Submitted:
14 June 2024
Posted:
17 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Characteristics of Study Participants and Their Tumors
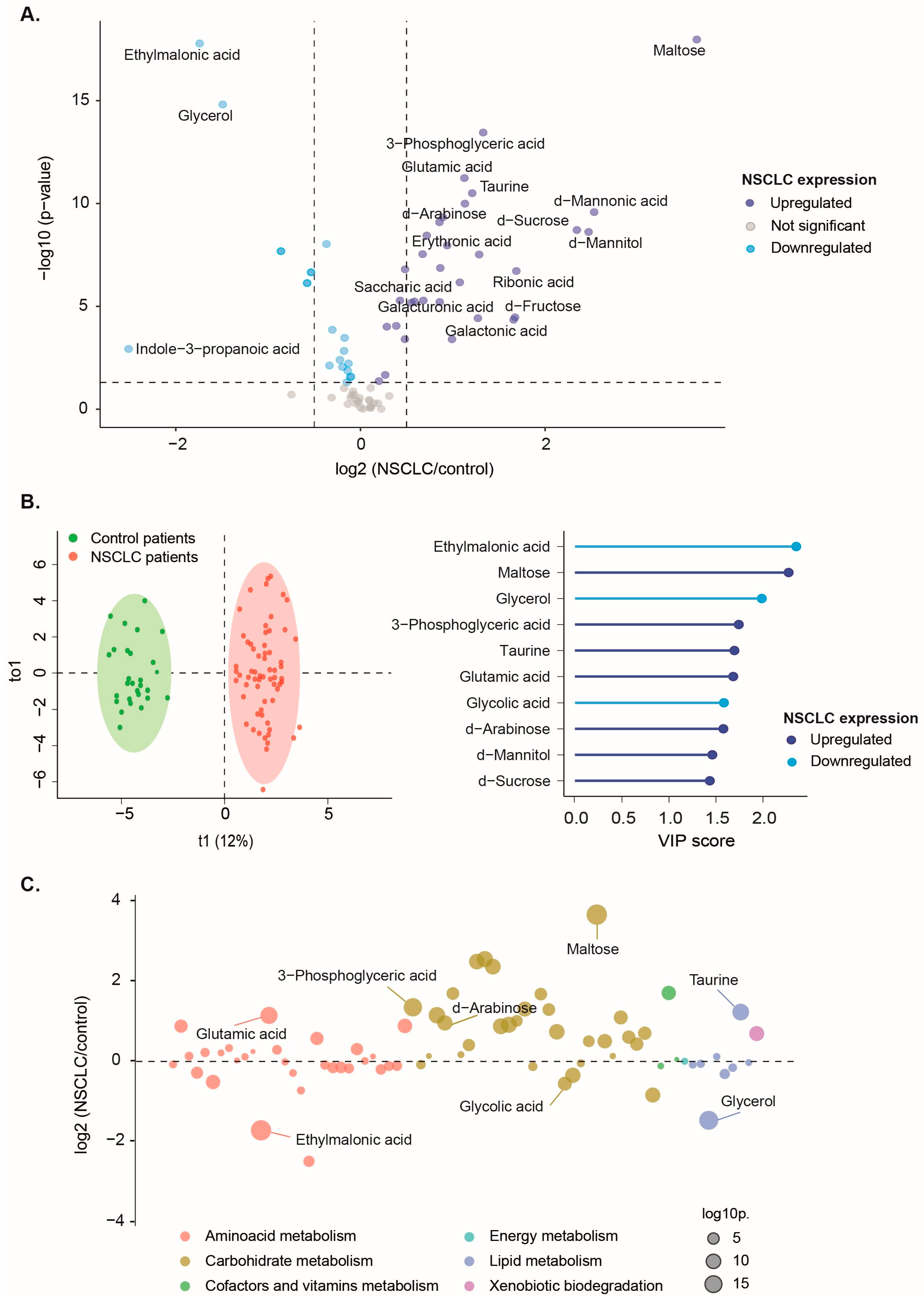
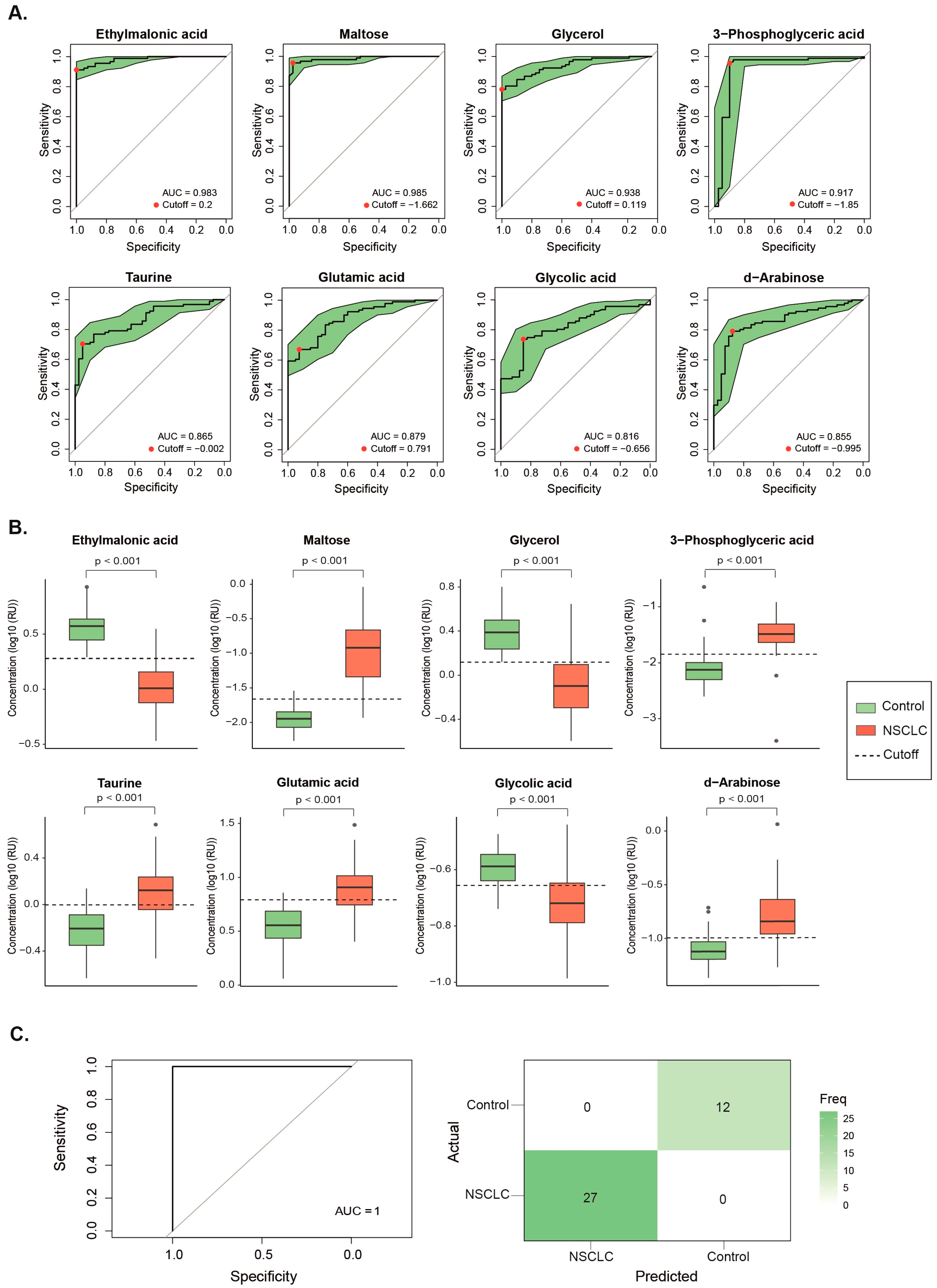
3.2. Baseline Metabolite Levels Effectively Differentiate NSCLC Patients from the Control Group
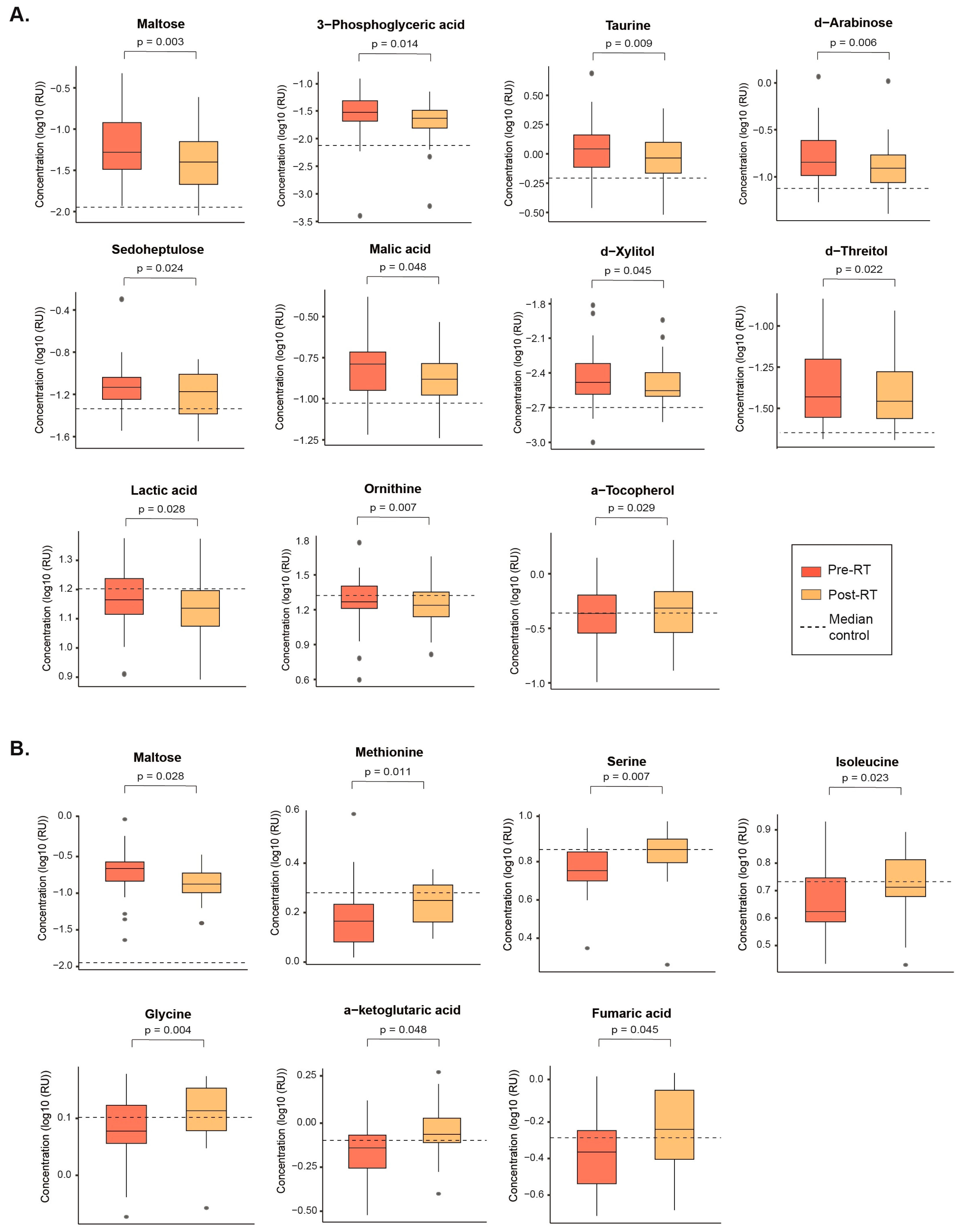
3.3. SBRT and CFRT Induce Distinct Changes in the Plasma Metabolome
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. /: 209–249. https. [CrossRef]
- Woodard, G.A.; Jones, K.D.; Jablons, D.M. Lung cancer staging and prognosis. Cancer Treat. Res. /: 47–75. https. [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A. ; Bolejack, V; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. /: 39–51. https. [CrossRef]
- Rodríguez De Dios, N.; Navarro-Martin, A.; Cigarral, C.; Chicas-Sett, R.; García, R.; Garcia, V.; Gonzalez, J.A.; Gonzalo, S.; Murcia-Mejía, M.; Robaina, R.; Sotoca, A.; Vallejo, C.; Valtueña, G.; Couñago, F. GOECP/SEOR radiotheraphy guidelines for non-small-cell lung cancer. World J. Clin. Oncol. 2022, 13, 237–266. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research, Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. 5. National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. [CrossRef]
- Jantus-Lewintre, E.; Usó, M.; Sanmartín, E.; Camps, C. Update on biomarkers for the detection of lung cancer. Lung Cancer (Auckl) 2012, 3, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kannampuzha, S.; Mukherjee, A.G.; Wanjari, U.R.; Gopalakrishnan, A.V.; Murali, R.; Namachivayam, A.; Renu, K.; Dey, A.; Vellingiri, B.; Madhyastha, H.; Ganesan, R. A systematic role of metabolomics, metabolic pathways, and chemical metabolism in lung cancer. Vaccines (Basel) 2023, 11, 381. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, K.; Zhang, X. Next-generation metabolomics in lung cancer diagnosis, treatment and precision medicine: mini review. Oncotarget 2017, 8, 115774–115786. [Google Scholar] [CrossRef] [PubMed]
