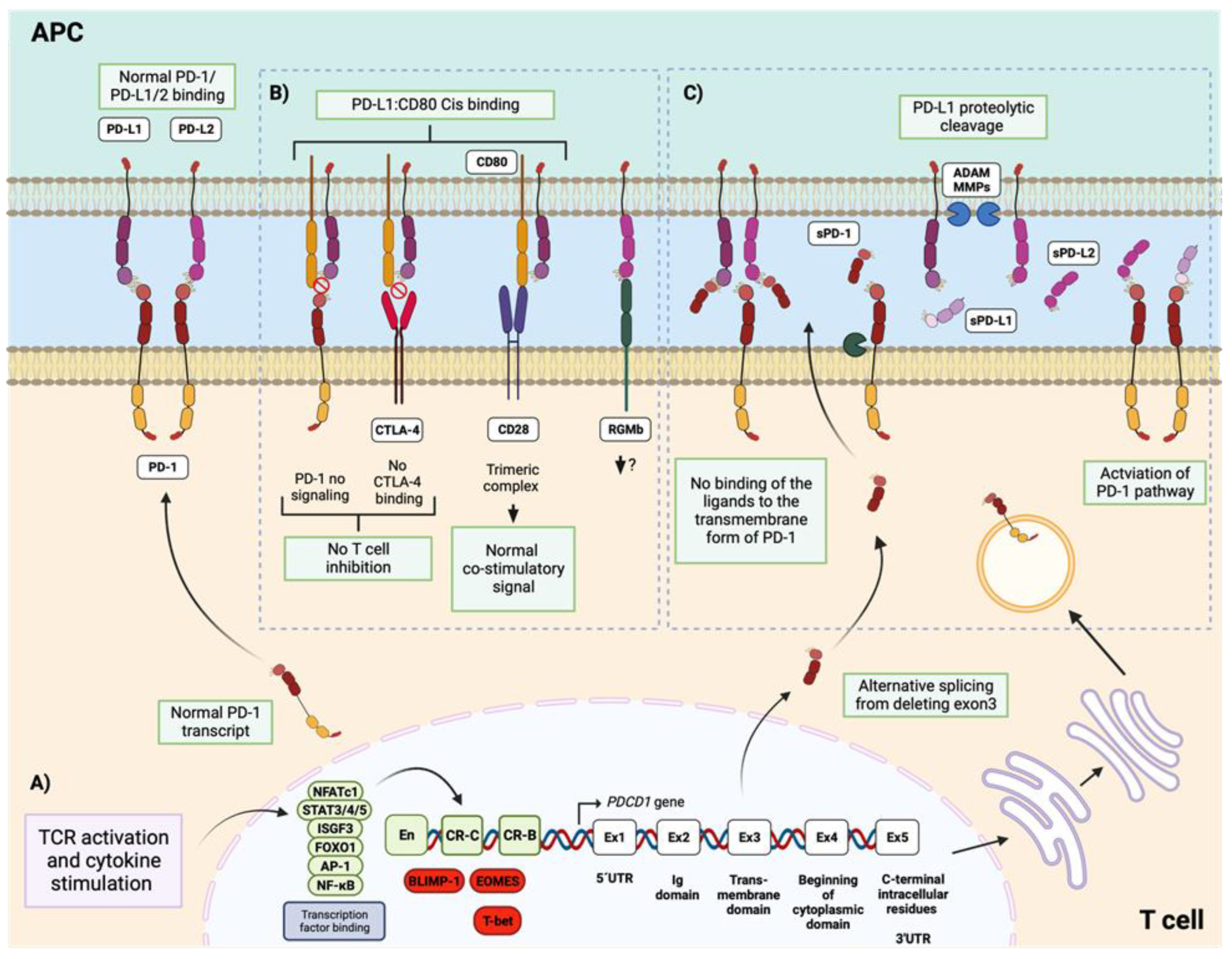
4. Regulation of PD-1 Expression and Its Relationship with the Autoimmune Response in SLE
4.1. Stimulation of PD-1 Expression
4.2. Repression of PD-1 Expression
5. PD-1 Ligands Emerged as a New and Important Focus of Study in the Field of SLE
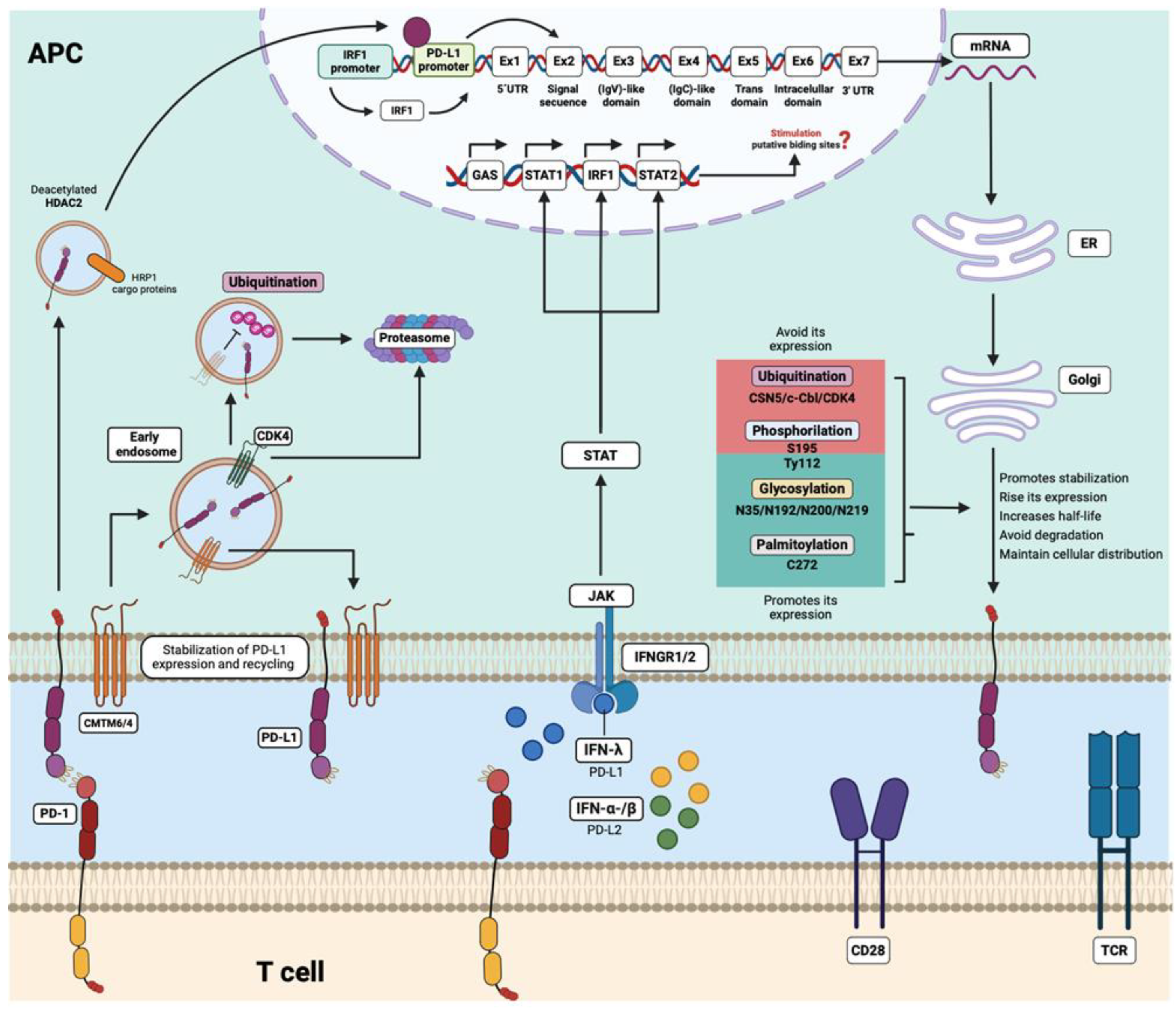
6. Regulation of PD-L1 and PD-L2 Expression and Its Association with the Autoimmune Response
7. Is It Possible that PD-1/PD-L1/2 Has also Another Important Role beyond Inhibition?
8. PD-1 as a Possible Therapeutic Target in SLE
8.1. Accessibility between PD-1 and Its Ligands
8.2. PD-1 Agonists
9. Conclusions
Author Contributions
Funding
Acknowledgments
Data Availability Statement
Conflicts of Interest
References
- Fugger L, Jensen LT, Rossjohn J. Challenges, Progress, and Prospects of Developing Therapies to Treat Autoimmune Diseases. Cell [Internet]. 2020 Apr 2 [cited 2023 Aug 8];181(1):63–80. Available from: http://www.cell.com/article/S0092867420302695/fulltext.
- Tsokos GC, Lo MS, Reis PC, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nature Reviews Rheumatology 2016 12:12 [Internet]. 2016 Nov 22 [cited 2024 May 13];12(12):716–30. Available from: https://www.nature.com/articles/nrrheum.2016.186.
- Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nature Reviews Rheumatology 2020 16:10 [Internet]. 2020 Sep 3 [cited 2024 Jan 8];16(10):565–79. Available from: https://www.nature.com/articles/s41584-020-0480-7.
- Burke KP, Patterson DG, Liang D, Sharpe AH. Immune checkpoint receptors in autoimmunity. Curr Opin Immunol. 2023 Feb 1;80:102283.
- Reynoso ED, Elpek KG, Francisco L, Bronson R, Bellemare-Pelletier A, Sharpe AH, et al. Intestinal Tolerance Is Converted to Autoimmune Enteritis upon PD-1 Ligand Blockade. The Journal of Immunology [Internet]. 2009 Feb 15 [cited 2023 Sep 21];182(4):2102–12. Available from:. [CrossRef]
- Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR. Programmed Death Ligand 1 Regulates a Critical Checkpoint for Autoimmune Myocarditis and Pneumonitis in MRL Mice. The Journal of Immunology [Internet]. 2008 Aug 15 [cited 2023 Sep 21];181(4):2513–21. Available from:. [CrossRef]
- Salama AD, Chitnis T, Imitola J, Akiba H, Tushima F, Azuma M, et al. Critical Role of the Programmed Death-1 (PD-1) Pathway in Regulation of Experimental Autoimmune Encephalomyelitis. Journal of Experimental Medicine [Internet]. 2003 Jul 7 [cited 2023 Sep 21];198(1):71–8. Available from: http://www.jem.org/cgi/doi/10.1084/jem.20022119.
- Ansari MJI, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, et al. The Programmed Death-1 (PD-1) Pathway Regulates Autoimmune Diabetes in Nonobese Diabetic (NOD) Mice. Journal of Experimental Medicine [Internet]. 2003 Jul 7 [cited 2023 Sep 21];198(1):63–9. Available from: http://www.jem.org/cgi/doi/10.1084/jem.20022125.
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity. 1999 Aug 1;11(2):141–51.
- Kroner A, Mehling M, Hemmer B, Rieckmann P, Toyka K V., Mäurer M, et al. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol [Internet]. 2005 Jul 1 [cited 2023 Sep 21];58(1):50–7. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ana.20514.
- Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Association of programmed cell death 1 polymorphisms and systemic lupus erythematosus: a meta-analysis. [Internet]. 2009 Jan 1 [cited 2023 Sep 21];18(1):9–15. Available from: https://journals.sagepub.com/doi/10.1177/0961203308093923?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed.
- Gao J, Gai N, Wang L, Liu K, Liu XH, Wei LT, et al. Meta-analysis of programmed cell death 1 polymorphisms with systemic lupus erythematosus risk. Oncotarget [Internet]. 2017 Mar 18 [cited 2023 Sep 22];8(22):36885–97. Available from: https://www.oncotarget.com/article/16378/text/.
- Lee YH, Bae SC, Kim JH, Song GG. Meta-analysis of genetic polymorphisms in programmed cell death 1. Associations with rheumatoid arthritis, ankylosing spondylitis, and type 1 diabetes susceptibility. Z Rheumatol [Internet]. 2015 Apr 1 [cited 2023 Sep 22];74(3):230–9. Available from: https://pubmed.ncbi.nlm.nih.gov/24942602/.
- Huang Y, Ba X, Han L, Wang H, Lin W, Chen Z, et al. T peripheral helper cells in autoimmune diseases: What do we know? Front Immunol. 2023;14.
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO Journal. 1992;11(11):3887–95.
- Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1-deficient mice: implication of PD-1 as a negative regulator for B cell responses. Vol. 10, International Immunology. 1998.
- Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med [Internet]. 2003 Dec [cited 2023 Jul 30];9(12):1477–83. Available from: https://pubmed.ncbi.nlm.nih.gov/14595408/.
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity. 1999;11:141–51.
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science [Internet]. 2001 Jan 12 [cited 2023 Jul 30];291(5502):319–22. Available from: https://pubmed.ncbi.nlm.nih.gov/11209085/.
- Jubel JM, Barbati ZR, Burger C, Wirtz DC, Schildberg FA. The Role of PD-1 in Acute and Chronic Infection. Front Immunol. 2020 Mar 24;11:524474.
- Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. New England Journal of Medicine [Internet]. 2016 Nov 3 [cited 2023 Sep 21];375(18):1767–78. Available from: https://www.nejm.org/doi/10.1056/NEJMra1514296.
- Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity [Internet]. 2016 May 17 [cited 2023 Sep 21];44(5):955–72. Available from: http://www.cell.com/article/S107476131630156X/fulltext.
- Chemnitz JM, Parry R V., Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol [Internet]. 2004 Jul 15 [cited 2023 Aug 3];173(2):945–54. Available from: https://pubmed.ncbi.nlm.nih.gov/15240681/.
- Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A [Internet]. 2001 Nov 20 [cited 2023 Aug 3];98(24):13866–71. Available from: https://pubmed.ncbi.nlm.nih.gov/11698646/.
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med [Internet]. 2000 Oct 2 [cited 2023 Aug 2];192(7):1027–34. Available from: https://pubmed.ncbi.nlm.nih.gov/11015443/.
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol [Internet]. 2001 Mar [cited 2023 Aug 2];2(3):261–8. Available from: https://pubmed.ncbi.nlm.nih.gov/11224527/.
- Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J Cell Physiol [Internet]. 2019 Feb 1 [cited 2023 Aug 11];234(2):1313–25. Available from: https://pubmed.ncbi.nlm.nih.gov/30191996/.
- Ahn E, Araki K, Hashimoto M, Li W, Riley JL, Cheung J, et al. Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci U S A [Internet]. 2018 May 1 [cited 2023 Aug 11];115(18):4749–54. Available from: https://pubmed.ncbi.nlm.nih.gov/29654146/.
- Fritz JM, Lenardo MJ. Development of immune checkpoint therapy for cancer. J Exp Med [Internet]. 2019 Jun 1 [cited 2023 Aug 11];216(6):1244–54. Available from: https://pubmed.ncbi.nlm.nih.gov/31068379/.
- Catakovic K, Klieser E, Neureiter D, Geisberger R. T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Communication and Signaling 2017 15:1 [Internet]. 2017 Jan 5 [cited 2023 Sep 21];15(1):1–16. Available from: https://biosignaling.biomedcentral.com/articles/10.1186/s12964-016-0160-z.
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nature Reviews Immunology 2015 15:8 [Internet]. 2015 Jul 24 [cited 2023 Sep 21];15(8):486–99. Available from: https://www.nature.com/articles/nri3862.
- Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol [Internet]. 2015 Apr 1 [cited 2023 Sep 21];36(4):265–76. Available from: http://www.cell.com/article/S1471490615000393/fulltext.