- Puchades-Carrasco, L.; Jantus-Lewintre, E.; Pérez-Rambla, C.; García-García, F.; Lucas, R.; Calabuig, S.; Blasco, A.; Dopazo, J.; Camps, C.; Pineda-Lucena, A. Serum metabolomic profiling facilitates the non-invasive identification of metabolic biomarkers associated with the onset and progression of non-small cell lung cancer. Oncotarget 2016, 7, 12904–12916. [Google Scholar] [CrossRef]
- Majem, B.; Nadal, E.; Muñoz-Pinedo, C. Exploiting metabolic vulnerabilities of non small cell lung carcinoma. Semin. Cell. Dev. Biol. 2020, 98, 54–62. [Google Scholar] [CrossRef]
- Holmes, E.; Wilson, I.D.; Nicholson, J.K. Metabolic phenotyping in health and disease. Cell 2008, 134, 714–717. [Google Scholar] [CrossRef]
- Puchades-Carrasco, L.; Pineda-Lucena, A. Metabolomics in pharmaceutical research and development. Curr. Opin. Biotechnol. 2015, 35, 73–77. [Google Scholar] [CrossRef]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; Mazzone, P.J.; Montuenga, L.M. Biomarkers in lung cancer screening: Achievements, promises, and challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef]
- Vanhove, K.; Derveaux, E.; Mesotten, L.; Thomeer, M.; Criel, M.; Mariën, H.; Adriaensens, P. Unraveling the rewired metabolism in lung cancer using quantitative NMR metabolomics. Int. J. Mol. Sci. 2022, 23, 5602. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wei, J.; Sun, R.; Jiang, W.; Chen, Y.; Shao, Y.; Gu, W. Stereotactic body radiation therapy versus conventional radiation therapy in pain relief for bone metastases: A systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 909–921. [Google Scholar] [CrossRef]