- Wakabayashi G, Lee YC, Luh F, Kuo CN, Chang WC, Yen Y. Development and clinical applications of cancer immunotherapy against PD-1 signaling pathway. J Biomed Sci [Internet]. 2019 Dec 5 [cited 2023 Sep 21];26(1):1–13. Available from: https://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-019-0588-8.
- Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci [Internet]. 2011 Jan 1 [cited 2023 Sep 22];1217(1):45–59. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1749-6632.2010.05919.x.
- Nishimura H, Honjo T, Minato N. Facilitation of β Selection and Modification of Positive Selection in the Thymus of Pd-1–Deficient Mice. Journal of Experimental Medicine [Internet]. 2000 Mar 6 [cited 2023 Sep 22];191(5):891–8. Available from: http://www.jem.org/cgi/current/full/191/5/891.
- Keir ME, Latchman YE, Freeman GJ, Sharpe AH. Programmed Death-1 (PD-1):PD-Ligand 1 Interactions Inhibit TCR-Mediated Positive Selection of Thymocytes. J Immunol [Internet]. 2005 Dec 12 [cited 2023 Sep 22];175(11):7372. Available from: /pmc/articles/PMC2779139/.
- Blank C, Brown I, Marks R, Nishimura H, Honjo T, Gajewski TF. Absence of Programmed Death Receptor 1 Alters Thymic Development and Enhances Generation of CD4/CD8 Double-Negative TCR-Transgenic T Cells. The Journal of Immunology [Internet]. 2003 Nov 1 [cited 2023 Sep 22];171(9):4574–81. Available from:. [CrossRef]
- Policheni AN, Teh CE, Robbins A, Tuzlak S, Strasser A, Gray DHD. PD-1 cooperates with AIRE-mediated tolerance to prevent lethal autoimmune disease. Proc Natl Acad Sci U S A [Internet]. 2022 Apr 12 [cited 2023 Sep 22];119(15):e2120149119. Available from: https://www.pnas.org/doi/abs/10.1073/pnas.2120149119.
- Probst HC, McCoy K, Okazaki T, Honjo T, Van Den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance in vivo through PD-1 and CTLA-4. Probst, H C; McCoy, K; Okazaki, T; Honjo, T; van den Broek, Maries (2005) Resting dendritic cells induce peripheral CD8+ T cell tolerance in vivo through PD-1 and CTLA-4 Nature Immunology, 6:280-286 [Internet]. 2005 [cited 2023 Oct 18];6(3):280–6. Available from: https://www.zora.uzh.ch/id/eprint/136849/.
- Wei X, Niu X. T follicular helper cells in autoimmune diseases. Vol. 134, Journal of Autoimmunity. Academic Press; 2023.
- Mesas-Fernández A, Bodner E, Hilke FJ, Meier K, Ghoreschi K, Solimani F. Interleukin-21 in autoimmune and inflammatory skin diseases. Vol. 53, European Journal of Immunology. John Wiley and Sons Inc; 2023.
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A Fundamental Role for Interleukin-21 in the Generation of T Follicular Helper Cells. Immunity [Internet]. 2008 Jul 18 [cited 2024 May 13];29(1):127–37. Available from: http://www.cell.com/article/S1074761308002744/fulltext.
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T Follicular Helper Cells Is Mediated by Interleukin-21 but Independent of T Helper 1, 2, or 17 Cell Lineages. Immunity [Internet]. 2008 Jul 18 [cited 2024 May 13];29(1):138–49. Available from: http://www.cell.com/article/S1074761308002732/fulltext.
- Zhou H, Hu B, Huang N, Mo X, Li W, Zhang B, et al. Aberrant T cell subsets and cytokines expression profile in systemic lupus erythematosus. Clin Rheumatol [Internet]. 2018 Sep 1 [cited 2023 Dec 2];37(9):2405–13. Available from: https://link.springer.com/article/10.1007/s10067-018-4124-0.
- Han L, Yang X, Yu Y, Wan W, Lv L, Zou H. Associations of circulating CXCR3–PD-1+CD4+T cells with disease activity of systemic lupus erythematosus. Mod Rheumatol [Internet]. 2019 May 4 [cited 2023 Dec 2];29(3):461–9. Available from:. [CrossRef]
- Zhang X, Lindwall E, Gauthier C, Lyman J, Spencer N, Alarakhia A, et al. Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. [Internet]. 2015 Feb 5 [cited 2023 Dec 2];24(9):909–17. Available from: https://journals.sagepub.com/doi/10.1177/0961203314567750?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed.
- Choi JY, Ho JHE, Pasoto SG, Bunin V, Kim ST, Carrasco S, et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: Association with disease activity. Arthritis and Rheumatology. 2015 Apr 1;67(4):988–99.
- Wang L, Zhao P, Ma L, Shan Y, Jiang Z, Wang J, et al. Increased Interleukin 21 and Follicular Helper T-like Cells and Reduced Interleukin 10+ B cells in Patients with New-onset Systemic Lupus Erythematosus. J Rheumatol [Internet]. 2014 Sep 1 [cited 2023 Dec 2];41(9):1781–92. Available from: https://www.jrheum.org/content/41/9/1781.
- Cao G, Chi S, Wang X, Sun J, Zhang Y. CD4+CXCR5+PD-1+ T Follicular Helper Cells Play a Pivotal Role in the Development of Rheumatoid Arthritis. Medical Science Monitor. 2019 Apr 25;25:3032–40.
- Liu R, Wu Q, Su D, Che N, Chen H, Geng L, et al. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res Ther [Internet]. 2012 Nov 23 [cited 2023 Dec 3];14(6):1–12. Available from: https://arthritis-research.biomedcentral.com/articles/10.1186/ar4100.
- Wang X, Yang C, Xu F, Qi L, Wang J, Yang P. Imbalance of circulating Tfr/Tfh ratio in patients with rheumatoid arthritis. Clin Exp Med [Internet]. 2019 Feb 1 [cited 2023 Dec 3];19(1):55–64. Available from: https://link.springer.com/article/10.1007/s10238-018-0530-5.
- Fonseca VR, Romão VC, Agua-Doce A, Santos M, López-Presa D, Ferreira AC, et al. The Ratio of Blood T Follicular Regulatory Cells to T Follicular Helper Cells Marks Ectopic Lymphoid Structure Formation While Activated Follicular Helper T Cells Indicate Disease Activity in Primary Sjögren’s Syndrome. Arthritis and Rheumatology. 2018 May 1;70(5):774–84.
- Kim JW, Lee J, Hong SM, Lee J, Cho M La, Park SH. Circulating CCR7loPD-1hi Follicular Helper T Cells Indicate Disease Activity and Glandular Inflammation in Patients with Primary Sjögren’s Syndrome. Immune Netw [Internet]. 2019 Aug 27 [cited 2023 Dec 4];19(4). Available from: . [CrossRef]
- Pontarini E, Murray-Brown WJ, Croia C, Lucchesi D, Conway J, Rivellese F, et al. Unique expansion of IL-21+ Tfh and Tph cells under control of ICOS identifies Sjögren’s syndrome with ectopic germinal centres and MALT lymphoma. Ann Rheum Dis [Internet]. 2020 Dec 1 [cited 2023 Dec 4];79(12):1588–99. Available from: https://ard.bmj.com/content/79/12/1588.
- Szabó K, Papp G, Szántó A, Tarr T, Zeher M. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjögren’s syndrome and systemic lupus erythematosus. Clin Exp Immunol [Internet]. 2015 Dec 22 [cited 2023 Dec 4];183(1):76–89. Available from:. [CrossRef]
- Szabó K, Jámbor I, Szántó A, Horváth IF, Tarr T, Nakken B, et al. The Imbalance of Circulating Follicular T Helper Cell Subsets in Primary Sjögren’s Syndrome Associates With Serological Alterations and Abnormal B-Cell Distribution. Front Immunol. 2021 Mar 19;12:639975.
- Haque R, Kim Y, Park K, Jang H, Kim SY, Lee H, et al. Altered distributions in circulating follicular helper and follicular regulatory T cells accountable for imbalanced cytokine production in multiple sclerosis. Clin Exp Immunol [Internet]. 2021 Jun 17 [cited 2023 Dec 4];205(1):75–88. Available from:. [CrossRef]
- Guo J, Zhao C, Wu F, Tao L, Zhang C, Zhao D, et al. T follicular helper-like cells are involved in the pathogenesis of experimental autoimmune encephalomyelitis. Front Immunol. 2018 May 7;9(MAY):348539.
- Ribeiro F, Romão VC, Rosa S, Jesus K, Água-Doce A, Barreira SC, et al. Different antibody-associated autoimmune diseases have distinct patterns of T follicular cell dysregulation. Scientific Reports 2022 12:1 [Internet]. 2022 Oct 21 [cited 2023 Dec 2];12(1):1–8. Available from: https://www.nature.com/articles/s41598-022-21576-8.
- Lin J, Yu Y, Ma J, Ren C, Chen W. PD-1+CXCR5−CD4+T cells are correlated with the severity of systemic lupus erythematosus. Rheumatology [Internet]. 2019 Dec 1 [cited 2023 Dec 2];58(12):2188–92. Available from:. [CrossRef]
- Makiyama A, Chiba A, Noto D, Murayama G, Yamaji K, Tamura N, et al. Expanded circulating peripheral helper T cells in systemic lupus erythematosus: association with disease activity and B cell differentiation. Rheumatology [Internet]. 2019 Oct 1 [cited 2023 Dec 2];58(10):1861–9. Available from:. [CrossRef]
- Sagrero-Fabela N, Ortíz-Lazareno PC, Salazar-Camarena DC, Cruz A, Cerpa-Cruz S, Muñoz-Valle JF, et al. BAFFR expression in circulating T follicular helper (CD4+CXCR5+PD-1+) and T peripheral helper (CD4+CXCR5−PD-1+) cells in systemic lupus erythematosus. [Internet]. 2023 Jul 17 [cited 2023 Sep 25]; Available from: https://journals.sagepub.com/doi/10.1177/09612033231189804?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed.
- Luo Q, Fu P, Guo Y, Fu B, Guo Y, Huang Q, et al. Increased TIGIT+PD-1+CXCR5-CD4+T cells are associated with disease activity in rheumatoid arthritis. Exp Ther Med [Internet]. 2022 Aug 31 [cited 2023 Dec 3];24(4). Available from: /pmc/articles/PMC9468811/.
- Zhao L, Li Z, Zeng X, Xia C, Xu L, Xu Q, et al. Circulating CD4+ FoxP3– CXCR5– CXCR3+ PD-1hi cells are elevated in active rheumatoid arthritis and reflect the severity of the disease. Int J Rheum Dis [Internet]. 2021 Aug 1 [cited 2023 Dec 3];24(8):1032–9. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/1756-185X.14170.
- Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017 542:7639 [Internet]. 2017 Feb 2 [cited 2023 Dec 3];542(7639):110–4. Available from: https://www.nature.com/articles/nature20810.
- Sakuragi T, Yamada H, Haraguchi A, Kai K, Fukushi J ichi, Ikemura S, et al. Autoreactivity of Peripheral Helper T Cells in the Joints of Rheumatoid Arthritis. The Journal of Immunology [Internet]. 2021 May 1 [cited 2023 Dec 3];206(9):2045–51. Available from:. [CrossRef]
- Chen W, Yang F, Lin J. Tph Cells Expanded in Primary Sjögren’s Syndrome. Front Med (Lausanne). 2022 Jun 9;9:900349.
- Dupré A, Pascaud J, Rivière E, Paoletti A, Ly B, Mingueneau M, et al. Association between T follicular helper cells and T peripheral helper cells with B-cell biomarkers and disease activity in primary Sjögren syndrome. RMD Open [Internet]. 2021 Mar 1 [cited 2023 Dec 4];7(1):e001442. Available from: https://rmdopen.bmj.com/content/7/1/e001442.
- Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nature Reviews Immunology 2015 16:2 [Internet]. 2015 Dec 21 [cited 2023 Oct 18];16(2):90–101. Available from: https://www.nature.com/articles/nri.2015.1.
- Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Vol. 19, Nature Immunology. Nature Publishing Group; 2018. p. 665–73.
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. Journal of Experimental Medicine [Internet]. 2009 Dec 21 [cited 2023 Oct 18];206(13):3015–29. Available from: www.jem.org/cgi/doi/10.1084/jem.20090847.
- Amarnath S, Mangus CW, Wang JCM, Wei F, He A, Kapoor V, et al. The PDL1-PD1 axis converts human T H1 cells into regulatory T cells. Sci Transl Med [Internet]. 2011 Nov 30 [cited 2023 Oct 18];3(111). Available from: https://www.science.org/doi/10.1126/scitranslmed.3003130.
- Zhao L, Zhou X, Zhou X, Wang H, Gu L, Ke Y, et al. Low expressions of PD-L1 and CTLA-4 by induced CD4+CD25+ Foxp3+ Tregs in patients with SLE and their correlation with the disease activity. Cytokine. 2020 Sep 1;133:155119.
- Ferreira RC, Castro Dopico X, Oliveira JJ, Rainbow DB, Yang JH, Trzupek D, et al. Chronic Immune Activation in Systemic Lupus Erythematosus and the Autoimmune PTPN22 Trp620 Risk Allele Drive the Expansion of FOXP3+ Regulatory T Cells and PD-1 Expression. Front Immunol. 2019 Nov 8;10.
- Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol [Internet]. 2008 Oct 1 [cited 2023 Aug 2];181(7):4832–9. Available from: https://pubmed.ncbi.nlm.nih.gov/18802087/.
- Austin JW, Lu P, Majumder P, Ahmed R, Boss JM. STAT3, STAT4, NFATc1, and CTCF regulate PD-1 through multiple novel regulatory regions in murine T cells. J Immunol [Internet]. 2014 May 15 [cited 2023 Aug 3];192(10):4876–86. Available from: https://pubmed.ncbi.nlm.nih.gov/24711622/.
- Wang G, Tajima M, Honjo T, Ohta A. STAT5 interferes with PD-1 transcriptional activation and affects CD8+ T-cell sensitivity to PD-1-dependent immunoregulation. Int Immunol [Internet]. 2021 Nov 1 [cited 2023 Aug 3];33(11):563–72. Available from: https://pubmed.ncbi.nlm.nih.gov/34453440/.
- Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, et al. IFN-α directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol [Internet]. 2011 Mar 1 [cited 2023 Aug 3];186(5):2772–9. Available from: https://pubmed.ncbi.nlm.nih.gov/21263073/.
- Xiao G, Deng A, Liu H, Ge G, Liu X. Activator protein 1 suppresses antitumor T-cell function via the induction of programmed death 1. Proc Natl Acad Sci U S A [Internet]. 2012 Sep 18 [cited 2023 Aug 3];109(38):15419–24. Available from: https://pubmed.ncbi.nlm.nih.gov/22949674/.
- Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH, Perry CJ, et al. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity [Internet]. 2014 Nov 20 [cited 2023 Aug 3];41(5):802–14. Available from: https://pubmed.ncbi.nlm.nih.gov/25464856/.
- Chi Z, Lu Y, Yang Y, Li B, Lu P. Transcriptional and epigenetic regulation of PD-1 expression. Cell Mol Life Sci [Internet]. 2021 Apr 1 [cited 2023 Aug 2];78(7):3239–46. Available from: https://pubmed.ncbi.nlm.nih.gov/33738533/.
- Tsokos GC, Lo MS, Reis PC, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Vol. 12, Nature Reviews Rheumatology. Nature Publishing Group; 2016. p. 716–30.
- Okada M, Chikuma S, Kondo T, Hibino S, Machiyama H, Yokosuka T, et al. Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell Rep [Internet]. 2017 Aug 1 [cited 2023 Dec 11];20(5):1017–28. Available from: http://www.cell.com/article/S2211124717309932/fulltext.