- Wirsdörfer, F.; de Leve, S.; Jendrossek, V. Combining radiotherapy and immunotherapy in lung cancer: Can we expect limitations due to altered normal tissue toxicity? Int. J. Mol. Sci. 2018, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Fort-Gallifa, I.; García-Heredia, A.; Hernández-Aguilera, A.; Simó, J.M.; Sepúlveda, J.; Martín-Paredero, V.; Camps, J.; Joven, J. Biochemical indices of oxidative stress and inflammation in the evaluation of peripheral artery disease. Free Radic. Biol. Med. 2016, 97, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Caterino, M.; Ruoppolo, M. Targeted metabolomics. In Metabolomics perspectives: From theory to practical application; Troisy, J., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 219–236. [Google Scholar] [CrossRef]
- Bräkling, S.; Hinterleitner, C.; Cappellin, L.; Vetter, M.; Mayer, I.; Benter, T.; Klee, S.; Kersten, H. Gas chromatography coupled to time-of-flight mass spectrometry using parallel electron and chemical ionization with permeation tube facilitated reagent ion control for material emission analysis. Rapid Commun. Mass Spectrom. 2023, 37, e9461. [Google Scholar] [CrossRef]
- Hofman, D.L.; van Buul, V.J.; Brouns, F.J. Nutrition, health, and regulatory aspects of digestible maltodextrins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Xie, Y.; Meng, W.Y.; Li, R.Z.; Wang, Y.W.; Qian, X.; Chan, C.; Yu, Z.F.; Fan, X.X.; Pan, H.D.; Xie, C.; Wu, Q.B.; Yan, P.Y.; Liu, L.; Tang, Y.J.; Yao, X.J.; Wang, M.F.; Leung, E.L. Early lung cancer diagnostic biomarker discovery by machine learning methods. Transl. Oncol. 2021, 14, 100907. [Google Scholar] [CrossRef]
- Tu, S.; Zhang, X.; Wan, H.; Xia, Y.; Liu, Z.; Yang, X.; Wan, F. Effect of taurine on cell proliferation and apoptosis human lung cancer A549 cells. Oncology Lett. 2018, 15, 5473–5480. [Google Scholar] [CrossRef]