- Pentcheva-Hoang T, Chen L, Pardoll DM, Allison JP. Programmed death-1 concentration at the immunological synapse is determined by ligand affinity and availability. Proc Natl Acad Sci U S A [Internet]. 2007 Nov 6 [cited 2023 Dec 11];104(45):17765–70. Available from: https://www.pnas.org/doi/abs/10.1073/pnas.0708767104.
- Mathieu M, Cotta-Grand N, Daudelin JF, Thébault P, Labrecque N. Notch signaling regulates PD-1 expression during CD8+ T-cell activation. Immunol Cell Biol [Internet]. 2013 Jan 1 [cited 2023 Sep 21];91(1):82–8. Available from: https://onlinelibrary.wiley.com/doi/full/10.1038/icb.2012.53.
- Lu P, Youngblood BA, Austin JJ, Mohammed AUR, Butler R, Ahmed R, et al. Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J Exp Med [Internet]. 2014 Mar [cited 2023 Aug 3];211(3):515–27. Available from: https://pubmed.ncbi.nlm.nih.gov/24590765/.
- Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MAA, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol [Internet]. 2011 May [cited 2023 Aug 3];12(7):663–71. Available from: https://pubmed.ncbi.nlm.nih.gov/21623380/.
- Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity [Internet]. 2009 Aug 21 [cited 2023 Aug 3];31(2):309–20. Available from: https://pubmed.ncbi.nlm.nih.gov/19664943/.
- Shankar EM, Che KF, Messmer D, Lifson JD, Larsson M. Expression of a broad array of negative costimulatory molecules and Blimp-1 in T cells following priming by HIV-1 pulsed dendritic cells. Molecular Medicine [Internet]. 2011 Mar 17 [cited 2023 Sep 30];17(3–4):229–40. Available from: https://molmed.biomedcentral.com/articles/10.2119/molmed.2010.00175.
- Che KF, Shankar EM, Muthu S, Zandi S, Sigvardsson M, Hinkula J, et al. p38 mitogen-activated protein kinase/signal transducer and activator of transcription-3 pathway signaling regulates expression of inhibitory molecules in T Cells activated by HIV-1-exposed dendritic cells. Molecular Medicine [Internet]. 2012 Aug 3 [cited 2023 Sep 30];18(8):1169–82. Available from: https://molmed.biomedcentral.com/articles/10.2119/molmed.2012.00103.
- Philips EA, Garcia-España A, Tocheva AS, Ahearn IM, Adam KR, Pan R, et al. The structural features that distinguish PD-L2 from PD-L1 emerged in placental mammals. Journal of Biological Chemistry [Internet]. 2020 Apr 3 [cited 2023 Dec 11];295(14):4372–80. Available from: http://www.jbc.org/article/S0021925817487047/fulltext.
- Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front Immunol [Internet]. 2016 [cited 2023 Aug 11];7(DEC). Available from: https://pubmed.ncbi.nlm.nih.gov/28018338/.
- Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, et al. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett [Internet]. 2002 Oct 21 [cited 2023 Aug 2];84(1):57–62. Available from: https://pubmed.ncbi.nlm.nih.gov/12161284/.
- Ibañez-Vega J, Vilchez C, Jimenez K, Guevara C, Burgos PI, Naves R. Cellular and molecular regulation of the programmed death-1/programmed death ligand system and its role in multiple sclerosis and other autoimmune diseases. J Autoimmun [Internet]. 2021 Sep 1 [cited 2023 Aug 11];123. Available from: https://pubmed.ncbi.nlm.nih.gov/34311143/.
- Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity [Internet]. 2018 Mar 20 [cited 2023 Aug 11];48(3):434–52. Available from: https://pubmed.ncbi.nlm.nih.gov/29562194/.
- Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol [Internet]. 2021 Dec 1 [cited 2023 Aug 11];14(1). Available from: https://pubmed.ncbi.nlm.nih.gov/33413496/.
- Nie X, Chen W, Zhu Y, Huang B, Yu W, Wu Z, et al. B7-DC (PD-L2) costimulation of CD4+ T-helper 1 response via RGMb. Cell Mol Immunol [Internet]. 2018 Oct 1 [cited 2023 Aug 11];15(10):888–97. Available from: https://pubmed.ncbi.nlm.nih.gov/28479601/.
- Muraro E, Romanò R, Fanetti G, Vaccher E, Turturici I, Lupato V, et al. Tissue and circulating PD-L2: moving from health and immune-mediated diseases to head and neck oncology. Crit Rev Oncol Hematol [Internet]. 2022 Jul 1 [cited 2023 Aug 11];175. Available from: https://pubmed.ncbi.nlm.nih.gov/35569724/.
- Mayoux M, Roller A, Pulko V, Sammicheli S, Chen S, Sum E, et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med [Internet]. 2020 Mar 11 [cited 2023 Aug 11];12(534). Available from: https://pubmed.ncbi.nlm.nih.gov/32161104/.
- Anton Van Der Merwe P, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) Binds Both CD28 and CTLA-4 with a Low Affinity and Very Fast Kinetics. J Exp Med [Internet]. 1997 [cited 2023 Sep 8];185(3):393–403. Available from: http://rupress.org/jem/article-pdf/185/3/393/1680533/5372.pdf.
- Butte MJ, Peña-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Mol Immunol. 2008 Aug 1;45(13):3567–72.
- Sugiura D, Maruhashi T, Okazaki IM, Shimizu K, Maeda TK, Takemoto T, et al. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science (1979) [Internet]. 2019 [cited 2023 Aug 8];364(6440):558–66. Available from: https://www.science.org.
- Zhao Y, Lee CK, Lin CH, Gassen RB, Xu X, Huang Z, et al. Article PD-L1:CD80 Cis-Heterodimer Triggers the Co-stimulatory Receptor CD28 While Repressing the Inhibitory PD-1 and CTLA-4 Pathways. [cited 2023 Aug 8]; Available from: . [CrossRef]
- Linnerbauer M, Beyer T, Nirschl L, Farrenkopf D, Lößlein L, Vandrey O, et al. PD-L1 positive astrocytes attenuate inflammatory functions of PD-1 positive microglia in models of autoimmune neuroinflammation. Nature Communications 2023 14:1 [Internet]. 2023 Sep 9 [cited 2023 Sep 22];14(1):1–17. Available from: https://www.nature.com/articles/s41467-023-40982-8.
- Raptopoulou AP, Bertsias G, Makrygiannakis D, Verginis P, Kritikos I, Tzardi M, et al. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum. 2010 Jul;62(7):1870–80.
- Sugiura D, Okazaki I mi, Maeda TK, Maruhashi T, Shimizu K, Arakaki R, et al. PD-1 agonism by anti-CD80 inhibits T cell activation and alleviates autoimmunity. Nat Immunol. 2022 Mar 1;23(3):399–410.
- Nielsen C, Ohm-Laursen L, Barington T, Husby S, Lillevang ST. Alternative splice variants of the human PD-1 gene. Cell Immunol. 2005 Jun 1;235(2):109–16.
- Gu D, Ao X, Yang Y, Chen Z, Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. Journal for ImmunoTherapy of Cancer 2018 6:1 [Internet]. 2018 Nov 27 [cited 2023 Sep 8];6(1):1–14. Available from: https://jitc.biomedcentral.com/articles/10.1186/s40425-018-0449-0.
- Király Z, Nagy E, Bokor L, Kovács A, Marschalkó M, Hidvégi B. The Possible Clinical Significance of a Decreased Serum Level of Soluble PD-L1 in Discoid Lupus Erythematosus, but Not in Subacute Cutaneous Lupus Erythematosus—A Pilot Study. Journal of Clinical Medicine 2023, Vol 12, Page 5648 [Internet]. 2023 Aug 30 [cited 2023 Oct 3];12(17):5648. Available from: https://www.mdpi.com/2077-0383/12/17/5648/htm.
- Hirahara S, Katsumata Y, Kawasumi H, Kawaguchi Y, Harigai M. Serum levels of soluble programmed cell death protein 1 and soluble programmed cell death protein ligand 2 are increased in systemic lupus erythematosus and associated with the disease activity. [Internet]. 2020 Apr 7 [cited 2023 Oct 3];29(7):686–96. Available from: https://journals.sagepub.com/doi/10.1177/0961203320916517?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed.
- Du Y, Nie L, Xu L, Wu X, Zhang S, Xue J. Serum levels of soluble programmed death-1 (sPD-1) and soluble programmed death ligand 1(sPD-L1) in systemic lupus erythematosus: Association with activity and severity. Scand J Immunol [Internet]. 2020 Jul 1 [cited 2023 Oct 3];92(1). Available from: https://pubmed.ncbi.nlm.nih.gov/32243638/.
- Xu L, Jiang L, Nie L, Zhang S, Liu L, Du Y, et al. Soluble programmed death molecule 1 (sPD-1) as a predictor of interstitial lung disease in rheumatoid arthritis. BMC Immunol [Internet]. 2021 Dec 1 [cited 2023 Oct 3];22(1):1–10. Available from: https://bmcimmunol.biomedcentral.com/articles/10.1186/s12865-021-00460-6.
- Greisen SR, Rasmussen TK, Stengaard-Pedersen K, Hetland ML, Horslev-Petersen K, Hvid M, et al. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand J Rheumatol [Internet]. 2014 [cited 2023 Oct 4];43(2):101–8. Available from: https://www.tandfonline.com/doi/abs/10.3109/03009742.2013.823517.
- Liu C, Jiang J, Gao L, Wang X, Hu X, Wu M, et al. Soluble PD-1 aggravates progression of collagen-induced arthritis through Th1 and Th17 pathways. Arthritis Res Ther [Internet]. 2015 Nov 25 [cited 2023 Oct 4];17(1):1–13. Available from: https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-015-0859-z.
- Chen Y, Wang Y, Xu L, Zhu W, Xu C, Xu M, et al. Influence of total glucosides of paeony on PD-1/PD-L1 expression in primary Sjögren’s syndrome. Int J Rheum Dis [Internet]. 2019 Feb 1 [cited 2023 Oct 4];22(2):200–6. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/1756-185X.13391.
- Qian S, Xu J, Zhao S, Yang P, Yang C. CMTM6: increased circulating level and up-regulated expression in labial salivary glands in patients with primary Sjogren’s syndrome. Clin Exp Immunol [Internet]. 2022 Jan 28 [cited 2023 Oct 4];207(1):65–71. Available from:. [CrossRef]
- Yoon T, Ahn SS, Jung SM, Song JJ, Park YB, Lee SW. Serum soluble programmed cell death protein 1 could predict the current activity and severity of antineutrophil cytoplasmic antibody-associated vasculitis: a monocentric prospective study. Clin Exp Rheumatol [Internet]. 2019 Mar 1 [cited 2023 Oct 4];37(2):116–21. Available from: https://www.clinexprheumatol.org/abstract.asp?a=13748.
- Bailly C, Thuru X, Quesnel B. Soluble programmed death ligand-1 (Spd-l1): A pool of circulating proteins implicated in health and diseases. Vol. 13, Cancers. MDPI; 2021.
- He XH, Xu LH, Liu Y. Identification of a novel splice variant of human PD-L1 mRNA encoding an isoform-lacking Igv-like domain. Acta Pharmacologica Sinica 2005 26:4 [Internet]. 2005 Apr [cited 2023 Oct 3];26(4):462–8. Available from: https://www.nature.com/articles/aps200570.
- He XH, Liu Y, Xu LH, Zeng YY. Cloning and Identification of Two Novel Splice Variants of Human PD-L2. Acta Biochim Biophys Sin (Shanghai) [Internet]. 2004 Apr 1 [cited 2023 Oct 3];36(4):284–9. Available from:. [CrossRef]
- Niu M, Liu Y, Yi M, Jiao D, Wu K. Biological Characteristics and Clinical Significance of Soluble PD-1/PD-L1 and Exosomal PD-L1 in Cancer. Front Immunol. 2022 Mar 21;13:827921.
- Bertsias GK, Nakou M, Choulaki C, Raptopoulou A, Papadimitraki E, Goulielmos G, et al. Genetic, immunologic, and immunohistochemical analysis of the programmed death 1/programmed death ligand 1 pathway in human systemic lupus erythematosus. Arthritis Rheum. 2009 Jan;60(1):207–18.
- Liu MF, Weng CT, Weng MY. Variable increased expression of program death-1 and program death-1 ligands on peripheral mononuclear cells is not impaired in patients with systemic lupus erythematosus. J Biomed Biotechnol. 2009;2009.
- Luo Q, Huang Z, Ye J, Deng Y, Fang L, Li X, et al. PD-L1-expressing neutrophils as a novel indicator to assess disease activity and severity of systemic lupus erythematosus. Arthritis Res Ther [Internet]. 2016 Feb 11 [cited 2023 Dec 2];18(1):1–11. Available from: https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-016-0942-0.
- Jia XY, Zhu Q qing, Wang YY, Lu Y, Li ZJ, Li BQ, et al. The role and clinical significance of programmed cell death- ligand 1 expressed on CD19+B-cells and subsets in systemic lupus erythematosus. Clinical Immunology. 2019 Jan 1;198:89–99.
- Zhu Q, Li Y, Zhang L, Wang M, Chen Z, Shi J, et al. Patients with systemic lupus erythematosus show increased proportions of CD19+CD20− B cells and secretion of related autoantibodies. Clin Rheumatol [Internet]. 2021 Jan 1 [cited 2023 Dec 2];40(1):151–65. Available from: https://link.springer.com/article/10.1007/s10067-020-05220-2.
- Rincon-Arevalo H, Wiedemann A, Stefanski AL, Lettau M, Szelinski F, Fuchs S, et al. Deep Phenotyping of CD11c+ B Cells in Systemic Autoimmunity and Controls. Front Immunol. 2021 Mar 12;12:635615.
- Shi H, Ye J, Teng J, Yin Y, Hu Q, Wu X, et al. Elevated serum autoantibodies against co-inhibitory PD-1 facilitate T cell proliferation and correlate with disease activity in new-onset systemic lupus erythematosus patients. Arthritis Res Ther [Internet]. 2017 Mar 9 [cited 2023 Dec 2];19(1):1–10. Available from: https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-017-1258-4.
- Tong M, Fang X, Yang J, Wu P, Guo Y, Sun J. Abnormal membrane-bound and soluble programmed death ligand 2 (PD-L2) expression in systemic lupus erythematosus is associated with disease activity. Immunol Lett. 2020 Nov 1;227:96–101.
- Xiong J, Yang J, Sun Y, Chen Y, Guo Y, Liu C, et al. Dysregulated PD-L2 is correlated with disease activity and inflammation in rheumatoid arthritis. Immunogenetics [Internet]. 2023 Oct 1 [cited 2023 Dec 4];75(5):425–31. Available from: https://link.springer.com/article/10.1007/s00251-023-01307-7.
- Guo Y, Walsh AM, Canavan M, Wechalekar MD, Cole S, Yin X, et al. Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PLoS One [Internet]. 2018 Feb 1 [cited 2023 Dec 4];13(2):e0192704. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0192704.
- Matsuda K, Miyoshi H, Hiraoka K, Hamada T, Yoshida S, Ishibashi Y, et al. Clinicopathological value of programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) expression in synovium of patients with rheumatoid arthritis. Clin Exp Med [Internet]. 2018 Nov 1 [cited 2023 Dec 4];18(4):487–94. Available from: https://link.springer.com/article/10.1007/s10238-018-0515-4.