- Wang, L.; Peng, W.; Wu, T.; Deng, P.; Zhao, Y.L. Increased glutamine anabolism sensitizes non-small cell lung cancer to gefitinib treatment. Cell. Death Discov. /: 24. https. [CrossRef]
- Vanhove, K.; Giesen, P.; Owokotomo, O.E.; Mesotten, L.; Louis, E.; Shkedy, Z.; Thomeer, M.; Adriaensens, P. The plasma glutamate concentration as a complementary tool to differentiate benign PET-positive lung lesions from lung cancer. BMC Cancer 2018, 18, 868. [Google Scholar] [CrossRef] [PubMed]
- Fahrmann, J.F.; Kim, K.; DeFelice, B.C.; Taylor, S.L.; Gandara, D.R.; Yoneda, K.Y.; Cooke, D.T.; Fiehn, O.; Kelly, K.; Miyamoto, S. Investigation of metabolomic blood biomarkers for detection of adenocarcinoma lung cancer. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.; Grapov, D.; Fahrmann, J.; DeFelice, B.; Rom, W.; Pass, H.; Kim, K.; Nguyen, U.; Taylor, S.L.; Gandara, D.R.; Kelly, K.; Fiehn, O.; Miyamoto, S. Metabolomic markers of altered nucleotide metabolism in early stage adenocarcinoma. Cancer Prev. Res. (Phila) 2015, 8, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Cao, Y.; Wang, Y.; Hu, C.; Hu, A.; Ruan, L.; Bo, Q.; Liu, Q.; Chen, W.; Tao, F.; Ren, M.; Ge, Y.; Chen, A.; Li, L. Plasma and tissue free amino acid profiles and their concentration correlation in patients with lung cancer. Asia Pac. J. Clin Nutr. [CrossRef]
- Lachaux, C.; Frazao, C.J.R.; Krauβer, F.; Morin, N.; Walther, T.; François, J.M. A new synthetic pathway for the bioproduction of glycolic acid from lignocellulosic sugars aimed at maximal carbon conservation. Front. Bioeng. Biotechnol. 2019, 7, 359. [Google Scholar] [CrossRef] [PubMed]
- Erez, A.; Shchelochkov, O.A.; Plon, S.E.; Scaglia, F.; Lee, B. Insights into the pathogenesis and treatment of cancer from inborn errors of metabolism. Am. J. Hum. Genet. 2011, 88, 402–421. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.A.; Wu, Q.; Chen, Z.; Zhang, W.; Zhou, Y.; Mao, K.; Li, J.; Li, Y.; Chen, J.; Huang, Y.; Huang, Y. High-resolution metabolomic biomarkers for lung cancer diagnosis and prognosis. Sci. Rep. 2021, 11, 11805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.; Qin, L.; Liu, Y.; Huang, S.; Dai, L.; Tao, J.; Pan, J.; Su, C.; Zhang, Y. Plasma metabolomics for the assessment of the progression of non-small cell lung cancer. Int. J. Biol. Markers. 2023, 38, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, J.; Ahmed, R.; Huang, G.; Reid, J.; Mandal, R.; Maksymuik, A.; Sitar, D.S.; Tappia, P.S.; Ramjiawan, B.; Joubert, P.; Russo, A.; Rolfo, C.D.; Wishart, D.S. A high-performing plasma metabolite panel for early-stage lung cancer detection. Cancers (Basel) 2020, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tomàs, E.; Arguís, M.; Arenas, M.; Fernández-Arroyo, S.; Murcia, M.; Sabater, S.; Torres, L.; Baiges-Gayà, G.; Hernández-Aguilera, A.; Camps, J.; Joven, J. Alterations in plasma concentrations of energy-balance-related metabolites in patients with lung, or head & neck, cancers: Effects of radiotherapy. J. Proteomics 2020, 213, 103605. [Google Scholar] [CrossRef]
- Arenas, M.; Rodríguez, E.; García-Heredia, A.; Fernández-Arroyo, S.; Sabater, S.; Robaina, R.; Gascón, M.; Rodríguez-Pla, M.; Cabré, N.; Luciano-Mateo, F.; Hernández-Aguilera, A.; Fort-Gallifa, I.; Camps, J.; Joven, J. Metabolite normalization with local radiotherapy following breast tumor resection. PLoS One 2018, 13, e0207474. [Google Scholar] [CrossRef]
- Abla, H.; Sollazzo, M.; Gasparre, G.; Iommarini, L.; Porcelli, A.M. The multifaceted contribution of α-ketoglutarate to tumor progression: An opportunity to exploit? Semin. Cell. Dev. Biol. 2020, 98, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Soga, T.; Pollard, P.J.; Adam, J. The emerging role of fumarate as an oncometabolite. Front. Oncol. 2012, 2, 85. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M.; Keller, M.A.; Breitenbach, M.; Brindle, K.M.; Rabinowitz, J.D.; Ralser, M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Kurhaluk, N. Tricarboxylic acid cycle intermediates and individual ageing. Biomolecules 2024, 14, 260. [Google Scholar] [CrossRef] [PubMed]
- Tomar, M.S.; Kumar, A.; Shrivastava, A. Mitochondrial metabolism as a dynamic regulatory hub to malignant transformation and anti-cancer drug resistance. Biochem. Biophys. Res. Commun. 2024, 694, 149382. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Xiang, S.; Ge, Y.; Zhang, Y.; Bao, X.; Su, X.; Shi, L.; Xia, Y.; Han, H.; Ying, J.; Lai, S.; Chen, J.; Zhu, X. L-arabinose exerts probiotic functions by improving gut microbiota and metabolism in vivo and in vitro. J. Funct. Foods 2024, 113, 106047. [Google Scholar] [CrossRef]
- Li, Z.; Cui, J. Targeting the lactic acid metabolic pathway for antitumor therapy. Mol. Ther. Oncolytics 2023, 31, 100740. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Kinj, R.; Bourhis, J. How stereotactic radiotherapy changed the landscape in cancer care. Cancers (Basel) 2023, 15, 1734. [Google Scholar] [CrossRef]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Gkika, E.; Firat, E.; Adebahr, S.; Graf, E.; Popp, I.; Radicioni, G.; Lo, S.S.; Nestle, U.; Nicolay, N.H.; Niedermann, G.; Duda, D.G.; Grosu, A.L. Systemic immune modulation by stereotactic radiotherapy in early-stage lung cancer. NPJ Precis. Oncol. 2023, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.E.; Adashek, J.J.; Lin, S.H.; Subbiah, V. The abscopal effect in patients with cancer receiving immunotherapy. Med. 2023, 4, 233–244. [Google Scholar] [CrossRef]
- Swamy, K. Stereotactic body radiotherapy immunological planning-A review with a proposed theoretical model. Front. Oncol. 2022, 12, 729250. [Google Scholar] [CrossRef] [PubMed]