- Moret FM, van der Wurff-Jacobs KMG, Bijlsma JWJ, Lafeber FPJG, van Roon JAG. Synovial T cell hyporesponsiveness to myeloid dendritic cells is reversed by preventing PD-1/PD-L1 interactions. Arthritis Res Ther [Internet]. 2014 Nov 30 [cited 2023 Dec 4];16(6):1–8. Available from: https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-014-0497-x.
- Wan B, Nie H, Liu A, Feng G, He D, Xu R, et al. Aberrant Regulation of Synovial T Cell Activation by Soluble Costimulatory Molecules in Rheumatoid Arthritis. The Journal of Immunology [Internet]. 2006 Dec 15 [cited 2023 Dec 4];177(12):8844–50. Available from:. [CrossRef]
- Greisen SR, Rasmussen TK, Stengaard-Pedersen K, Hetland ML, Horslev-Petersen K, Hvid M, et al. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand J Rheumatol [Internet]. 2014 [cited 2023 Dec 4];43(2):101–8. Available from: https://www.tandfonline.com/doi/abs/10.3109/03009742.2013.823517.
- Bommarito D, Hall C, Taams LS, Corrigall VM. Inflammatory cytokines compromise programmed cell death-1 (PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1. Clin Exp Immunol [Internet]. 2017 May 9 [cited 2023 Dec 4];188(3):455–66. Available from:. [CrossRef]
- Kobayashi M, Kawano S, Hatachi S, Kurimoto C, Okazaki T, Iwai Y, et al. Enhanced expression of programmed death-1 (PD-1)/PD-L1 in salivary glands of patients with Sjögren’s syndrome. J Rheumatol. 2005;32(11).
- Loureiro-Amig J, Franco-Jarav C, Perurena-Priet J, Palacio C, Martínez-Vall F, Soláns-Laqu R. Serum CXCL13, BAFF, IL-21 and IL-22 levels are related to disease activity and lymphocyte profile in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2021;39(6):S131–9.
- Nishikawa A, Suzuki K, Kassai Y, Gotou Y, Takiguchi M, Miyazaki T, et al. Identification of definitive serum biomarkers associated with disease activity in primary Sjögren’s syndrome. Arthritis Res Ther [Internet]. 2016 May 14 [cited 2023 Dec 5];18(1):1–10. Available from: https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-016-1006-1.
- Linnerbauer M, Beyer T, Nirschl L, Farrenkopf D, Lößlein L, Vandrey O, et al. PD-L1 positive astrocytes attenuate inflammatory functions of PD-1 positive microglia in models of autoimmune neuroinflammation. Nature Communications 2023 14:1 [Internet]. 2023 Sep 9 [cited 2023 Dec 7];14(1):1–17. Available from: https://www.nature.com/articles/s41467-023-40982-8.
- Trabattoni D, Saresella M, Pacei M, Marventano I, Mendozzi L, Rovaris M, et al. Costimulatory Pathways in Multiple Sclerosis: Distinctive Expression of PD-1 and PD-L1 in Patients with Different Patterns of Disease. The Journal of Immunology [Internet]. 2009 Oct 15 [cited 2023 Dec 7];183(8):4984–93. Available from:. [CrossRef]
- Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep [Internet]. 2017 May 9 [cited 2023 Sep 29];19(6):1189–201. Available from: http://www.cell.com/article/S2211124717305259/fulltext.
- Horita H, Law A, Hong S, Middleton K. Identifying Regulatory Posttranslational Modifications of PD-L1: A Focus on Monoubiquitinaton. Neoplasia (United States). 2017 Apr 1;19(4):346–53.
- Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nature Communications 2016 7:1 [Internet]. 2016 Aug 30 [cited 2023 Dec 10];7(1):1–11. Available from: https://www.nature.com/articles/ncomms12632.
- Yao H, Lan J, Li C, Shi H, Brosseau JP, Wang H, et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nature Biomedical Engineering 2019 3:4 [Internet]. 2019 Mar 25 [cited 2023 Dec 10];3(4):306–17. Available from: https://www.nature.com/articles/s41551-019-0375-6.
- Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nature Reviews Molecular Cell Biology 2007 8:1 [Internet]. 2007 Jan [cited 2023 Dec 10];8(1):74–84. Available from: https://www.nature.com/articles/nrm2084.
- Yu X, Li W, Young KH, Li Y. Posttranslational Modifications in PD-L1 Turnover and Function: From Cradle to Grave. Biomedicines 2021, Vol 9, Page 1702 [Internet]. 2021 Nov 16 [cited 2023 Dec 10];9(11):1702. Available from: https://www.mdpi.com/2227-9059/9/11/1702/htm.
- Burr ML, Sparbier CE, Chan YC, Williamson JC, Woods K, Beavis PA, et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 2017 549:7670 [Internet]. 2017 Aug 16 [cited 2023 Sep 29];549(7670):101–5. Available from: https://www.nature.com/articles/nature23643.
- Mezzadra R, Sun C, Jae LT, Gomez-Eerland R, De Vries E, Wu W, et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature 2017 549:7670 [Internet]. 2017 Aug 16 [cited 2023 Sep 29];549(7670):106–10. Available from: https://www.nature.com/articles/nature23669.
- Davis NA, Lareau CA, White BC, Pandey A, Wiley G, Montgomery CG, et al. Encore: Genetic Association Interaction Network Centrality Pipeline and Application to SLE Exome Data. Genet Epidemiol [Internet]. 2013 Sep 1 [cited 2023 Sep 29];37(6):614–21. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/gepi.21739.
- Zeisbrich M, Chevalier N, Sehnert B, Rizzi M, Venhoff N, Thiel J, et al. CMTM6-Deficient Monocytes in ANCA-Associated Vasculitis Fail to Present the Immune Checkpoint PD-L1. Front Immunol. 2021 May 24;12:673912.
- Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science [Internet]. 2017 Mar 31 [cited 2023 Aug 11];355(6332):1428–33. Available from: https://pubmed.ncbi.nlm.nih.gov/28280247/.
- Zamani MR, Aslani S, Salmaninejad A, Javan MR, Rezaei N. PD-1/PD-L and autoimmunity: A growing relationship. Cell Immunol [Internet]. 2016 Dec 1 [cited 2023 Aug 11];310:27–41. Available from: https://pubmed.ncbi.nlm.nih.gov/27660198/.
- Tocheva AS, Peled M, Strazza M, Adam KR, Lerrer S, Nayak S, et al. Quantitative phosphoproteomic analysis reveals involvement of PD-1 in multiple T cell functions. Journal of Biological Chemistry. 2020 Dec 25;295(52):18036–50.
- Strazza M, Bukhari S, Tocheva AS, Mor A. PD-1-induced proliferating T cells exhibit a distinct transcriptional signature. Immunology [Internet]. 2021 Nov 1 [cited 2023 Oct 10];164(3):555–68. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/imm.13388.
- Lerrer S, Tocheva AS, Bukhari S, Adam K, Mor A. PD-1-stimulated T cell subsets are transcriptionally and functionally distinct. iScience. 2021 Sep 24;24(9):103020.
- Haynes NM, Allen CDC, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in Follicular Th Cell Positioning and Appearance of a Programmed Cell Death Gene-1High Germinal Center-Associated Subpopulation. The Journal of Immunology [Internet]. 2007 Oct 15 [cited 2023 Oct 7];179(8):5099–108. Available from:. [CrossRef]
- Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010 Jun;11(6):535–42.
- Ding BB, Bi E, Chen H, Yu JJ, Ye BH. IL-21 and CD40L Synergistically Promote Plasma Cell Differentiation through Upregulation of Blimp-1 in Human B Cells. The Journal of Immunology [Internet]. 2013 Feb 15 [cited 2023 Oct 7];190(4):1827–36. Available from:. [CrossRef]
- Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science (1979). 2012 Apr 27;336(6080):485–9.
- Shi J, Hou S, Fang Q, Liu X, Liu X, Correspondence HQ, et al. PD-1 Controls Follicular T Helper Cell Positioning and Function Article PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity [Internet]. 2018 [cited 2023 Sep 8];49:264–74. Available from: . [CrossRef]
- Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011 Jun 24;34(6):932–46.
- Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, et al. The Common γ-Chain Cytokines IL-2, IL-7, IL-15, and IL-21 Induce the Expression of Programmed Death-1 and Its Ligands. The Journal of Immunology [Internet]. 2008 Nov 15 [cited 2023 Oct 7];181(10):6738–46. Available from:. [CrossRef]
- Haymaker C, Wu R, Bernatchez C, Radvanyi L. PD-1 and BTLA and CD8+ T-cell “exhaustion” in cancer: “Exercising” an alternative viewpoint. Oncoimmunology [Internet]. 2012 Aug 8 [cited 2024 May 14];1(5):735. Available from: /pmc/articles/PMC3429577/.
- Dolff S, Abdulahad WH, Westra J, Doornbos-van der Meer B, Limburg PC, Kallenberg CGM, et al. Increase in IL-21 producing T-cells in patients with systemic lupus erythematosus. Arthritis Res Ther [Internet]. 2011 Sep 29 [cited 2024 Jan 9];13(5):1–10. Available from: https://arthritis-research.biomedcentral.com/articles/10.1186/ar3474.
- Paluch C, Santos AM, Anzilotti C, Cornall RJ, Davis SJ. Immune checkpoints as therapeutic targets in autoimmunity. Vol. 9, Frontiers in Immunology. Frontiers Media S.A.; 2018.
- Liao W, Zheng H, Wu S, Zhang Y, Wang W, Zhang Z, et al. The Systemic Activation of Programmed Death 1-PD-L1 Axis Protects Systemic Lupus Erythematosus Model from Nephritis. Am J Nephrol. 2017 Nov 1;46(5):371–9.
- Bryan CM, Rocklin GJ, Bick MJ, Ford A, Majri-Morrison S, Kroll A V, et al. Computational design of a synthetic PD-1 agonist. Proc Natl Acad Sci U S A [Internet]. 2021; Available from: . [CrossRef]
- Curnock AP, Bossi G, Kumaran J, Bawden LJ, Figueiredo R, Tawar R, et al. Cell-targeted PD-1 agonists that mimic PD-L1 are potent T cell inhibitors. JCI Insight. 2021 Oct 22;6(20).
- Guo Q, Chen C, Wu Z, Zhang W, Wang L, Yu J, et al. Engineered PD-1/TIGIT dual-activating cell-membrane nanoparticles with dexamethasone act synergistically to shape the effector T cell/Treg balance and alleviate systemic lupus erythematosus. Biomaterials. 2022 Jun 1;285.
- Khramova T, Beduleva L, Sidorov A, Terentiev A, Menshikov I. Regulatory Rheumatoid Factor is Specific to PD-1 and Uses PD-1 Pathway to Control CD4 T Lymphocytes. Immunol Invest. 2023;
- Wang B, Chen C, Liu X, Zhou S, Xu T, Wu M. The effect of combining PD-1 agonist and low-dose Interleukin-2 on treating systemic lupus erythematosus. Vol. 14, Frontiers in Immunology. Frontiers Media S.A.; 2023.
| Disease | Evaluation | Finding | Reference |
|---|---|---|---|
| SLE | Tissue expression | PD-1 and PD-L1 were identified in the kidney of lupus nephritis patients. | Bertsias, George K et al. 2009 [122] |
| Cell expression | Increased percentages of PD-1-expressing CD3+ T cells and PD-1-expressing CD19+ B cells in SLE. High expression of PD-L1-expressing CD19+B cells and PD-L2-expressing CD14+ monocytes. |
Liu, Ming-Fei et al. 2009 [123] |
|
| Elevated frequency of PD-L1-expressing neutrophils in SLE. This percentage decreased after receiving a 15-day regular treatment with corticosteroids and immunosuppressive drugs. | Luo, Qing et al. 2016 [124] | ||
| PD-L1 was significantly higher in CD19+ cells of SLE patients with active disease and LN. The expression of PD-L1 was increased in double-negative B cells (DN) and plasma cells (PC). The percentage of CD19+PD-L1+ cells correlated with the disease activity index and the T follicular helper cells (Tfh) frequency. |
Jia, Xiao-Yun et al. 2019 [125] |
||
| Higher frequency of PD-1, PD-L1, PD-L2, and CD86 in CD19+CD20- B than in CD19+CD20+ B cells. | Zhu, Qingqing et al. 2021 [126] |
||
| Higher expression of PD-1 and PD-L1 in CD11c+ B cells. | Rincon-Arevalo, Hector et al. 2021 [127] | ||
| Soluble levels | Increased serum levels of anti-PD-1 IgG in new-onset SLE patients. | Shi, Hui et al. 2017 [128] | |
| Higher serum levels of sPD-1 and sPD-L1. | Du, Yan et al. 2020 [111] | ||
| Higher serum levels of sPD-1 and sPD-L2 in SLE patients with high disease activity. sPD-L1 was not elevated in SLE patients. |
Hirahara, Shinya et al. 2020 [110] | ||
| Higher expression of membrane-bound PD-L2 on monocytes. Lower serum levels of sPD-L2. |
Tong, Min et al. 2020 [129] | ||
| RA | Tissue expression | PD-L2 was highly expressed on macrophages in the synovial tissue. | Xiong, Jian et al. 2023 [130] |
| Low PD-L1 expression in RA synovial tissue | Guo, Yanxia et al. 2018 [131] | ||
| PD-1 expression on synovium infiltrating lymphocytes. PD-L1 expression on synovial lining cells. |
Matsuda, Kotaro et al. 2018 [132] | ||
| Cell expression | Increased percentages of PD-L2-expressing CD14+ monocytes. | Xiong, Jian et al. 2023 [130] | |
| Increased PD-L1 expression on synovial fluid mDCs compared with peripheral blood mDCs | Moret, Frederique M et al. 2014 [133] | ||
| Soluble levels | Lower sPD-L2 in the serum of RA patients. | Xiong, Jian et al. 2023 [130] | |
| sPD-1 levels are increased in ACPA+ but not ACPA- early RA | Guo, Yanxia et al. 2018 [131] | ||
| Elevated sPD-1 and sPD-L1 levels in serum and synovial fluid of RA | Wan, Bing et al. 2006 [134] | ||
| Higher sPD-1 in early and chronic RA | Greisen, S R et al. 2014 [135] | ||
| Elevated sPD-1 in RA serum | Bommarito, D et al. 2017 [ 136] | ||
| pSS | Tissue expression |
PD-1 expression on infiltrating lymphocytes in the salivary gland from 52% of SS patients. PD-L1 expression on ductal and acinar epithelial cells from 68% of SS patients. |
Kobayashi, Masaya et al. 2005 [137] |
| PD-1 and PD-L1 expression in labial glands were higher than in non-pSS controls | Qian, Sirui et al. 2022 [116] | ||
| Cell expression | Increased expression of PD-L1 in CD11c+ B cells of pSS patients | Rincon-Arevalo, Hector et al. 2021 [127] | |
| Serum levels | Lower sPD-L2 serum levels in pSS patients | Loureiro-Amigo, Jose et al. 2021 [138] | |
| Elevated sPD-L2 serum levels in pSS patients | Nishikawa, Ayumi et al. 2016 [139] | ||
| Elevated sPD-1 and sPD-L1 serum levels in pSS patients | Qian, Sirui et al. 2022 [116] | ||
| MS | Tissue expression | Astrocytes in white matter lesions from MS patients upregulate PD-L1 in response to aryl hydrocarbon receptor and interferon signaling | Linnerbauer, Mathias et al. 2023 [140] |
| Cell expression | Frequency of PD-L1-expressing CD19+ B cells, and PD-L1+/IL-10+CD14+ monocytes are higher in stable multiple sclerosis patients compared to acute MS (AMS) patients | Trabattoni, Daria et al. 2009 [141] | |
| Serum levels | No information |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