| Control group | All NSCLC patients | CFRT | SBRT | p-value | |
|---|---|---|---|---|---|
| Cancer characteristics | n = 99 | n = 38 | n = 61 | ||
| Patient characteristics | n= 40 | n= 91 | n= 38 | n= 53 | |
| Age (years) | 69.50 [65.0-74.0] | 73.00 [66.5-79.0] | 71.50 [66.8-77.8] | 74.00 [67.0-79.0] | * |
| Women | 12 (30.0) | 39 (42.9) | 8 (21.1) | 31 (58.5) | # |
| Smoking habit | 9 (22.5) | 69 (76.7) | 34 (91.9) | 35 (66.0) | * # |
| Alcohol habit | 20 (50.0) | 20 (22.0) | 11 (28.9) | 9 (17.0) | * |
| Diabetes mellitus | 3 (7.5) | 27 (30.0) | 11 (29.7) | 16 (30.2) | * |
| Arterial hypertension | 13 (32.5) | 56 (62.2) | 21 (56.8) | 35 (66.0) | * |
| Dyslipidemia | 6 (15.0) | 43 (47.3) | 19 (50.0) | 24 (45.3) | * |
| Non-cancer pulmonary disease | NA | 58 (63.7) | 31 (81.6) | 27 (50.9) | NS |
| Cardiovascular disease | NA | 34 (37.4) | 20 (52.6) | 14 (26.4) | # |
| Histology | NS | ||||
| Adenocarcinoma | NA | 44 (44.4) | 15 (39.5) | 29 (47.5) | |
| Squamous cell carcinoma | NA | 42 (42.4) | 21 (55.3) | 21 (34.4) | |
| Others | NA | 8 (8.1) | 2 (5.3) | 6 (9.8) | |
| Not determined | NA | 5 (5.1) | - | 5 (8.2) | |
| Stage | # | ||||
| Ia | NA | 60 (60.6) | 13 (34.2) | 47 (77.0) | |
| Ib | NA | 15 (15.2) | 6 (15.8) | 9 (14.8) | |
| IIa | NA | 1 (1.0) | 1 (2.6) | 0 (0.0) | |
| IIb | NA | 8 (8.1) | 3 (7.9) | 5 (8.2) | |
| IIIa | NA | 5 (5.1) | 5 (13.2) | 0 (0.0) | |
| IIIb | NA | 8 (8.1) | 8 (21.1) | 0 (0.0) | |
| IIIc | NA | 1 (1.0) | 1 (2.6) | 0 (0.0) | |
| IV | NA | 1 (1.0) | 1 (2.6) | 0 (0.0) | |
| Tumor location | NS | ||||
| RUL | NA | 27 (27.6) | 10 (26.3) | 17 (28.3) | |
| RML | NA | 5 (5.1) | 2 (5.3) | 3 (5.0) | |
| RLL | NA | 21 (21.4) | 11 (28.9) | 10 (16.7) | |
| LUL | NA | 27 (27.6) | 11 (28.9) | 16 (26.7) | |
| LLL | NA | 18 (18.4) | 4 (10.5) | 14 (23.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





